Abstract
This study aims to determine whether male sex has adverse effect on mortality and morbidities in very low birth weight infants (VLBWI) <30 weeks of gestation and to ascertain this sex effect, stratified by gestational age, adjusting for perinatal risk factors. This is a population-based study from Korean Neonatal Network for VLBWI born at 23+0 and 29+6 weeks of gestation between January 2013 and December 2014. The primary outcome was gestation-specific sex difference in the occurrence of mortality, combined morbidities, and individual morbidity. A total of 2228 VLBWI were enrolled (males, 51.7%). Mortality was not different between sexes. The risk of bronchopulmonary dysplasia and combined morbidities was significantly higher in males ≤25 weeks of gestation (odds ratio [OR] 2.08, 95% confidence interval [CI] 1.35–3.20 and OR 2.00, CI 1.19–3.39, respectively). Males had a significantly higher incidence of periventricular leukomalacia at 23 and 29 weeks of gestation. The risk of severe retinopathy of prematurity was higher in females >25 weeks of gestation. Although both sexes have similar risk for mortality, male sex remains an independent risk for major morbidities, especially at ≤25 weeks of gestation. The risk of each outcome for males has a specific pattern with increasing gestational age.
Introduction
Preterm birth is defined by the World Health Organization (WHO) as all births before 37 completed weeks of gestation1. Infants were divided according to birth weight into very low birth weight infants (VLBWIs; infants born with <1,500 g) and extremely low birth weight infants (infants born with <1,000 g)2. Approximately 15 million infants are born preterm each year, representing a preterm birth rate of 11.1%3. In Korea4, preterm birth accounts for approximately 8% of live birth, similar to East Asia (7.4%) and Western countries (8.6%)3. Complications of preterm birth are the single largest direct cause of neonatal deaths, responsible for 35% of the world’s 3.1 million deaths a year5, with VLBWI being a main cause of neonatal death and major complications6. To date, a number of guidelines for clinicians and parents on neonatal outcomes at various gestational ages have been developed7–9. Many factors are known to affect the mortality and morbidities of preterm infants. Among them, higher birth weight, higher gestational age, and the use of antenatal steroid have been consistently linked to better prognosis10. However, information on gestation-specific sex effect on outcomes is very limited10–12. Sex difference in clinical study is important because hormonal, physiological, and developmental differences between males and females could lead to sex-specific outcomes13. Previous studies have shown that the male sex is associated with an increased risk of mortality in preterm infants10, 14–16. Male infants are also associated with worse respiratory outcomes, such as respiratory distress syndrome and bronchopulmonary dysplasia (BPD), as well as intraventricular hemorrhage (IVH) and retinopathy of prematurity (ROP)10, 13, 17–20. Later surfactant production and higher density of androgen receptors in male fetus contribute to increased alveolar resistance and the modulation of bronchiole budding in the early fetal lung21–23, and these effects may continue and worsen the respiratory outcome in males after birth. Recently, a few studies have not found any difference in sex and mortality, due to progress in neonatal intensive care11, 24. Although gestational age is the most powerful factor for neonatal outcomes, only a few studies showed the sex effects, adjusted by gestational age10, 12. Furthermore, as far as we know, there were no sex studies that considered various perinatal factors including antenatal steroid use, maternal diabetes, chorioamnionitis, intrauterine growth restriction (IUGR), and multiple births in preterm infants.
The present study is a retrospective observational study based on the Korean Neonatal Network (KNN), which is a nationwide database on VLBWI across South Korea. The aim of the present study was to investigate whether male sex has disadvantage on mortality and short-term morbidities in preterm infants. Because mortality and major morbidities frequently occur at gestational age ≤29 weeks, with lower risk from 30 weeks onward24–26, we focused on VLBWIs born at <30 weeks of gestation. We aimed to ascertain sex effect, stratified by gestational age in this population, adjusting for perinatal risk factors that may affect neonatal outcomes. Furthermore, because previous studies have reported that preterm infants born at ≤25 weeks of gestation showed especially higher mortality and morbidities9, 11, 25, 27, 28, we performed a subgroup analysis according to gestational age group, from 23 to 25 weeks of gestation and from 26 to 29 weeks of gestation.
Results
Population
A total of 2228 VLBWIs (males, n = 1151, 51.7%; females, n = 1077, 41.3%) with gestational age of <30 weeks were registered to the KNN during the study period. Severe congenital anomaly was noted in 65 infants (33 males and 32 females). In male infants, there were 6 congenital heart diseases, 4 genitourinary tract defects, 2 central nervous system anomalies, 12 digestive organ anomalies, 1 pulmonary abnormality, 2 chromosomal anomalies, and 5 other anomalies. In female infants, there were 12 congenital heart diseases, 1 genitourinary tract defect, 2 central nervous system anomalies, 10 digestive organ anomalies, 1 pulmonary abnormality, 1 chromosomal anomaly, and 5 other anomalies. The number of male and female infants in the two gestational age groups and at individual gestational age is shown in Fig. 1.
Figure 1.
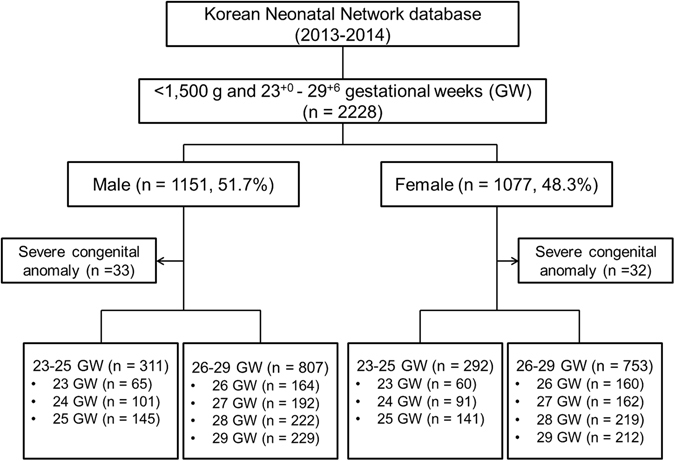
Study population from the Korean Neonatal Network database.
Infants’ characteristics
There was no significant sex difference in gestational age, delivery mode, outborn status, Apgar scores, length of hospital stay, and duration on ventilation. The rate of IUGR was significantly higher in female infants (P = 0.026), but in the subgroup analysis, this significance disappeared. Overall, male infants had higher birth weight (P < 0.001), height (P < 0.001), and head circumference (P = 0.001) at birth and at discharge (all Ps < 0.001). In the subgroup analysis, the 23–25 weeks of gestation group showed that male infants had higher weight and head circumference at birth (P < 0.001 and P = 0.002, respectively) and at discharge (all Ps = 0.028). In the 26–29 weeks of gestation group, male infants had higher birth weight, height, and head circumference at birth (all Ps < 0.001) and at discharge (P = 0.009, P = 0.007, and P = 0.018, respectively) (Table 1).
Table 1.
Infants’ characteristics and sex difference according to gestational age group.
| Variables | Total enrolled infants | 23–25 weeks of gestation group | 26–29 weeks of gestation group | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Male (n = 1118) | Female (n = 1045) | P | Male (n = 311) | Female (n = 292) | P | Male (n = 807) | Female (n = 753) | P | |
| Gestational age (weeks) | 27+1 (2+0) | 27+1 (2+0) | 0.971 | 24+5 (0+2) | 24+5 (0+2) | 0.752 | 28+0 (1+2) | 28+0 (1+2) | 0.971 |
| Cesarean section | 773 (69.1) | 738 (70.6) | 0.453 | 211 (67.8) | 190 (65.1) | 0.470 | 562 (69.6) | 548 (72.8) | 0.172 |
| Outborn | 47 (4.2) | 37 (3.5) | 0.425 | 10 (3.2) | 11 (3.8) | 0.712 | 37 (4.6) | 26 (3.5) | 0.256 |
| Intrauterine growth restriction | 63 (5.6) | 84 (8.0) | 0.026 | 19 (6.1) | 26 (8.9) | 0.192 | 44 (5.5) | 58 (7.7) | 0.072 |
| Birth weight (g) | 1009 (261) | 946 (259) | <0.001 | 746 (136) | 700 (137) | <0.001 | 1111 (224) | 1041 (230) | <0.001 |
| Height at birth (cm) | 35.5 (3.4) | 34.8 (3.4) | <0.001 | 32.2 (2.3) | 31.8 (2.2) | 0.087 | 36.6 (3.0) | 35.8 (3.1) | <0.001 |
| Head circumference at birth (cm) | 25.1 (2.2) | 24.5 (2.2) | 0.001 | 22.8 (1.3) | 22.4 (1.6) | 0.002 | 25.8 (1.9) | 25.2 (1.8) | <0.001 |
| Weight at discharge (g) | 2553 (954) | 2418 (907) | <0.001 | 2384 (1287) | 2164 (1154) | 0.028 | 2619 (781) | 2516 (770) | 0.009 |
| Height at discharge (cm) | 45.0 (5.1) | 44.2 (5.4) | <0.001 | 43.7 (6.6) | 42.5 (7.0) | 0.057 | 45.4 (4.3) | 44.8 (4.5) | 0.007 |
| Head circumference at discharge (cm) | 32.1 (3.3) | 31.6 (3.5) | <0.001 | 31.4 (4.6) | 30.5 (4.8) | 0.028 | 32.3 (2.7) | 32.0 (2.8) | 0.018 |
| Apgar scores 1 min | 3.3 (1.6) | 3.4 (1.8) | 0.278 | 3.2 (1.6) | 3.1 (1.7) | 0.413 | 4.4 (1.9) | 4.6 (1.8) | 0.070 |
| Apgar scores 5 min | 4.1 (1.9) | 4.1 (1.9) | 0.775 | 5.6 (1.8) | 5.3 (2.0) | 0.064 | 6.7 (1.7) | 6.8 (1.6) | 0.292 |
| Length of stay (days) | 76 (44) | 76 (43) | 0.939 | 88 (62) | 84 (57) | 0.383 | 72 (33) | 74 (37) | 0.306 |
| Duration on ventilation (days) | 43.8 (39.7) | 41.9 (38.8) | 0.241 | 63.2 (50.1) | 52.5 (43.0) | 0.133 | 36.4 (31.8) | 35.8 (35.3) | 0.736 |
Data are presented as n (%) or mean (SD).
Maternal characteristics
The prevalence of maternal diabetes, histologic chorioamnionitis, multiple births, and the use of antenatal steroid were not different between sexes (Table 2). The mothers of the female infants in the 26–29 weeks of gestation group had a higher rate of maternal hypertension (P = 0.044).
Table 2.
Infants’ sex difference in maternal records according to gestational age group.
| Variables | Total study population | 23–25 weeks of gestation group | 26–29 weeks of gestation group | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Male (n = 1118) | Female (n = 1045) | P | Male (n = 311) | Female (n = 292) | P | Male (n = 807) | Female (n = 753) | P | |
| Multiple births | 367 (32.8) | 336 (32.2) | 0.738 | 126 (40.5) | 101 (34.6) | 0.133 | 241 (29.9) | 235 (31.2) | 0.564 |
| Maternal diabetes | 98 (8.8) | 73 (7.0) | 0.125 | 17 (5.5) | 14 (4.8) | 0.709 | 81 (10.0) | 59 (7.8) | 0.709 |
| Maternal hypertension | 117 (10.5) | 139 (13.3) | 0.041 | 17 (5.5) | 19 (6.5) | 0.590 | 100 (12.4) | 120 (15.9) | 0.044 |
| Chorioamnionitis | 386 (34.5) | 351 (33.6) | 0.646 | 151 (48.6) | 129 (44.2) | 0.282 | 235 (29.1) | 222 (29.5) | 0.875 |
| Antenatal steroid | 881 (78.8) | 825 (78.9) | 0.934 | 235 (75.6) | 217 (74.3) | 0.724 | 646 (80.0) | 608 (80.7) | 0.730 |
Data are presented as n (%).
Mortality and morbidities
Mortality was not different between male and female infants. In all enrolled infants, males had a higher prevalence of combined morbidity (P = 0.011). Males also showed a higher occurrence of BPD (P = 0.012), IVH grade ≥3 (P = 0.049), and cystic periventricular leukomalacia (PVL) (P = 0.014) (Table 3). In the analysis according to individual gestational age, the odds ratio (OR) of the male versus female sex for combined morbidities was the highest at 23 weeks of gestation (OR 5.0, 95% confidence interval [CI] 1.1–55.7, P = 0.038). This OR tended to decrease and then lost significance from 26 weeks of gestation. BPD showed a similar pattern; the OR of males on BPD was significant at 24 and 25 weeks of gestation (OR 2.7, 95% CI 1.2–6.3, P = 0.020 and OR 1.9, 95% CI 1.1–3.3, P = 0.004, respectively), and this significance disappeared from 26 weeks of gestation. Males had a significantly higher incidence of PVL at 23 and 29 weeks of gestation (OR 4.0, 95% CI 1.1–15.5, P = 0.042 and OR 2.4, 95% CI 1.1–5.0, P = 0.026, respectively). In contrast, males had a lower incidence of ROP at 26 and 27 weeks of gestation (OR 0.5, 95% CI 0.3–0.9, P = 0.026 and OR 0.4, 95% CI 0.2–0.9, P = 0.022, respectively). There was no significant sex difference in IVH grade ≥3 and necrotizing enterocolitis (NEC) at any gestation age (Table 4).
Table 3.
Sex difference for the occurrence of mortality and the prevalence of major neonatal morbidities, stratified by gestational age group.
| Variables | Subgroup | Male (n, %) | Female (n, %) | P (χ 2) | Adjusted OR* (95% CI) | P* |
|---|---|---|---|---|---|---|
| Mortality | Total | 179/1118 (16.0) | 165/1045 (15.8) | 0.888 | 1.04 (0.82–1.31) | 0.756 |
| 23–25 wk | 108/311 (34.7) | 96/292 (32.9) | 0.631 | 1.13 (0.79–1.59) | 0.508 | |
| 26–29 wk | 71/807 (8.8) | 69/753 (9.2) | 0.801 | 0.97 (0.68–1.39) | 0.872 | |
| BPD | Total | 419/951 (44.1) | 346/888 (39.0) | 0.027 | 1.28 (1.06–1.54) | 0.012 |
| 23–25 wk | 155/210 (73.8) | 113/196 (57.7) | 0.001 | 2.08 (1.35–3.20) | 0.001 | |
| 26–29 wk | 264/741 (35.6) | 233/692 (33.7) | 0.437 | 1.12 (0.90–1.40) | 0.308 | |
| IVH, grade ≥ 3 | Total | 176/1082 (16.3) | 130/1002 (13.0) | 0.034 | 1.28 (1.01–1.65) | 0.049 |
| 23–25 wk | 99/292 (33.9) | 73/268 (27.2) | 0.088 | 1.38 (0.95–2.01) | 0.096 | |
| 26–29 wk | 77/790 (9.7) | 57/734 (7.8) | 0.172 | 1.27 (0.88–1.84) | 0.196 | |
| PVL | Total | 140/1073 (13.0) | 95/994 (9.6) | 0.013 | 1.42 (1.07–1.87) | 0.014 |
| 23–25 wk | 47/285 (16.5) | 33/262 (12.6) | 0.198 | 1.27 (0.78–2.08) | 0.334 | |
| 26–29 wk | 93/788 (11.8) | 62/732 (8.5) | 0.032 | 1.47 (1.04–2.06) | 0.028 | |
| ROP, grade ≥ 3 | Total | 159/952 (16.7) | 170/902 (18.8) | 0.227 | 0.89 (0.70–1.13) | 0.343 |
| 23–25 wk | 105/209 (50.2) | 84/202 (41.6) | 0.078 | 1.37 (0.92–2.04) | 0.123 | |
| 26–29 wk | 54/743 (7.3) | 86/700 (12.3) | 0.001 | 0.59 (0.41–0.85) | 0.004 | |
| NEC | Total | 109/1116 (9.8) | 81/1039 (7.8) | 0.107 | 1.34 (0.99–1.81) | 0.062 |
| 23–25 wk | 52/311 (16.7) | 41/290 (14.1) | 0.382 | 1.27 (0.80–2.01) | 0.305 | |
| 26–29 wk | 57/805 (7.1) | 40/749 (5.3) | 0.156 | 1.44 (0.94–2.20) | 0.094 | |
| Combined morbidities | Total | 521/941 (55.4) | 444/882 (50.3) | 0.032 | 1.27 (1.06–1.54) | 0.011 |
| 23–25 wk | 179/208 (86.1) | 143/191 (74.9) | 0.005 | 2.00 (1.19–3.39) | 0.009 | |
| 26–29 wk | 342/733 (46.7) | 301/691 (43.6) | 0.240 | 1.18 (0.95–1.46) | 0.128 |
*Values are odds ratio and 95% confidence interval. Values are adjusted by gestational age, histologic chorioamnionitis, intrauterine growth restriction, maternal diabetes, maternal hypertension, multiple births, delivery mode, and the use of antenatal steroid.
Table 4.
Sex difference for the occurrence of mortality and the prevalence of major neonatal morbidities, stratified by individual gestational age.
| GA (weeks) | Mortality | BPD | IVH, grade ≥ 3 | PVL | ROP, grade ≥ 3 | NEC | Combined morbidities | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR* (95% CI) | P | OR* (95% CI) | P | OR* (95% CI) | P | OR* (95% CI) | P | OR* (95% CI) | P | OR* (95% CI) | P | OR* (95% CI) | P | |
| 23 | 0.93 (0.44–2.07) | 0.821 | 2.86 (0.57–14.54) | 0.266 | 2.41 (0.97–5.30) | 0.057 | 4.05 (1.06–15.5) | 0.042 | 2.90 (0.83–10.02) | 0.082 | 1.98 (0.73–5.40) | 0.121 | 5.00 (1.10–55.67) | 0.038 |
| 24 | 1.15 (0.62–2.15) | 0.993 | 2.72 (1.18–6.25) | 0.020 | 1.50 (0.77–2.66) | 0.183 | 1.33 (0.50–3.54) | 0.538 | 1.36 (0.65–2.86) | 0.298 | 0.97 (0.61–2.13) | 0.959 | 1.68 (0.58–4.90) | 0.383 |
| 25 | 1.14 (0.63–2.04) | 0.423 | 1.86 (1.05–3.29) | 0.004 | 1.04 (0.58–1.93) | 0.721 | 0.98 (0.49–1.93) | 0.802 | 1.20 (0.68–2.11) | 0.333 | 1.34 (0.64–2.75) | 0.854 | 2.09 (1.07–4.09) | 0.012 |
| 26 | 1.11 (0.62–1.97) | 0.781 | 1.26 (0.77–2.05) | 0.315 | 0.884 (0.47–1.65) | 0.657 | 1.29 (0.67–2.48) | 0.193 | 0.50 (0.27–0.92) | 0.026 | 1.20 (0.56–2.57) | 0.580 | 1.20 (0.71–2.03) | 0.640 |
| 27 | 1.03 (0.48–2.23) | 0.634 | 1.06 (0.67–1.68) | 0.951 | 1.72 (0.84–3.53) | 0.109 | 1.31 (0.64–2.68) | 0.562 | 0.41 (0.19–0.88) | 0.022 | 1.79 (0.83–3.89) | 0.132 | 1.06 (0.67–1.67) | 0.966 |
| 28 | 0.81 (0.35–1.86) | 0.368 | 1.32 (0.86–2.03) | 0.272 | 1.06 (0.46–2.46) | 0.782 | 1.40 (0.70–2.80) | 0.238 | 1.07 (0.49–2.31) | 0.936 | 1.83 (0.70–4.76) | 0.165 | 1.48 (0.99–2.23) | 0.239 |
| 29 | 1.08 (0.39–3.00) | 0.406 | 1.01 (0.61–1.65) | 0.845 | 2.44 (0.74–8.05) | 0.267 | 2.36 (1.11–5.02) | 0.026 | 0.44 (0.13–1.54) | 0.134 | 1.01 (0.27–4.00) | 0.892 | 0.99 (0.64–1.55) | 0.929 |
*Values are adjusted odds ratio and 95% confidence interval. Values are adjusted by histologic chorioamnionitis, intrauterine growth restriction, maternal diabetes, maternal hypertension, multiple births, delivery mode, and the use of antenatal steroid according to individual gestational age (GA).
Subgroup analysis demonstrated that male infants ≤25 weeks of gestation had a higher incidence of combined morbidities (OR 2.0, 95% CI 1.2–3.4, P = 0.009) and BPD (OR 2.1, 95% CI 1.4–3.2, P = 0.001). In the 26–29 weeks of gestation group, female infants had a higher incidence of ROP (OR 0.6, 95% CI 0.4–0.9, P = 0.004), and male infants had a higher incidence of PVL (OR 1.5, 95% CI 1.0–2.1, P = 0.028) (Table 3).
Discussion
In the present study, male VLBWIs born <30 weeks of gestation had similar incidence of mortality compared with female infants but had higher prevalence of combined morbidities and higher occurrence of BPD, severe IVH, and PVL. In contrast to previous studies15, 16, 29, the present study performed subgroup analysis according to gestational age and could suggest that male infants have a higher risk for BPD and combined morbidities under 25 weeks of gestation. Furthermore, male infants present specific pattern of risk for each outcome with increasing gestational age.
To date, many studies have confirmed a survival advantage for female infants compared with male infants10, 14–16, 30. Only a few studies did not find any difference in mortality between sexes11, 24; a Canadian cohort study11 suggested that there was no significant sex difference in mortality at >24 weeks of gestation, but survival was higher in female infants born at 24 weeks of gestation. Ray and Platt24 reported no effect of sex on mortality either for all gestations or among infants at 23–25 weeks of gestation. Our finding of no sex difference in mortality adds weight to those studies. Several possible reasons can be considered. The use of antenatal steroids and exogenous surfactant may have improved survival rate, more notably in male than in female infants. The KNN registry does not include information on stillbirth after onset of labor or delivery room death of live-born infants. This might affect our mortality results because the male disadvantage for adverse outcomes commences from early pregnancy31. Our data showed that female infants have significantly lower birth weight compared with male infants at the same gestational age. This finding was also observed in other studies10, 12, 32, and recently, a preterm growth chart for each sex has been provided33. Many previous studies that reported sex difference were stratified by birth weight14, 15, which might lead to the comparison of more mature female infants with less mature male infants, resulting in female advantage for survival. However, not all sex differences were eliminated in the present study, and female infants continue to have lower incidences of major morbidities. Previous studies have shown increased risk for BPD and IVH in male infants born preterm10, 13, 20. The present study analyzed sex difference, stratified by gestational age, and we found that the risk for combined morbidities and BPD was higher in male infants with ≤25 weeks of gestation compared with female infants of same gestational age; the OR of males gradually decreased with increasing gestational age and then lost significance from 26 weeks of gestation. Severe ROP has a similar pattern; however, it showed opposite OR results as of 25 weeks of gestation. These patterns suggest that 25 weeks of gestation seems to be a divided time point of male disadvantage for adverse neonatal outcomes. The subgroup analysis supported it with more detailed data – male infants in the 23–25 weeks of gestation group demonstrated a doubling of risk for combined morbidities and BPD, whereas the 26–29 weeks of gestation group showed a similar risk between sexes. For severe IVH, PVL, and NEC, male infants had generally higher risk throughout gestations compared with female infants, in terms of OR. The sex benefit of females is multifactorial and has an effect on prenatal and postnatal development13, 19, 34. To date, several animal studies had suggested possible pathologies for female advantage in neonatal outcomes. A different hormonal milieu in females is associated with increased organ maturation, compared with males35. In mice, males are known to have a higher arrest in alveolization and pulmonary angiogenesis and more inflammation compared with females13. Better antioxidant defense mechanism in female, including higher expression of glutathione peroxidase and superoxide dismutase36, 37, might contribute to female advantage in lung or brain injury in the perinatal period. A study reported that after resuscitation, the blood-brain barrier is better preserved and neuronal injury is lower in female piglets compared with male piglets19. These results could explain the increased incidence of BPD, IVH, or PVL in male infants of the present study but could not explain the gestation-specific sex effect on major outcomes. Until now, studies for gestation specific-sex difference are very limited10, 12, 38. Binet et al.10 reported the sex effect on short-term outcomes of extremely premature infants (≤27 weeks of gestation) born in Canada and found that the prevalence of BPD for infants born between 24 and 26 weeks were higher in males. Although their study is different from ours in study population and statistics, their finding is generally consistent with the present study. Ito et al.38 stated that male sex was a disadvantage for BPD in preterm infants at ≥26 weeks of gestation by using a 10-year database from the neonatal research network in Japan. As the medical situations and the study period were different in this database, the results seen were different from those in our study. In contrast to Binet et al.10 and Ito et al.38, the present study adjusted the perinatal factors that might affect neonatal outcomes and demonstrated that male sex is an independent risk factor of major morbidities, especially in infants at ≤25 weeks of gestation. Interestingly, in contrast to previous studies17, 39, 40, the present study found that severe ROP occurred more often in female infants after 26 weeks of gestation, and this remains after adjusting for perinatal risk factors including IUGR, maternal hypertension, use of antenatal steroid, and so on. At present, the explanation for the better outcome of ROP in male infants at this gestational period is unclear. Insulin-like growth factor 1 (IGF-1) is critical to normal vascular development, and the duration of low IGF-1 concentrations is strongly correlated with severity of ROP41, 42. Additional investigations for IGF-1 and sex according to gestations are warranted.
The strength of our study is that it is based on a large, geographical cohort, including approximately 70% of VLBWI in South Korea; therefore, the present study has greater relevance than a single-centered study. Although the present study was a retrospective study, data collection was done prospectively using the same strict guidelines of the KNN. However, we could not evaluate the long-term neurological outcome. Previous studies have demonstrated a correlation between short-term morbidities and long-term neurological outcomes43, 44.
Conclusion
Male VLBWIs born at <30 weeks of gestation have similar incidence of mortality compared with female infants, which remains after adjusting for perinatal risk factors that may affect neonatal outcomes. However, male infants showed increased risk for combined morbidity, BPD, severe IVH, and PVL. Each outcome for males has a specific risk pattern with increasing gestational age. Subgroup analysis showed that the risk for combined morbidities and BPD is higher in males ≤25 weeks of gestation. In contrast, severe ROP occurred more often in females >25 weeks of gestation. Our results showed that sex difference, stratified by gestational age, is a major factor for neonatal outcomes of preterm infants. A guideline on neonatal outcomes that includes male disadvantage, especially for BPD and combined morbidities in infants ≤25 weeks of gestation, will help clinicians’ understanding and parents’ education.
Methods
This is a population-based study of VLBWIs born 23+0 and 29+6 weeks of gestation between January 2013 and December 2014 and admitted to a neonatal intensive care unit (NICU) participating in the KNN6. Infants with severe congenital anomaly were excluded. The KNN is a nationwide database on VLBWI from 69 hospitals across South Korea. VLBWIs in these hospitals accounted for approximately 70% of VLBWIs in South Korea. Data were collected at each participating center and entered into a data program, and the study variables were defined according to the KNN manual. The present study was approved by the KNN data management committee.
Ethics statement
The KNN registry was approved by the institutional review board (IRB) at each participating hospital, and informed consent was obtained from the parents at enrollment by the NICUs participating in the KNN. All methods were carried out in accordance with the IRB-approved protocol and in compliance with relevant guidelines and regulations.
Primary outcome
The primary outcome was gestation-specific sex difference in the occurrence of mortality and the prevalence of combined morbidities among five outcomes and individual morbidity.
To identify gestation-specific sex outcomes, outcomes were tested at individual gestational age from 23+0 to 29+6 weeks of gestation. Furthermore, we divided the enrolled infants into two gestational age groups: the 23+0–25+6 weeks of gestation group and the 26+0–29+6 weeks of gestation group.
Data collection
The infants’ data were collected from the KNN database (Fig. 1). Sex, gestational age, anthropometry measurements (including weight, height, and head circumference at birth and at discharge), delivery mode, Apgar score, outborn status, and IUGR were included. Length of hospital stay and duration on mechanical ventilation were also reviewed. Maternal data including multiple births, diabetes, hypertension, histological chorioamnionitis, and use of antenatal steroid were recorded. The following outcomes were collected for each sex: mortality during NICU hospitalization, BPD, IVH grade ≥3, PVL, ROP grade ≥3, and NEC.
Definitions
VLBWI was defined infants born <1,500 g according to the WHO2. BPD was defined as a requirement for supplemental oxygen at 36 weeks of postmenstrual age45. IVH was defined using Papile’s criteria by cranial ultrasonography46. Because imaging was conducted using the individual policy of each hospital, the worst grade among several cranial ultrasonography results during hospitalization was adopted. PVL included only cystic PVL revealed by cranial ultrasonography or magnetic resonance imaging which was performed during hospitalization. NEC was defined according to Bell’s criteria (stage 2 or higher)47. ROP was defined according to the international classification of ROP48, and the maximum stage of ROP was adopted. To investigate the prevalence of combined morbidities, the present study developed the variable ‘combined morbidities’, which was defined as having one and more morbidities among the five morbidities. To develop the variable of combined morbidities, the value of each morbidity (0: no present, 1: present) was added, and the ‘0’ value in the sum was use to designate no combined morbidities. IUGR was defined as birth weight less than the 10th percentile for the gestational age based on a sex-specific growth chart33.
Statistical analysis
Statistical analyses were performed using SPSS. Data are expressed as number (%) with OR and 95% CI or mean (standard deviation). Univariate analyses of categorical variables were performed by χ 2 test, and t test was used for continuous variables. Adjusted sex difference in mortality and morbidities was tested by multiple logistic regression. Multiple logistic regression was performed at individual gestational age and then for the two gestational age groups. Criteria for entry and removal were P < 0.05 and P > 0.10, respectively. The fit of models was checked with the Hosmer-Lemeshow goodness-of-fit test49. A P value < 0.05 with two-tailed comparisons was considered significant.
Data Availability
The datasets analyzed during the current study are not publicly available due to the policy of Research of Korea Centers for Disease Control and Prevention but are available from the corresponding author on reasonable request.
Acknowledgements
This research was supported by a fund (2016-ER6307-00#) by Research of Korea Centers for Disease Control and Prevention.
Author Contributions
S.S.Y. conceptualized and designed the study, drafted the initial manuscript, and approved the final manuscript as submitted. K.K.A. carried out the initial analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted. C.S.J. and P.E.A. conceptualized and designed the study, and supervised data collection, critically reviewed the manuscript, and approved the final manuscript as submitted.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths. Modifications recommended by FIGO as amended October 14, 1976. Acta Obstet Gynecol Scand. 1977;56:247–253. [PubMed] [Google Scholar]
- 2.UNICEF. Low birthweight country, regional and global estimates. (2004).
- 3.Blencowe H, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 4.Shin SM, et al. Low birth weight, very low birth weight rates and gestational age-specific birth weight distribution of Korea newborn infant. J Korean Med Sci. 2005;20:182–187. doi: 10.3346/jkms.2005.20.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blencowe H, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10(Suppl 1):S2. doi: 10.1186/1742-4755-10-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang YS, Park HY, Park WS. The Korean Neonatal Network: An Overview. J Korean Med Sci. 2015;30(Suppl 1):S3–S11. doi: 10.3346/jkms.2015.30.S1.S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batton DG. Committee on, F. & Newborn. Clinical report–Antenatal counseling regarding resuscitation at an extremely low gestational age. Pediatrics. 2009;124:422–427. doi: 10.1542/peds.2009-1060. [DOI] [PubMed] [Google Scholar]
- 8.Sheldon T. Dutch doctors change policy on treating preterm babies. British Medical Journal. 2001;322:1383–1383. doi: 10.1136/bmj.322.7299.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkinson AR, et al. Management of babies born extremely preterm at less than 26 weeks of gestation: a framework for clinical practice at the time of birth. Arch Dis Child Fetal Neonatal Ed. 2009;94:F2–5. doi: 10.1136/adc.2008.143321. [DOI] [PubMed] [Google Scholar]
- 10.Binet ME, et al. Role of gender in morbidity and mortality of extremely premature neonates. Am J Perinatol. 2012;29:159–166. doi: 10.1055/s-0031-1284225. [DOI] [PubMed] [Google Scholar]
- 11.Jones HP, et al. Actuarial survival of a large Canadian cohort of preterm infants. BMC Pediatr. 2005;5:40. doi: 10.1186/1471-2431-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kent AL, Wright IM, Abdel-Latif ME, New South W. Australian Capital Territory Neonatal Intensive Care Units Audit, G. Mortality and adverse neurologic outcomes are greater in preterm male infants. Pediatrics. 2012;129:124–131. doi: 10.1542/peds.2011-1578. [DOI] [PubMed] [Google Scholar]
- 13.Lingappan K, Jiang W, Wang L, Moorthy B. Sex-specific differences in neonatal hyperoxic lung injury. Am J Physiol Lung Cell Mol Physiol. 2016;311:L481–493. doi: 10.1152/ajplung.00047.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Battin M, Ling EW, Whitfield MF, Mackinnon M, Effer SB. Has the outcome for extremely low gestational age (ELGA) infants improved following recent advances in neonatal intensive care? Am J Perinatol. 1998;15:469–477. doi: 10.1055/s-2007-994068. [DOI] [PubMed] [Google Scholar]
- 15.Tucker J, McGuire W. Epidemiology of preterm birth. BMJ. 2004;329:675–678. doi: 10.1136/bmj.329.7467.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weng YH, Yang CY, Chiu YW. Neonatal outcomes in relation to sex differences: a national cohort survey in Taiwan. Biol Sex Differ. 2015;6:30. doi: 10.1186/s13293-015-0052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darlow BA, et al. Prenatal risk factors for severe retinopathy of prematurity among very preterm infants of the Australian and New Zealand Neonatal Network. Pediatrics. 2005;115:990–996. doi: 10.1542/peds.2004-1309. [DOI] [PubMed] [Google Scholar]
- 18.Kaltofen T, Haase M, Thome UH, Laube M. Male Sex is Associated with a Reduced Alveolar Epithelial Sodium Transport. PLoS One. 2015;10:e0136178. doi: 10.1371/journal.pone.0136178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Semenas E, et al. Sex differences in cerebral injury after severe haemorrhage and ventricular fibrillation in pigs. Acta Anaesthesiol Scand. 2010;54:343–353. doi: 10.1111/j.1399-6576.2009.02125.x. [DOI] [PubMed] [Google Scholar]
- 20.Tioseco JA, Aly H, Essers J, Patel K, El-Mohandes AA. Male sex and intraventricular hemorrhage. Pediatr Crit Care Med. 2006;7:40–44. doi: 10.1097/01.PCC.0000192341.67078.61. [DOI] [PubMed] [Google Scholar]
- 21.Fleisher B, Kulovich MV, Hallman M, Gluck L. Lung profile: sex differences in normal pregnancy. Obstet Gynecol. 1985;66:327–330. [PubMed] [Google Scholar]
- 22.Kimura Y, et al. Expression of androgen receptor and 5alpha-reductase types 1 and 2 in early gestation fetal lung: a possible correlation with branching morphogenesis. Clin Sci (Lond) 2003;105:709–713. doi: 10.1042/CS20030236. [DOI] [PubMed] [Google Scholar]
- 23.Townsel CD, Emmer SF, Campbell WA, Hussain N. Gender Differences in Respiratory Morbidity and Mortality of Preterm Neonates. Front Pediatr. 2017;5:6. doi: 10.3389/fped.2017.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ray B, Platt MP. Mortality of twin and singleton livebirths under 30 weeks’ gestation: a population-based study. Arch Dis Child Fetal Neonatal Ed. 2009;94:F140–143. doi: 10.1136/adc.2008.143016. [DOI] [PubMed] [Google Scholar]
- 25.Draper ES, et al. Variability in Very Preterm Stillbirth and In-Hospital Mortality Across Europe. Pediatrics. 2017 doi: 10.1542/peds.2016-1990. [DOI] [PubMed] [Google Scholar]
- 26.Nuytten A, et al. Evidence-Based Neonatal Unit Practices and Determinants of Postnatal Corticosteroid-Use in Preterm Births below 30 Weeks GA in Europe. A Population-Based Cohort Study. PLoS One. 2017;12:e0170234. doi: 10.1371/journal.pone.0170234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schindler T, et al. Causes of death in very preterm infants cared for in neonatal intensive care units: a population-based retrospective cohort study. BMC Pediatr. 2017;17:59. doi: 10.1186/s12887-017-0810-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith LK, et al. Variability in the management and outcomes of extremely preterm births across five European countries: a population-based cohort study. Arch Dis Child Fetal Neonatal Ed. 2017 doi: 10.1136/archdischild-2016-312100. [DOI] [PubMed] [Google Scholar]
- 29.Bacak SJ, Baptiste-Roberts K, Amon E, Ireland B, Leet T. Risk factors for neonatal mortality among extremely-low-birth-weight infants. Am J Obstet Gynecol. 2005;192:862–867. doi: 10.1016/j.ajog.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 30.Itabashi K, et al. Mortality rates for extremely low birth weight infants born in Japan in 2005. Pediatrics. 2009;123:445–450. doi: 10.1542/peds.2008-0763. [DOI] [PubMed] [Google Scholar]
- 31.Wells JC. Natural selection and sex differences in morbidity and mortality in early life. J Theor Biol. 2000;202:65–76. doi: 10.1006/jtbi.1999.1044. [DOI] [PubMed] [Google Scholar]
- 32.Rizzo G, et al. The effect of fetal sex on customized fetal growth charts. J Matern Fetal Neonatal Med. 2016;29:3768–3775. doi: 10.3109/14767058.2016.1149565. [DOI] [PubMed] [Google Scholar]
- 33.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. doi: 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seaborn T, Simard M, Provost PR, Piedboeuf B, Tremblay Y. Sex hormone metabolism in lung development and maturation. Trends Endocrinol Metab. 2010;21:729–738. doi: 10.1016/j.tem.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Ingemarsson I. Gender aspects of preterm birth. BJOG. 2003;110(Suppl 20):34–38. doi: 10.1046/j.1471-0528.2003.00022.x. [DOI] [PubMed] [Google Scholar]
- 36.Malorni W, Campesi I, Straface E, Vella S, Franconi F. Redox features of the cell: a gender perspective. Antioxid Redox Signal. 2007;9:1779–1801. doi: 10.1089/ars.2007.1596. [DOI] [PubMed] [Google Scholar]
- 37.Tondreau MY, Boucher E, Simard M, Tremblay Y, Bilodeau JF. Sex-specific perinatal expression of glutathione peroxidases during mouse lung development. Mol Cell Endocrinol. 2012;355:87–95. doi: 10.1016/j.mce.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 38.Ito M, Tamura M, Namba F, Neonatal Research Network of, J. Role of sex in morbidity and mortality of very premature neonates. Pediatr Int. 2017 doi: 10.1111/ped.13320. [DOI] [PubMed] [Google Scholar]
- 39.Nodgaard H, Andreasen H, Hansen H, Sorensen HT. Risk factors associated with retinopathy of prematurity (ROP) in northern Jutland, Denmark 1990–1993. Acta Ophthalmol Scand. 1996;74:306–310. doi: 10.1111/j.1600-0420.1996.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 40.Wheatley CM, Dickinson JL, Mackey DA, Craig JE, Sale MM. Retinopathy of prematurity: recent advances in our understanding. Br J Ophthalmol. 2002;86:696–700. doi: 10.1136/bjo.86.6.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hellstrom A, et al. IGF-I is critical for normal vascularization of the human retina. J Clin Endocrinol Metab. 2002;87:3413–3416. doi: 10.1210/jcem.87.7.8629. [DOI] [PubMed] [Google Scholar]
- 42.Hellstrom A, et al. Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics. 2003;112:1016–1020. doi: 10.1542/peds.112.5.1016. [DOI] [PubMed] [Google Scholar]
- 43.Bassler D, et al. Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: added role of neonatal infection. Pediatrics. 2009;123:313–318. doi: 10.1542/peds.2008-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saldir M, Sarici SU, Bakar EE, Ozcan O. Neurodevelopmental status of preterm newborns at infancy, born at a tertiary care center in Turkey. Am J Perinatol. 2010;27:121–128. doi: 10.1055/s-0029-1224863. [DOI] [PubMed] [Google Scholar]
- 45.Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics. 1988;82:527–532. [PubMed] [Google Scholar]
- 46.Papile LA, Munsick-Bruno G, Schaefer A. Relationship of cerebral intraventricular hemorrhage and early childhood neurologic handicaps. J Pediatr. 1983;103:273–277. doi: 10.1016/S0022-3476(83)80366-7. [DOI] [PubMed] [Google Scholar]
- 47.Bell MJ, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.An international classification of retinopathy of prematurity. The Committee for the Classification of Retinopathy of Prematurity. Arch Ophthalmol102, 1130–1134 (1984). [DOI] [PubMed]
- 49.Hosmer DW, Hosmer T, Le Cessie S, Lemeshow S. A comparison of goodness-of-fit tests for the logistic regression model. Stat Med. 1997;16:965–980. doi: 10.1002/(SICI)1097-0258(19970515)16:9<965::AID-SIM509>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are not publicly available due to the policy of Research of Korea Centers for Disease Control and Prevention but are available from the corresponding author on reasonable request.


