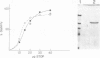Abstract
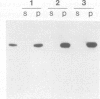
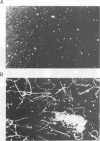
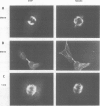
STOP (Stable Tubule Only Polypeptide) is a neuronal microtubule associated protein of 145 kd that stabilizes microtubules indefinitely to in vitro disassembly induced by cold temperature, millimolar calcium or by drugs. We have produced monoclonal antibodies against STOP. Using an antibody affinity column, we have produced a homogeneously pure 145 kd protein which has STOP activity as defined by its ability to induce cold stability and resistance to dilution induced disassembly in microtubules in vitro. Western blot analysis, using a specific monoclonal antibody, demonstrates that STOP recycles quantitatively with microtubules through three assembly cycles in vitro. Immunofluorescence analysis demonstrates that STOP is specifically associated with microtubules of mitotic spindles in neuronal cells. Further, and most interestingly, STOP at physiological temperature appears to be preferentially distributed on the distinct microtubule subpopulations that display cold stability; kinetochore-to-pole microtubules and telophase midbody microtubules. The observed distribution suggests that STOP induces the observed cold stability of these microtubule subpopulations in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asnes C. F., Wilson L. Isolation of bovine brain microtubule protein without glycerol: polymerization kinetics change during purification cycles. Anal Biochem. 1979 Sep 15;98(1):64–73. doi: 10.1016/0003-2697(79)90706-1. [DOI] [PubMed] [Google Scholar]
- Baas P. W., Heidemann S. R. Microtubule reassembly from nucleating fragments during the regrowth of amputated neurites. J Cell Biol. 1986 Sep;103(3):917–927. doi: 10.1083/jcb.103.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke O., Forer A. Evidence for four classes of microtubules in individual cells. J Cell Sci. 1967 Jun;2(2):169–192. doi: 10.1242/jcs.2.2.169. [DOI] [PubMed] [Google Scholar]
- Black M. M., Cochran J. M., Kurdyla J. T. Solubility properties of neuronal tubulin: evidence for labile and stable microtubules. Brain Res. 1984 Mar 19;295(2):255–263. doi: 10.1016/0006-8993(84)90974-0. [DOI] [PubMed] [Google Scholar]
- Bray D., Bunge M. B. Serial analysis of microtubules in cultured rat sensory axons. J Neurocytol. 1981 Aug;10(4):589–605. doi: 10.1007/BF01262592. [DOI] [PubMed] [Google Scholar]
- Brinkley B. R., Cartwright J., Jr Cold-labile and cold-stable microtubules in the mitotic spindle of mammalian cells. Ann N Y Acad Sci. 1975 Jun 30;253:428–439. doi: 10.1111/j.1749-6632.1975.tb19218.x. [DOI] [PubMed] [Google Scholar]
- Deery W. J., Means A. R., Brinkley B. R. Calmodulin-microtubule association in cultured mammalian cells. J Cell Biol. 1984 Mar;98(3):904–910. doi: 10.1083/jcb.98.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinsmore J. H., Sloboda R. D. Calcium and calmodulin-dependent phosphorylation of a 62 kd protein induces microtubule depolymerization in sea urchin mitotic apparatuses. Cell. 1988 Jun 3;53(5):769–780. doi: 10.1016/0092-8674(88)90094-3. [DOI] [PubMed] [Google Scholar]
- Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70(A):419–439. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- Garel J. R., Job D., Margolis R. L. Model of anaphase chromosome movement based on polymer-guided diffusion. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3599–3603. doi: 10.1073/pnas.84.11.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M. L., Margulies D. H., Scharff M. D. A simple method for polyethylene glycol-promoted hybridization of mouse myeloma cells. Somatic Cell Genet. 1977 Mar;3(2):231–236. doi: 10.1007/BF01551818. [DOI] [PubMed] [Google Scholar]
- Gundersen G. G., Khawaja S., Bulinski J. C. Postpolymerization detyrosination of alpha-tubulin: a mechanism for subcellular differentiation of microtubules. J Cell Biol. 1987 Jul;105(1):251–264. doi: 10.1083/jcb.105.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidemann S. R., Hamborg M. A., Thomas S. J., Song B., Lindley S., Chu D. Spatial organization of axonal microtubules. J Cell Biol. 1984 Oct;99(4 Pt 1):1289–1295. doi: 10.1083/jcb.99.4.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidemann S. R., Joshi H. C., Schechter A., Fletcher J. R., Bothwell M. Synergistic effects of cyclic AMP and nerve growth factor on neurite outgrowth and microtubule stability of PC12 cells. J Cell Biol. 1985 Mar;100(3):916–927. doi: 10.1083/jcb.100.3.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job D., Fischer E. H., Margolis R. L. Rapid disassembly of cold-stable microtubules by calmodulin. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4679–4682. doi: 10.1073/pnas.78.8.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job D., Margolis R. L. Isolation from bovine brain of a superstable microtubule subpopulation with microtubule seeding activity. Biochemistry. 1984 Jun 19;23(13):3025–3031. doi: 10.1021/bi00308a028. [DOI] [PubMed] [Google Scholar]
- Job D., Pabion M., Margolis R. L. Generation of microtubule stability subclasses by microtubule-associated proteins: implications for the microtubule "dynamic instability" model. J Cell Biol. 1985 Nov;101(5 Pt 1):1680–1689. doi: 10.1083/jcb.101.5.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job D., Rauch C. T., Fischer E. H., Margolis R. L. Recycling of cold-stable microtubules: evidence that cold stability is due to substoichiometric polymer blocks. Biochemistry. 1982 Feb 2;21(3):509–515. doi: 10.1021/bi00532a015. [DOI] [PubMed] [Google Scholar]
- Job D., Rauch C. T., Fischer E. H., Margolis R. L. Regulation of microtubule cold stability by calmodulin-dependent and -independent phosphorylation. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3894–3898. doi: 10.1073/pnas.80.13.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job D., Rauch C. T., Margolis R. L. High concentrations of STOP protein induce a microtubule super-stable state. Biochem Biophys Res Commun. 1987 Oct 14;148(1):429–434. doi: 10.1016/0006-291x(87)91129-6. [DOI] [PubMed] [Google Scholar]
- Jones D. H., Gray E. G., Barron J. Cold stable microtubules in brain studied in fractions and slices. J Neurocytol. 1980 Aug;9(4):493–504. doi: 10.1007/BF01204838. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Mitchison T. J., Kirschner M. W. Polewards chromosome movement driven by microtubule depolymerization in vitro. Nature. 1988 Feb 11;331(6156):499–504. doi: 10.1038/331499a0. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Job D., Pabion M., Rauch C. T. Sliding of STOP proteins on microtubules: a model system for diffusion-dependent microtubule motility. Ann N Y Acad Sci. 1986;466:306–321. doi: 10.1111/j.1749-6632.1986.tb38402.x. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Rauch C. T., Job D. Purification and assay of a 145-kDa protein (STOP145) with microtubule-stabilizing and motility behavior. Proc Natl Acad Sci U S A. 1986 Feb;83(3):639–643. doi: 10.1073/pnas.83.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Opposite end assembly and disassembly of microtubules at steady state in vitro. Cell. 1978 Jan;13(1):1–8. doi: 10.1016/0092-8674(78)90132-0. [DOI] [PubMed] [Google Scholar]
- Mitchison T. J. Polewards microtubule flux in the mitotic spindle: evidence from photoactivation of fluorescence. J Cell Biol. 1989 Aug;109(2):637–652. doi: 10.1083/jcb.109.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins J. M., McIntosh J. R. Isolation and initial characterization of the mammalian midbody. J Cell Biol. 1982 Sep;94(3):654–661. doi: 10.1083/jcb.94.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. B., Vallee R. B., Borisy G. G. Identity and polymerization-stimulatory activity of the nontubulin proteins associated with microtubules. Biochemistry. 1977 Jun 14;16(12):2598–2605. doi: 10.1021/bi00631a004. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. Immunofluorescence and immunocytochemical procedures with affinity purified antibodies: tubulin-containing structures. Methods Cell Biol. 1982;24:97–132. doi: 10.1016/s0091-679x(08)60650-0. [DOI] [PubMed] [Google Scholar]
- Pabion M., Job D., Margolis R. L. Sliding of STOP proteins on microtubules. Biochemistry. 1984 Dec 18;23(26):6642–6648. doi: 10.1021/bi00321a055. [DOI] [PubMed] [Google Scholar]
- Paturle L., Wehland J., Margolis R. L., Job D. Complete separation of tyrosinated, detyrosinated, and nontyrosinatable brain tubulin subpopulations using affinity chromatography. Biochemistry. 1989 Mar 21;28(6):2698–2704. doi: 10.1021/bi00432a050. [DOI] [PubMed] [Google Scholar]
- Piperno G., LeDizet M., Chang X. J. Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J Cell Biol. 1987 Feb;104(2):289–302. doi: 10.1083/jcb.104.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirollet F., Rauch C. T., Job D., Margolis R. L. Monoclonal antibody to microtubule-associated STOP protein: affinity purification of neuronal STOP activity and comparison of antigen with activity in neuronal and nonneuronal cell extracts. Biochemistry. 1989 Jan 24;28(2):835–842. doi: 10.1021/bi00428a064. [DOI] [PubMed] [Google Scholar]
- Poenie M., Alderton J., Steinhardt R., Tsien R. Calcium rises abruptly and briefly throughout the cell at the onset of anaphase. Science. 1986 Aug 22;233(4766):886–889. doi: 10.1126/science.3755550. [DOI] [PubMed] [Google Scholar]
- Rasmussen C. D., Means A. R. Calmodulin is required for cell-cycle progression during G1 and mitosis. EMBO J. 1989 Jan;8(1):73–82. doi: 10.1002/j.1460-2075.1989.tb03350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatten G., Simerly C., Asai D. J., Szöke E., Cooke P., Schatten H. Acetylated alpha-tubulin in microtubules during mouse fertilization and early development. Dev Biol. 1988 Nov;130(1):74–86. doi: 10.1016/0012-1606(88)90415-0. [DOI] [PubMed] [Google Scholar]
- Schulze E., Kirschner M. Microtubule dynamics in interphase cells. J Cell Biol. 1986 Mar;102(3):1020–1031. doi: 10.1083/jcb.102.3.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheir-Neiss G., Lai M. H., Morris N. R. Identification of a gene for beta-tubulin in Aspergillus nidulans. Cell. 1978 Oct;15(2):639–647. doi: 10.1016/0092-8674(78)90032-6. [DOI] [PubMed] [Google Scholar]
- Sweet S. C., Rogers C. M., Welsh M. J. Calmodulin stabilization of kinetochore microtubule structure to the effect of nocodazole. J Cell Biol. 1988 Dec;107(6 Pt 1):2243–2251. doi: 10.1083/jcb.107.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombes R. M., Borisy G. G. Intracellular free calcium and mitosis in mammalian cells: anaphase onset is calcium modulated, but is not triggered by a brief transient. J Cell Biol. 1989 Aug;109(2):627–636. doi: 10.1083/jcb.109.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Soll D. R., Gibbons I. R. One-dimensional diffusion of microtubules bound to flagellar dynein. Cell. 1989 Dec 1;59(5):915–925. doi: 10.1016/0092-8674(89)90614-4. [DOI] [PubMed] [Google Scholar]
- Wehland J., Weber K. Turnover of the carboxy-terminal tyrosine of alpha-tubulin and means of reaching elevated levels of detyrosination in living cells. J Cell Sci. 1987 Sep;88(Pt 2):185–203. doi: 10.1242/jcs.88.2.185. [DOI] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M. J., Dedman J. R., Brinkley B. R., Means A. R. Calcium-dependent regulator protein: localization in mitotic apparatus of eukaryotic cells. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1867–1871. doi: 10.1073/pnas.75.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M. J., Dedman J. R., Brinkley B. R., Means A. R. Tubulin and calmodulin. Effects of microtubule and microfilament inhibitors on localization in the mitotic apparatus. J Cell Biol. 1979 Jun;81(3):624–634. doi: 10.1083/jcb.81.3.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieve G., Solomon F. Proteins specifically associated with the microtubules of the mammalian mitotic spindle. Cell. 1982 Feb;28(2):233–242. doi: 10.1016/0092-8674(82)90341-5. [DOI] [PubMed] [Google Scholar]