Abstract Abstract
A new species of algae-scraping cyprinid of the genus Capoeta Valenciennes, 1842 is described from the Kheyroud River, located in the southern part of the Caspian Sea basin in Iran. The species differs from other members of this genus by a combination of the following characters: one pair of barbels; predorsal length equal to postdorsal length; maxillary barbel slightly smaller than eye’s horizontal diameter and reach to posterior margin of orbit; intranasal length slightly shorter than snout length; lateral line with 46–54 scales; 7–9 scales between dorsal-fin origin and lateral line, and 6–7 scales between anal-fin origin and lateral line.
Keywords: Algae-scraping cyprinid, Caspian Sea, inland freshwater, Iran, taxonomy
Introduction
Cyprinid fishes of the genus Capoeta Valenciennes, 1842 have a wide distribution throughout western Asia from Anatolia to the Levant, Transcaucasia, the Tigris and Euphrates basins, Turkmenistan, and northern Afghanistan (Bănărescu 1999; Levin et al. 2012; Ghanavi et al. 2016; Jouladeh-Roudbar et al. 2016). This genus has at least 28 species, of which the following 15 species are present in Iran: Capoeta aculeata (Valenciennes, 1844); C. alborzensis Jouladeh-Roudbar, Eagderi, Ghanavi & Doadrio, 2016; C. anamisensis Zareian, Esmaeili & Freyhof, 2016; C. barroisi Lortet, 1894; C. buhsei Kessler, 1877; C. capoeta (Güldenstaedt, 1773); C. coadi Alwan, Zareian, & Esmaeili, 2016; C. damascina (Valenciennes, 1842); C. fusca Nikolskii, 1897; Capoeta gracilis (Keyserling, 1861); C. heratensis (Keyserling, 1861); C. mandica Bianco & Bănărescu, 1982; C. saadii (Heckel, 1847), C. trutta (Heckel, 1843), and C. umbla (Heckel, 1843) (Jouladeh-Roudbar et al. 2015a,b; Alwan et al. 2016; Zareian et al. 2016; Jouladeh-Roudbar et al. 2016). Of these species, eight are endemic to Iran and three have been described recently based on the results of molecular studies (Alwan et al. 2016; Jouladeh-Roudbar et al. 2016; Zareian et al. 2016).
Capoeta species mainly inhabit fast flowing streams and rivers, but some species may also be found in lakes and springs (Turan et al. 2006). The members of this genus possess a fusiform body with small to moderately large scales and an inferior mouth (Coad 2017). Their lower lip bears a keratinized edge and lower lip is restricted to the corner of mouth (Howes 1982; Turan et al. 2006; Coad 2017). The dorsal fin is short with the last unbranched ray thickened, and has serrations posteriorly (serrations sometimes reduced to absent).
The populations of the genus Capoeta from the southern Caspian Sea basin are considered as belonging to two different species: C. gracilis and C. capoeta (Esmaeili et al. 2010; Jouladeh-Roudbar et al. 2015b). Capoeta gracilis was originally described from rivers near Esfahan, central Iran (Esfahan basin) and C. capoeta from Tiflis (Caspian Sea basin), Georgia (the Caspian Sea basin) (Güldenstädt 1773; Temminck and Schlegel 1843; Coad 2017). Several authors have considered C. gracilis as subspecies of C. capoeta, both with allopatric distribution. Capoeta c. gracilis was restricted to rivers between the Sefid and Atrak rivers in the southern part of the Caspian basin in Iran and C. c. capoeta to the Kura-Aras basin in western part of the Caspian basin (Bianco and Banarescu 1982). Furthermore, Bănărescu (1999) restricted the distribution of C. c. gracilis to the Urmia Lake basin and the Sefid River in southern part of the Caspian basin (and also to the lower Kura River in Azerbaijan) while C. capoeta aff. gracilis (an unnamed subspecies related to C. c. gracilis) was considered to inhabit the rest of the Iranian Caspian shore (Jouladeh-Roudbar et al. 2015). Posterior works have considered C. gracilis as a valid species but its distribution has been controversial (Esmaeili et al. 2014).
Currently, molecular studies have shown a high genetic differentiation in the populations of southern Caspian basins considered previously as C. gracilis or C. c. aff. gracilis and this led to the consideration of these populations as an undescribed species (Levin et al. 2012; Ghanavi et al. 2016). The presence of C. capoeta in both the Caspian Sea and Urmia Lake basins was also confirmed based on molecular and morphological data (Ghasemi et al. 2015; Ghanavi et al. 2016).
Previous phylogenetic and phylogeographic studies based on molecular mitochondrial data recognized three main clades within the genus Capoeta, Mesopotamian clade, Aralo-Caspian clade, and Anatolian-Iranian clade (Levin et al. 2012; Ghanavi et al. 2016). The Aralo-Caspian clade is composed by four valid species i.e. C. capoeta, C. heratensis, C. fusca and C. alborzensis in the Iranian freshwater basins (Ghanavi et al. 2016; Jouladeh-Roudbar et al. 2016). A detailed study of the populations of Aralo-Caspian clade in Iran, found some populations of the genus Capoeta, which were not identified as any described species (Ghanavi et al. 2016). Among them were populations distributed in the southern Caspian Sea basin, traditionally identified as C. gracilis (Jouladeh-Roudbar et al. 2015b). Our collection of the genus Capoeta from the southern Caspian Sea basin revealed the presence of two species, i.e. C. capoeta and an undescribed species (considered as Capoeta sp.1 in Ghanavi et al. 2016) that differ molecularly and morphologically from other described Capoeta species including species from the Esfahan basin (Alwan et al. 2016; Ghanavi et al. 2016). According to our intensive samplings from the Esfahan basin, only two species i.e. C. aculeata and C. coadi were found. Therefore, the main goal of this work is to study morphologically the populations of the collected Capoeta specimens from the southern Caspian Sea basin, north of Iran, previously assigned to C. gracilis, and to compare them with the remaining species of this genus from Iran, and based on differences found, they are described as a new species herein.
Materials and methods
Approximately 150 specimens of the genus Capoeta were collected by electrofishing at 14 sites covering most of its distribution area in southern Caspian Basin (Figure 1, Table 1). Fin clips stored in 96% ethanol and deposited in the Tissue and DNA Collection of the Ichtyological Museum of Natural Resources Faculty – University of Tehran (IMNRF-UT). The fish were killed with overdoses of MS222, were fixed in 10% formalin, and were later preserved in the Ichthyology collection of IMNRF-UT, Iran. For morphometric purposes and to have a base for molecular studies 23 individuals of C. capoeta and C. fusca from the Urmia Lake and Hari River basins, respectively, were also analysed.
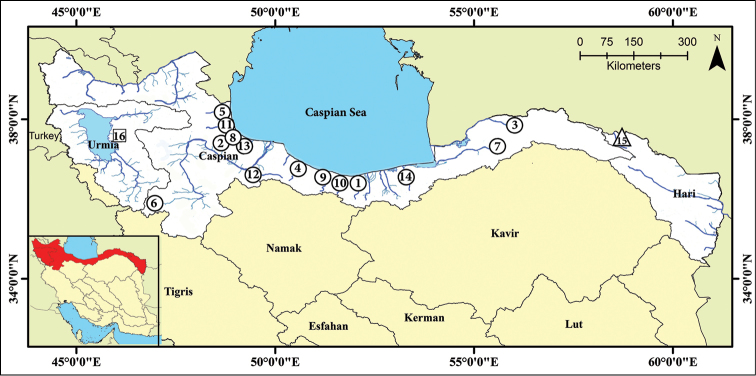
Figure 1.
Map of the southern Caspian Sea basin and sampling points. Numbers of the sampling sites correspond to the numbers of sampling sites in Table 1, circle: Capoeta razii sp. n., triangle: C. fusca, square: C. capoeta.
Table 1.
Sampling sites and coordinates. Numbers in the first column (Loc) correspond to numbers on the sampling map in Figure 1.
| Loc. | River | Locality | Species | GPS Coordinates | Alt. (m) |
|---|---|---|---|---|---|
| 1 | Angueta Rud | Sangetab | Capoeta razii sp. n. | 36°28'37"N, 42°13'31"E | 44 |
| 2 | Asalem | Asalem | 37°42'53"N, 48°55'44"E | 104 | |
| 3 | Atrak | Maraveh Tappeh | 37°54'30"N, 55°57'10"E | 198 | |
| 4 | Chalk Rud | Katalom | 36°52'19"N, 50°46'17"E | -20 | |
| 5 | Choobar Rud | Choobar | 38°10'36"N, 48°52'54"E | -7 | |
| 6 | Ghezel Ozan | Nesareh | 35°52'12"N, 47°04'54"E | 1732 | |
| 7 | Golestan | Tangrah | 37°22'55"N, 55°51'12"E | 564 | |
| 8 | Karrgan Rud | Talesh | 37°48'02"N, 48°53'04"E | 71 | |
| 9 | Kelar Abad Rud | Kelar Abad | 36°42'05"N, 51°13'10"E | -15 | |
| 10 | Kheyr Rud | Chalos | 36°36'35"N, 51°33'45"E | 34 | |
| 11 | Khushavar Rud | Khushavar | 38°01'51"N, 48°53'31"E | 17 | |
| 12 | Sefid Rud | Lowshan | 36°38'13."N, 49°29'17"E | 307 | |
| 13 | Shafa Rud | Punel | 37°31'52"N, 49°06'36"E | 246 | |
| 14 | Tajan | Payin Hular (Sari) | 36°29'12"N, 53°05'10"E | 90 | |
| 15 | Ghale Chay | Ajab Shir | C. capoeta | 37°29'25"N, 45°59'57"E | |
| 16 | Segonbadan | Farooj | C. fusca | 37°14'46"N, 58°08'01"E |
Morphological examinations. Thirty morphometric measurements and thirteen meristic character countings were performed using a digital caliper to the nearest 0.1 mm and stereomicroscope, respectively (Tables 4–8). Measurements follow Kottelat and Freyhof (2007). Fin ray counts separate unbranched and branched rays. The last two branched rays articulated on a single pterygiophore in dorsal and anal-fins are noted as “1”.
Table 4.
Morphometric data of Capoeta razii sp. n. (holotype, IMNRF-UT-1072-9; paratypes, IMNRF-1072, 14 specimens) C. capoeta (IMNRF-UT-1067, 15 specimens) and C. fusca (IMNRF-UT-1065, 8 specimens).
| Characters | Holotype | C. razii sp. n. | C. capoeta | C. fusca | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range | Mean | SD | Range | Mean | SD | Range | Mean | SD | ||
| Standard length (mm) | 142.6 | 90.7–184.2 | 66.5–157.3 | 47.2–124.2 | ||||||
| In percent of standard length (SL) | ||||||||||
| Body depth maximal | 23.7 | 23.1–25.5 | 23.9 | 0.7 | 23.4–26.9 | 25.2 | 1.0 | 24.4–27.1 | 26.0 | 0.9 |
| Caudal peduncle depth | 12.1 | 11.1–12.9 | 11.9 | 0.5 | 10.1–12.6 | 11.7 | 0.7 | 11.1–13.5 | 12.5 | 0.8 |
| Predorsal length | 52.3 | 50.2–53.1 | 51.8 | 0.9 | 50.8–55.5 | 52.9 | 1.2 | 52.6–55.0 | 53.8 | 0.9 |
| Postdorsal length | 51.8 | 49.9–54.2 | 51.7 | 1.2 | 47.6–55.1 | 51.9 | 2.1 | 48.9–52.3 | 50.6 | 1.2 |
| Prepelvic length | 55.1 | 55–58.7 | 56.1 | 1.1 | 54.3–61.3 | 57.1 | 1.9 | 55.2–58.6 | 57.3 | 1.2 |
| Preanal length | 75.9 | 76.4–79.6 | 77.6 | 1.0 | 74.9–79.7 | 77.5 | 1.4 | 76.7–79.9 | 78.4 | 1.3 |
| Caudal peduncle length | 18.9 | 16.1–19.4 | 17.4 | 1.1 | 14.7–20.0 | 17.2 | 1.4 | 14.2–17.9 | 16.1 | 1.3 |
| Dorsal fin base length | 11.3 | 12.1–15.4 | 13.6 | 0.9 | 12.7–16.7 | 14.5 | 1.4 | 14.9–18.0 | 16.5 | 0.9 |
| Dorsal fin depth | 17.7 | 16.2–21 | 18.9 | 1.2 | 18.5–22.2 | 20.5 | 0.9 | 18.7–26.1 | 22.3 | 2.2 |
| Anal fin base length | 7.3 | 6.8–8.3 | 7.5 | 0.4 | 6.0–9.1 | 7.7 | 0.8 | 8.1–10.1 | 9.1 | 0.7 |
| Anal fin depth | 16.8 | 15–20.4 | 17.7 | 1.4 | 14.4–18 | 16.2 | 1.0 | 17.1–19.9 | 18.7 | 0.8 |
| Pectoral fin length | 20.5 | 17.8–21.3 | 19.5 | 1.1 | 15.4–20.6 | 18.7 | 1.9 | 18.3–24.2 | 21.2 | 2.1 |
| Pelvic fin length | 16.7 | 14.1–17.5 | 16.0 | 1.0 | 14.2–17.3 | 16.0 | 0.9 | 15.9–19.9 | 18.1 | 1.2 |
| Pectoral – pelvic-fin origin distance | 32.3 | 30.6–36.1 | 32.8 | 1.4 | 31.4–37.0 | 34.2 | 1.7 | 29.5–34.5 | 32.3 | 1.8 |
| Pelvic – anal-fin origin distance | 20.6 | 21–24.2 | 22.2 | 1.0 | 18.7–23.0 | 21.5 | 1.2 | 20.1–23.9 | 22.1 | 1.4 |
| Body width | 16.3 | 15.1–17 | 16.0 | 0.6 | 16.3–18.4 | 17.2 | 0.6 | 16.6–18.7 | 17.6 | 0.7 |
| Caudal peduncle width | 3.6 | 2.8–4.1 | 3.4 | 0.5 | 3.1–4.2 | 3.7 | 0.3 | 5.5–7.0 | 6.3 | 0.5 |
| Head length (HL) | 22.5 | 20.5–24 | 23.0 | 1.0 | 19.8–25.9 | 22.6 | 1.8 | 25.0–28.6 | 26.2 | 1.7 |
| As percentage of head length (HL) | ||||||||||
| Snout length | 26.2 | 26.2–31.6 | 28.7 | 1.4 | 24.7–29.8 | 27.1 | 1.6 | 28.2–33.1 | 30.6 | 1.9 |
| Eye horizontal diameter | 20.1 | 17.1–26.7 | 23.3 | 2.7 | 17.4–22.7 | 19.4 | 1.7 | 15.4–23.7 | 19.3 | 2.9 |
| Postorbital distance | 53.5 | 46.4–54.4 | 50.7 | 2.2 | 47.9–60.8 | 56.2 | 3.4 | 48.1–54.2 | 52.2 | 2.0 |
| Head depth at nape | 78.3 | 70.1–82.9 | 76.4 | 3.5 | 67.5–87.5 | 79.4 | 5.2 | 70.3–76.1 | 72.6 | 2.0 |
| Head depth at eye | 50.2 | 45.7–53 | 51.1 | 2.0 | 44.8–56.8 | 52.7 | 3.2 | 47.0–53.4 | 51.2 | 1.9 |
| Head length at nape | 90.1 | 88.9–97 | 92.2 | 2.4 | 83.8–98.6 | 92.9 | 3.9 | 87.9–96.3 | 91.5 | 3.1 |
| Head width | 67.6 | 61.6–73.1 | 65.9 | 3.1 | 62.3–77.3 | 70.0 | 5.4 | 54.9–69.7 | 60.7 | 4.7 |
| Inter orbital | 42.5 | 34.3–46 | 42.8 | 2.9 | 41.4–52.2 | 46.2 | 3.4 | 35.7–40.1 | 37.0 | 1.4 |
| Inter nasal | 26.1 | 20.2–26 | 24.7 | 1.8 | 24.0–31.3 | 28.0 | 2.2 | 17.1–23.6 | 20.7 | 1.8 |
| Mouth width | 35.6 | 28.7–37.9 | 34.2 | 2.9 | 31.4–41.3 | 36.0 | 2.9 | 26.6–38.9 | 31.3 | 4.7 |
| Barbel length | 13.0 | 14–21.6 | 17.2 | 2.4 | 9.3–16.2 | 13.2 | 1.8 | 9.9–17.3 | 13.6 | 2.9 |
Table 8.
Range of meristic features of Iranian Capoeta species.
| No. | Species | LL | ALL | BLL | CPS | TGR | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Capoeta alborzensis | 39–44 | 6–8 | 5–8 | 16–17 | 19–22 | This study |
| 2 | Capoeta aculeata | 39–43 | 7–8 | 5–7 | 16–20 | 19–23 | This study |
| 3 | Capoeta razii sp. n. | 46–54 | 7–9 | 6–7 | 17–18 | 15–21 | This study |
| 4 | Capoeta anamisensis | 56–67 | 11–12 | 6–8 | – | 21–25 | Zareian et al. 2016 |
| 5 | Capoeta barroisi | 76–84 | 14–16 | 10–13 | – | 26–29 | Turan et al. 2006 |
| 6 | Capoeta buhsei | 80–89 | 13–15 | 11–13 | 29–31 | 11–13 | This study |
| 7 | Capoeta capoeta | 51–58 | 9–11 | 7–8 | 19–23 | 17–29 | This study |
| 8 | Capoeta coadi | 68–75 | 12–15 | 9–10 | 25–29 | 15–18 | This study |
| 9 | Capoeta damascina | 64–82 | 12–17 | 8–12 | 23–30 | 17–25 | Alwan, 2011 |
| 10 | Capoeta fusca | 46–54 | 8–10 | 8–9 | 19–26 | 16–18 | This study |
| 11 | Capoeta heratensis | 55–61 | 9–12 | 7–9 | 22–25 | 21–24 | This study |
| 12 | Capoeta mandica | 58–68 | 12–13 | 8–10 | 27–33 | 23–27 | Alwan et al. 2016 |
| 13 | Capoeta saadi | 61–78 | 9–14 | 6–10 | – | 12–17 | Alwan, 2011 |
| 14 | Capoeta trutta | 65–82 | 9–14 | 9–12 | 27–31 | 20–30 | This study |
| 15 | Capoeta umbla | 90–102 | 18–23 | 12–14 | 33–36 | 18–20 | This study |
An allometric method was used to remove size-dependent variation in morphometric characters using following formula (Elliott et al. 1995): Madj = M(Ls/L0)b, where M is the original measurement, Madj the size adjusted measurement, L0 the standard length of the fish, Ls the overall mean of the standard length for all fish from all samples in each analysis, and b was estimated for each character from the observed data as the slope of the regression of log M on log L0 using all fish in any group. The adjusted morphometric characters of the studied populations were analysed using Principal Component Analysis (PCA) and compared by Non-Parametric Multivariate Analysis of Variance (NPMANOVA) based on the P-values obtained from permutation test with 1000 replicates in PAST software (version 2.14). The meristic characters of the studied populations were analysed using Correspondence Analysis (CA), and compared by Non-Parametric Multivariate Analysis Of Variance (NPMANOVA) based on the Bonferoni-corrected P-values obtained from permutation test with 1000 replicates in PAST software (version 2.14).
Molecular data analysis. To analyse the molecular composition we studied the complete mitochondrial cytochrome b gene of all species of Aralo-Caspian group which include an unnamed population from Caspian Sea basin (Levin et al. 2012; Ghanavi et al. 2016). In this study, we considered sequences obtained from previous studies and deposited in GenBank (Table 2) (Levin et al. 2012; Ghanavi et al. 2016; Zareian et al. 2016; Jouladeh-Roudbar et al. 2016). Sequences were aligned using Geneious software (Geneious v. 10.0.2, Biomatters, http://www.geneious.com/), and visually verified to maximize positional homology. Sequences of Luciobarbus capito (Güldenstädt, 1773), L. brachycephalus (Kessler, 1872) and L. subquincunciatus (Günther, 1868) species were chosen as outgroup based on their phylogenetic relationship to genus Capoeta (Levin et al. 2012; Yang et al. 2015; Ghanavi et al. 2016). Uncorrected pairwise genetic distances (p-distances) between species (Table 3) were calculated with Mega 6 (Tamura et al. 2013). A bootstrapping process was implemented with 1000 repetitions. Jmodeltest 2.1.4 (Darriba et al. 2012) selected TrN+I as the best evolutionary model. RAxML (Stamatakis 2006) implemented in GENEIOUS software was used to estimate the maximum-likelihood (ML) tree. Bayesian inference was conducted with MrBAYES v. 3.2.2 (Ronquist et al. 2012). Two simultaneous analyses were run on 2*107 generations, each with four MCMC chains sampling tree every 2000 generations. Convergence was checked on Tracer 1.6 (Rambaut and Drummond 2013). After discarding the first 10% of generations as burn-in, we obtained the 50% majority rule consensus tree and the posterior probabilities. The species delimitation methodology used was Bayesian Poisson tree process (bPTP) model which is based on a distance-based tree (Zhang et al. 2013). bPTP were accessed at Exelixis Labs (http://sco.h-its.org/exelixis/web/software/PTP/index.html). Haplotype genealogies were visualized by HaploView v. 4.2 (Barrett et al. 2005).
Table 2.
List of species used for molecular analysis for Cyt b and GenBank accession number.
| KU312380 | Capoeta anamisensis | KU167903 | Capoeta razii sp. n. | JF798266 | Capoeta aculeata |
| KU312381 | KU167905 | KM459640 | |||
| JF798279 | Capoeta barroisi | KM459627 | KM459638 | ||
| KM459651 | Capoeta mandica | KM459628 | KM459637 | ||
| KM459649 | KU167933 | JF798267 | |||
| KM459650 | KM459630 | KM459631 | Capoeta saadii | ||
| AF145949 | Capoeta trutta | KU167922 | KM459639 | ||
| KM459673 | KU167934 | KM459641 | |||
| JF798332 | KU167932 | KU167952 | Capoeta damascina | ||
| KU167893 | Capoeta heratensis | KU167913 | KU167953 | ||
| JF798317 | KU167911 | KU167954 | |||
| JF798318 | KU167912 | KM459624 | Capoeta buhsei | ||
| JF798319 | KU167918 | KM459623 | |||
| JF798316 | KM459696 | Capoeta alborzensis | JF798283 | ||
| KU167894 | KY365754 | KM459634 | Capoeta coadi | ||
| KU167936 | Capoeta capoeta | KY365752 | JF798285 | ||
| KU167937 | KY365753 | KM459633 | |||
| KU167938 | KM459695 | AF145937 | Luciobarbus subquincunciatus | ||
| KU312371 | Capoeta fusca | KM459688 | KP712171 | Luciobarbus capito | |
| KU312372 | KM459687 | AY004729 | Luciobarbus brachycephalus |
Table 3.
Estimates of evolutionary divergence over sequence pairs between Capoeta razii sp. n. and other Iranian Capoeta species.
| species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | L. subquincunciatus | – | |||||||||||||||
| 2 | L. capito | 9.5 | – | ||||||||||||||
| 3 | L. brachycephalus | 8.6 | 3.3 | – | |||||||||||||
| 4 | C. barroisi | 8.6 | 9.0 | 8.6 | – | ||||||||||||
| 5 | C. trutta | 9.7 | 9.3 | 9.2 | 1.2 | – | |||||||||||
| 6 | C. mandica | 9.6 | 9.0 | 8.7 | 1.3 | 1.1 | – | ||||||||||
| 7 | C. anamisensis | 9.2 | 8.4 | 8.6 | 1.6 | 1.4 | 1.5 | – | |||||||||
| 8 | C. saadii | 9.8 | 8.7 | 9.0 | 7.6 | 7.9 | 8.0 | 8.2 | – | ||||||||
| 9 | C. damascina | 9.2 | 8.3 | 8.8 | 7.5 | 7.9 | 7.9 | 8.3 | 2.8 | – | |||||||
| 10 | C. buhsei | 9.6 | 8.6 | 9.3 | 8.1 | 8.4 | 8.4 | 8.7 | 2.6 | 2.2 | – | ||||||
| 11 | C. coadi | 9.6 | 8.6 | 9.4 | 7.8 | 8.1 | 8.0 | 8.7 | 2.7 | 2.1 | 1.4 | – | |||||
| 12 | C. fusca | 8.8 | 8.9 | 8.5 | 8.5 | 8.9 | 9.1 | 8.9 | 6.5 | 6.4 | 5.7 | 6.3 | – | ||||
| 13 | C. alborzensis | 8.7 | 8.2 | 8.6 | 7.9 | 8.3 | 8.5 | 8.3 | 5.6 | 5.4 | 5.3 | 5.5 | 1.6 | – | |||
| 14 | C. aculeata | 9.3 | 8.8 | 8.8 | 8.2 | 8.6 | 8.8 | 8.7 | 6.1 | 5.9 | 5.9 | 6.0 | 2.2 | 1.3 | – | ||
| 15 | C. heratensis | 10.1 | 9.1 | 9.7 | 9.1 | 9.3 | 9.5 | 9.0 | 6.2 | 5.9 | 5.8 | 6.5 | 2.5 | 2.2 | 2.6 | – | |
| 16 | C. capoeta | 9.0 | 8.5 | 8.6 | 7.9 | 8.4 | 8.6 | 7.9 | 5.9 | 5.6 | 5.8 | 5.9 | 2.3 | 1.8 | 2.0 | 2.6 | – |
| 17 | C. razii sp. n. | 9.5 | 9.1 | 9.3 | 8.4 | 8.8 | 9.1 | 8.8 | 6.0 | 5.8 | 5.8 | 5.9 | 2.2 | 1.4 | 1.8 | 2.5 | 2.1 |
Abbreviations
SL standard length;
HL lateral head length;
IMNRFI-UT Ichtyological Museum of Natural Resources Faculty.
Results
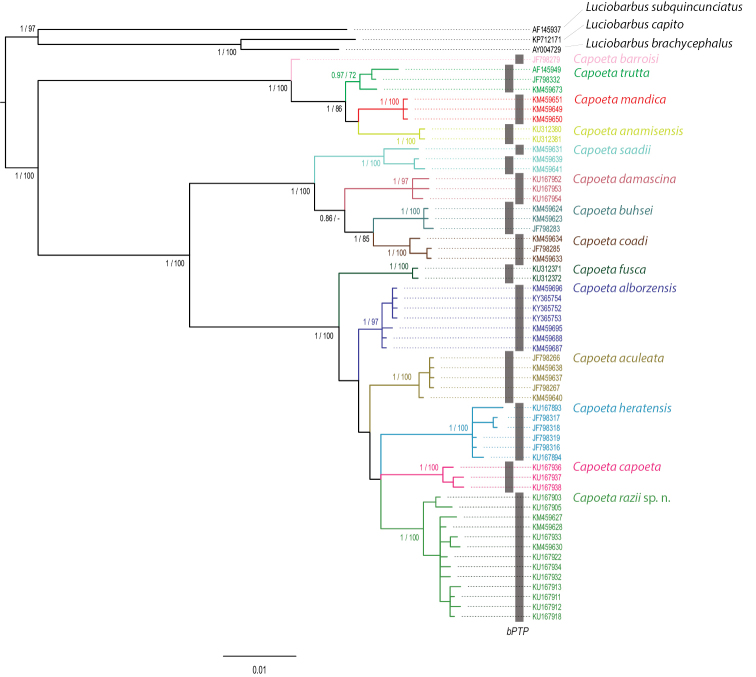
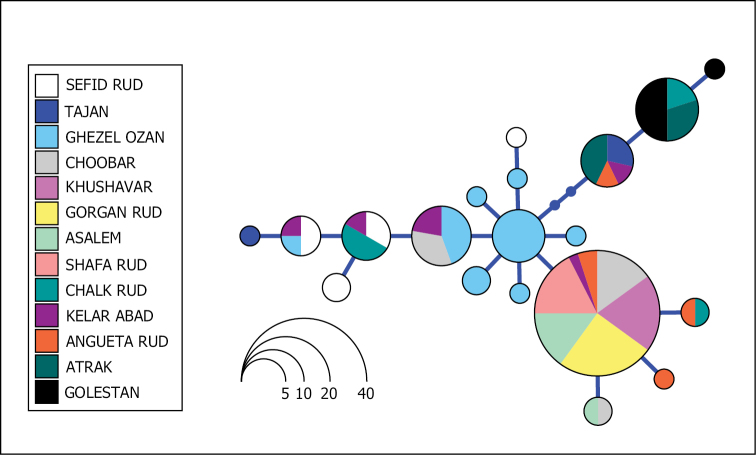
Based on the results, from the 1040 bp of complete mitochondrial cytochrome b genes, 793 positions were conserved and 195 were parsimony informative. Genetic distances between species are listed in Table 3. The Bayesian and ML analyses yielded similar topologies with well-supported nodes (Figure 2). The reconstructed topology was also in agreement with previously published higher-level phylogenies that included Capoeta and the three main clades, Aralo-Caspian, Anatolian-Iranian, and Mesopotamian were recovered (Levin et al. 2012; Ghanavi et al. 2016; Jouladeh-Roudbar et al. 2016). Based on molecular phylogeny, the differentiation of populations from Caspian Sea basin from the other described species is shown. The species delimitation methodology also supports these populations to be considered as a different species from the other populations included in the study (Figure 2). The haplotype network does not show any geographical patterns between the different populations of the suggested species in the closely located but independent rivers of the Caspian Sea basin (Figure 3).
Figure 2.
Capoeta genus; Values at nodes correspond to BI posterior probability/ML bootstrap. Grey bars represent the species delimitations performed with bPTP software.
Figure 3.
Haplotype networks of available specimens of Caspian Sea basin. Each independent river system is represented by a different colour. Data from Ghanavi et al. 2016.
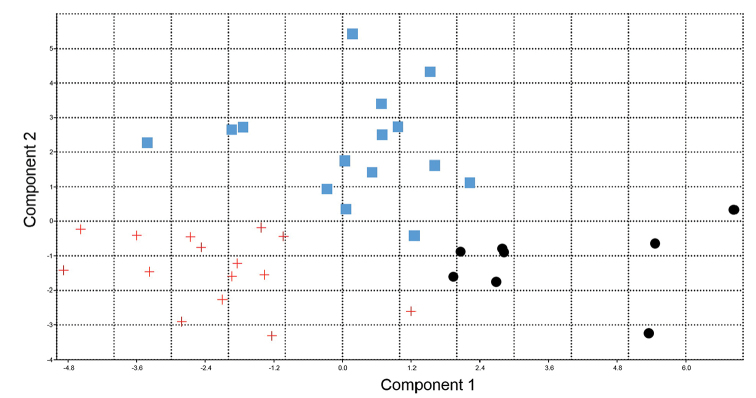
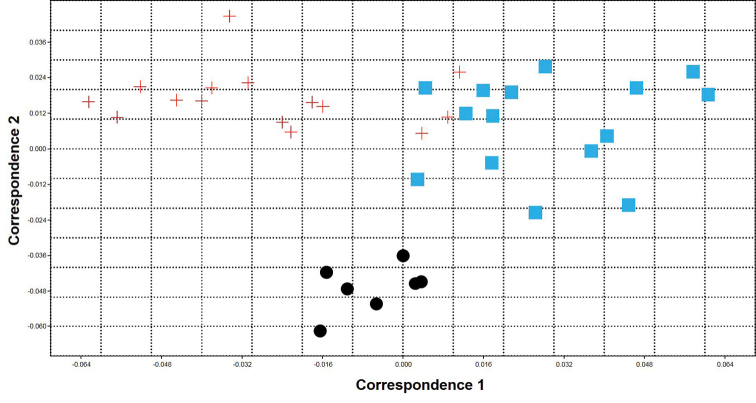
The result of PCA analysis showed that all specimens explained 45.79% of morphometric variations by the first two PC axes extracted from the variance-covariance matrix (PC1=27.60% and PC2=18.19%). Plotting of first and second PCs displayed a complete segregation of the three populations. In addition, NPMANOVA showed significant differences between all studied populations in terms of the morphometric characters (P<0.001) (Figure 11). The result of CA showed that all specimens explained 63.1% of morphometric variations by the first two CA (PCA1=35.82% and CA2=27.28%). Plotting of first and second CAs displayed a complete segregation of the three populations. In addition, NPMANOVA showed significant differences between all studied populations in terms of the morphometric characters (P<0.0001) (Figure 12).
Figure 11.
Principal component analysis of relative morphometric characters of the Capoeta razii sp. n. (+) C. fusca (•) and C. capoeta (◾) populations.
Figure 12.
Correspondence analysis of meristic characters of the Capoeta razii sp. n. (+) C. fusca (•) and C. capoeta (◾) populations.
Capoeta razii
http://zoobank.org/948BD913-A0DF-4371-97F6-B707CE56CFD6
Figure 4.
Capoeta razii sp. n., IMNRF-UT-1072-9, holotype, SL: 142.6 mm, Iran: Mazandaran Prov., Chalos city, Kheyroud River, Caspian Sea basin.
Figure 5.
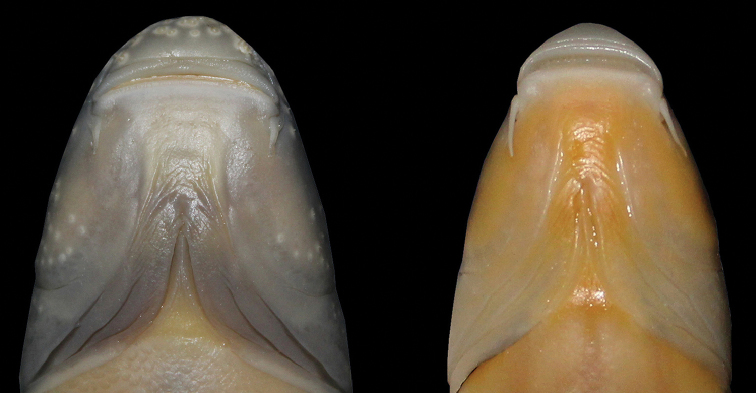
Ventral view of Head. Capoeta razii sp. n. (right, IMNRF-UT-1072-11, SL: 109 mm) and C. capoeta (left, IMNRF-UT-1067-6, SL: 110 mm).
Figure 6.
Capoeta razii sp. n., paratypes; A IMNRF-UT-4, SL: 130 mm B IMNRF-UT-12, SL: 115 mm C IMNRF-UT-3, SL: 99 mm.
Figure 7.
Last simple dorsal-fin rays, Capoeta razii sp. n. (Below, IMNRF-UT-1066-9, SL: 116) and C. capoeta (Above, IMNRF-UT-1067-13, SL: 121 mm).
Holotype.
IMNRF-UT-1072-9, holotype, 142.6 mm SL. Iran: Mazandaran Prov., Chalus city, Kheyroud River (Figure 8), Caspian Sea basin, 36°36'35"N, 51°33'45"E, S. Eagderi & A. Jouladeh-Roudbar, November 2016.
Figure 8.
Kheyroud River, near Chalos city, Caspian Sea basin, type locality of Capoeta razii sp. n.
Paratypes.
IMNRF-UT-1072, 14 specimens, 90.7–184.2 mm SL; data same as holotype.
Diagnosis.
Capoeta razii sp. n. is distinguished from the other species of Capoeta in Iran by a following combination of characters, none of them unique. One pair of barbels; pre-dorsal length equal to postdorsal length; maxillary barbel slightly smaller than eye’s horizontal diameter and reach to posterior margin of orbit; intranasal length slightly shorter than snout length; lateral line with 46–54 scales, 7–9 scales between dorsal-fin origin and lateral line and 6–7 scales between anal-fin origin and lateral line.
Table 5.
Number of scales above lateral line (ALL), below lateral line (BLL), Number Dorsal Soft Rays (DSR)/Hard (DHR), Anal Soft Rays (ASR)/Anal Hard Rays (AHR), pelvic (PLR) fin rays and Number Gill rakers on the lower limb (LOL) in Capoeta razii sp. n. and C. capoeta.
| Species | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Mod | Mean | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ALL | |||||||||||
| Capoeta razii sp. n. | 3 | 10 | 2 | 8 | 7.9 | 0.6 | |||||
| Capoeta capoeta | 3 | 10 | 2 | 9 | 8.9 | 0.6 | |||||
| BLL | |||||||||||
| Capoeta razii sp. n. | 10 | 5 | 6 | 6.3 | 0.5 | ||||||
| Capoeta capoeta | 12 | 3 | 7 | 7.2 | 0.4 | ||||||
| DHR | |||||||||||
| Capoeta razii sp. n. | 1 | 14 | 4 | 3.9 | 0.3 | ||||||
| Capoeta capoeta | 7 | 8 | 4 | 3.6 | 0.5 | ||||||
| DSR | |||||||||||
| Capoeta razii sp. n. | 2 | 13 | 8 | 7.9 | 0.4 | ||||||
| Capoeta capoeta | 3 | 12 | 8 | 7.8 | 0.4 | ||||||
| AHR | |||||||||||
| Capoeta razii sp. n. | 15 | 3 | 3.0 | 0.0 | |||||||
| Capoeta capoeta | 15 | 3 | 3.0 | 0.0 | |||||||
| ASR | |||||||||||
| Capoeta razii sp. n. | 15 | 6 | 6.0 | 0.0 | |||||||
| Capoeta capoeta | 15 | 6 | 6.0 | 0.0 | |||||||
| PLR | |||||||||||
| Capoeta razii sp. n. | 1 | 10 | 4 | 9 | 9.2 | 0.6 | |||||
| C. capoeta | 9 | 6 | 9 | 9.3 | 0.6 | ||||||
| LOL | |||||||||||
| Capoeta razii sp. n. | 4 | 12 | 1 | 5 | 4.9 | 0.5 | |||||
| Capoeta capoeta | 2 | 11 | 2 | 5 | 5.0 | 0.5 | |||||
Table 6.
Number of pectoral (PFR), caudal fin rays (DFR), total gill rakers (TGR) and circum-pendicular scales (CPS) in Capoeta razii sp. n. and C. capoeta.
| Species | 15 | 16 | 17 | 18 | 19 | 20 | 21 | Mod | Mean | SD |
|---|---|---|---|---|---|---|---|---|---|---|
| PFR | ||||||||||
| Capoeta razii sp. n. | 2 | 7 | 2 | 1 | 17 | 17.4 | 1.1 | |||
| Capoeta capoeta | 6 | 5 | 2 | 2 | 18 | 18.9 | 1.3 | |||
| CFR | ||||||||||
| Capoeta razii sp. n. | 1 | 14 | 19 | 18.9 | 0.3 | |||||
| Capoeta capoeta | 10 | 5 | 19 | 19.3 | 0.5 | |||||
| CPS | ||||||||||
| Capoeta razii sp. n. | 6 | 9 | 18 | 17.6 | 0.5 | |||||
| Capoeta capoeta | 10 | 3 | 2 | 18 | 18.5 | 0.7 | ||||
| TGR | ||||||||||
| Capoeta razii sp. n. | 1 | 2 | 8 | 2 | 1 | 1 | 18 | 18.1 | 1.4 | |
| Capoeta capoeta | 2 | 6 | 7 | 21 | 20.3 | 0.7 | ||||
Description.
See Figure 4 for general appearance and Tables 4–7 for morphometric and meristic data. Body is moderately deepened and compressed laterally. Greatest body depth occurs at the level of dorsal-fin origin. Dorsal profile of the head is convex. Predorsal length is equal to post-dorsal length. Dorsal profile of the body is convex without any keel in the front of dorsal-fin origin. Snout is rounded with a triangular view in ventral. Mouth is almost straight. Upper and lower lips are adnate to jaws. Lower jaw has a strong keratinized edge. Rostral cap is well developed and usually overlaps with upper lip. One set of maxillary barbels that are short, slightly smaller than eye’s horizontal diameter, reaching to posterior margin of orbit. Intranasal length is slightly shorter than snout length. Pelvic axillary scales are triangular, well developed, and pointed. Dorsal fin has 3–4 unbranched and 7–8 branched rays, its outer margin is straight or slightly concave. Last unbranched dorsal-fin ray is thickened and serrated, distally flexible, and with 15–25 serrae on its posterior margin, with serrations along 50–70% of its posterior margin, denticles are long and narrowly spaced but not strongly developed. Last unbranched dorsal-fin ray slightly shorter than first branched ray, and the tip is soft. Pelvic fins are inserted under posterior of the first branched dorsal-fin base. Caudal fin is deeply forked with pointed and equal size of lobes. Pectoral fin has 16–19 branched rays. Pelvic fin has 1 unbranched and 9–10 branched rays. Anal fin has 2–3 unbranched rays, 6 branched rays and its outer margin is usually convex or straight. There are 15–21 gill rakers on the outer side of the first arch. There are 17–18 circum-peduncular scales. Lateral line is complete, with 46–54 scales. There are 7–9 scales between the dorsal-fin origin and lateral line and 6–7 are located between the anal-fin origin and lateral line.
Table 7.
Number of total lateral-line scales in Capoeta razii sp. n. and C. capoeta.
| Species | Total lateral line Scales | Mod | Mean | SD | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 46 | 47 | 48 | 49 | 50 | 51 | 52 | 53 | 54 | 55 | 56 | 57 | 58 | ||||
| Capoeta razii sp. n. | 2 | 1 | 4 | 2 | 2 | - | 2 | - | 2 | 48 | 49.1 | 2.3 | ||||
| Capoeta capoeta | 4 | 5 | 4 | 2 | 56 | 56.3 | 1.0 | |||||||||
Colouration.
In life, the upper part of the body is golden brown, olive-green, or silver, and the belly is whitish up to the lateral line. The head is dark-brown or olive-green on top and the cheeks are pale brown to white (Figure 4). Anal, pelvic, and pectoral fins are hyaline or light brown, and dorsal and caudal fins have a narrow black line on rays. In specimen smaller than 50 mm SL, minute black spots are present on flanks.
When preserved, the dorsum is dark brown on back and flanks, and yellowish white on belly (Figure 6). Dorsum of the head is dark brown, and the cheeks beige. Fins are often light brown and pelvic and anal fins may be yellowish to hyaline. Dorsal and caudal fins are darker than lower fins. Peritoneum is black.
Distribution and habitat.
Capoeta razii is found in many rivers and streams of the southern Caspian Sea basin. It is one of the most abundant species in the Caspian Sea basin along with the members of the genus Alburnoides Jeitteles, 1861. At the Kheyroud River (type locality), the current was medium to fast, river width was between 3–14 m and the maximum depth was around one meter, the stream bed was composed of cobbles and gravel, and the riparian vegetation type was deciduous forests. Following fish species: Poticola iranicus Vasil’eva, Mousavi-Sabet & Vasil’ev 2015, Alburnoides taberstanensis Mousavi-Sabet, Anvarifar & Azizi, 2015, Alburnus chalcoides (Güldenstädt 1772), Barbus cyri De Filippi 1865, Squalius turcicus De Filippi 1865, Luciobarbus capito Güldenstädt 1773, L. mursa Güldenstädt 1773, Cobitis faridpaki Mousavi-Sabet, Vasil’eva, Vatandoust & Vasil’ev 2011, co-exist with C. razii in type locality. Capoeta razii is known from most of rivers and streams between Atrak and Kote komeh (Near Astara city) rivers in southern Caspian Sea basin.
Etymology.
The new species is named in honour of Abū Bakr Muhammad ibn Zakariyyā al-Rāzī, a Persian polymath, physician, alchemist, and philosopher, for his important contributions in the history of medicine. He also discovered numerous compounds including Ethanol.
Remarks.
Capoeta razii sp. n. is distinguished from C. aculeata and C. alborzensis by a smaller scale size and a higher number of total lateral line scales (46–54 vs. 39–44).
Capoeta razii sp. n. is distinguished from C. fusca, by a smaller caudal peduncle width (2.8–4.1 vs. 5.5–7.0 %SL), a smaller head length (20.5–24.0 vs. 25.0–28.6 %SL), and the presence of numerous minute scales on the caudal fin base extending distally onto the fin membranes for more than half the fin ray length (vs. absence of minute scales on the caudal fin base) (Figure 10).
Figure 10.
Live specimen of Capoeta fusca, IMNRF-UT-1065-1, SL: 124 mm, Iran: North Khorasan prov.: Near Farooj town, at segonbadan village, Qanat-e Segonbadan, Hari basin.
Capoeta razii sp. n. is distinguished from C. anamisensis, C. barroisi, C. buhsei, C. Capoeta, C. coadi, C. damascina, C. heratensis, C. mandica, C. saadi and C. umbla by a larger scale size, a fewer number of total lateral line scales (46–54 vs. 55–102).
Figure 9.
Uncatalogued live specimen of Capoeta capoeta. Iran: Ajab Shir town, Ghale Chay River, Urmia basin.
Comparative material.
– Capoeta aculeata: IMNRF-UT-1058, 9. 53–116 mm SL, Iran: Fars prov.: Tange Boragh village, Kor River, Kor basin, 37°14'46"N, 58°08'01"E, Aug 2014, S. Eagderi & H. Mossavi-Sabet. – Capoeta alborzensis.: IMNRF-1063, 7. 50–153mm SL, Iran: Tehran prov.: Nam River, tributary of Hableh River, near Arjomand village, 35°48'00"N, 52°30'57"E; IMNRF-UT-2063, 23, 46–163mm SL, Iran: Tehran prov.: Nam River, tributary of Hableh River, Kavir basin, near Harandeh village, 35°42'41"N, 52°40'19"E, S. Eagderi & A. Jouladeh-Roudbar, September 2014. – Capoeta buhsei: IMNRF-UT-1075, 12. 103.9–211.8 mm SL, Iran: Markazi prov.: Tafresh town, at Khalife kandy village, Mazlaghan Chay River, Namak basin, 34°45'34"N, 49°56'50"E, Nov 2016, A. Rahmani, M. A. Jahazi, R. Rahbar-zare, A. Jouladeh-Roudbar. – Capoeta capoeta: IMNRF-UT-1067, 15. 66–157 mm SL, Iran: Tabriz prov.: Near Ajab shir city, Ghale Chay River, Urmia Lake basin, 37°29'25"N, 45°59'57"E, Nov 2016, T. Hosseinpour, M. Ahmadian & A. Jouladeh-Roudbar. – Capoeta coadi: IMNRF-UT- 1074, 15. 125.7–194.7 mm SL, Iran: Chaharmahal and Bakhtiari prov.: Near Joneghan town, at Darkesh varkesh village, Behesht Abad River, Tigris basin, 32°05'22"N, 50°39'54"E, Aug 2016, T. Hosseinpour, A. Soleymani & A. Jouladeh-Roudbar. – Capoeta fusca: IMNRF-UT-1065, 8. 46–121 mm SL, Iran: North Khorasan prov.: Near Farooj town, at segonbadan village, Qanat-e Segonbadan, Hari basin, 37°14'46"N, 58°08'01"E, Jun 2016, S. Eagderi & A. Jouladeh-Roudbar. – Capoeta heratensis: IMNRF-UT-1064, 15. 116–161 mm SL, Iran: Khorasan-e Razavi prov.: Near Sarakhs, at Pole-e Khaton, Hari River, Hari basin, 35°56'51"N, 61°08'51"E, Jun 2016, S. Eagderi & A. Jouladeh-Roudbar. – Capoeta trutta: IMNRF-UT- 1073, 15. 54.1–165.2 mm SL, Iran: Kermanshah prov.: Songhor to Satar road, Tape Esmail village, Gavehroud River, Tigris basin, 34°56'01"N, 47°12'49"E, Aug 2016, T. Hosseinpour, A. Soleymani & A. Jouladeh-Roudbar. – Capoeta umbla: IMNRF-UT-1077, 15. 107.3–175.9 mm SL, Iran: Kurdistan prov.: Near Sardasht town, Barisu village, Little Zab River, Tigris, 36°08'48"N, 45°32'17"E, May 2016, S. Eagderi, H. Porbagher, P. Jalili & A. Jouladeh-Roudbar.
Supplementary Material
Acknowledgments
Authors wish to thank P. Rahimi, D. Corona-Santiago, P. Jalili and T. Hosseinpour for assistance in the laboratory and fish collection, and E. Elahi for proofreading this work. We thank the anonymous reviewers for their meticulous reading of our manuscript and their insightful comments and recommendations. This research was funded by Tehran University and the Spanish Ministry of Economy and Competitiveness (project number CGL2016-75262-P).
Citation
Jouladeh-Roudbar A, Eagderi S, Ghanavi HR, Doadrio I (2017) A new species of the genus Capoeta Valenciennes, 1842 from the Caspian Sea basin in Iran (Teleostei, Cyprinidae). ZooKeys 682: 137–155. https://doi.org/10.3897/zookeys.682.12670
References
- Alwan N. (2011) Systematics, taxonomy, phylogeny and zoogeography of the Capoeta damascina species complex (Pisces: Teleostei: Cyprinidae) inferred from comparative morphology and molecular markers. PhD thesis, Goethe Universität, Frankfurt a.M., Germany. [Google Scholar]
- Alwan N, Zareian H, Esmaeili HR. (2016) Capoeta coadi, a new species of cyprinid fish from the Karun River drainage, Iran based on morphological and molecular evidences (Teleostei, Cyprinidae). ZooKeys 572: 155–180. https://doi.org/10.3897/zookeys.572.7377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bănărescu PM. (1999) The Freshwater Fishes of Europe. Volume 5. Cyprinidae 2. Part I: Rhodeus to Capoeta. AULA-Verlag, Wiebelsheim, 427 pp. [Google Scholar]
- Barrett JC, Fry B, Maller MJ, Daly MJ. (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21(2): 263–265. https://doi.org/10.1093/bioinformatics/bth457 [DOI] [PubMed] [Google Scholar]
- Bianco PG, Banarescu P. (1982) A contribution to the knowledge of the Cyprinidae of Iran (Pisces, Cypriniformes). Cybium 6(2): 75–96. [Google Scholar]
- Coad BW. (2017) Freshwater Fishes of Iran. http://www.briancoad.com [accessed on 15 February 2017]
- Elliott NG, Haskard K, Koslow JA. (1995) Morphometric analysis of orange roughy (Hoplostethus atlanticus) off the continental slope of Southern Australia. Journal of Fish Biology 46(2): 202–220. https://doi.org/10.1111/j.1095-8649.1995.tb05962.x [Google Scholar]
- Esmaeili HR, Coad BW, Gholamifard A, Nazari N, Teimory A. (2010) Annotated checklist of the freshwater fishes of Iran. Zoosystematica Rossica 19(2): 361–386. [Google Scholar]
- Esmaeili HR, Coad BW, Mehraban H, Masoudi M, Khaefi R, Abbasi K, Mostavavi H, Vatandoust S. (2014) An updated checklist of fishes of the Caspian Sea basin of Iran with a note on their zoogeography. Iranian Journal of Ichthyology 1(3): 152–184. [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D. (2012) jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9(8): 772–772. https://doi.org/10.1038/nmeth.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanavi HR, Gonzalez EG, Doadrio I. (2016) Phylogenetic relationships of freshwater fishes of the genus Capoeta (Actinopterygii, Cyprinidae) in Iran. Ecology and Evolution 6(22): 8205–8222. https://doi.org/10.1002/ece3.2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi H, Jouladeh-Roudbar A, Eagderi S, Abbasi K, Vatandoust S, Esmaeili HR. (2015) Ichthyofauna of Urmia basin: Taxonomic diversity, distribution and conservation. Iranian Journal of Ichthyology 2(3): 177–193. [Google Scholar]
- Güldenstädt JA von. (1773) Cyprinus capoeta et Cyprinus mursa. Novi Commentarii Academiae Scientiarum Imperialis Petropolitanae 17: 507–521 [Google Scholar]
- Howes G. (1982) Anatomy and evolution of the jaws in the Semiplotine carps with a review of the genus Cyprinion Heckel, 1843 (Teleostei: Cyprinidae). Bulletin of the British Museum (Natural History) Zoology 42(4): 299–335. [Google Scholar]
- Jouladeh-Roudbar A, Eagderi S, Esmaeili H. (2015a) Fishes of the Dasht-e Kavir basin of Iran: an updated checklist. International Journal of Aquatic Biology 6: 263–73. [Google Scholar]
- Jouladeh-Roudbar A, Vatandoust S, Eagderi S, Jafari-Kenari S, Mousavi-Sabet H. (2015b) Freshwater fishes of Iran; an updated checklist. Aquaculture, Aquarium, Conservation and Legislation, International Journal of the Bioflux Society (AACL Bioflux) 8: 855–909. [Google Scholar]
- Jouladeh-Roudbar A, Eagderi S, Ghanavi HR, Doadrio I. (2016) Taxonomic review of the genus Capoeta Valenciennes, 1842 (Actinopterygii, Cyprinidae) from central Iran with the description of a new species FishTaxa 1(3): 166–175 [Google Scholar]
- Kottelat M, Freyhof J. (2007) Handbook of European freshwater fishes. Publications Kottelat, 646 pp. [Google Scholar]
- Levin BA, Freyhof J, Lajbner Z, Perea S, Abdoli A, Gaffaroğlu M, Doadrio I. (2012) Phylogenetic relationships of the algae scraping cyprinid genus Capoeta (Teleostei: Cyprinidae). Molecular Phylogenetics and Evolution 62(1): 542–549. https://doi.org/10.1016/j.ympev.2011.09.004 [DOI] [PubMed] [Google Scholar]
- Rambaut A, Drummond AJ. (2013) Tracer v1. 5 Available fromRice WR (1989) Analyzing tables of statistical tests. Evolution 43(1): 223–225. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Huelsenbeck JP. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61(3): 539–542. https://doi.org/10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22(21): 2688–2690. https://doi.org/10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729. https://doi.org/10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temminck CJ, Schlegel H. (1846) Pisces, Part 13. In: von Siebold PF (Ed.) Fauna Japonica, Leiden, 227–247. [Google Scholar]
- Turan D, Kottelat M, Ekmekci FG, Imamoglu HO. (2006) A review of Capoeta tinca, with descriptions of two new species from Turkey (Teleostei: Cyprinidae). Revue Suisse de Zoologie 113(2): 421–436. https://doi.org/10.5962/bhl.part.80358 [Google Scholar]
- Yang L, Sado T, Vincent Hirt M, Pasco-Viel E, Arunachalam M, Li J, Mayden RL. (2015) Phylogeny and polyploidy: Resolving the classification of cyprinine fishes (Teleostei: Cypriniformes). Molecular Phylogenetics and Evolution 85: 97–116. https://doi.org/10.1016/j.ympev.2015.01.014 [DOI] [PubMed] [Google Scholar]
- Zareian H, Esmaeili H, Freyhof J. (2016) Capoeta anamisensis, a new species from the Minab and Hasan Langhi River drainages in Iran (Teleostei: Cyprinidae). Zootaxa 4083: 126–142. https://doi.org/10.11646/zootaxa.4083.1.7 [DOI] [PubMed] [Google Scholar]
- Zhang J, Kapli P, Pavlidis P, Stamatakis A. (2013) A general species delimitation method with applications to phylogenetic placements. Bioinformatics 29: 2869–2876. https://doi.org/10.1093/bioinformatics/btt499 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.