Abstract
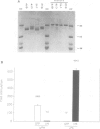
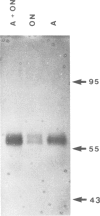
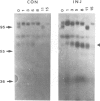
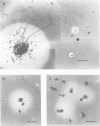
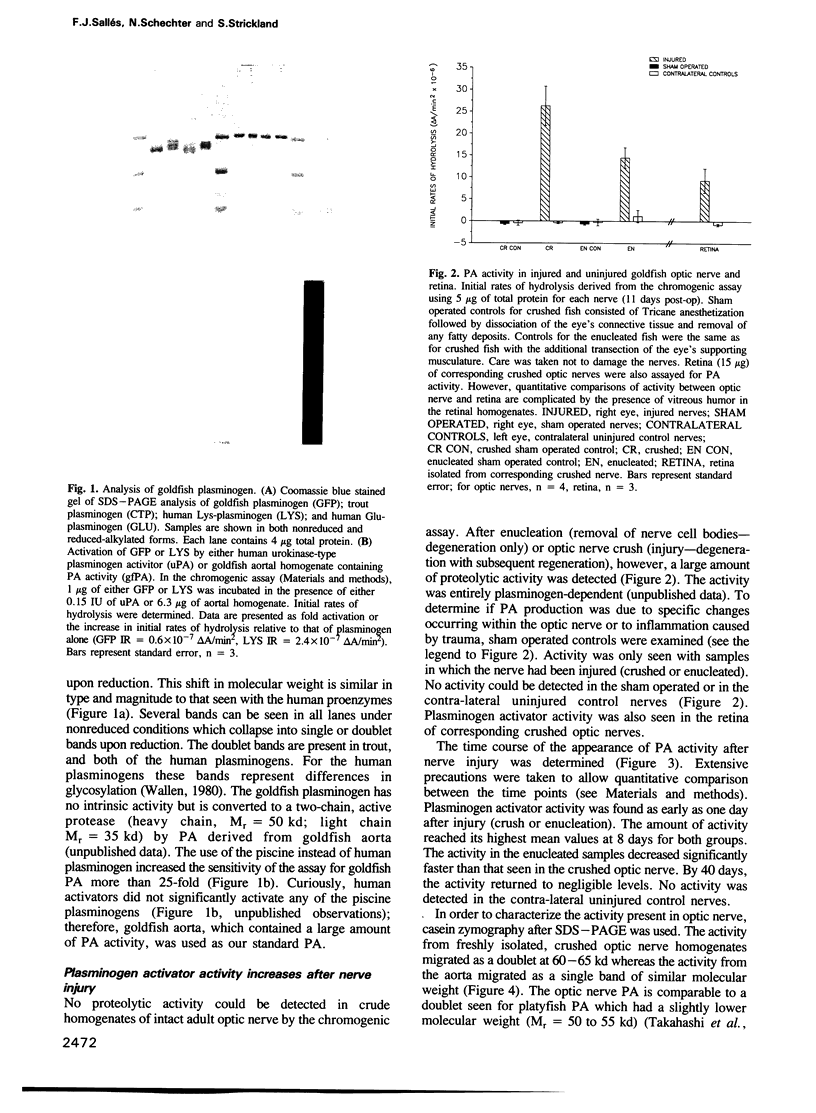
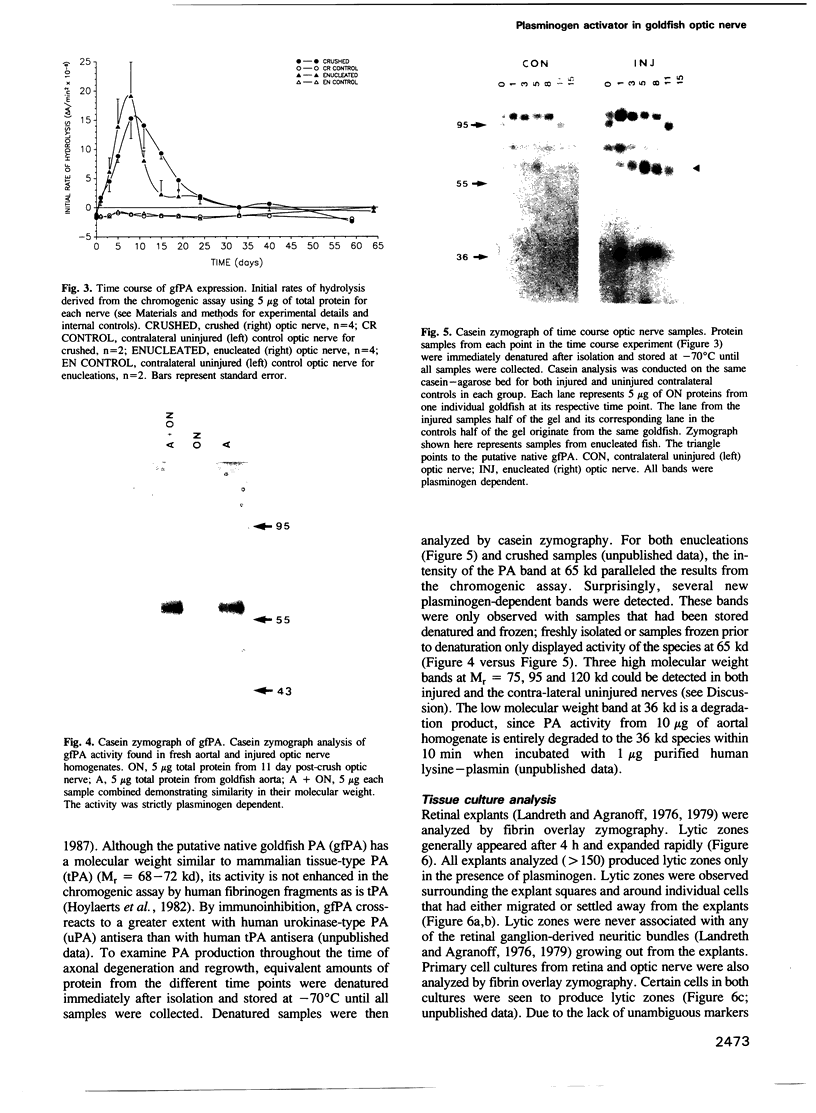
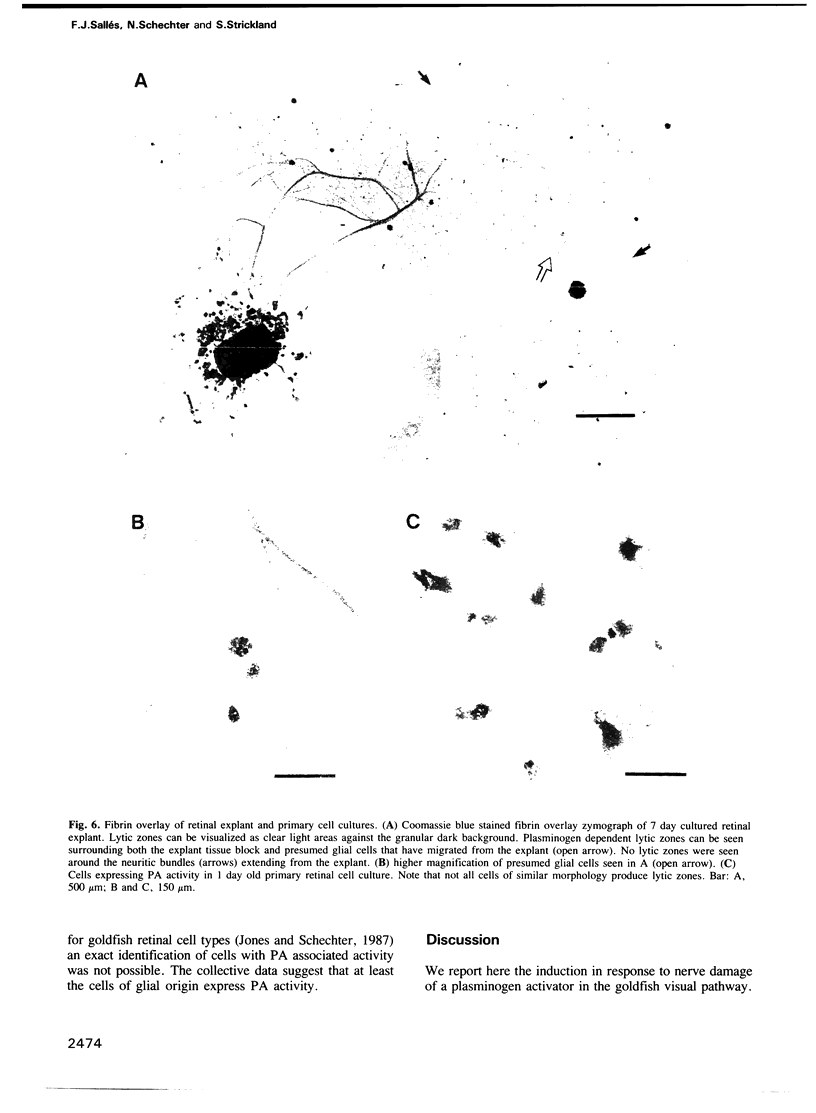
The use of purified piscine plasminogen in a chromogenic solution assay enabled us to detect plasminogen activator (PA) activity in crude homogenates of goldfish optic nerve following nerve injury. In contrast, no activity was detected in the homogenates of uninjured nerve. Under conditions allowing regeneration of the optic axons (optic nerve crush), PA activity peaked 8 days after crush, and decreased to undetectable levels by 60 days. Under conditions allowing only degeneration of the axons (enucleation), the activity peaked at 8 days but decreased more rapidly. Casein zymography of samples after fractionation in SDS-PAGE showed that PA activity migrated as a doublet at Mr = 60-65 kd. Using this assay, activity was also observed in uninjured control nerves. This plasminogen-dependent activity migrated as three bands of higher molecular weight (Mr = 75, 95 and 120 kd) and was undetectable in solution assays of unfractionated extracts, suggesting complex formation with an inhibitor(s). Fibrin overlay assay of retinal explants and isolated primary cells in culture suggest that the goldfish PA is associated with the glial cells of the goldfish visual pathway.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATTARDI D. G., SPERRY R. W. Preferential selection of central pathways by regenerating optic fibers. Exp Neurol. 1963 Jan;7:46–64. doi: 10.1016/0014-4886(63)90093-1. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A., Valinsky J. E. Production of plasminogen activator in cultures of superior cervical ganglia and isolated Schwann cells. Proc Natl Acad Sci U S A. 1985 May;82(10):3519–3523. doi: 10.1073/pnas.82.10.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Gordon P., Strickland S. Interaction of heparin with plasminogen activators and plasminogen: effects on the activation of plasminogen. Biochemistry. 1986 Jul 15;25(14):4033–4040. doi: 10.1021/bi00362a007. [DOI] [PubMed] [Google Scholar]
- Baron-Van Evercooren A., Leprince P., Rogister B., Lefebvre P. P., Delree P., Selak I., Moonen G. Plasminogen activators in developing peripheral nervous system, cellular origin and mitogenic effect. Brain Res. 1987 Nov;433(1):101–108. doi: 10.1016/0165-3806(87)90068-x. [DOI] [PubMed] [Google Scholar]
- Bignami A., Cella G., Chi N. H. Plasminogen activators in rat neural tissues during development and in Wallerian degeneration. Acta Neuropathol. 1982;58(3):224–228. doi: 10.1007/BF00690805. [DOI] [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Gloor S., Odink K., Guenther J., Nick H., Monard D. A glia-derived neurite promoting factor with protease inhibitory activity belongs to the protease nexins. Cell. 1986 Dec 5;47(5):687–693. doi: 10.1016/0092-8674(86)90511-8. [DOI] [PubMed] [Google Scholar]
- Granelli-Piperno A., Reich E. A study of proteases and protease-inhibitor complexes in biological fluids. J Exp Med. 1978 Jul 1;148(1):223–234. doi: 10.1084/jem.148.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther J., Nick H., Monard D. A glia-derived neurite-promoting factor with protease inhibitory activity. EMBO J. 1985 Aug;4(8):1963–1966. doi: 10.1002/j.1460-2075.1985.tb03878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantaï D., Rao J. S., Festoff B. W. Serine proteases and serpins: their possible roles in the motor system. Rev Neurol (Paris) 1988;144(11):680–687. [PubMed] [Google Scholar]
- Hawkins R. L., Seeds N. W. Protease inhibitors influence the direction of neurite outgrowth. Brain Res Dev Brain Res. 1989 Feb 1;45(2):203–209. doi: 10.1016/0165-3806(89)90039-4. [DOI] [PubMed] [Google Scholar]
- Heacock A. M., Agranoff B. W. Enhanced labeling of a retinal protein during regeneration of optic nerve in goldfish. Proc Natl Acad Sci U S A. 1976 Mar;73(3):828–832. doi: 10.1073/pnas.73.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoylaerts M., Rijken D. C., Lijnen H. R., Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982 Mar 25;257(6):2912–2919. [PubMed] [Google Scholar]
- Johns P. R., Easter S. S., Jr Growth of the adult goldfish eye. II. Increase in retinal cell number. J Comp Neurol. 1977 Dec 1;176(3):331–341. doi: 10.1002/cne.901760303. [DOI] [PubMed] [Google Scholar]
- Jones P. S., Schechter N. Distribution of specific intermediate-filament proteins in the goldfish retina. J Comp Neurol. 1987 Dec 1;266(1):112–121. doi: 10.1002/cne.902660109. [DOI] [PubMed] [Google Scholar]
- Jones P. S., Tesser P., Borchert J., Schechter N. Monoclonal antibodies differentiate neurofilament and glial filament proteins in the goldfish visual pathway: probes for monitoring neurite outgrowth from retinal explants. J Neurosci. 1989 Feb;9(2):454–465. doi: 10.1523/JNEUROSCI.09-02-00454.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon N. Schwann cell proliferation and localized proteolysis: expression of plasminogen-activator activity predominates in the proliferating cell populations. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7216–7220. doi: 10.1073/pnas.81.22.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystosek A., Seeds N. W. Peripheral neurons and Schwann cells secrete plasminogen activator. J Cell Biol. 1984 Feb;98(2):773–776. doi: 10.1083/jcb.98.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystosek A., Seeds N. W. Plasminogen activator release at the neuronal growth cone. Science. 1981 Sep 25;213(4515):1532–1534. doi: 10.1126/science.7197054. [DOI] [PubMed] [Google Scholar]
- Krystosek A., Seeds N. W. Plasminogen activator secretion by granule neurons in cultures of developing cerebellum. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7810–7814. doi: 10.1073/pnas.78.12.7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landreth G. E., Agranoff B. W. Explant culture of adult goldfish retina: a model for the study of CNS regeneration. Brain Res. 1979 Jan 26;161(1):39–55. doi: 10.1016/0006-8993(79)90194-x. [DOI] [PubMed] [Google Scholar]
- Landreth G. E., Agranoff B. W. Explant culture of adult goldfish retina: effect of prior optic nerve crush. Brain Res. 1976 Dec 17;118(2):299–303. doi: 10.1016/0006-8993(76)90714-9. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- McQuarrie I. G., Grafstein B. Protein synthesis and fast axonal transport in regenerating goldfish retinal ganglion cells. Brain Res. 1982 Mar 11;235(2):213–223. doi: 10.1016/0006-8993(82)91001-0. [DOI] [PubMed] [Google Scholar]
- Meyer R. L. Evidence from thymidine labeling for continuing growth of retina and tectum in juvenile goldfish. Exp Neurol. 1978 Mar;59(1):99–111. doi: 10.1016/0014-4886(78)90204-2. [DOI] [PubMed] [Google Scholar]
- Murray M. Regeneration of retinal axons into the goldfish optic tectum. J Comp Neurol. 1976 Jul 15;168(2):175–195. doi: 10.1002/cne.901680202. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Quigley J. P., Kellerman G. M., Reich E. Fibrinolysis associated with oncogenic transformation. Requirement of plasminogen for correlated changes in cellular morphology, colony formation in agar, and cell migration. J Exp Med. 1973 Nov 1;138(5):1056–1064. doi: 10.1084/jem.138.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson P. H. On the importance of being inhibited, or saying no to growth cones. Neuron. 1988 Jun;1(4):263–267. doi: 10.1016/0896-6273(88)90074-8. [DOI] [PubMed] [Google Scholar]
- Pittman R. N. Release of plasminogen activator and a calcium-dependent metalloprotease from cultured sympathetic and sensory neurons. Dev Biol. 1985 Jul;110(1):91–101. doi: 10.1016/0012-1606(85)90067-3. [DOI] [PubMed] [Google Scholar]
- Quitschke W., Jones P. S., Schechter N. Survey of intermediate filament proteins in optic nerve and spinal cord: evidence for differential expression. J Neurochem. 1985 May;44(5):1465–1476. doi: 10.1111/j.1471-4159.1985.tb08784.x. [DOI] [PubMed] [Google Scholar]
- SPERRY R. W. CHEMOAFFINITY IN THE ORDERLY GROWTH OF NERVE FIBER PATTERNS AND CONNECTIONS. Proc Natl Acad Sci U S A. 1963 Oct;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappino A. P., Huarte J., Belin D., Vassalli J. D. Plasminogen activators in tissue remodeling and invasion: mRNA localization in mouse ovaries and implanting embryos. J Cell Biol. 1989 Nov;109(5):2471–2479. doi: 10.1083/jcb.109.5.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreq H., Miskin R. Plasminogen activator in the developing rat cerebellum: biosynthesis and localization in granular neurons. Brain Res. 1983 Dec;313(2):149–158. doi: 10.1016/0165-3806(83)90212-2. [DOI] [PubMed] [Google Scholar]
- Soreq H., Miskin R., Zutra A., Littauer U. Z. Modulation in the levels and localization of plasminogen activator in differentiating neuroblastoma cells. Brain Res. 1983 Apr;283(2-3):257–269. doi: 10.1016/0165-3806(83)90182-7. [DOI] [PubMed] [Google Scholar]
- Strickland S., Reich E., Sherman M. I. Plasminogen activator in early embryogenesis: enzyme production by trophoblast and parietal endoderm. Cell. 1976 Oct;9(2):231–240. doi: 10.1016/0092-8674(76)90114-8. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Wakamatsu Y., Ozato K., Wakayama Y. Fish plasminogen activators: their identification and characterization. Cell Struct Funct. 1987 Feb;12(1):11–22. doi: 10.1247/csf.12.11. [DOI] [PubMed] [Google Scholar]
- Tesser P., Jones P. S., Schechter N. Elevated levels of retinal neurofilament mRNA accompany optic nerve regeneration. J Neurochem. 1986 Oct;47(4):1235–1243. doi: 10.1111/j.1471-4159.1986.tb00745.x. [DOI] [PubMed] [Google Scholar]
- Toshniwal P. K., Tiku M. L., Tiku K., Skosey J. L. Secretion of plasminogen activator by cerebral astrocytes and its modulation. J Neurol Sci. 1987 Sep;80(2-3):307–321. doi: 10.1016/0022-510x(87)90165-1. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Gordon S., Reich E. Secretion of plasminogen activator by stimulated macrophages. J Exp Med. 1974 Apr 1;139(4):834–850. doi: 10.1084/jem.139.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valinsky J. E., Le Douarin N. M. Production of plasminogen activator by migrating cephalic neural crest cells. EMBO J. 1985 Jun;4(6):1403–1406. doi: 10.1002/j.1460-2075.1985.tb03793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J. D., Dayer J. M., Wohlwend A., Belin D. Concomitant secretion of prourokinase and of a plasminogen activator-specific inhibitor by cultured human monocytes-macrophages. J Exp Med. 1984 Jun 1;159(6):1653–1668. doi: 10.1084/jem.159.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrall S., Seeds N. W. Characterization of 125I-tissue plasminogen activator binding to cerebellar granule neurons. J Cell Biol. 1989 Jul;109(1):265–271. doi: 10.1083/jcb.109.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlwend A., Belin D., Vassalli J. D. Plasminogen activator-specific inhibitors in mouse macrophages: in vivo and in vitro modulation of their synthesis and secretion. J Immunol. 1987 Aug 15;139(4):1278–1284. [PubMed] [Google Scholar]
- Zurn A. D., Nick H., Monard D. A glia-derived nexin promotes neurite outgrowth in cultured chick sympathetic neurons. Dev Neurosci. 1988;10(1):17–24. doi: 10.1159/000111951. [DOI] [PubMed] [Google Scholar]