Abstract
Background
The epidemiological evidence for a dose-response relationship between tea consumption and risk of cognitive disorders is sparse. The aim of the study was to summarize the evidence for the association of tea consumption with risk of cognitive disorders and assess the dose-response relationship.
Methods
We searched electronic databases of Pubmed, Embase, and Cochrane Library (from 1965 to Jan 19, 2017) for eligible studies that published in the international journals. A random-effects model was used to pool the most adjusted odds ratios (ORs) and the corresponding 95% confidence intervals (CIs).
Results
Seventeen studies involving 48,435 participants were included in our study. The meta-analysis showed that a higher tea consumption was associated with a significant reduction in the risk of cognitive disorders (OR=0.73, 95% CI: 0.65-0.82). When considering the specific types of tea consumption, the significantly inverse association is only found in green tea consumption (OR=0.64, 95% CI: 0.53-0.77) but not in black/oolong tea consumption (OR=0.75, 95% CI: 0.55-1.01). Dose-response meta-analysis indicated that tea consumption is linearly associated with a reduced risk of cognitive disorders. An increment of 100 ml/day, 300 ml/day, and 500 ml/day of tea consumption was associated with a 6% (OR=0.94, 95% CI: 0.92-0.96), 19% (OR=0.81, 95% CI: 0.74-0.88), and 29% (OR=0.71, 95% CI: 0.62-0.82) lower risk of cognitive disorders.
Conclusions
Tea consumption is inversely and linearly related to the risk of cognitive disorders. More studies are needed to further confirm our findings.
Keywords: tea consumption, cognitive disorders, dose-response, meta-analysis
INTRODUCTION
In the rapidly aging societies around the world, cognition-related diseases, such as mild cognitive impairment, cognitive decline, dementia and Alzheimer's disease (AD), are gradually increasing [1, 2]. The number of people with dementia worldwide is about 35.6 million as announced by WHO and this number will be doubled by 2030, tripled by 2050 [3]. Interventions against early cognitive disorders may be particularly effective to reduce the social burden of AD and other types of dementia. It has been indicated that the intake of certain diet and nutrients, such as fruit and vegetable [4], Mediterranean diet [5], omega-3 fatty acids [6], vitamin C [7], vitamin E [8], milk [9], coffee [10], and light to moderate amounts of alcohol [11], is related to the reduced risk of cognitive disorders and dementia.
A number of epidemiological studies have found that tea consumption may also improve mental performance [12] and reduce the progression of cognitive dysfunction [13, 14]. An animal study has shown that oolong and green teas could reduce the deteriorations of cognitive ability, brain degenerative changes and aging process in senescence accelerated-prone mice P8 [15]. Experimental studies indicated that the anti-oxidative and anti-inflammatory effects of tea and its components, such as catechins and theanine, may contribute to neuroprotection [16–20].
The strength of the association between tea consumption and the risk of cognitive disorders remains uncertain due to the differences in participants and methodological methods used in the previous studies. In the current study, we conducted a systematic review and dose-response meta-analysis to quantitatively assess the association between tea consumption and the risk of cognitive disorders.
RESULTS
Description of the included studies
Of the 407 citations identified from the database searches, 17 studies met the inclusion criteria and were finally included in the meta-analysis, including six cohort studies [21–26], three case-control studies [27–29] and eight cross-sectional studies [3, 30–36]. The study selection process is shown in Figure 1. Among the included studies, twelve were from Asia, two were from Europe, two were from North America, and one was from Australia, involving a total of 48,435 participants. The characteristics of these studies are summarized in Table 1. All included studies measured tea consumption by self-administered questionnaire or self-reported food frequency questionnaire. The diagnosis of cognitive disorders was based on standard criterion in all the articles. Most of the included studies adjusted for potential confounding factors. We recorded relative risks of cognitive disorders according to the highest vs. the lowest category of tea consumption. The results of quality evaluation for each study are presented in detail in the Supplementary Materials (Supplementary Tables 3–5).
Figure 1. Flowchart for the selection of eligible studies.
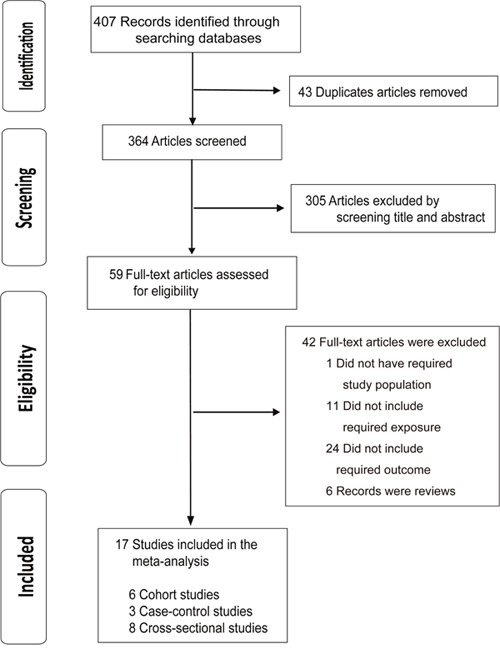
Table 1. Characteristics of included studies in the systematic review and meta-analysis.
| Study | Country | Study design | Sample size | Mean age (years) | Follow-up duration (years) | Exposure variable | Disease type | Disease ascertainment | Adjustment |
|---|---|---|---|---|---|---|---|---|---|
| Broe et al, 1990 | Australia | Case-control | 340 | 77.5 | - | All tea | AD | NINCDS-ADRDA | Age, sex and the general practice of origin |
| Chen et al, 2012 | China | Prospective nested case-control study | 5691 | 89.2 | 3 | All tea | Cognitive decline | MMSE | None |
| Dai et al, 2006 | The United States | Cohort | 1589 | 71.8 | 6.4 | All tea | AD | NINCDS-ADRDA | Years of education, gender, regular physical activity, BMI, baseline CASI score, olfaction diagnostic group, total energy intake, intake of saturated, monounsaturated, and polyunsaturated fatty acids, ApoE genotype, smoking status, alcohol drinking, supplementation of vitamin C, vitamin E, and multivitamin, tea drinking, fruit and vegetable juice drinking, dietary intake of vitamin C, vitamin E, and β-carotene. |
| Eskelinen et al, 2009 | Finland | Cohort | 1409 | 50.2 | 21 | All tea | Dementia, AD | MMSE, DSM-IV and NINCDS-ADRDA | Age, sex, education, follow-up time, community of residence, midlife smoking, systolic blood pressure, serum total cholesterol, BMI, and physical activity. |
| Forster et al, 1995 | The United Kingdom | Case-control | 218 | 55.9 | - | All tea | AD | NINCDS-ADRDA | None. |
| Huang et al, 2009 | China | Cross-sectional | 681 | 93.5 | - | All tea | Cognitive impairment | MMSE | Age, sex, sleep habits, educational level, religion habits, and temperament. |
| Kitamura et al, 2016 | Japan | Cross-sectional | 1143 | 68.9 | - | All tea; green tea | Cognitive impairment | MMSE | Age, BMI, history of stroke, history of myocardial infarction, walking time, alcohol intake, and fruit consumption. |
| Kuriyama et al, 2006 | Japan | Cross-sectional | 1003 | 74.7 | - | All tea and green tea | Cognitive impairment | MMSE | Age, sex, energy intake, intake of nondietary vitamin C or E, fish consumption, green or yellow vegetable consumption, mild leisure-time physical activity, vigorous leisure-time physical activity, smoking, and alcohol use. |
| Lindsay et al, 2002 | Canada | Cohort | 4088 | 73.3 | 5 | All tea | AD | DSM-IV | Age, sex, and education. |
| Ng et al, 2008 | Singapore | Cross-sectional | 2607 | 66 | - | All tea; green tea; black and oolong tea | Cognitive impairment, cognitive decline | MMSE | Age, sex, education, smoking, alcohol consumption, BMI, hypertension, diabetes, heart disease, stroke, depression, APOEε4, physical activities, social and productive activities, vegetable and fruit consumption, fish consumption, and coffee consumption. |
| Noguchi-Shinohara et al, 2014 | Japan | Cohort | 490 | 71.2 | 4.9 | Green tea; black tea | Dementia, cognitive decline | MMSE, CDR and DSM-III-R | Age, sex, history of hypertension, diabetes mellitus, typerlipidemia, education, APOE ε4 carrier status, alcohol drinking, smoking, physical activities and/or hobbies, and coffee consumption. |
| Shen et al, 2015 | China | Cross-sectional | 9375 | 70 | - | All tea; green tea | Cognitive impairment | CCM and MMSE | Age, sex, race, education, marriage, tea concentration, tea categories, physical examinations, family status, disease situation, behavioral risk factors, dietary intake, nutrition supplement, depression and ADL. |
| Tomata et al, 2016 | Japan | Cohort | 13645 | 73.8 | 5.7 | Green tea; black tea; oolong tea | Dementia | LTCI system and cognitive function score | Age, sex, history of disease, educational level, smoking, alcohol drinking, BMI, psychological distress score, time spent walking, social support, participation in community activities, motor function score, consumption volume of specific foods coffee consumption, and energy intake. |
| Wang et al, 2014 | China | Cohort | 223 | 70.9 | 2 | Green tea | Cognitive decline | MMSE | Age and gender. |
| Wang et al. 2016 | China | Cross-sectional | 1005 | 72.7 | - | All tea | Cognitive impairment | Clinical diagnosis and MMSE | Not mention. |
| Wu et al, 2011 | Taiwan | Cross-sectional | 2119 | 73.3 | - | All tea | Cognitive impairment | MMSE | Age, gender, educational level, marital status, social support, hyperlipidemia, stroke, physical function, depressive symptoms, self-rated health, cigarette smoking, leisure-time physical activity, fruits and vegetables consumption, coffee intake, multivitamin intake, and BMI. |
| Yao et al, 2010 | China | Cross-sectional | 2809 | 70.6 | - | All tea | Cognitive impairment | MMSE | None. |
Abbreviations: AD, Alzheimer's disease; ADL, Activities of Daily Living; BMI: body mass index; CASI, Cognitive Abilities Screening Instrument; CDR, Clinical Dementia Rating Scale; DSM-III-R, Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; LTCI, Long-term Care Insurance; MMSE, Mini-Mental State Examination; NINCDS-ADRDA, National Institute of Neurological and Communicative Diseases and Stroke-Alzheimer Disease and Related Disorders Association.
All types of tea consumption and risk of cognitive disorders
Figure 2 shows the results of meta-analysis of relative risk according to the highest vs. lowest category of all types of tea consumption. The summary result showed that high tea consumption was associated with a reduced risk of cognitive disorders (OR=0.73, 95% CI: 0.65-0.82), with some heterogeneity (I2=51.4%, p=0.001). In the stratified analysis by the type of outcome, the pooled ORs and 95% CIs of higher tea consumption were 0.66 (0.58-0.76) for cognitive impairment, 0.67 (0.43-1.03) for cognitive decline, 0.80 (0.59-1.09) for dementia, and 1.12 (0.87-1.45) for Alzheimer's disease. Subgroup analyses by study design showed that the inverse association between tea consumption and risk of cognitive disorders was found in both cohort studies (OR=0.84, 95% CI: 0.74-0.95) and cross-sectional studies (OR=0.66, 95% CI: 0.58-0.75) (Table 2). Sensitive analysis showed that the inverse association was not materially changed in the leave-one-out analyses by omitting one study in turn, with pooled ORs range from 0.71 (95% CI: 0.63-0.80) to 0.0.75 (95 CI: 0.66-0.84) (Supplementary Figure 1).
Figure 2. Relative risk of cognitive disorders according to the highest vs. lowest category of all types of tea consumption.
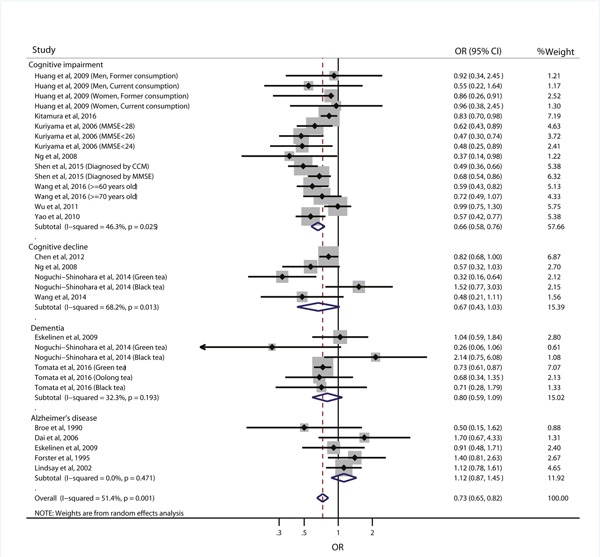
Table 2. Results of subgroup analyses by study design.
| Exposure variable | Study design | Number of estimates | Pooled OR (95% CI), I2 statistics (%), and P-value for the heterogeneity Q test |
|---|---|---|---|
| All types of tea consumption | Cohort | 12 | 0.84 (0.74-0.95); I2=56.6%, P=0.01 |
| Cross-sectional | 16 | 0.66 (0.58-0.75); I2=43.4%, P=0.03 | |
| Green tea consumption | Cohort | 3 | 0.46 (0.23-0.95); I2=70.9%, P=0.03 |
| Cross-sectional | 8 | 0.66 (0.53-0.83); I2=60.6%, P=0.01 | |
| Black/oolong tea consumption | Cohort | 4 | 1.08 (0.63-1.84); I2=41.2%, P=0.16 |
| Cross-sectional | 3 | 0.61 (0.49-0.75); I2=0.0%, P=0.52 | |
| An increment of 100 ml/day of tea consumption | Cohort | 6 | 0.96 (0.93-0.99); I2=34.8%, P=0.18 |
| Cross-sectional | 9 | 0.92 (0.89-0.95); I2=50.9%, P=0.04 | |
| An increment of 300 ml/day of tea consumption | Cohort | 6 | 0.91 (0.84-0.99); I2=63.2%, P=0.02 |
| Cross-sectional | 9 | 0.77 (0.72-0.83); I2=27.1%, P=0.20 | |
| An increment of 500 ml/day of tea consumption | Cohort | 6 | 0.84 (0.61-0.98); I2=67.8%, P=0.01 |
| Cross-sectional | 9 | 0.69 (0.63-0.75); I2=0.0%, P=0.45 |
Four estimates from two individual studies were available for assessing the relative risk for men and women, separately. The pooled results also showed a trend of reduced risk of cognitive disorders in both men and women by higher tea consumption, but did not reach statistical significances (OR=0.65, 95% CI: 0.38-1.11 and OR=0.63, 95% CI: 0.38-1.07, respectively) (Supplementary Figure 2).
Different types of tea consumption and risk of cognitive disorders
Eleven estimates from seven individual studies were available for analysis of green tea consumption and the risk of cognitive disorders and seven estimates from four individual studies were available for analysis of black/oolong tea consumption and risk of cognitive disorders. The pooled analyses showed that higher green tea consumption was associated with a reduced risk of cognitive disorders (OR=0.64, 95% CI: 0.53-0.77) (Figure 3A); however, there was no significant association between higher black/oolong tea consumption and risk of cognitive disorders (OR=0.75, 95% CI: 0.55-1.01) (Figure 3B). Subgroup analyses by study design showed that the inverse association between green tea consumption and risk of cognitive disorders was found in both cohort studies (OR=0.46, 95% CI: 0.23-0.95) and cross-sectional studies (OR=0.66, 95% CI: 0.53-0.83); higher black/oolong tea consumption was associated with a reduced risk of cognitive disorders in cross-sectional studies (OR=0.61, 95% CI: 0.49-0.75) (Table 2).
Figure 3.
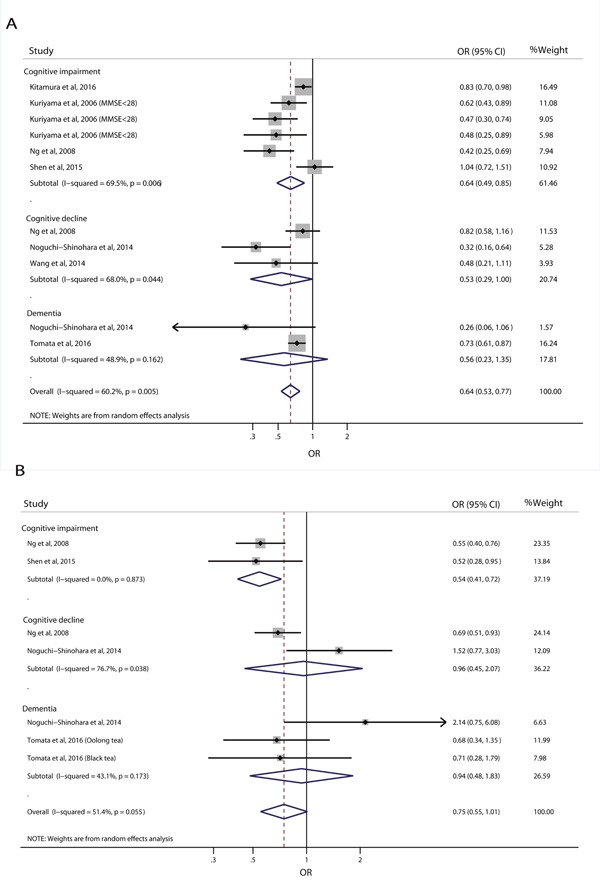
Relative risk of cognitive disorders according to the highest vs. lowest category of green tea (A) and black/oolong tea (B) consumption.
Dose-response analyses
Fifteen estimates from eight individual studies were included in the dose-response meta-analyses. Other studies were not included because there were only two categories of tea consumption in these studies, and dose-response meta-analysis requires data for the distribution of cases and person-time across at least three categories of exposure [37]. As shown in Figure 4, there was a linear relationship between tea consumption and risk of cognitive disorders (p for linear trend=0.042; p for non-linear trend=0.236). The dose-response meta-analyses showed that an increment of 100 ml/day, 300 ml/day, and 500 ml/day of tea consumption was associated with a 6% (OR=0.94, 95% CI: 0.92-0.96), 19% (OR=0.81, 95% CI: 0.74-0.88), and 29% (OR=0.71, 95% CI: 0.62-0.82) lower risk of cognitive disorders, respectively (Figure 5). The inverse association was not materially changed when subgrouped by study design (Table 2).
Figure 4. Dose-response relationship between tea consumption and risk of cognitive disorders.
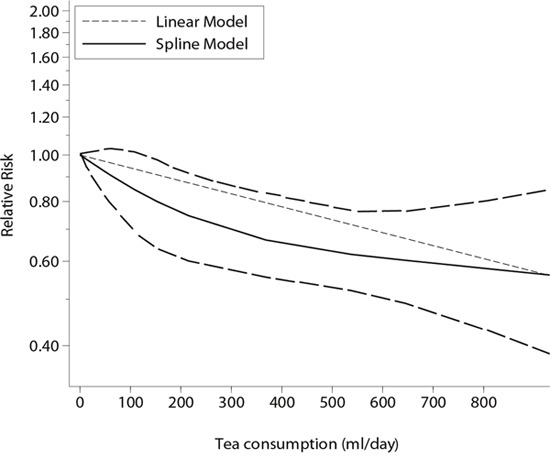
Figure 5.
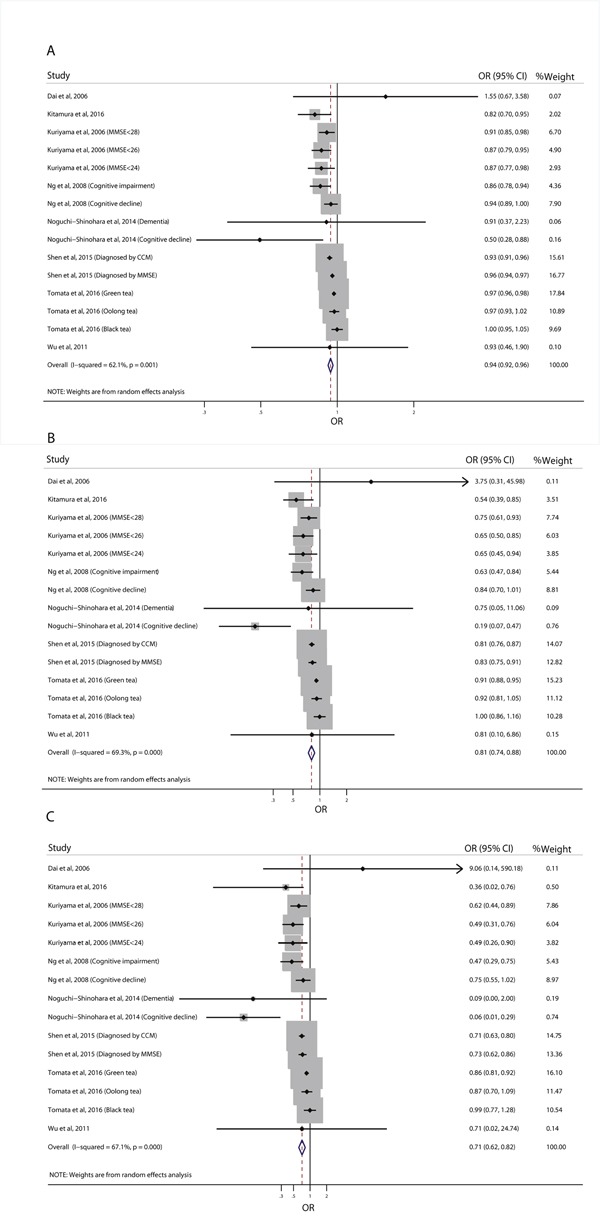
Relative risk of cognitive disorders for an increment of 100 ml/day (A), 300 ml/day (B), and 500 ml/day (C) of tea consumption.
Publication bias
Visual assessment of funnel plot (Figure 6) showed that the studies were distributed fairly symmetrically about the combined effect size in the meta-analysis of all types of tea consumption and risk of cognitive disorders, which suggests little publication bias. Egger's regression test (p=0.678) and Begg-Mazumdar test (p=0.747) further confirmed that there was no potential publication bias.
Figure 6. Funnel plot to explore publication bias.
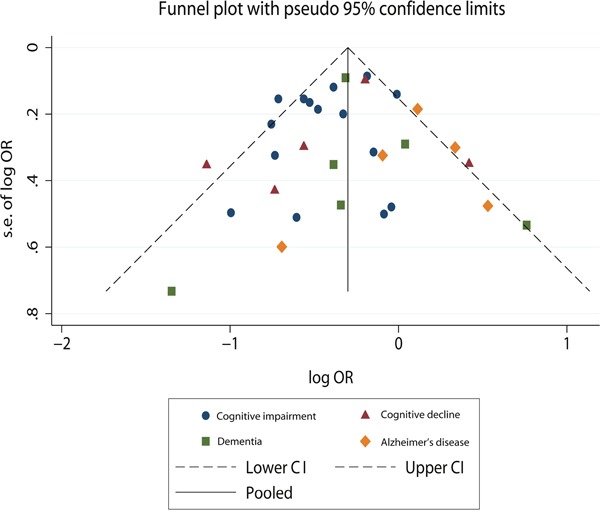
The vertical line is at the mean effect size.
DISCUSSION
Tea is a commonly consumed beverage not only in Asia but also in other parts of the world and has been reported to have unlimited health benefits. To our knowledge, this study is the first dose-response meta-analysis to evaluate the effect of tea consumption on the risk of cognitive disorders. The findings from our meta-analysis indicated that tea consumption is inversely associated with the risk of cognitive disorders. However, when considering the specific types of tea consumption, the significantly inverse association is only found in green tea consumption but not in black/oolong tea consumption. Overall, the risk decreases by 6%, 19%, and 29% for every 100 ml/day, 300ml/day, and 500ml/day increase in tea consumption, respectively.
A previous meta-analysis from Kim et al. [38] found that caffeine intake from coffee or tea was not associated with the risk of cognitive disorders. When subgrouped by caffeine source, caffeine intake from tea also showed no association with cognitive disorders. However, in their study, they used caffeine intake from tea as the exposure variable of interest, while our study used the overall tea consumption as the exposure. Systematic reviews from Arab et al. [39] and Panza et al. [40] assessed epidemiologic evidence of the relationship between tea, coffee, or caffeine consumption and cognitive decline. However, the included studies that specifically for tea consumption were rare and pooled analyses were not conducted in these two reviews. In addition, although they found the estimates of cognitive decline were lower among consumers, there is a lack of a distinct dose response. The findings of our study extend the results of a previous cohort study [41], which found that tea consumption is significantly associated with a lower risk of incident functional disability, such as stroke, cognitive impairment, and osteoporosis. In Feng et al's study [14], verbal fluency test was used as measure of cognitive function. This study found that daily and occasional tea drinkers had significantly higher verbal fluency scores compared with non-drinkers, and regular tea drinking was associated with better cognitive function in oldest-old Chinese. In Ide et al.'s study [13], they found that green tea consumption was not only effective in improving cognitive function, but also effective in reducing the progression of cognitive dysfunction.
Arab et al.'s study [42] found that some levels of tea consumption modestly reduced rates of cognitive decline among the women but not men. Thus, sex may be an important factor that impacts the association between tea consumption and risk of cognitive disorders. However, there were very limited studies that focused on this association for men and women separately. In the current meta-analysis, only four estimates from two individual studies were available for assessing the relative risks for men and women, separately; and our results showed that neither male nor female tea consumers showed a significant association with the risk of cognitive disorders. This may be due to the very limited studies included in the meta-analyses, which led to low statistical powers. Therefore, more studies are needed to further assess the association between tea consumption and risk of cognitive disorders in difference sexes.
There are several plausible mechanisms to explain the protective effect of tea consumption against cognitive disorders. Many studies have shown that oxidative stress, which occurs by oxidizing macromolecules such as proteins, lipids, and DNA, is an important pathogenic factor in cognitive diseases including AD [43–46]. Tea is enriched in polyphenols and the major tea-related polyphenols present in green tea are catechins, which are polyphenolic compounds with high antioxidant capacities [47], and they have shown effects on anti-aging [48], anti-diabetic [49, 50], anti-stroke [51], and anti-cancer [52] in various studies. Polyphenols contained in green tea have also been reported to have anti-amyloidogenic effects, and could be a key molecule for the development of preventives and therapeutics for AD [53, 54]. Coimbra et al.'s study [20] showed that green tea can significantly reduce the serum levels of malonyldialdehyde and malonyldialdehyde+4-hydroxy-2(E)-nonenal, the lipid peroxidation products, and reduce the oxidative stress within the erythrocyte. L-theanine, a free amino acid naturally found in tea, can also play a neuroprotective role [55]. Caffeine, another important component of tea leaf, has been known for its refreshing effect and is beneficial for performance improvement on attention tasks when combined with L-theanine [56, 57]. Chen et al. [58] demonstrated that one mechanism implicated in the pathogenesis of AD is blood-brain barrier dysfunction and caffeine exerts protective effects against AD at least in part by keeping the blood-brain barrier intact. Furthermore, previous studies supported the biological effects of caffeine on brain function [59], including modulation of white matter lesions and/or microvascular ischemic lesions [60]. Another potential mechanism for long-term neuroprotective effect of caffeine may involve blockade of adenosine A2A receptors [61], which may attenuate injury caused by β-amyloid, the toxic peptide that accumulates in the brain of patients with AD [62, 63]. In fact, both acute and long-term caffeine intake were shown to suppress β-amyloid levels in plasma and brain of AD transgenic mice [64, 65] and memory restoration and reversal of AD pathology in mice with preexisting β-amyloid burden [66]. In addition, tea consumption has shown to reduce the hypertension risk [67] and decrease the serum concentrations of total cholesterol (TC) and low density lipoprotein cholesterol (LDL-C) [68]; hypertension and dyslipidemia are well known to be the risk factors for atherosclerosis [69, 70], and atherosclerosis is in turn associated with cognitive dysfunction. Thus, tea consumption may reduce the risk of cognitive disorders indirectly by reducing the relevant health problems on atherosclerosis.
In our study, green tea consumption but not in black/oolong tea consumption was associated with reduced risk of cognitive disorders. This may be due to the fact that the nutritional and functional components are not quite the same between green tea and black/oolong tea. For example, the levels of catechin (epigallocatechin gallate [ECGC]) are highest in green tea, followed in order by oolong tea and black tea [71]. Moreover, green tea contains ascorbic acid and high intake of ascorbic acid is related to the reduced risk of AD [72]; however, black tea do not contain ascorbic acid [22]. The differences in the protective effects of cognitive function between green tea and black/oolong tea need to be further clarified by more mechanistic studies.
The strengths of the present meta-analysis include less influence exerted by small-study bias, greatest extent of control for confounding factors, a moderate-to-high quality of studies included in the meta-analysis, and no evidence of publication bias. Moreover, we performed dose-response meta-analysis and found a linear relationship between tea consumption and risk of cognitive disorders.
However, our study has several limitations. First, we found a mild heterogeneity within the studies, which could be explained by the difference in study designs, study populations, measure methods of tea and cognitive disorders, and analytical strategies. Second, the association was evaluated by using the most adjusted model in each included study; however, some important confounding factors, such as lifestyle (diets, hobbies, physical activities, etc.), cultural differences, lifestyle-related diseases (diabetes mellitus, hypertension, dyslipiedemia, etc.) and ApoE status were not adjusted in some of the included studies. In addition, we cannot exclude chance, residual or unmeasured confounding as the alternative explanation of our results. Thus, our results must be explained with caution. Third, in most of the included studies, the consumption of tea was measured using a self-administered questionnaire; and thus, misclassification is inevitable. Fourth, although the overall analysis was based on a large number of studies, few studies were available according to tea subtypes, study populations from Europe and US, and genders, which have led to unstable results in or restriction to secondary analysis. Finally, our conclusions are based on results from observational studies. Especially, as the number of available cohort studies was limited, we also included cross-sectional studies in our meta-analysis to increase the statistical power. Thus, a causal association between tea consumption and cognitive disorders cannot be determined with the present data alone.
In conclusion, the results of our meta-analysis, involving 17 independent observational studies, provide significant evidence of an inverse and a linear relationship of tea consumption with the occurrence of cognitive disorders. A greater increment of tea drinking led to a greater magnitude in risk reduction. Further well-designed long-term randomized controlled trials (RCTs) are needed to confirm our findings.
MATERIALS AND METHODS
Search strategy and eligibility criteria
The guidelines published by the Meta-analysis of Observational Studies in Epidemiology (MOOSE) group was followed to complete the meta-analysis (Supplementary Table 1) [73]. Eligible studies that published in the international journals were searched in electronic databases of Pubmed, Embase, and Cochrane Library (from 1965 to Jan 19, 2017) by two investigators. We used the following MeSH and free-text terms in the search strategy: “Tea [Mesh]”, “Cognitive Dysfunction [Mesh]”, “Alzheimer Disease [Mesh]”, “Dementia [Mesh]”, “tea consumption”, “tea intake”, “tea”, “cognitive decline”, “cognitive impairment”, “cognitive disorder”, “dementia”, “Alzheimer disease”, and “Alzheimer's disease”. The search was restricted to studies in human beings and publications in English language. The reference lists of identified articles and relevant reviews were also checked for potentially eligible studies.
Studies that met the following criteria were included in our meta-analysis: (1) examination of tea consumption as the variable of interest; (2) determination of incidence of cognitive impairment, cognitive decline, dementia, or Alzheimer's disease as the outcome of interest; and (3) reporting the relative risks (RR) or odds ratios (OR) of cognitive disorders, and 95% confidence intervals (CI), or sufficient data with which to calculate these, according to the different levels of tea consumption. Studies about animal experiment, mechanistic research and review research were excluded.
Data extraction and study quality evaluation
The following data from each included study were extracted by two investigators: first author, publication year, country, study design, sample size, mean age of the participants, follow-up duration of cohort studies, disease type of the outcome (cognitive impairment, cognitive decline, dementia, or Alzheimer's disease), exposure variable (tea type), exposure variable ascertainment method, disease ascertainment methods, categories of tea consumption, risk estimates with CIs, and confounding factors adjusted for. We included the most adjusted estimate when a study reported more than one risk estimate. Two investigators assessed the quality of each study, using the Newcastle-Ottawa Scale (NOS) recommended by Wells and colleagues [74]. The NOS score of each included study ranges from 1 to 9 stars for cohort and case-control studies and 1 to 5 stars for cross-sectional studies.
Statistical analyses
We performed meta-analyses of risk estimates for cognitive disorders comparing the highest category of exposure to tea consumption with the lowest category. The results of studies using cognitive impairment, cognitive decline, dementia and Alzheimer's disease as the outcomes are presented separately. If a single study reported results for different populations (men and women), different types of tea consumption (green tea and black tea) or different assessment methods of cognitive disorders but did not report the overall results, the results for each population, type of tea consumption and assessment method of cognitive disorders were calculated as a different study [75]. We first conducted a meta-analysis of all types of tea consumption and the risk of cognitive disorders, and then we conducted meta-analyses for this association in different sexes and types of tea consumption, respectively. Subsequently, we conducted a dose-response meta-analysis from the correlated natural log of ORs across categories of tea consumption [76, 77], to estimate linear trends of OR for cognitive disorders per 100, 300, and 500 ml/day increment in tea consumption. As studies reported results for tea consumption in cups, we derived milliliter by assuming that the average cup equals to 215 ml [32]. We converted the level of consumption category based on the calculated midpoint of tea consumption if the study did not report the median of exposure category. If the maximum dose was fixed unlimitedly (e.g. ≥2 cups/d), we assumed that the mean was 25% larger than the lower level of the specific category [78]. Supplementary Table 2 summaries the definition of tea consumption and the means of conversion of categories within each study.
We used a random effects model to estimate the pooled ORs and 95% CIs to take into account the heterogeneities between studies. χ2 test and I2 statistic were used to evaluate the heterogeneity between studies. Sensitivity analysis was performed for the main meta-analysis by removing each individual study from the meta-analysis [79]. We visually assessed publication bias for the main meta-analysis by using funnel plots. Then we used Egger's regression test [80] and Begg-Mazumdar test [81] to further assess publication bias. All statistical analyses were performed using Stata Version 12.0 software (Stata Corp, College Station, TX).
SUPPLEMENTARY FIGURES AND TABLES
Footnotes
CONFLICTS OF INTEREST
No conflicts of interest was declared by the authors.
REFERENCES
- 1.Ritchie K, Touchon J. Mild cognitive impairment: conceptual basis and current nosological status. Lancet. 2000;355:225–228. doi: 10.1016/S0140-6736(99)06155-3. [DOI] [PubMed] [Google Scholar]
- 2.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9:63–75.e2. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Shen W, Xiao Y, Ying X, Li S, Zhai Y, Shang X, Li F, Wang X, He F, Lin J. Tea Consumption and Cognitive Impairment: A Cross-Sectional Study among Chinese Elderly. PLoS One. 2015;10:e0137781. doi: 10.1371/journal.pone.0137781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology. 2006;67:1370–1376. doi: 10.1212/01.wnl.0000240224.38978.d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu L, Sun D. Adherence to Mediterranean diet and risk of developing cognitive disorders: An updated systematic review and meta-analysis of prospective cohort studies. Sci Rep. 2017;7:41317. doi: 10.1038/srep41317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu S, Ding Y, Wu F, Li R, Hou J, Mao P. Omega-3 fatty acids intake and risks of dementia and Alzheimer's disease: a meta-analysis. Neurosci Biobehav Rev. 2015;48:1–9. doi: 10.1016/j.neubiorev.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Paleologos M, Cumming RG, Lazarus R. Cohort study of vitamin C intake and cognitive impairment. Am J Epidemiol. 1998;148:45–50. doi: 10.1093/oxfordjournals.aje.a009559. [DOI] [PubMed] [Google Scholar]
- 8.Grodstein F, Chen J, Willett WC. High-dose antioxidant supplements and cognitive function in community-dwelling elderly women. Am J Clin Nutr. 2003;77:975–984. doi: 10.1093/ajcn/77.4.975. [DOI] [PubMed] [Google Scholar]
- 9.Wu L, Sun D. Meta-Analysis of Milk Consumption and the Risk of Cognitive Disorders. Nutrients. 2016:8. doi: 10.3390/nu8120824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugiyama K, Tomata Y, Kaiho Y, Honkura K, Sugawara Y, Tsuji I. Association between Coffee Consumption and Incident Risk of Disabling Dementia in Elderly Japanese: The Ohsaki Cohort 2006 Study. J Alzheimers Dis. 2016;50:491–500. doi: 10.3233/JAD-150693. [DOI] [PubMed] [Google Scholar]
- 11.Ilomaki J, Jokanovic N, Tan EC, Lonnroos E. Alcohol Consumption, Dementia and Cognitive Decline: An Overview of Systematic Reviews. Curr Clin Pharmacol. 2015;10:204–212. doi: 10.2174/157488471003150820145539. [DOI] [PubMed] [Google Scholar]
- 12.Gardner EJ, Ruxton CH, Leeds AR. Black tea--helpful or harmful? A review of the evidence. Eur J Clin Nutr. 2007;61:3–18. doi: 10.1038/sj.ejcn.1602489. [DOI] [PubMed] [Google Scholar]
- 13.Ide K, Yamada H, Takuma N, Park M, Wakamiya N, Nakase J, Ukawa Y, Sagesaka YM. Green tea consumption affects cognitive dysfunction in the elderly: a pilot study. Nutrients. 2014;6:4032–4042. doi: 10.3390/nu6104032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng L, Li J, Ng TP, Lee TS, Kua EH, Zeng Y. Tea drinking and cognitive function in oldest-old Chinese. J Nutr Health Aging. 2012;16:754–758. doi: 10.1007/s12603-012-0077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan YC, Hosoda K, Tsai CJ, Yamamoto S, Wang MF. Favorable effects of tea on reducing the cognitive deficits and brain morphological changes in senescence-accelerated mice. J Nutr Sci Vitaminol (Tokyo) 2006;52:266–273. doi: 10.3177/jnsv.52.266. [DOI] [PubMed] [Google Scholar]
- 16.Jaewoong L, Yongkyoung L, Ban JO, Taeyoul H, Yun YP, Han SB, Kiwan O, Hong JT. Green tea (−)-epigallocatechin-3-gallate inhibits β-amyloid-induced cognitive dysfunction through modification of secretase activity via inhibition of ERK and NF-κB pathways in mice. Journal of Nutrition. 2009;139:1987–1993. doi: 10.3945/jn.109.109785. [DOI] [PubMed] [Google Scholar]
- 17.Kim TI, Lee YK, Park SG, Choi IS, Ban JO, Park HK, Nam SY, Yun YW, Han SB, Oh KW, Hong JT. l-Theanine, an amino acid in green tea, attenuates beta-amyloid-induced cognitive dysfunction and neurotoxicity: reduction in oxidative damage and inactivation of ERK/p38 kinase and NF-kappaB pathways. Free Radic Biol Med. 2009;47:1601–1610. doi: 10.1016/j.freeradbiomed.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Lee YJ, Choi DY, Yun YP, Han SB, Oh KW, Hong JT. Epigallocatechin-3-gallate prevents systemic inflammation-induced memory deficiency and amyloidogenesis via its anti-neuroinflammatory properties. J Nutr Biochem. 2013;24:298–310. doi: 10.1016/j.jnutbio.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Biasibetti R, Tramontina AC, Costa AP, Dutra MF, Quincozes-Santos A, Nardin P, Bernardi CL, Wartchow KM, Lunardi PS, Goncalves CA. Green tea (−)epigallocatechin-3-gallate reverses oxidative stress and reduces acetylcholinesterase activity in a streptozotocin-induced model of dementia. Behav Brain Res. 2013;236:186–193. doi: 10.1016/j.bbr.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 20.Coimbra S, Castro E, Rocha-Pereira P, Rebelo I, Rocha S, Santos-Silva A. The effect of green tea in oxidative stress. Clin Nutr. 2006;25:790–796. doi: 10.1016/j.clnu.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 21.Eskelinen MH, Ngandu T, Tuomilehto J, Soininen H, Kivipelto M. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis. 2009;16:85–91. doi: 10.3233/JAD-2009-0920. [DOI] [PubMed] [Google Scholar]
- 22.Noguchi-Shinohara M, Yuki S, Dohmoto C, Ikeda Y, Samuraki M, Iwasa K, Yokogawa M, Asai K, Komai K, Nakamura H, Yamada M. Consumption of green tea, but not black tea or coffee, is associated with reduced risk of cognitive decline. PLoS One. 2014;9:e96013. doi: 10.1371/journal.pone.0096013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai Q, Borenstein AR, Wu Y, Jackson JC, Larson EB. Fruit and vegetable juices and Alzheimer's disease: the Kame Project. Am J Med. 2006;119:751–759. doi: 10.1016/j.amjmed.2006.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, McDowell I. Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 25.Wang G, Tang HD, Zhuang JP, Xu XH, Liu LH, Li B, Wang LL, Xu ZM, Cheng Q, Chen SD. Risk factors for cognitive decline in elderly people: findings from the two-year follow-up study in a Shanghai urban community. J Alzheimers Dis. 2014;39:891–897. doi: 10.3233/JAD-131514. [DOI] [PubMed] [Google Scholar]
- 26.Tomata Y, Sugiyama K, Kaiho Y, Honkura K, Watanabe T, Zhang S, Sugawara Y, Green Tsuji I. Tea Consumption and the Risk of Incident Dementia in Elderly Japanese: The Ohsaki Cohort 2006 Study. Am J Geriatr Psychiatry. 2016;24:881–889. doi: 10.1016/j.jagp.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Broe GA, Henderson AS, Creasey H, McCusker E, Korten AE, Jorm AF, Longley W, Anthony JC. A case-control study of Alzheimer's disease in Australia. Neurology. 1990;40:1698–1707. doi: 10.1212/wnl.40.11.1698. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Huang Y, Cheng HG. Lower intake of vegetables and legumes associated with cognitive decline among illiterate elderly Chinese: A 3-year cohort study. J Nutr Health Aging. 2012;16:549–552. doi: 10.1007/s12603-012-0023-2. [DOI] [PubMed] [Google Scholar]
- 29.Forster DP, Newens AJ, Kay DW, Edwardson JA. Risk factors in clinically diagnosed presenile dementia of the Alzheimer type: a case-control study in northern England. J Epidemiol Community Health. 1995;49:253–258. doi: 10.1136/jech.49.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang CQ, Dong BR, Zhang YL, Wu HM, Liu QX. Association of cognitive impairment with smoking, alcohol consumption, tea consumption, and exercise among Chinese nonagenarians/centenarians. Cogn Behav Neurol. 2009;22:190–196. doi: 10.1097/WNN.0b013e3181b2790b. [DOI] [PubMed] [Google Scholar]
- 31.Kuriyama S, Hozawa A, Ohmori K, Shimazu T, Matsui T, Ebihara S, Awata S, Nagatomi R, Arai H, Tsuji I. Green tea consumption and cognitive function: a cross-sectional study from the Tsurugaya Project 1. Am J Clin Nutr. 2006;83:355–361. doi: 10.1093/ajcn/83.2.355. [DOI] [PubMed] [Google Scholar]
- 32.Ng TP, Feng L, Niti M, Kua EH, Yap KB. Tea consumption and cognitive impairment and decline in older Chinese adults. Am J Clin Nutr. 2008;88:224–231. doi: 10.1093/ajcn/88.1.224. [DOI] [PubMed] [Google Scholar]
- 33.Wu MS, Lan TH, Chen CM, Chiu HC, Lan TY. Socio-demographic and health-related factors associated with cognitive impairment in the elderly in Taiwan. BMC Public Health. 2011;11:22. doi: 10.1186/1471-2458-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao YH, Xu RF, Tang HD, Jiang GX, Wang Y, Wang G, Chen SD, Cheng Q. Cognitive impairment and associated factors among the elderly in the Shanghai suburb: findings from a low-education population. Neuroepidemiology. 2010;34:245–252. doi: 10.1159/000297751. [DOI] [PubMed] [Google Scholar]
- 35.Wang T, Xiao S, Chen K, Yang C, Dong S, Cheng Y, Li X, Wang J, Zhu M, Yang F, Li G, Su N, Liu Y, et al. Prevalence, Incidence, Risk and Protective Factors of Amnestic Mild Cognitive Impairment in the Elderly in Shanghai. Curr Alzheimer Res. 2016 doi: 10.2174/1567205013666161122094208. [DOI] [PubMed] [Google Scholar]
- 36.Kitamura K, Watanabe Y, Nakamura K, Sanpei K, Wakasugi M, Yokoseki A, Onodera O, Ikeuchi T, Kuwano R, Momotsu T, Narita I, Endo N. Modifiable Factors Associated with Cognitive Impairment in 1,143 Japanese Outpatients: The Project in Sado for Total Health (PROST) Dement Geriatr Cogn Dis Extra. 2016;6:341–349. doi: 10.1159/000447963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alexander DD, Cushing CA, Lowe KA, Sceurman B, Roberts MA. Meta-analysis of animal fat or animal protein intake and colorectal cancer. Am J Clin Nutr. 2009;89:1402–1409. doi: 10.3945/ajcn.2008.26838. [DOI] [PubMed] [Google Scholar]
- 38.Kim YS, Kwak SM, Myung SK. Caffeine intake from coffee or tea and cognitive disorders: a meta-analysis of observational studies. Neuroepidemiology. 2015;44:51–63. doi: 10.1159/000371710. [DOI] [PubMed] [Google Scholar]
- 39.Arab L, Khan F, Lam H. Epidemiologic evidence of a relationship between tea, coffee, or caffeine consumption and cognitive decline. Adv Nutr. 2013;4:115–122. doi: 10.3945/an.112.002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panza F, Solfrizzi V, Barulli MR, Bonfiglio C, Guerra V, Osella A, Seripa D, Sabba C, Pilotto A, Logroscino G. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: a systematic review. J Nutr Health Aging. 2015;19:313–328. doi: 10.1007/s12603-014-0563-8. [DOI] [PubMed] [Google Scholar]
- 41.Tomata Y, Kakizaki M, Nakaya N, Tsuboya T, Sone T, Kuriyama S, Hozawa A, Tsuji I. Green tea consumption and the risk of incident functional disability in elderly Japanese: the Ohsaki Cohort 2006 Study. Am J Clin Nutr. 2012;95:732–739. doi: 10.3945/ajcn.111.023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arab L, Biggs ML, O'Meara ES, Longstreth WT, Crane PK, Fitzpatrick AL. Gender differences in tea, coffee, and cognitive decline in the elderly: the Cardiovascular Health Study. J Alzheimers Dis. 2011;27:553–566. doi: 10.3233/JAD-2011-110431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gholami M, Roushan MH, Mahjoub S, Bijani A. How is total antioxidant status in plasma of Patients with brucellosis? Caspian J Intern Med. 2011;3:363–367. [PMC free article] [PubMed] [Google Scholar]
- 44.Cai ZY, Yan Y. Pathway and mechanism of oxidative stress in Alzheimer's disease. J Med Colleges PLA. 2007;22:320–324. [Google Scholar]
- 45.Mahjoub S, Davary S, Moazezi Z, Qujeq D. Hypolipidemic Effects of Ethanolic and Aqueous Extracts of Urtica Dioica in Rats. World Appl Sci. 2012;17:1345–1348. [Google Scholar]
- 46.Alizadeh M, Mahjoub S, Esmaelzadeh S, Hajian K, Basirat Z, Ghasemi M. Evaluation of oxidative stress in endometriosis: A case-control study. Caspian J Intern Med. 2015;6:25–29. [PMC free article] [PubMed] [Google Scholar]
- 47.Haque AM, Hashimoto M, Katakura M, Hara Y, Shido O. Green tea catechins prevent cognitive deficits caused by Abeta1-40 in rats. J Nutr Biochem. 2008;19:619–626. doi: 10.1016/j.jnutbio.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 48.Luczaj W, Waszkiewicz E, Skrzydlewska E, Roszkowska-Jakimiec W. Green tea protection against age-dependent ethanol-induced oxidative stress. J Toxicol Environ Health A. 2004;67:595–606. doi: 10.1080/15287390490425579. [DOI] [PubMed] [Google Scholar]
- 49.Wu LY, Juan CC, Ho LT, Hsu YP, Hwang LS. Effect of green tea supplementation on insulin sensitivity in Sprague-Dawley rats. J Agric Food Chem. 2004;52:643–648. doi: 10.1021/jf030365d. [DOI] [PubMed] [Google Scholar]
- 50.Tsuneki H, Ishizuka M, Terasawa M, Wu JB, Sasaoka T, Kimura I. Effect of green tea on blood glucose levels and serum proteomic patterns in diabetic (db/db) mice and on glucose metabolism in healthy humans. BMC Pharmacol. 2004;4:18. doi: 10.1186/1471-2210-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arab L, Liu W, Elashoff D. Green and black tea consumption and risk of stroke: a meta-analysis. Stroke. 2009;40:1786–1792. doi: 10.1161/STROKEAHA.108.538470. [DOI] [PubMed] [Google Scholar]
- 52.Setiawan VW, Zhang ZF, Yu GP, Lu QY, Li YL, Lu ML, Wang MR, Guo CH, Yu SZ, Kurtz RC, Hsieh CC. Protective effect of green tea on the risks of chronic gastritis and stomach cancer. Int J Cancer. 2001;92:600–604. doi: 10.1002/ijc.1231. [DOI] [PubMed] [Google Scholar]
- 53.Yamada M, Ono K, Hamaguchi T, Noguchi-Shinohara M. Natural Phenolic Compounds as Therapeutic and Preventive Agents for Cerebral Amyloidosis. Adv Exp Med Biol. 2015;863:79–94. doi: 10.1007/978-3-319-18365-7_4. [DOI] [PubMed] [Google Scholar]
- 54.Ono K, Yoshiike Y, Takashima A, Hasegawa K, Naiki H, Yamada M. Potent anti-amyloidogenic and fibril-destabilizing effects of polyphenols in vitro: implications for the prevention and therapeutics of Alzheimer's disease. J Neurochem. 2003;87:172–181. doi: 10.1046/j.1471-4159.2003.01976.x. [DOI] [PubMed] [Google Scholar]
- 55.Nathan PJ, Lu K, Gray M, Oliver C. The neuropharmacology of L-theanine(N-ethyl-L-glutamine): a possible neuroprotective and cognitive enhancing agent. J Herb Pharmacother. 2006;6:21–30. [PubMed] [Google Scholar]
- 56.Dodd FL, Kennedy DO, Riby LM, Haskell-Ramsay CF. A double-blind, placebo-controlled study evaluating the effects of caffeine and L-theanine both alone and in combination on cerebral blood flow, cognition and mood. Psychopharmacology (Berl) 2015;232:2563–2576. doi: 10.1007/s00213-015-3895-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haskell CF, Kennedy DO, Milne AL, Wesnes KA, Scholey AB. The effects of L-theanine, caffeine and their combination on cognition and mood. Biol Psychol. 2008;77:113–122. doi: 10.1016/j.biopsycho.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Chen X, Ghribi O, Geiger JD. Caffeine protects against disruptions of the blood-brain barrier in animal models of Alzheimer's and Parkinson's diseases. J Alzheimers Dis. 2010;20:S127–141. doi: 10.3233/JAD-2010-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ritchie K, Artero S, Portet F, Brickman A, Muraskin J, Beanino E, Ancelin ML, Carriere I. Caffeine, cognitive functioning, and white matter lesions in the elderly: establishing causality from epidemiological evidence. J Alzheimers Dis. 2010;20:S161–166. doi: 10.3233/JAD-2010-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gelber RP, Petrovitch H, Masaki KH, Ross GW, White LR. Coffee intake in midlife and risk of dementia and its neuropathologic correlates. J Alzheimers Dis. 2011;23:607–615. doi: 10.3233/JAD-2010-101428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 62.Vercambre MN, Berr C, Ritchie K, Kang JH. Caffeine and cognitive decline in elderly women at high vascular risk. J Alzheimers Dis. 2013;35:413–421. doi: 10.3233/JAD-122371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dall'Igna OP, Fett P, Gomes MW, Souza DO, Cunha RA, Lara DR. Caffeine and adenosine A(2a) receptor antagonists prevent beta-amyloid (25-35)-induced cognitive deficits in mice. Exp Neurol. 2007;203:241–245. doi: 10.1016/j.expneurol.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 64.Arendash GW, Schleif W, Rezai-Zadeh K, Jackson EK, Zacharia LC, Cracchiolo JR, Shippy D, Tan J. Caffeine protects Alzheimer's mice against cognitive impairment and reduces brain beta-amyloid production. Neuroscience. 2006;142:941–952. doi: 10.1016/j.neuroscience.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 65.Cao C, Cirrito JR, Lin X, Wang L, Verges DK, Dickson A, Mamcarz M, Zhang C, Mori T, Arendash GW, Holtzman DM, Potter H. Caffeine suppresses amyloid-beta levels in plasma and brain of Alzheimer's disease transgenic mice. J Alzheimers Dis. 2009;17:681–697. doi: 10.3233/JAD-2009-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arendash GW, Mori T, Cao C, Mamcarz M, Runfeldt M, Dickson A, Rezai-Zadeh K, Tane J, Citron BA, Lin X, Echeverria V, Potter H. Caffeine reverses cognitive impairment and decreases brain amyloid-beta levels in aged Alzheimer's disease mice. J Alzheimers Dis. 2009;17:661–680. doi: 10.3233/JAD-2009-1087. [DOI] [PubMed] [Google Scholar]
- 67.Li W, Yang J, Zhu XS, Li SC, Ho PC. Correlation between tea consumption and prevalence of hypertension among Singaporean Chinese residents aged 40 years. J Hum Hypertens. 2016;30:11–17. doi: 10.1038/jhh.2015.45. [DOI] [PubMed] [Google Scholar]
- 68.Zheng XX, Xu YL, Li SH, Liu XX, Hui R, Huang XH. Green tea intake lowers fasting serum total and LDL cholesterol in adults: a meta-analysis of 14 randomized controlled trials. Am J Clin Nutr. 2011;94:601–610. doi: 10.3945/ajcn.110.010926. [DOI] [PubMed] [Google Scholar]
- 69.Arnett DK, Tyroler HA, Burke G, Hutchinson R, Howard G, Heiss G. Hypertension and subclinical carotid artery atherosclerosis in blacks and whites. The Atherosclerosis Risk in Communities Study. ARIC Investigators. Arch Intern Med. 1996;156:1983–1989. [PubMed] [Google Scholar]
- 70.Etgen T, Sander D, Bickel H, Forstl H. Mild cognitive impairment and dementia: the importance of modifiable risk factors. Dtsch Arztebl Int. 2011;108:743–750. doi: 10.3238/arztebl.2011.0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin YS, Tsai YJ, Tsay JS, Lin JK. Factors affecting the levels of tea polyphenols and caffeine in tea leaves. J Agric Food Chem. 2003;51:1864–1873. doi: 10.1021/jf021066b. [DOI] [PubMed] [Google Scholar]
- 72.Engelhart MJ, Geerlings MI, Ruitenberg A, van Swieten JC, Hofman A, Witteman JC, Breteler MM. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287:3223–3229. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- 73.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 74.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: University of Ottawa; 2011. [Google Scholar]
- 75.Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev. 2007;16:2533–2547. doi: 10.1158/1055-9965.EPI-07-0708. [DOI] [PubMed] [Google Scholar]
- 76.Orsini N. Generalized least squares for trend estimation of summarized dose–response data. Stata Journal. 2006;6:40–57. [Google Scholar]
- 77.Greenland S, Longnecker MP. Methods for Trend Estimation from Summarized Dose-Response Data, with Applications to Meta-Analysis. Am J Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 78.Ding M, Bhupathiraju SN, Chen M, van Dam RM, Hu FB. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: a systematic review and a dose-response meta-analysis. Diabetes Care. 2014;37:569–586. doi: 10.2337/dc13-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tobias A. Assessing the influence of a single study in meta-analysis. Stata Tech Bull. 1999;47:15–17. [Google Scholar]
- 80.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


