Abstract
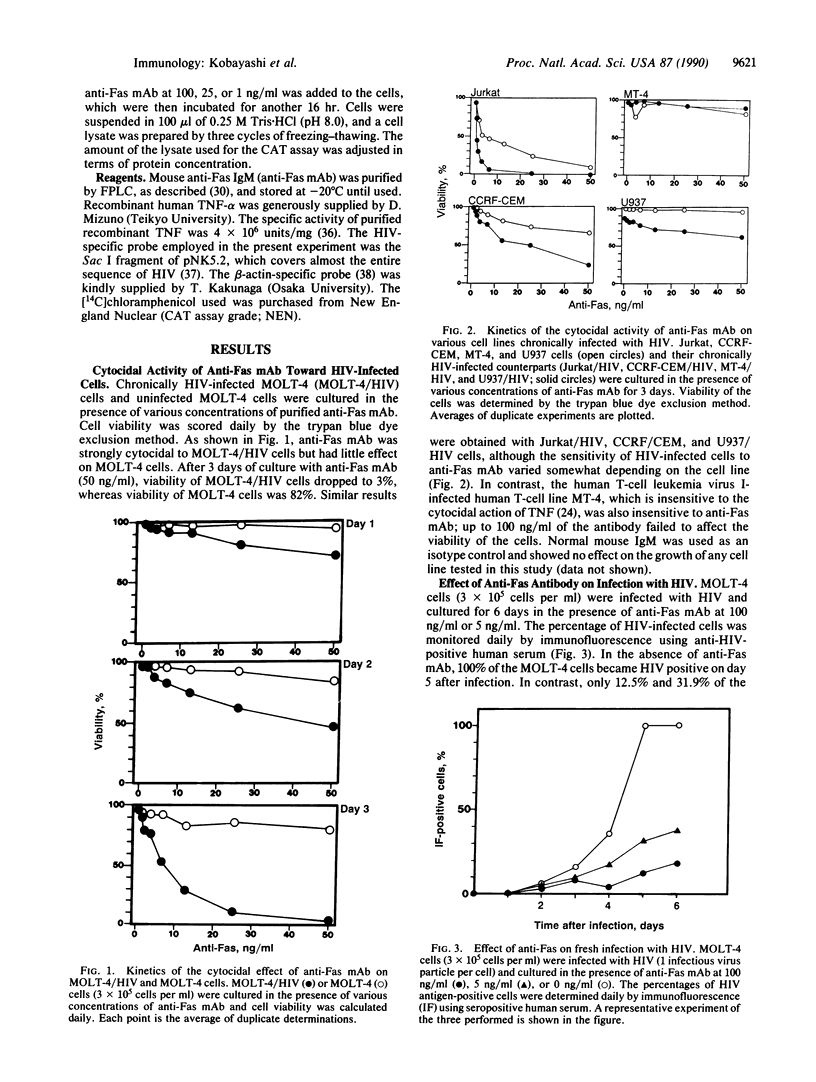
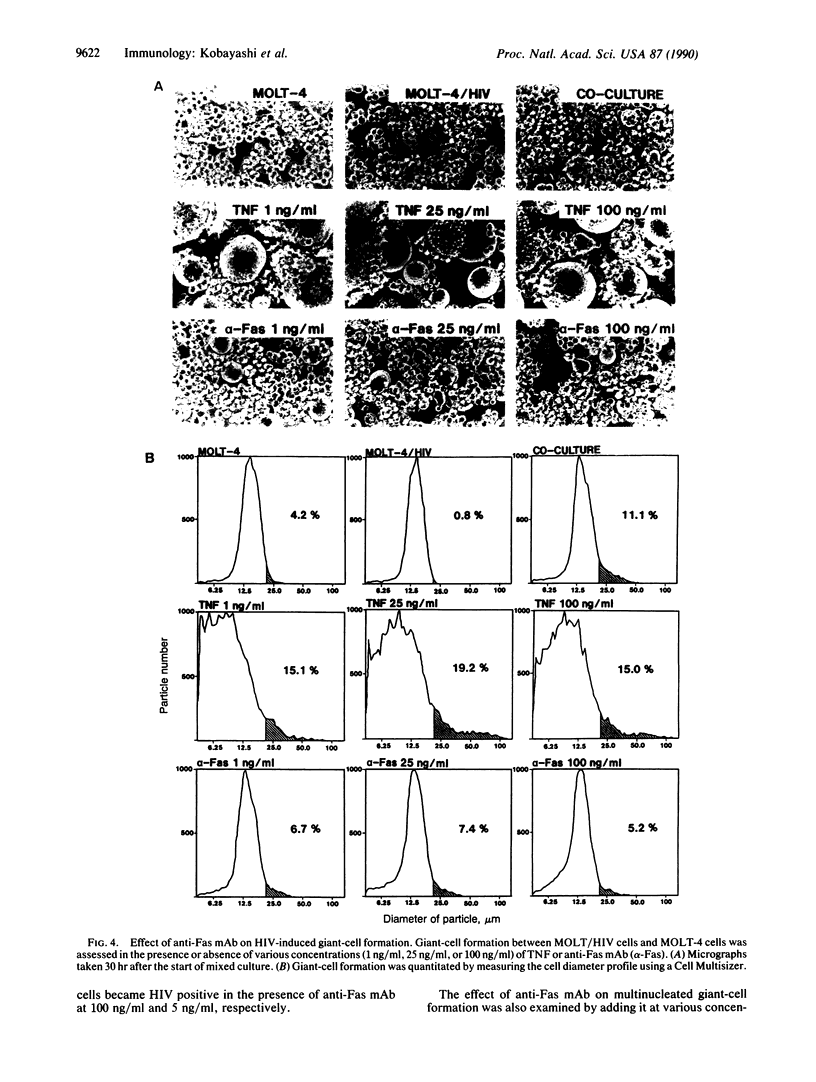
A cytotoxic monoclonal antibody (anti-Fas mAb) against the 200-kDa cell surface Fas antigen, which is associated with the tumor necrosis factor (TNF) receptor, was examined for its in vitro activity on human immunodeficiency virus (HIV)-infected cells. It was found that both TNF and anti-Fas mAb selectively killed the chronically HIV-infected cells. Uninfected cells were less sensitive to the antibody than those infected with HIV. When the cells were cultured in the presence of anti-Fas mAb immediately after the HIV infection, the spread of HIV-infected cells was suppressed by the antibody. TNF augmented both the synthesis of HIV-specific mRNA in HIV-infected cells and formation of multinucleated giant cells. In contrast, the anti-Fas mAb did not augment HIV replication or enhance the HIV-induced formation of syncytia. The results indicated that anti-Fas mAb mimicks the cytocidal action of TNF but does not augment HIV replication.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Fisher S., Levo Y., Holtmann H., Hahn T., Wallach D. Cachectin/tumour-necrosis-factor production by cancer patients. Lancet. 1985 Nov 23;2(8465):1190–1190. doi: 10.1016/s0140-6736(85)92713-8. [DOI] [PubMed] [Google Scholar]
- Balkwill F., Osborne R., Burke F., Naylor S., Talbot D., Durbin H., Tavernier J., Fiers W. Evidence for tumour necrosis factor/cachectin production in cancer. Lancet. 1987 Nov 28;2(8570):1229–1232. doi: 10.1016/s0140-6736(87)91850-2. [DOI] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Folks T. M., Clouse K. A., Justement J., Rabson A., Duh E., Kehrl J. H., Fauci A. S. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2365–2368. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada S., Koyanagi Y., Yamamoto N. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science. 1985 Aug 9;229(4713):563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- Hinuma S., Naruo K., Tsukamoto K., Sugamura K., Hinuma Y. Production of cytotoxic factor(s) in human T cell lines transformed by a human retrovirus. Biochem Biophys Res Commun. 1985 Aug 15;130(3):1052–1058. doi: 10.1016/0006-291x(85)91722-x. [DOI] [PubMed] [Google Scholar]
- Kawakami M., Pekala P. H., Lane M. D., Cerami A. Lipoprotein lipase suppression in 3T3-L1 cells by an endotoxin-induced mediator from exudate cells. Proc Natl Acad Sci U S A. 1982 Feb;79(3):912–916. doi: 10.1073/pnas.79.3.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N., Hamamoto Y., Koyanagi Y., Chen I. S., Yamamoto N. Effect of interleukin-1 on the augmentation of human immunodeficiency virus gene expression. Biochem Biophys Res Commun. 1989 Dec 15;165(2):715–721. doi: 10.1016/s0006-291x(89)80025-7. [DOI] [PubMed] [Google Scholar]
- Kobayashi N., Hamamoto Y., Yamamoto N. Is TNF involved in the progression of AIDS? Virus Genes. 1990 Jul;4(2):183–190. doi: 10.1007/BF00678409. [DOI] [PubMed] [Google Scholar]
- Kobayashi N., Hamamoto Y., Yamamoto N. Production of tumor necrosis factors by human T cell lines infected with HTLV-1 may cause their high susceptibility to human immunodeficiency virus infection. Med Microbiol Immunol. 1990;179(2):115–122. doi: 10.1007/BF00198532. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Hamamoto Y., Kobayashi N., Yamamoto N. Serum level of TNF alpha in HIV-infected individuals. AIDS. 1990 Feb;4(2):169–170. [PubMed] [Google Scholar]
- Koff W. C., Fann A. V. Human tumor necrosis factor-alpha kills herpesvirus-infected but not normal cells. Lymphokine Res. 1986 Summer;5(3):215–221. [PubMed] [Google Scholar]
- Kondo L. L., Rosenau W., Wara D. W. Role of lymphotoxin in antibody-dependent cell-mediated cytotoxicity (ADCC). J Immunol. 1981 Mar;126(3):1131–1133. [PubMed] [Google Scholar]
- Lähdevirta J., Maury C. P., Teppo A. M., Repo H. Elevated levels of circulating cachectin/tumor necrosis factor in patients with acquired immunodeficiency syndrome. Am J Med. 1988 Sep;85(3):289–291. doi: 10.1016/0002-9343(88)90576-1. [DOI] [PubMed] [Google Scholar]
- Matsuyama T., Hamamoto Y., Kobayashi S., Kurimoto M., Minowada J., Kobayashi N., Yamamoto N. Enhancement of human immunodeficiency virus production by natural lymphotoxin. Med Microbiol Immunol. 1988;177(4):181–187. doi: 10.1007/BF00211217. [DOI] [PubMed] [Google Scholar]
- Matsuyama T., Hamamoto Y., Soma G., Mizuno D., Yamamoto N., Kobayashi N. Cytocidal effect of tumor necrosis factor on cells chronically infected with human immunodeficiency virus (HIV): enhancement of HIV replication. J Virol. 1989 Jun;63(6):2504–2509. doi: 10.1128/jvi.63.6.2504-2509.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama T., Hamamoto Y., Yoshida T., Kido Y., Kobayashi S., Kobayashi N., Yamamoto N. Effect of culture supernatant of MT-2 cells on human immunodeficiency virus-producing cells, MOLT-4/HIVHTLV-IIIB cells. Jpn J Cancer Res. 1988 Feb;79(2):156–159. doi: 10.1111/j.1349-7006.1988.tb01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama T., Yoshiyama H., Hamamoto Y., Yamamoto N., Soma G., Mizuno D., Kobayashi N. Enhancement of HIV replication and giant cell formation by tumor necrosis factor. AIDS Res Hum Retroviruses. 1989 Apr;5(2):139–146. doi: 10.1089/aid.1989.5.139. [DOI] [PubMed] [Google Scholar]
- Mestan J., Digel W., Mittnacht S., Hillen H., Blohm D., Möller A., Jacobsen H., Kirchner H. Antiviral effects of recombinant tumour necrosis factor in vitro. 1986 Oct 30-Nov 5Nature. 323(6091):816–819. doi: 10.1038/323816a0. [DOI] [PubMed] [Google Scholar]
- Michihiko S., Yamamoto N., Shinozaki F., Shimada K., Soma G., Kobayashi N. Augmentation of in-vitro HIV replication in peripheral blood mononuclear cells of AIDS and ARC patients by tumour necrosis factor. Lancet. 1989 May 27;1(8648):1206–1207. doi: 10.1016/s0140-6736(89)92788-8. [DOI] [PubMed] [Google Scholar]
- Nakajima-Iijima S., Hamada H., Reddy P., Kakunaga T. Molecular structure of the human cytoplasmic beta-actin gene: interspecies homology of sequences in the introns. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6133–6137. doi: 10.1073/pnas.82.18.6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T., Matsuyama T., Mori S., Hamamoto Y., Kobayashi N., Yamamoto N., Josephs S. F., Wong-Staal F., Shimotohno K. Augmentation of human immunodeficiency virus type 1 gene expression by tumor necrosis factor alpha. AIDS Res Hum Retroviruses. 1989 Apr;5(2):131–138. doi: 10.1089/aid.1989.5.131. [DOI] [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Osborn L., Kunkel S., Nabel G. J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Rosenberg Z. F., Fauci A. S. Induction of expression of HIV in latently or chronically infected cells. AIDS Res Hum Retroviruses. 1989 Feb;5(1):1–4. doi: 10.1089/aid.1989.5.1. [DOI] [PubMed] [Google Scholar]
- Scuderi P., Sterling K. E., Lam K. S., Finley P. R., Ryan K. J., Ray C. G., Petersen E., Slymen D. J., Salmon S. E. Raised serum levels of tumour necrosis factor in parasitic infections. Lancet. 1986 Dec 13;2(8520):1364–1365. doi: 10.1016/s0140-6736(86)92007-6. [DOI] [PubMed] [Google Scholar]
- Sodroski J. G., Rosen C. A., Haseltine W. A. Trans-acting transcriptional activation of the long terminal repeat of human T lymphotropic viruses in infected cells. Science. 1984 Jul 27;225(4660):381–385. doi: 10.1126/science.6330891. [DOI] [PubMed] [Google Scholar]
- Soma G., Tsuji Y., Tanabe Y., Noguchi K., Kitahara-Tanabe N., Gatanaga T., Inagawa H., Kawakami M., Mizuno D. Biological activities of novel recombinant tumor necrosis factor having N-terminal amino acid sequences derived from cytotoxic factors produced by THP-1 cells. J Biol Response Mod. 1988 Dec;7(6):587–595. [PubMed] [Google Scholar]
- Sugarman B. J., Aggarwal B. B., Hass P. E., Figari I. S., Palladino M. A., Jr, Shepard H. M. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985 Nov 22;230(4728):943–945. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- Tochikura T. S., Nakashima H., Tanabe A., Yamamoto N. Human immunodeficiency virus (HIV)-induced cell fusion: quantification and its application for the simple and rapid screening of anti-HIV substances in vitro. Virology. 1988 Jun;164(2):542–546. doi: 10.1016/0042-6822(88)90570-3. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Palombella V. J., Henriksen-DeStefano D., Swenson C., Feinman R., Hirai M., Tsujimoto M. Fibroblast growth enhancing activity of tumor necrosis factor and its relationship to other polypeptide growth factors. J Exp Med. 1986 Mar 1;163(3):632–643. doi: 10.1084/jem.163.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware C. F., Granger G. A. Mechanisms of lymphocyte-mediated cytotoxicity. III. Characterization of the mechanism of inhibition of the human alloimmune lymphocyte-mediated cytotoxic reaction by polyspecific anti-lymphotoxin sera in vitro. J Immunol. 1981 May;126(5):1934–1940. [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Tumour necrosis factors alpha and beta inhibit virus replication and synergize with interferons. 1986 Oct 30-Nov 5Nature. 323(6091):819–822. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]
- Yonehara S., Ishii A., Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989 May 1;169(5):1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama H., Kobayashi N., Matsui T., Nakashima H., Kajii T., Yamato K., Kotani S., Miyoshi I., Yamamoto N. Transmission and genetic shift of human immunodeficiency virus (HIV) in vivo. Mol Biol Med. 1987 Dec;4(6):385–396. [PubMed] [Google Scholar]





