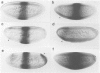
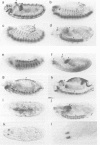
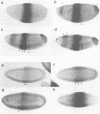
Abstract
Krüppel (Kr), a gap gene of Drosophila, shows complex spatial patterns of expression during the different stages of embryogenesis. In order to identify cis-acting sequences required for normal Kr gene expression, we analysed the expression patterns of fusion gene constructs in transgenic embryos. In these constructs, bacterial lacZ expression was placed under the control of Kr sequences in front of a basal promoter. We identified cis-acting Kr control units which drive beta-galactosidase expression in 10 known locations of Kr expression in early and late embryos. More than one cis-regulatory element drives the expression in the anterior domain at the blastoderm stage, in the nervous system, the midline precursor cells and in the amino-serosa. In addition, two cis-acting elements direct the first zygotic expression of Kr in a striped subpattern within the central region of the blastoderm embryo. Both elements respond to alterations in the activities of maternal organizer genes known to be required for Kr expression in establishing the thoracic and anterior abdominal segments in the wild-type embryo.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akam M. The molecular basis for metameric pattern in the Drosophila embryo. Development. 1987 Sep;101(1):1–22. [PubMed] [Google Scholar]
- Gaul U., Jäckle H. Analysis of maternal effect mutant combinations elucidates regulation and function of the overlap of hunchback and Krüppel gene expression in the Drosophila blastoderm embryo. Development. 1989 Nov;107(3):651–662. doi: 10.1242/dev.107.3.651. [DOI] [PubMed] [Google Scholar]
- Gaul U., Jäckle H. Pole region-dependent repression of the Drosophila gap gene Krüppel by maternal gene products. Cell. 1987 Nov 20;51(4):549–555. doi: 10.1016/0092-8674(87)90124-3. [DOI] [PubMed] [Google Scholar]
- Gaul U., Seifert E., Schuh R., Jäckle H. Analysis of Krüppel protein distribution during early Drosophila development reveals posttranscriptional regulation. Cell. 1987 Aug 14;50(4):639–647. doi: 10.1016/0092-8674(87)90037-7. [DOI] [PubMed] [Google Scholar]
- Harbecke R., Janning W. The segmentation gene Krüppel of Drosophila melanogaster has homeotic properties. Genes Dev. 1989 Jan;3(1):114–122. doi: 10.1101/gad.3.1.114. [DOI] [PubMed] [Google Scholar]
- Harding K., Levine M. Gap genes define the limits of antennapedia and bithorax gene expression during early development in Drosophila. EMBO J. 1988 Jan;7(1):205–214. doi: 10.1002/j.1460-2075.1988.tb02801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiromi Y., Gehring W. J. Regulation and function of the Drosophila segmentation gene fushi tarazu. Cell. 1987 Sep 11;50(6):963–974. doi: 10.1016/0092-8674(87)90523-x. [DOI] [PubMed] [Google Scholar]
- Hülskamp M., Schröder C., Pfeifle C., Jäckle H., Tautz D. Posterior segmentation of the Drosophila embryo in the absence of a maternal posterior organizer gene. Nature. 1989 Apr 20;338(6217):629–632. doi: 10.1038/338629a0. [DOI] [PubMed] [Google Scholar]
- Ingham P. W. The molecular genetics of embryonic pattern formation in Drosophila. Nature. 1988 Sep 1;335(6185):25–34. doi: 10.1038/335025a0. [DOI] [PubMed] [Google Scholar]
- Irish V., Lehmann R., Akam M. The Drosophila posterior-group gene nanos functions by repressing hunchback activity. Nature. 1989 Apr 20;338(6217):646–648. doi: 10.1038/338646a0. [DOI] [PubMed] [Google Scholar]
- Knipple D. C., Seifert E., Rosenberg U. B., Preiss A., Jäckle H. Spatial and temporal patterns of Krüppel gene expression in early Drosophila embryos. Nature. 1985 Sep 5;317(6032):40–44. doi: 10.1038/317040a0. [DOI] [PubMed] [Google Scholar]
- Macdonald P. M., Struhl G. A molecular gradient in early Drosophila embryos and its role in specifying the body pattern. Nature. 1986 Dec 11;324(6097):537–545. doi: 10.1038/324537a0. [DOI] [PubMed] [Google Scholar]
- Mullins M. C., Rio D. C., Rubin G. M. cis-acting DNA sequence requirements for P-element transposition. Genes Dev. 1989 May;3(5):729–738. doi: 10.1101/gad.3.5.729. [DOI] [PubMed] [Google Scholar]
- Muriel W. J., Cole J., Lehmann A. R. Molecular analysis of ouabain-resistant mutants of the mouse lymphoma cell line L5178Y. Mutagenesis. 1987 Sep;2(5):383–389. doi: 10.1093/mutage/2.5.383. [DOI] [PubMed] [Google Scholar]
- Nauber U., Pankratz M. J., Kienlin A., Seifert E., Klemm U., Jäckle H. Abdominal segmentation of the Drosophila embryo requires a hormone receptor-like protein encoded by the gap gene knirps. Nature. 1988 Dec 1;336(6198):489–492. doi: 10.1038/336489a0. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., Frohnhöfer H. G., Lehmann R. Determination of anteroposterior polarity in Drosophila. Science. 1987 Dec 18;238(4834):1675–1681. doi: 10.1126/science.3686007. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980 Oct 30;287(5785):795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Pankratz M. J., Hoch M., Seifert E., Jäckle H. Krüppel requirement for knirps enhancement reflects overlapping gap gene activities in the Drosophila embryo. Nature. 1989 Sep 28;341(6240):337–340. doi: 10.1038/341337a0. [DOI] [PubMed] [Google Scholar]
- Pankratz M. J., Seifert E., Gerwin N., Billi B., Nauber U., Jäckle H. Gradients of Krüppel and knirps gene products direct pair-rule gene stripe patterning in the posterior region of the Drosophila embryo. Cell. 1990 Apr 20;61(2):309–317. doi: 10.1016/0092-8674(90)90811-r. [DOI] [PubMed] [Google Scholar]
- Rothe M., Nauber U., Jäckle H. Three hormone receptor-like Drosophila genes encode an identical DNA-binding finger. EMBO J. 1989 Oct;8(10):3087–3094. doi: 10.1002/j.1460-2075.1989.tb08460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Struhl G. Differing strategies for organizing anterior and posterior body pattern in Drosophila embryos. Nature. 1989 Apr 27;338(6218):741–744. doi: 10.1038/338741a0. [DOI] [PubMed] [Google Scholar]
- Tautz D., Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989 Aug;98(2):81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Weigel D., Jürgens G., Klingler M., Jäckle H. Two gap genes mediate maternal terminal pattern information in Drosophila. Science. 1990 Apr 27;248(4954):495–498. doi: 10.1126/science.2158673. [DOI] [PubMed] [Google Scholar]
- Weigel D., Seifert E., Reuter D., Jäckle H. Regulatory elements controlling expression of the Drosophila homeotic gene fork head. EMBO J. 1990 Apr;9(4):1199–1207. doi: 10.1002/j.1460-2075.1990.tb08227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieschaus E., Nusslein-Volhard C., Kluding H. Krüppel, a gene whose activity is required early in the zygotic genome for normal embryonic segmentation. Dev Biol. 1984 Jul;104(1):172–186. doi: 10.1016/0012-1606(84)90046-0. [DOI] [PubMed] [Google Scholar]