Abstract
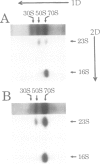
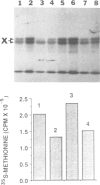
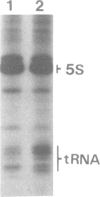
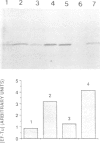
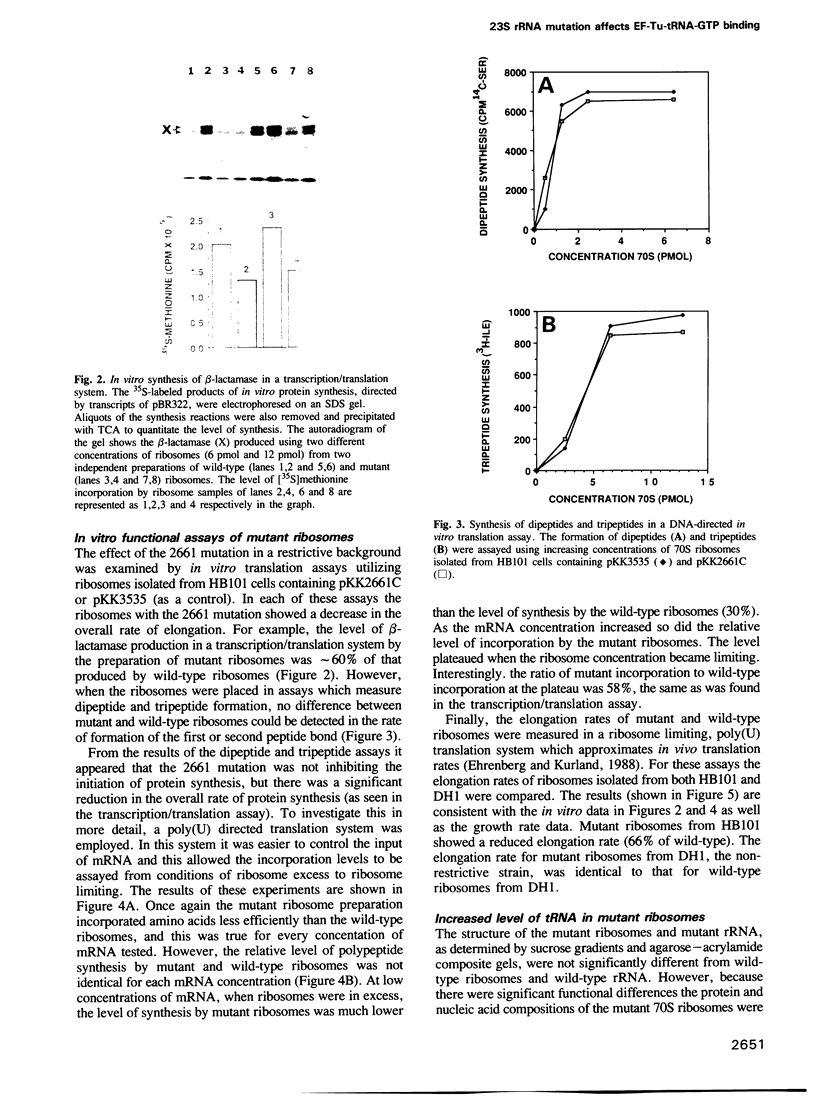
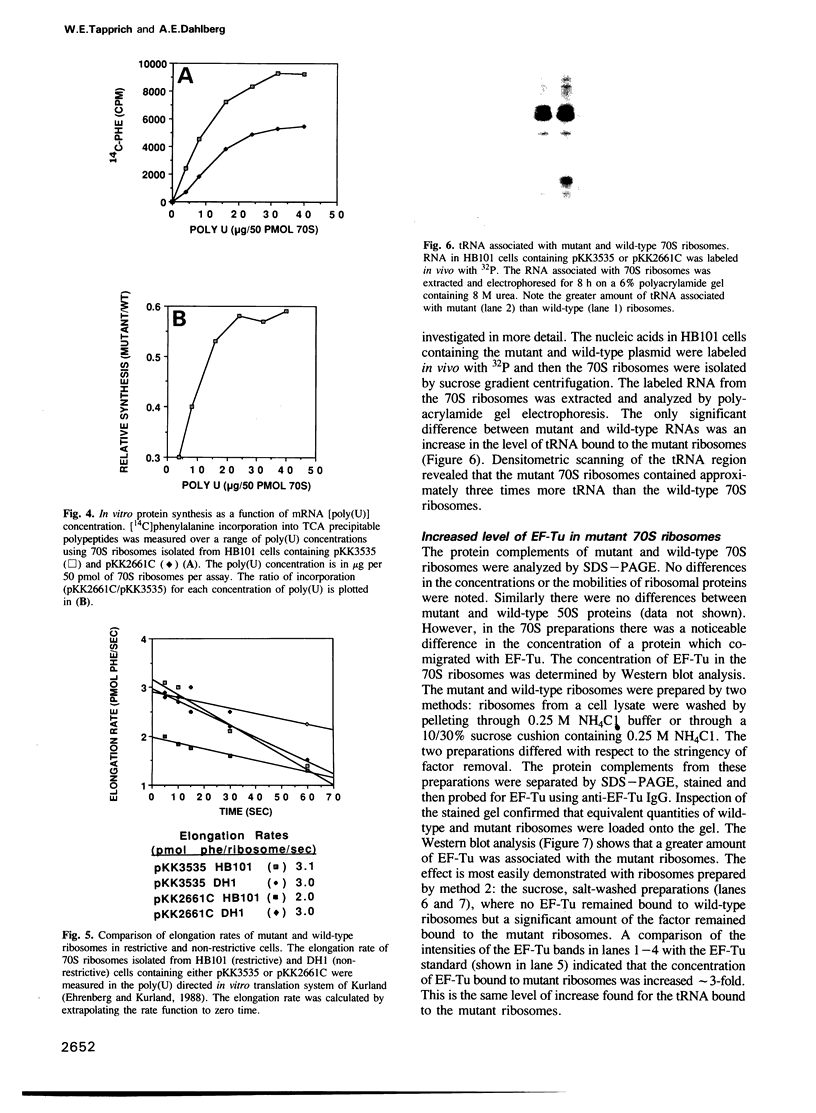
A single base substitution mutation from guanine to cytosine was constructed at position 2661 of Escherichia coli 23S rRNA and cloned into the rrnB operon of the multi-copy plasmid pKK3535. The mutant plasmid was transformed into E.coli to determine the effect of the mutation on cell growth as well as the structural and functional properties of the mutant ribosomes in vivo and in vitro. The results show that the mutant ribosomes have a slower elongation rate and an altered affinity for EF-Tu-tRNA-GTP ternary complex. This supports previous findings which indicated that position 2661 is part of a region of 23S rRNA that forms a recognition site for binding of the ternary complex in the ribosomal A site. Combinations of the 2661 mutation with various mutations in ribosomal protein S12 also demonstrate that elements of both ribosomal subunits work in concert to form this binding site.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohman K., Ruusala T., Jelenc P. C., Kurland C. G. Kinetic impairment of restrictive streptomycin-resistant ribosomes. Mol Gen Genet. 1984;198(2):90–99. doi: 10.1007/BF00328706. [DOI] [PubMed] [Google Scholar]
- Brosius J., Ullrich A., Raker M. A., Gray A., Dull T. J., Gutell R. R., Noller H. F. Construction and fine mapping of recombinant plasmids containing the rrnB ribosomal RNA operon of E. coli. Plasmid. 1981 Jul;6(1):112–118. doi: 10.1016/0147-619x(81)90058-5. [DOI] [PubMed] [Google Scholar]
- Cenatiempo Y., Robakis N., Reid B. R., Weissbach H., Brot N. In vitro expression of Escherichia coli ribosomal protein L 10 gene: tripeptide synthesis as a measure of functional mRNA. Arch Biochem Biophys. 1982 Oct 15;218(2):572–578. doi: 10.1016/0003-9861(82)90381-2. [DOI] [PubMed] [Google Scholar]
- Chan Y. L., Endo Y., Wool I. G. The sequence of the nucleotides at the alpha-sarcin cleavage site in rat 28 S ribosomal ribonucleic acid. J Biol Chem. 1983 Nov 10;258(21):12768–12770. [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Dahlberg A. E. The functional role of ribosomal RNA in protein synthesis. Cell. 1989 May 19;57(4):525–529. doi: 10.1016/0092-8674(89)90122-0. [DOI] [PubMed] [Google Scholar]
- Diaz I., Ehrenberg M., Kurland C. G. How do combinations of rpsL- and miaA- generate streptomycin dependence? Mol Gen Genet. 1986 Feb;202(2):207–211. doi: 10.1007/BF00331638. [DOI] [PubMed] [Google Scholar]
- Duisterwinkel F. J., De Graaf J. M., Schretlen P. J., Kraal B., Bosch L. A mutant elongation factor Tu which does not immobilize the ribosome upon binding of kirromycin. Eur J Biochem. 1981 Jun;117(1):7–12. doi: 10.1111/j.1432-1033.1981.tb06295.x. [DOI] [PubMed] [Google Scholar]
- Ehrenberg M., Kurland C. G. Measurement of translational kinetic parameters. Methods Enzymol. 1988;164:611–631. doi: 10.1016/s0076-6879(88)64073-0. [DOI] [PubMed] [Google Scholar]
- Endo Y., Mitsui K., Motizuki M., Tsurugi K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J Biol Chem. 1987 Apr 25;262(12):5908–5912. [PubMed] [Google Scholar]
- Endo Y., Tsurugi K. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J Biol Chem. 1987 Jun 15;262(17):8128–8130. [PubMed] [Google Scholar]
- Endo Y., Tsurugi K. The RNA N-glycosidase activity of ricin A-chain. The characteristics of the enzymatic activity of ricin A-chain with ribosomes and with rRNA. J Biol Chem. 1988 Jun 25;263(18):8735–8739. [PubMed] [Google Scholar]
- Endo Y., Wool I. G. The site of action of alpha-sarcin on eukaryotic ribosomes. The sequence at the alpha-sarcin cleavage site in 28 S ribosomal ribonucleic acid. J Biol Chem. 1982 Aug 10;257(15):9054–9060. [PubMed] [Google Scholar]
- Fernandez-Puentes C., Vazquez D. Effects of some proteins that inactivate the eukaryotic ribosome. FEBS Lett. 1977;78(1):143–146. doi: 10.1016/0014-5793(77)80292-5. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Sinsheimer R. L. Use of Brij lysis as a general method to prepare polyribosomes from Escherichia coli. Biochim Biophys Acta. 1967 Dec 19;149(2):489–495. doi: 10.1016/0005-2787(67)90176-1. [DOI] [PubMed] [Google Scholar]
- Hausner T. P., Atmadja J., Nierhaus K. H. Evidence that the G2661 region of 23S rRNA is located at the ribosomal binding sites of both elongation factors. Biochimie. 1987 Sep;69(9):911–923. doi: 10.1016/0300-9084(87)90225-2. [DOI] [PubMed] [Google Scholar]
- Hobden A. N., Cundliffe E. The mode of action of alpha sarcin and a novel assay of the puromycin reaction. Biochem J. 1978 Jan 15;170(1):57–61. doi: 10.1042/bj1700057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemiolo D. K., Steen R., Stark M. J., Dahlberg A. E. Analysis of plasmid-coded ribosomal RNA maxicell techniques. Methods Enzymol. 1988;164:691–706. doi: 10.1016/s0076-6879(88)64078-x. [DOI] [PubMed] [Google Scholar]
- Kung H. F., Redfield B., Treadwell B. V., Eskin B., Spears C., Weissbach H. DNA-directed in vitro synthesis of beta-galactosidase. Studies with purified factors. J Biol Chem. 1977 Oct 10;252(19):6889–6894. [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D., Robertson J. M., Noller H. F. Interaction of elongation factors EF-G and EF-Tu with a conserved loop in 23S RNA. Nature. 1988 Jul 28;334(6180):362–364. doi: 10.1038/334362a0. [DOI] [PubMed] [Google Scholar]
- Montanaro L., Sperti S., Stirpe F. Inhibition by ricin of protein synthesis in vitro. Ribosomes as the target of the toxin. Biochem J. 1973 Nov;136(3):677–683. doi: 10.1042/bj1360677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noller H. F. Structure of ribosomal RNA. Annu Rev Biochem. 1984;53:119–162. doi: 10.1146/annurev.bi.53.070184.001003. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Petrullo L. A., Gallagher P. J., Elseviers D. The role of 2-methylthio-N6-isopentenyladenosine in readthrough and suppression of nonsense codons in Escherichia coli. Mol Gen Genet. 1983;190(2):289–294. doi: 10.1007/BF00330653. [DOI] [PubMed] [Google Scholar]
- Robakis N., Cenatiempo Y., Meza-Basso L., Brot N., Weissbach H. A coupled DNA-directed in vitro system to study gene expression based on di- and tripeptide formation. Methods Enzymol. 1983;101:690–706. doi: 10.1016/0076-6879(83)01048-4. [DOI] [PubMed] [Google Scholar]
- Ruusala T., Andersson D., Ehrenberg M., Kurland C. G. Hyper-accurate ribosomes inhibit growth. EMBO J. 1984 Nov;3(11):2575–2580. doi: 10.1002/j.1460-2075.1984.tb02176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinovich O., Montelaro R. C. Reversible staining and peptide mapping of proteins transferred to nitrocellulose after separation by sodium dodecylsulfate-polyacrylamide gel electrophoresis. Anal Biochem. 1986 Aug 1;156(2):341–347. doi: 10.1016/0003-2697(86)90263-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler D. G., Davies J. E. Specific cleavage of ribosomal RNA caused by alpha sarcin. Nucleic Acids Res. 1977 Apr;4(4):1097–1110. doi: 10.1093/nar/4.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperti S., Montanaro L., Mattioli A., Stirpe F. Inhibition by ricin of protein synthesis in vitro: 60 S ribosomal subunit as the target of the toxin. Biochem J. 1973 Nov;136(3):813–815. doi: 10.1042/bj1360813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark M. J., Gourse R. L., Dahlberg A. E. Site-directed mutagenesis of ribosomal RNA. Analysis of ribosomal RNA deletion mutants using maxicells. J Mol Biol. 1982 Aug 15;159(3):417–439. doi: 10.1016/0022-2836(82)90292-3. [DOI] [PubMed] [Google Scholar]
- Tapio S., Isaksson L. A. Antagonistic effects of mutant elongation factor Tu and ribosomal protein S12 on control of translational accuracy, suppression and cellular growth. Biochimie. 1988 Feb;70(2):273–281. doi: 10.1016/0300-9084(88)90071-5. [DOI] [PubMed] [Google Scholar]
- Tapio S., Kurland C. G. Mutant EF-Tu increases missense error in vitro. Mol Gen Genet. 1986 Oct;205(1):186–188. doi: 10.1007/BF02428051. [DOI] [PubMed] [Google Scholar]
- Tapprich W. E., Goss D. J., Dahlberg A. E. Mutation at position 791 in Escherichia coli 16S ribosomal RNA affects processes involved in the initiation of protein synthesis. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4927–4931. doi: 10.1073/pnas.86.13.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao K., Uchiumi T., Endo Y., Ogata K. Ricin and alpha-sarcin alter the conformation of 60S ribosomal subunits at neighboring but different sites. Eur J Biochem. 1988 Jun 15;174(3):459–463. doi: 10.1111/j.1432-1033.1988.tb14120.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vester B., Garrett R. A. The importance of highly conserved nucleotides in the binding region of chloramphenicol at the peptidyl transfer centre of Escherichia coli 23S ribosomal RNA. EMBO J. 1988 Nov;7(11):3577–3587. doi: 10.1002/j.1460-2075.1988.tb03235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarucki-Schulz T., Jerez C., Goldberg G., Kung H. F., Huang K. H., Brot N., Weissbach H. DNA-directed in vitro synthesis of proteins involved in bacterial transcription and translation. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6115–6119. doi: 10.1073/pnas.76.12.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis using M13-derived vectors: an efficient and general procedure for the production of point mutations in any fragment of DNA. Nucleic Acids Res. 1982 Oct 25;10(20):6487–6500. doi: 10.1093/nar/10.20.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]