Abstract
T cell dysfunction in cancer comes in many forms, with two new varieties reported in this issue. Daley et al. find that T cells expressing γδ T cell receptors (TCR) promote pancreatic tumor growth by inhibiting activation of T cells with conventional TCRs. Singer et al. characterize dysfunctional tumor infiltrating lymphocytes to reveal a role for zinc homeostasis in anti-tumor immunity.
For the immune system to mount an adequate response to cancer it must overcome a slew of obstacles. T cells that recognize tumor antigens must be sufficiently activated by antigen presenting cells, then seek out and destroy tumor cells. However, within the harsh tumor microenvironment, numerous factors dampen and suppress anti-tumor T cell responses. For example, a complex combination of hypoxia, nutrient imbalances, acidic pH, immunosuppressive cytokines (such as TGF-β and IL-10), lipids (such as PGE2), lactate and other metabolites are well-known to inhibit tumor-infiltrating lymphocytes (TILs). Additionally, co-inhibitory receptor:ligand pairs (such as PD-1:PD-L1, CTLA-4:B7, and TIM-3:Galectin-9) expressed on tumor cells and tumor-infiltrating immune cells are currently at the center of attention as drugs developed to block these molecular interactions (termed immune checkpoint inhibitors) have demonstrated considerable clinical benefit for numerous cancer types. The heterogeneous nature of individual tumors and cancer types and the development of acquired resistance to most therapy, make it unlikely that one form of therapy will reinvigorate the immune system to effectively combat all cancers. Thus, identifying new avenues to circumvent immune cell dysfunction in adjunct with current cancer therapies is needed to increase overall patient survival. In this issue of Cell, two groups report on the discovery of new mediators of T cell dysfunction in cancer. Daley et al. (2016) describe an unexpected role for γδ T cells in blocking the activity of conventional αβ T cells in pancreatic tumors through PD-1:PD-L1 interactions. Singer et al. (2016) characterize a genetic signature of dysfunctional CD8 T cells that led to the identification of metallothioneins (MTs) and the zinc-finger transcription factor GATA3 in suppressing the function of tumor-infiltrating lymphocytes. Both of these studies shed light on new biological targets that may lead to new anti-cancer treatments (Figure 1).
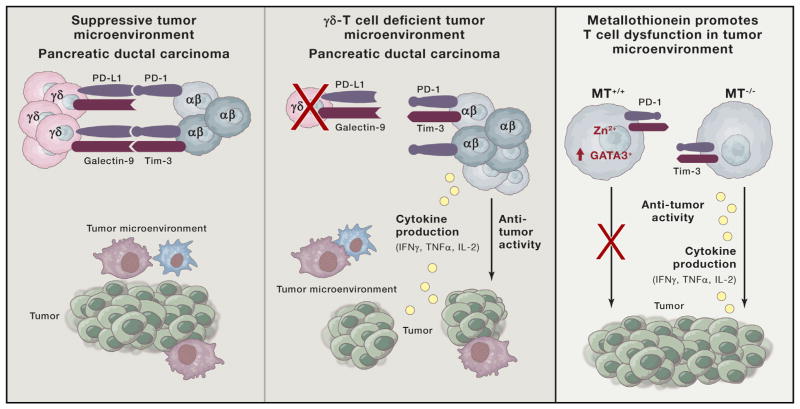
Figure 1. Releasing Brakes on Tumor T Cells.
(Left and center) γδ T cells, in concert with myeloid-derived suppresor cells and tumor-associated macrophages, foster a suppressive tumor microenvironment in pancreatic cancer. γδ T cells promote tumor growth through upregulation of co-inhibitory molecules PD-L1 and Galectin-9, directly blocking αβ CD4 and CD8 T cell activation. Deletion of γδ T cells through various means (i.e., CCR2, CCR5, CCR6, and TCRδ-genetic deficiency) restores αβ T cell cytokine production, co-stimulatory molecule expression, and control of tumor growth. Therapeutic benefit of PD-L1 or Galectin-9 antibody blockade primarily hinges upon targeting γδ T cells, in turn promoting increased T cell infiltration and control of tumor growth. (Right) Metallothioneins (MTs) regulate metabolism of Zn2+ and other trace minerals. Intracellular accumulation of Zn2+ promotes T cell dysfunction in the tumor microenvironment and increases activity of zinc-finger transcription factors. Genetic deletion of Gata3 reverses metallothionein-dependent T cell loss of cytokine production, independent of the expression of classic cell surface markers of T cell dysfunction.
Pancreatic cancer is a devastating form of cancer with extremely poor survival rates that can significantly benefit from identification of new therapeutic targets. Daley et al. show that the majority of T cells infiltrating pancreatic ductal carcinoma in humans and a mouse model of pancreatic cancer possess the γδ T cell receptor (TCR), not the conventional αβ TCR. γδ T cells makeup a relatively small subset of T cells in the circulation but are found in greater proportions within the intestinal mucosa and skin epithelium where their role in barrier protection is well characterized. γδ T cells are reported to mount potent responses against tumors, but other studies have demonstrated their ability to be tumor promoting. In the current study, the authors find that the γδ T cells infiltrate the pancreas and suppress activation of αβ T cells. γδ T cells in the pancreas secrete a combination of anti-inflammatory and pro-inflammatory cytokines, such as IL- 10 and IL-17, IFN-γ, TNF-α, and IL-13. The γδ T cells also express high levels of PD-L1 and Galectin-9, which the authors find directly inhibit the activation of αβ T cells. Removal of γδ T cells from the tumor microenvironment enables αβ T cells to better infiltrate the tumor and control tumor growth. These findings not only strengthen the rationale for expanding PD-1/PD-L1 blockade therapy to pancreatic cancer but also suggest that targeting γδ T cells through antibody depletion may be another therapeutic option.
In the report by Singer et al. (2016), a series of computational methods is used to identify genes unique to dysfunctional CD8+ tumor-infiltrating lymphocytes. By comparing RNA profiles of populations of dysfunctional T cells (as well as individual single T cells within) to functional antiviral CD8 T cells, they are able to better distinguish genes associated with T cell dysfunction from those associated with T cell activation. This has been challenging because in some cases the inhibitory receptors that help define dysfunctional T cells (e.g., PD-1, TIM-3, LAG-3, and TIGIT) are also found on functional activated T cells. Thus, population-based analyses of T cells distinguished by these markers alone may preclude the identification of genes driving T cell dysfunction in tumors. Using single-cell gene expression analysis of tumor-infiltrating lymphocytes the authors found that the T cell “activation” and “dysfunction” gene modules negatively correlate with each other and are exclusively enriched in distinct populations of CD8 T cells. These findings suggest that while dysfunctional or “exhausted” T cells arise from activated T cells, they acquire a distinct transcriptional state that is no longer dependent on the module of activation genes. Furthermore, zeroing in on the dysfunctional gene modules led to the discovery of new regulators of T cell dysfunction, including metallothioneins and the zinc-dependent transcription factor Gata3. The authors find that metallothioneins are highly expressed in dysfunctional T cells and genetic deletion of MT1 and MT2 restores their cytokine production. Interestingly, tumors grew slower in metallothionein-deficient mice in spite of relatively high amounts of PD-1 and Tim-3 on tumor-infiltrating lymphocytes.
As metallothionein levels are regulated by Zn2+ and are important for its binding and transport the authors postulate that increased metallothionein and intracellular Zn2+ affects zinc-dependent transcription factors involved in T cell dysfunction. It is worth noting, though, that the role of metallothioneins in T cells may not be limited to Zn2+ as they also bind other metal cations such as Cu2+. Profiling the transcription factors differentially expressed between the dysfunctional and activation gene modules identified GATA3 and Helios (IKZF2), both zinc-finger transcription factors. Indeed, a small subset of tumor-infiltrating lymphocytes express higher levels of GATA3, and upon activation these cells produced IL-10 and made less IFN-γ and IL-2 than GATA3− cells. Deletion of Gata3 in tumor-specific CD8 T cells increased IFN-γ and IL-2 production, decreased IL-10, and suppressed tumor growth. Interestingly, GATA3 counter-regulates T-bet in CD4 T cells, and given that T-bet restrains the terminal stages of CD8 T cell exhaustion (Kao et al., 2011), switches in GATA3 expression in CD8 T cells may signify and promote this transition.
While these findings reveal new factors in regulating T cell dysfunction in tumors, many questions remain. For example, what induces high amounts of PD-L1 on pancreatic γδ T cells? Possibly IL-27, as it has been shown to induce PD-L1 expression on CD4 T cells and inhibit Th17 mediated autoimmunity (Hirahara et al., 2012). What type of TCR antigens or other stress-related ligands for NKG2D or Toll-like-receptors in the pancreatic tumor microenvironment drive γδ T cell accumulation and their suppressive functions? Alternatively, perturbed metabolic properties of the malignant pancreas or its close proximity to the duodenum may promote pancreatic infiltration of γδ T cells.
Additionally, what programs T cell dysfunction aside from persistent antigen? This study demonstrates a role for GATA3 in promoting T cell dysfunction. However, it remains undetermined if GATA3 is a core regulator of the dysfunctional gene modules or if deletion of metallothionein suppresses the activity of GATA3 or other zinc-finger transcription factors (e.g., IKZF2) due to zinc irregularities. IKZF2 also represses IL-2 production and is required for Ly49+ CD8+ Tregs (Kim et al., 2015), potentially drawing a parallel between these cells and tumor-infiltrating lymphocytes. Other regulators of CD8 T cell dysfunction in tumors include PGE2 and adenosine, and both signal downstream through cAMP and PKA. Interestingly, the dysfunctional gene module contains the PGE2 receptor Ptger4, in agreement with our prior work demonstrating Ptger2 and Ptger4 contribute to CD8 T cell dysfunction (Chen et al., 2015). Moreover, blockade of PGE2 signaling boosts the therapeutic effects of PD-1 blockade in cancer and chronic viral infection (Chen et al., 2015; Zelenay et al., 2015). Furthermore, metallothionein expression can be enhanced by adenosine (Xiong et al., 1992) and cAMP/PKA signaling, providing another link between these pathways in lymphocyte dysfunction.
Lastly, it will be important to link the dysfunctional gene program(s) to T cell metabolism because decreased glycolysis and mitochondrial function are core to T cell dysfunction (Chang and Pearce, 2016). PD-1 can suppress T cell glycolysis, and conversely, increasing hypoxia inducible factor-1 alpha (HIF-1α) activity boosts glycolysis as well as effector functions in CD8 T cells in chronic viral infection and tumors (Doedens et al., 2013). Perhaps, HIF-1α counteracts GATA3 and IKZF2 to promote “activation” gene expression and metabolic states optimal for CD8 T cell effector function. In relation, hypoxic stress can paradoxically increase production of reactive oxygen species and possibly infiltrating lymphocytes combat this in hypoxic tumor microenvironments by importing Zn2+ as an antioxidant. However, this in turn, may increase metallothionein expression and further suppress T cell function and mitochondrial respiration. Thus, while signaling through co-inhibitory receptors may set the stage for T cell dysfunction, the eventual loss of bioenergetic potential and alterations of intracellular metabolites and ions may ultimately remodel the transcriptional and epigenetic landscape that manifests in CD8 T cell dysfunction in tumors.
Supplementary Material
References
- Chang CH, Pearce EL. Nat Immunol. 2016;17:364–368. doi: 10.1038/ni.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JH, Perry CJ, Tsui YC, Staron MM, Parish IA, Dominguez CX, Rosenberg DW, Kaech SM. Nat Med. 2015;21:327–334. doi: 10.1038/nm.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley D, Zambirinis C, Seifert L, Akkad N, Mohan N, Werba G, Barilla R, Torres-Hernandez A, Hundeyin M, Mani VRK. Cell. 2016;166:1485–1499. doi: 10.1016/j.cell.2016.07.046. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E, Johnson RS, Goldrath AW. Nat Immunol. 2013;14:1173–1182. doi: 10.1038/ni.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirahara K, Ghoreschi K, Yang XP, Takahashi H, Laurence A, Vahedi G, Sciumè G, Hall AO, Dupont CD, Francisco LM, et al. Immunity. 2012;36:1017–1030. doi: 10.1016/j.immuni.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C, Oestreich KJ, Paley MA, Crawford A, Angelosanto JM, Ali MA, Intlekofer AM, Boss JM, Reiner SL, Weinmann AS, Wherry EJ. Nat Immunol. 2011;12:663–671. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Barnitz RA, Kreslavsky T, Brown FD, Moffett H, Lemieux ME, Kaygusuz Y, Meissner T, Holderried TA, Chan S, et al. Science. 2015;350:334–339. doi: 10.1126/science.aad0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M, Wang C, Cong L, Marjanovic ND, Kowalczyk MS, Zhang H, Nyman J, Sakuishi K, Kurtulus S, Gennert D, et al. Cell. 2016;166:1500–1511. doi: 10.1016/j.cell.2016.08.052. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Garrett SH, Arizono K, Brady FO. Proc Soc Exp Biol Med. 1992;201:59–65. doi: 10.3181/00379727-201-43480. [DOI] [PubMed] [Google Scholar]
- Zelenay S, van der Veen AG, Böttcher JP, Snelgrove KJ, Rogers N, Acton SE, Chakravarty P, Girotti MR, Marais R, Quezada SA, et al. Cell. 2015;162:1257–1270. doi: 10.1016/j.cell.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.