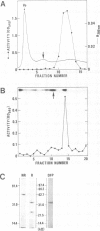
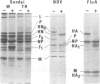
Abstract
Host cell proteases responsible for activation of viral fusion glycoproteins are an important determinant for spread and tropism of various animal viruses. Exemplifying such proteases for the first time, we isolated an endoprotease from chick embryo, that activates para- and orthomyxovirus fusion glycoproteins by cleaving their precursor proteins at a specific, single arginine site. The protease is a calcium dependent serine protease consisting of two subunits, the 33 kd catalytic chain and the 23 kd chain possibly required for Ca2+ binding, and was found to be highly homologous, if not identical, to the blood clotting factor X(FX), a member of the prothrombin family. Its high efficiency and specificity in cleavage reactions was attributable to the properties characteristic of FX. Its role in vivo was strongly supported by cleavage inhibition in ovo highly selective for this virus group with a specific peptide inhibitor against FX.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard G., Davis G. B. Activation of Sendai virus infectivity by an enzyme in chicken amniotic fluid. J Gen Virol. 1983 Apr;64(Pt 4):813–823. doi: 10.1099/0022-1317-64-4-813. [DOI] [PubMed] [Google Scholar]
- Bloom J. W., Mann K. G. Metal ion induced conformational transitions of prothrombin and prothrombin fragment 1. Biochemistry. 1978 Oct 17;17(21):4430–4438. doi: 10.1021/bi00614a012. [DOI] [PubMed] [Google Scholar]
- Blumberg B. M., Giorgi C., Rose K., Kolakofsky D. Sequence determination of the Sendai virus fusion protein gene. J Gen Virol. 1985 Feb;66(Pt 2):317–331. doi: 10.1099/0022-1317-66-2-317. [DOI] [PubMed] [Google Scholar]
- Blumberg B., Giorgi C., Roux L., Raju R., Dowling P., Chollet A., Kolakofsky D. Sequence determination of the Sendai virus HN gene and its comparison to the influenza virus glycoproteins. Cell. 1985 May;41(1):269–278. doi: 10.1016/0092-8674(85)90080-7. [DOI] [PubMed] [Google Scholar]
- Bosch F. X., Garten W., Klenk H. D., Rott R. Proteolytic cleavage of influenza virus hemagglutinins: primary structure of the connecting peptide between HA1 and HA2 determines proteolytic cleavability and pathogenicity of Avian influenza viruses. Virology. 1981 Sep;113(2):725–735. doi: 10.1016/0042-6822(81)90201-4. [DOI] [PubMed] [Google Scholar]
- Bosch F. X., Orlich M., Klenk H. D., Rott R. The structure of the hemagglutinin, a determinant for the pathogenicity of influenza viruses. Virology. 1979 May;95(1):197–207. doi: 10.1016/0042-6822(79)90414-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Furie B. C., Blumenstein M., Furie B. Metal binding sites of a gamma-carboxyglutamic acid-rich fragment of bovine prothrombin. J Biol Chem. 1979 Dec 25;254(24):12521–12530. [PubMed] [Google Scholar]
- Furie B., Bing D. H., Feldmann R. J., Robison D. J., Burnier J. P., Furie B. C. Computer-generated models of blood coagulation factor Xa, factor IXa, and thrombin based upon structural homology with other serine proteases. J Biol Chem. 1982 Apr 10;257(7):3875–3882. [PubMed] [Google Scholar]
- Furie B., Furie B. C. The molecular basis of blood coagulation. Cell. 1988 May 20;53(4):505–518. doi: 10.1016/0092-8674(88)90567-3. [DOI] [PubMed] [Google Scholar]
- Glickman R. L., Syddall R. J., Iorio R. M., Sheehan J. P., Bratt M. A. Quantitative basic residue requirements in the cleavage-activation site of the fusion glycoprotein as a determinant of virulence for Newcastle disease virus. J Virol. 1988 Jan;62(1):354–356. doi: 10.1128/jvi.62.1.354-356.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman J. J., Nestorowicz A., Mitchell S. J., Corino G. L., Selleck P. W. Characterization of the sites of proteolytic activation of Newcastle disease virus membrane glycoprotein precursors. J Biol Chem. 1988 Sep 5;263(25):12522–12531. [PubMed] [Google Scholar]
- Homma M., Ouchi M. Trypsin action on the growth of Sendai virus in tissue culture cells. 3. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J Virol. 1973 Dec;12(6):1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R. T., Rott R., Klenk H. D. Influenza viruses cause hemolysis and fusion of cells. Virology. 1981 Apr 15;110(1):243–247. doi: 10.1016/0042-6822(81)90030-1. [DOI] [PubMed] [Google Scholar]
- Itoh M., Shibuta H., Homma M. Single amino acid substitution of Sendai virus at the cleavage site of the fusion protein confers trypsin resistance. J Gen Virol. 1987 Nov;68(Pt 11):2939–2944. doi: 10.1099/0022-1317-68-11-2939. [DOI] [PubMed] [Google Scholar]
- Kettner C., Shaw E. The selective affinity labeling of factor Xa by peptides of arginine chloromethyl ketone. Thromb Res. 1981 Jun 1;22(5-6):645–652. doi: 10.1016/0049-3848(81)90062-1. [DOI] [PubMed] [Google Scholar]
- Kido H., Fukusen N., Katunuma N., Morita T., Iwanaga S. Tryptase from rat mast cells converts bovine prothrombin to thrombin. Biochem Biophys Res Commun. 1985 Oct 30;132(2):613–619. doi: 10.1016/0006-291x(85)91177-5. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Rott R., Orlich M., Blödorn J. Activation of influenza A viruses by trypsin treatment. Virology. 1975 Dec;68(2):426–439. doi: 10.1016/0042-6822(75)90284-6. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Rott R. The molecular biology of influenza virus pathogenicity. Adv Virus Res. 1988;34:247–281. doi: 10.1016/S0065-3527(08)60520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mann K. G., Jenny R. J., Krishnaswamy S. Cofactor proteins in the assembly and expression of blood clotting enzyme complexes. Annu Rev Biochem. 1988;57:915–956. doi: 10.1146/annurev.bi.57.070188.004411. [DOI] [PubMed] [Google Scholar]
- Mann K. G., Nesheim M. E., Tracy P. B., Hibbard L. S., Bloom J. W. Assembly of the prothrombinase complex. Biophys J. 1982 Jan;37(1):106–107. doi: 10.1016/S0006-3495(82)84624-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu M., Homma M. Trypsin action on the growth of Sendai virus in tissue culture cells. V. An activating enzyme for Sendai virus in the chorioallantoic fluid of the embryonated chicken egg. Microbiol Immunol. 1980;24(2):113–122. doi: 10.1111/j.1348-0421.1980.tb00569.x. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Hamaguchi M., Toyoda T. Molecular biology of Newcastle disease virus. Prog Vet Microbiol Immunol. 1989;5:16–64. [PubMed] [Google Scholar]
- Nagai Y., Klenk H. D. Activation of precursors to both glycoporteins of Newcastle disease virus by proteolytic cleavage. Virology. 1977 Mar;77(1):125–134. doi: 10.1016/0042-6822(77)90412-3. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Klenk H. D., Rott R. Proteolytic cleavage of the viral glycoproteins and its significance for the virulence of Newcastle disease virus. Virology. 1976 Jul 15;72(2):494–508. doi: 10.1016/0042-6822(76)90178-1. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Ogura H., Klenk H. Studies on the assembly of the envelope of Newcastle disease virus. Virology. 1976 Feb;69(2):523–538. doi: 10.1016/0042-6822(76)90482-7. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Shimokata K., Yoshida T., Hamaguchi M., Iinuma M., Maeno K., Matsumoto T., Klenk H. D., Rott R. The spread of a pathogenic and an apathogenic strain of Newcastle disease virus in the chick embryo as depending on the protease sensitivity of the virus glycoproteins. J Gen Virol. 1979 Nov;45(2):263–272. doi: 10.1099/0022-1317-45-2-263. [DOI] [PubMed] [Google Scholar]
- Poser J. W., Price P. A. A method for decarboxylation of gamma-carboxyglutamic acid in proteins. Properties of the decarboxylated gamma-carboxyglutamic acid protein from calf bone. J Biol Chem. 1979 Jan 25;254(2):431–436. [PubMed] [Google Scholar]
- Robertson J. S., Bootman J. S., Newman R., Oxford J. S., Daniels R. S., Webster R. G., Schild G. C. Structural changes in the haemagglutinin which accompany egg adaptation of an influenza A(H1N1) virus. Virology. 1987 Sep;160(1):31–37. doi: 10.1016/0042-6822(87)90040-7. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Schuy W., Garten W., Linder D., Klenk H. D. The carboxyterminus of the hemagglutinin-neuraminidase of Newcastle disease virus is exposed at the surface of the viral envelope. Virus Res. 1984;1(5):415–426. doi: 10.1016/0168-1702(84)90027-3. [DOI] [PubMed] [Google Scholar]
- Shuman M. A. Thrombin-cellular interactions. Ann N Y Acad Sci. 1986;485:228–239. doi: 10.1111/j.1749-6632.1986.tb34585.x. [DOI] [PubMed] [Google Scholar]
- Tashiro M., Ciborowski P., Reinacher M., Pulverer G., Klenk H. D., Rott R. Synergistic role of staphylococcal proteases in the induction of influenza virus pathogenicity. Virology. 1987 Apr;157(2):421–430. doi: 10.1016/0042-6822(87)90284-4. [DOI] [PubMed] [Google Scholar]
- Tashiro M., Homma M. Evidence of proteolytic activation of Sendai virus in mouse lung. Arch Virol. 1983;77(2-4):127–137. doi: 10.1007/BF01309262. [DOI] [PubMed] [Google Scholar]
- Tashiro M., Homma M. Pneumotropism of Sendai virus in relation to protease-mediated activation in mouse lungs. Infect Immun. 1983 Feb;39(2):879–888. doi: 10.1128/iai.39.2.879-888.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda T., Sakaguchi T., Hirota H., Gotoh B., Kuma K., Miyata T., Nagai Y. Newcastle disease virus evolution. II. Lack of gene recombination in generating virulent and avirulent strains. Virology. 1989 Apr;169(2):273–282. doi: 10.1016/0042-6822(89)90152-9. [DOI] [PubMed] [Google Scholar]
- Toyoda T., Sakaguchi T., Imai K., Inocencio N. M., Gotoh B., Hamaguchi M., Nagai Y. Structural comparison of the cleavage-activation site of the fusion glycoprotein between virulent and avirulent strains of Newcastle disease virus. Virology. 1987 May;158(1):242–247. doi: 10.1016/0042-6822(87)90261-3. [DOI] [PubMed] [Google Scholar]
- Walz D. A., Kipfer R. K., Jones J. P., Olson R. E. Purification and properties of chicken prothrombin. Arch Biochem Biophys. 1974 Oct;164(2):527–535. doi: 10.1016/0003-9861(74)90063-0. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Rott R. Influenza virus A pathogenicity: the pivotal role of hemagglutinin. Cell. 1987 Aug 28;50(5):665–666. doi: 10.1016/0092-8674(87)90321-7. [DOI] [PubMed] [Google Scholar]
- White J. M., Littman D. R. Viral receptors of the immunoglobulin superfamily. Cell. 1989 Mar 10;56(5):725–728. doi: 10.1016/0092-8674(89)90674-0. [DOI] [PubMed] [Google Scholar]
- Winter G., Fields S., Gait M. J., Brownlee G. G. The use of synthetic oligodeoxynucleotide primers in cloning and sequencing segment of 8 influenza virus (A/PR/8/34). Nucleic Acids Res. 1981 Jan 24;9(2):237–245. doi: 10.1093/nar/9.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]