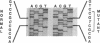Abstract
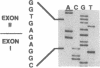
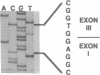
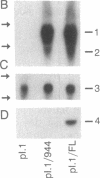
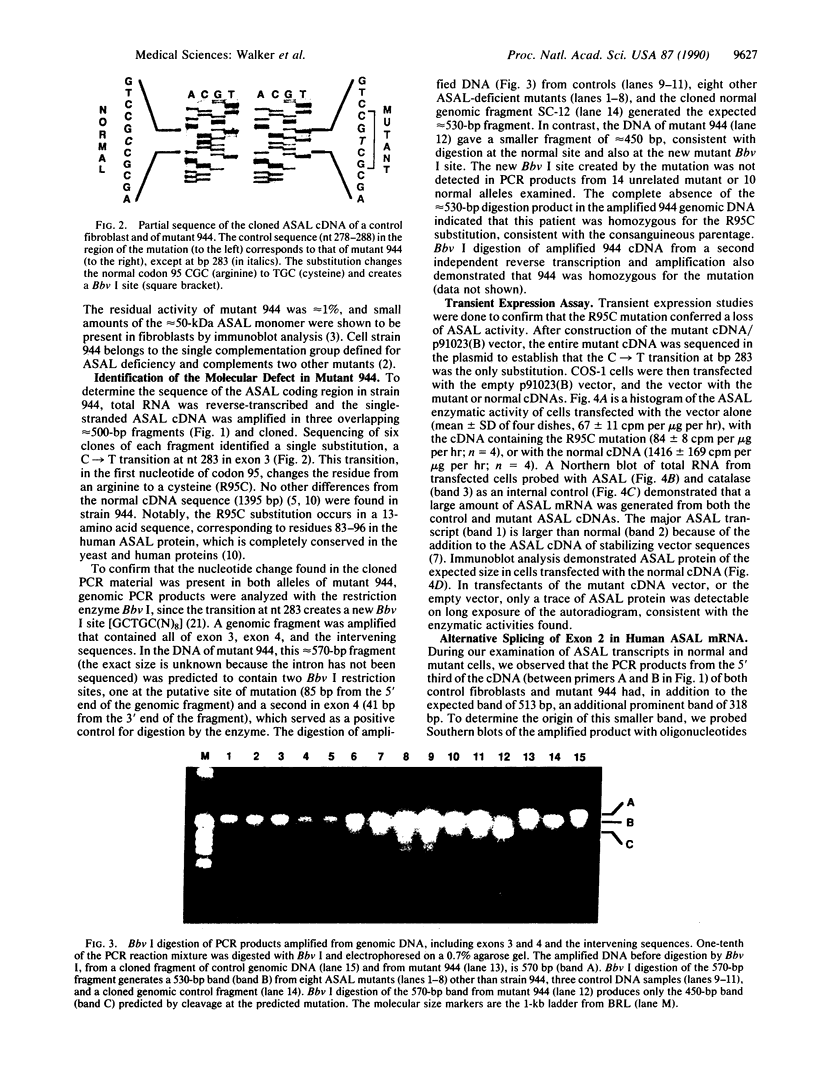
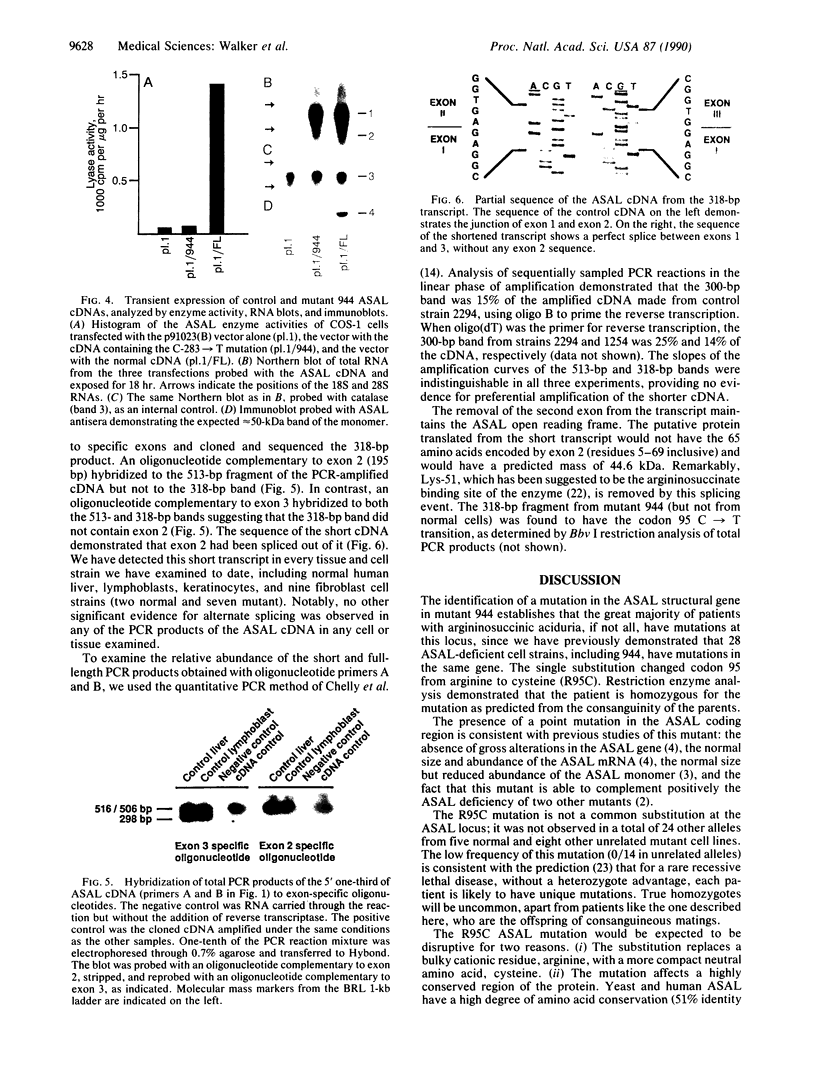
Argininosuccinic acid lyase (ASAL) deficiency is a clinically heterogeneous autosomal recessive urea cycle disorder. We previously established by complementation analysis that 28 ASAL-deficient patients have heterogeneous mutations in a single gene. To prove that the ASAL structural gene is the affected locus, we sequenced polymerase chain reaction-amplified ASAL cDNA of a representative mutant from the single complementation group. Fibroblast strain 944 (approximately 1% of residual ASAL activity), from a late-onset patient who was the product of a consanguineous mating, had only a single base-pair change in the coding region, a C-283----T transition at a CpG dinucleotide in exon 3. This substitution converts Arg-95 to Cys (R95C), occurs in a stretch of 13 residues that is identical in yeast and human ASAL, and was present in both of the patient's alleles but not in 14 other mutant or 10 normal alleles. Expression in COS cells demonstrated that the R95C mutation produces normal amounts of ASAL mRNA but little protein and less than 1% ASAL activity. We observed that amplified cDNA from mutant 944 and normal cells (liver, keratinocytes, lymphoblasts, and fibroblasts) contained, in addition to the expected 5' 513-base-pair band, a prominent 318-base-pair ASAL band formed by the splicing of exon 2 from the transcript. The short transcript maintains the ASAL reading frame but removes Lys-51, a residue that may be essential for catalysis, since it binds the argininosuccinate substrate. We conclude (i) that the identification of the R95C mutation in strain 944 demonstrates that virtually all ASAL deficiency results from defects in the ASAL structural gene and (ii) that minor alternative splicing of the coding region occurs at the ASAL locus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chelly J., Kaplan J. C., Maire P., Gautron S., Kahn A. Transcription of the dystrophin gene in human muscle and non-muscle tissue. Nature. 1988 Jun 30;333(6176):858–860. doi: 10.1038/333858a0. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Freytag S. O., Beaudet A. L., Bock H. G., O'Brien W. E. Molecular structure of the human argininosuccinate synthetase gene: occurrence of alternative mRNA splicing. Mol Cell Biol. 1984 Oct;4(10):1978–1984. doi: 10.1128/mcb.4.10.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingeras T. R., MIlazzo J. P., Roberts R. J. A computer assisted method for the determination of restriction enzyme recognifion sites. Nucleic Acids Res. 1978 Nov;5(11):4105–4127. doi: 10.1093/nar/5.11.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B., Sokoloff P., Martres M. P., Riou J. F., Emorine L. J., Schwartz J. C. Alternative splicing directs the expression of two D2 dopamine receptor isoforms. Nature. 1989 Dec 21;342(6252):923–926. doi: 10.1038/342923a0. [DOI] [PubMed] [Google Scholar]
- Henthorn P., Zervos P., Raducha M., Harris H., Kadesch T. Expression of a human placental alkaline phosphatase gene in transfected cells: use as a reporter for studies of gene expression. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6342–6346. doi: 10.1073/pnas.85.17.6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambert M. A., Simard L. R., Ray P. N., McInnes R. R. Molecular cloning of cDNA for rat argininosuccinate lyase and its expression in rat hepatoma cell lines. Mol Cell Biol. 1986 May;6(5):1722–1728. doi: 10.1128/mcb.6.5.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusty C. J., Ratner S. Reaction of argininosuccinase with bromomesaconic acid: role of an essential lysine in the active site. Proc Natl Acad Sci U S A. 1987 May;84(10):3176–3180. doi: 10.1073/pnas.84.10.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubasa T., Takiguchi M., Amaya Y., Matsuda I., Mori M. Structure of the rat argininosuccinate lyase gene: close similarity to chicken delta-crystallin genes. Proc Natl Acad Sci U S A. 1989 Jan;86(2):592–596. doi: 10.1073/pnas.86.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes R. R., Shih V., Chilton S. Interallelic complementation in an inborn error of metabolism: genetic heterogeneity in argininosuccinate lyase deficiency. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4480–4484. doi: 10.1073/pnas.81.14.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. A., Looney J. E., Brody L. C., Steel G., Suchanek M., Engelhardt J. F., Willard H. F., Valle D. Human ornithine-delta-aminotransferase. cDNA cloning and analysis of the structural gene. J Biol Chem. 1988 Oct 5;263(28):14288–14295. [PubMed] [Google Scholar]
- O'Brien W. E., McInnes R., Kalumuck K., Adcock M. Cloning and sequence analysis of cDNA for human argininosuccinate lyase. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7211–7215. doi: 10.1073/pnas.83.19.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatigorsky J., O'Brien W. E., Norman B. L., Kalumuck K., Wistow G. J., Borras T., Nickerson J. M., Wawrousek E. F. Gene sharing by delta-crystallin and argininosuccinate lyase. Proc Natl Acad Sci U S A. 1988 May;85(10):3479–3483. doi: 10.1073/pnas.85.10.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson A., Hill W. G. Identity of different mutations for deleterious genes. Nature. 1983 Jan 13;301(5896):176–177. doi: 10.1038/301176a0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. G., Lin C. R., Amara S. G., Stolarsky L., Roos B. A., Ong E. S., Evans R. M. Calcitonin mRNA polymorphism: peptide switching associated with alternative RNA splicing events. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1717–1721. doi: 10.1073/pnas.79.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Simard L., O'Brien W. E., McInnes R. R. Argininosuccinate lyase deficiency: evidence for heterogeneous structural gene mutations by immunoblotting. Am J Hum Genet. 1986 Jul;39(1):38–51. [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd S., McGill J. R., McCombs J. L., Moore C. M., Weider I., Naylor S. L. cDNA sequence, interspecies comparison, and gene mapping analysis of argininosuccinate lyase. Genomics. 1989 Jan;4(1):53–59. doi: 10.1016/0888-7543(89)90314-5. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]