Abstract Abstract
Longidorus piceicola, a new geographical and host record from Romania, was described and illustrated on the basis of two populations originating from a coniferous and a deciduous forest. The main morphological characters of specimens from Romania correspond very well with the type material collected from the soil around Picea abies L. (Slovakia) except for the shorter body and tail. The D2-D3 fragment of 28S rDNA from both populations was amplified and sequenced, and the sequences were identical to L. piceicola sequence from Slovakia. The partial 18S-ITS1-5.8S-ITS2 rDNA regions from one of the populations were sequenced for the first time. The evolutionary relationships between L. piceicola and the closest species L. intermedius based on D2-D3 sequence divergence and single-nucleotide polymorphisms are discussed. Although having very low sequence dissimilarity (0.3–0.9 %) both species have distinct morphology and biology. Longidorus piceicola differs from L. intermedius in having a much longer odontostyle, body, distance anterior end - guide ring, a wider lip region, more ventromedian supplements (11 vs 5–7) in the male, and develops through four rather than three juvenile stages. Furthermore, L. piceicola occurs more frequently in association with conifers, while L. intermedius is found mainly in oak forests.
Keywords: D2–D3 expansion region rDNA, ITS, juvenile stages, new record, phylogeny, SNPs
Introduction
Longidorus piceicola Lišková, Robbins & Brown, 1997 was originally described from Slovakia (Lišková et al. 1997) in association with Picea abies L. Subsequently, it was recovered from different localities in Bosnia and Herzegovina, Serbia and Montenegro (Barsi and Lamberti 2001), and Poland (Kornobis and Peneva 2011) in forests dominated by coniferous trees. Here two new findings of this species in Romania are reported. The aims of this paper are to characterize morphologically and molecularly the populations recovered and to discuss the phylogenetic relationships with the most closely related species.
Materials and methods
Sampling and processing
Specimens were collected from the rhizosphere of a Larix decidua Mill. forest near to Bran, Braşov County, Romania (45.3050N, 25.2156E), ca 760 m a.s.l. on 15.10.2013, and from the soil around roots of deciduous trees (Quercus sp., Tilia sp., and Fraxinus sp.), Cernica forest, Ilfov County (44.2637N, 26.16514E) and ca 60 m a.s.l. on 4.08.2014. Nematodes were isolated from soil samples by a decanting and sieving technique (Cobb 1918); specimens recovered were heat killed at 55 °C for two minutes, fixed in a 4 % formalin/1 % glycerol mixture, processed to anhydrous glycerol (Seinhorst 1959), and mounted on glass microscope slides. Drawings were prepared using an Olympus BX51 compound microscope with differential interference contrast (DIC). Photographs were taken using an Axio Imager.M2-Carl Zeiss compound microscope equipped with a digital camera (ProgRes C7) and specialised software (CapturePro Software 2.8). Measurements were made using an Olympus BX41 light microscope, a digitising tablet (CalComp Drawing Board III, GTCO CalCom Peripherals, Scottsdale, AZ, USA), and computer Digitrak 1.0f programme, (Philip Smith, Scottish Crop Research Institute, Dundee, UK) and a Leica DMLB microscope with a Leica DFC 295 camera and LAS V 4.2 software.
DNA extraction, amplification and sequencing
The genomic DNA extraction, amplification, and sequencing of single specimens of L. piceicola from both populations in Romania were carried out independently in two laboratories: one at the Institute for Sustainable Plant Protection, Bari, Italy and the other at the Institute of Biodiversity and Ecosystem Research, Sofia, Bulgaria. Both protocols are presented separately below.
Institute for Sustainable Plant Protection (Bari Unit): specimens (Cernica locality) for molecular analysis were kept in DESS solution (Yoder et al. 2006) before extraction. Genomic DNA was extracted from six individual female nematodes as described by De Luca et al. (2004). The crude DNA isolated from each individual nematode was directly amplified. The partial 18S-ITS1-5.8S-ITS2 regions were amplified using the forward primer TW81 (5’-GTTTCCGTAGGTGAACCTGC-3’) and the reverse primer AB28 (5’-ATATGCTTAAGTTCAGCGGGT-3’) (Subbotin et al. 2001) and the D2-D3 expansion segments of 28S rDNA was amplified using the D2A (5’-ACAAGTACCGTGAGGGAAAGTTG-3’) and D3B (5’-TCGGAAGGAACCAGCTACTA-3’) primers (De Ley et al. 1999). PCR cycling conditions used for amplification were: an initial denaturation at 94°C for 5 min, followed by 35 cycles of denaturation at 94°C for 50s, annealing at 55°C for 50s and extension at 72°C for 1 min and a final step at 72°C for 7 min. The size of amplification products was determined by comparison with the molecular weight marker ladder 100 (Fermentas, St. Leon-Rot, Germany) following electrophoresis of 10 ml on a 1 % agarose gel. PCR products of the ITS and D2-D3 regions were purified for cloning and sequencing using the protocol provided by the manufacturer (High Pure PCR elution kit, Roche, Germany). Purified ITS fragments were cloned in TA cloning vector (Invitrogen) and several clones were sequenced using an ABI Prism 377 sequencer (PE Applied Biosystem, Foster City, CA).
Institute of Biodiversity and Ecosystem Research: Genomic DNA was extracted from two single female worms L. piceicola from Bran locality using a standard nematode digestion protocol (Holterman et al. 2006). The D2–D3 expansion segments of the 28S rRNA gene were amplified using the same primers D2A and D3B (De Ley et al. 1999). Each PCR reaction was performed under the following conditions: initial denaturation 94°C for 5 min; 40 cycles (denaturation 94°C for 30 secs; primer annealing 50°C for 30 secs; extension 72°C for 1 min), and final extension 72°C for 10 min. For further details, see Nedelchev et al. (2014). The amplified products were sequenced by Eurofins MWG Operon, Germany.
Sequence and phylogenetic analysis
The sequences of the L. piceicola have been deposited in GenBank with the following accession numbers: KY086070 and LT669801 for D2-D3 expansion domains of 28S rRNA gene; LT669802 and LT669803 for the ITS region. The D2-D3 and ITS sequences were compared with those of other nematode species available at the GenBank sequence database using BLASTN similarity search tool revealing similar results for both regions. The closest D2-D3 sequences to L. piceicola were aligned using ClustalX 2.1 (Larkin et al. 2007). The estimates of evolutionary divergence between the sequences of L. piceicola and L. intermedius Kozlowska & Seinhorst, 1979 (numbers of base differences and p-distances) and Single Nucleotide Polymorphism (SNP) variations (six transitions and four transversions) were performed with MEGA7 (Kumar et al. 2016). Furthermore, sequences revealing the highest similarity to L. piceicola were used for phylogenetic analyses; however only a midpoint rooted tree based on a reduced number of sequences (26) comprising several related species was presented here. The multiple sequence alignments used for phylogeny reconstructions were carried out using GUIDANCE2 Server (http://guidance.tau.ac.il) with the default settings (Sela et al. 2015). Bayesian Inference (BI) algorithm implemented in MrBayes 3.2.5 was used for phylogenetic relationships reconstruction (Huelsenbeck and Ronquist 2001; Ronquist et al. 2012). For further details, see Lazarova et al. (2016).
Results
Longidorus piceicola
Lišková, Robbins & Brown, 1997
Material examined.
Eleven females and 21 juveniles, two females and one juvenile from Cernica forest, Ilfov County, Romania on slide numbers NE 35–37 stored at the reference collection of the National Phytosanitary Laboratory, Voluntari, Romania, 9 females and 20 juveniles - at the personal collection of the first author; nine females and 30 juveniles from Bran, Braşov County, Romania, stored in the nematode collection of IBER, Bulgaria, slide numbers N2-29/2/1-19.
Description.
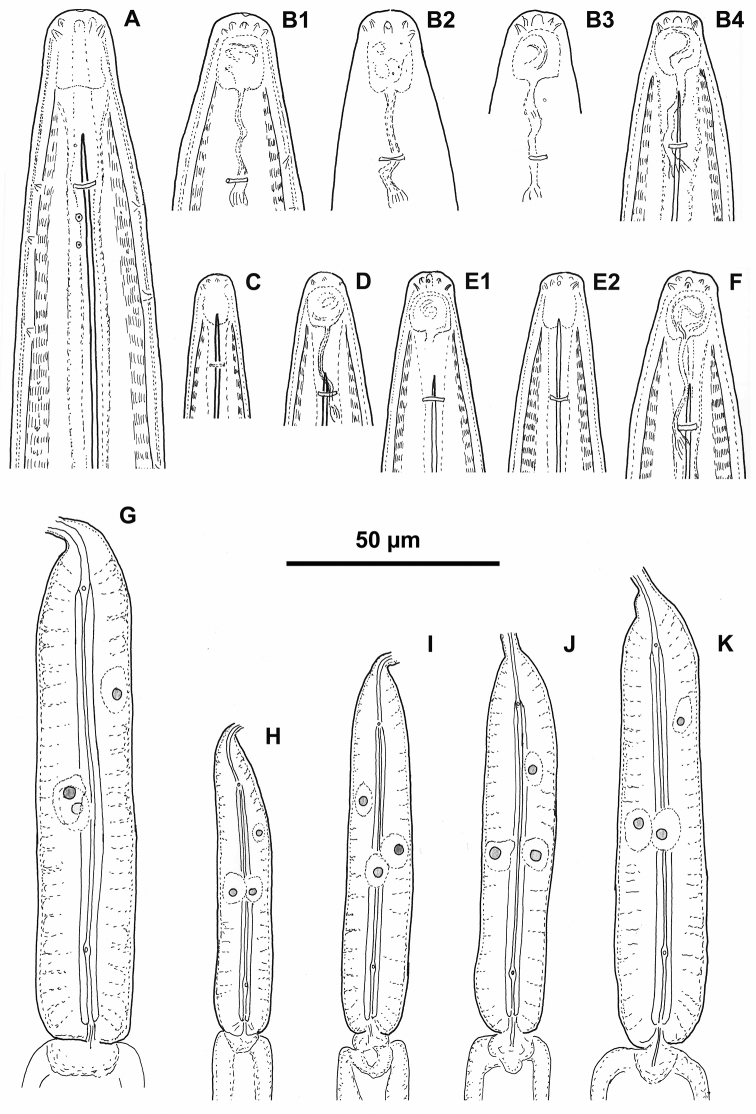
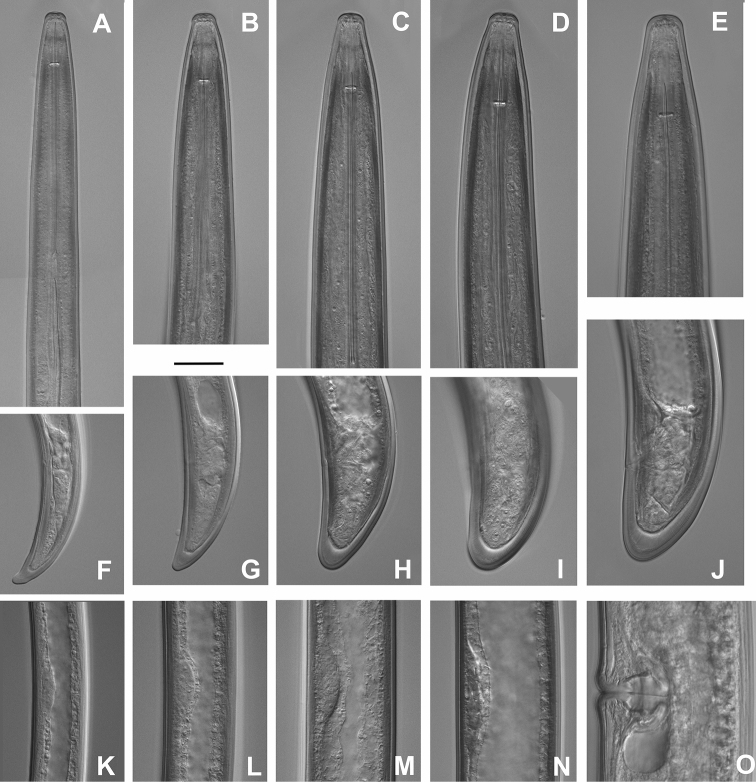
Figure 1.
Longidorus piceicola Female and juveniles: A Neck region – female B1–B4, C Head end with amphidial fovea B1–B3 females, B4 juvenile 4th stage (B2 right and B3 left) C, D, E1, E2, F Anterior ends of first- to fourth-stage juveniles G–K Pharyngeal bulb of female (G) and first- to fourth-stage juveniles (H–K).
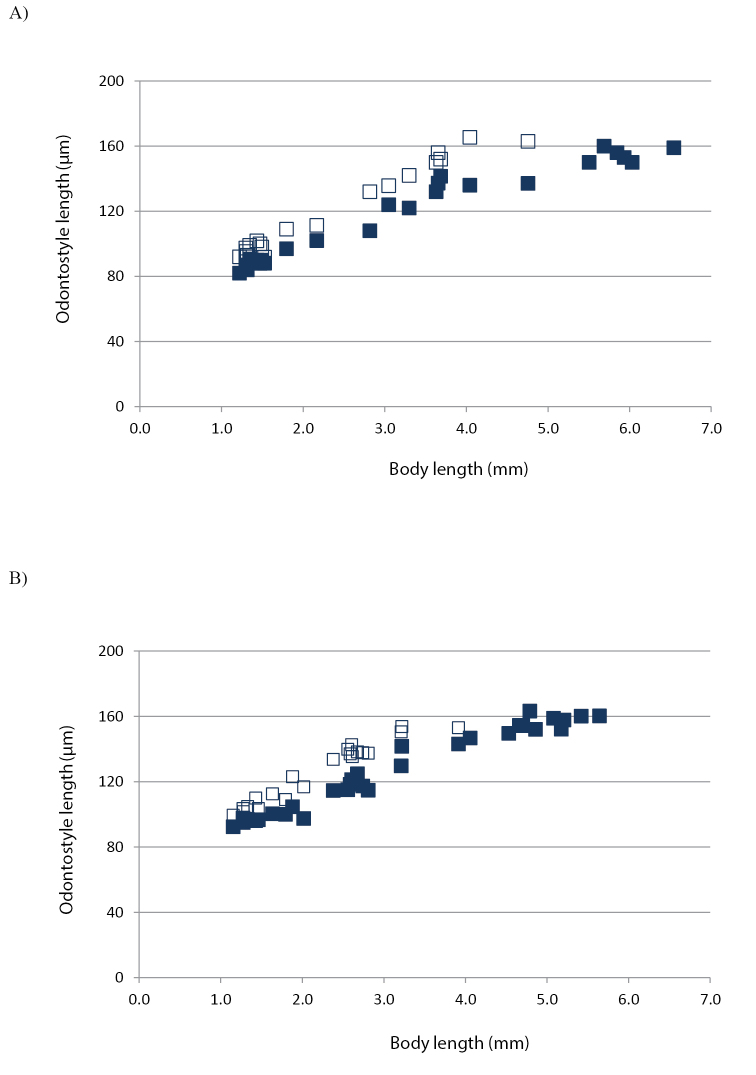
Figure 7.
Scatter plot of odontostyle (■) and replacement odontostyle (□) against body length of Longidorus piceicola juveniles (J1 to J4) and females from A Cernica forest, Ilfov county and B Bran locality, Braşov county.
Table 1.
Measurements of females and juveniles (J) of Longidorus piceicola from Bran, Braşov County, Romania (mean ± standard deviation, with range). All measurements in micrometers except for body length (mm).
| Character | Females | J1 | J2 | J3 | J4 |
|---|---|---|---|---|---|
| n | 9 | 6 | 4 | 8 | 3 |
| L | 4.90±0.47 4.05–5.64 | 1.32±0.11 1.15–1.47 | 1.83±0.16 1.63–2.02 | 2.62±0.13 2.38–2.81 | 3.21, 3.91, 3.22 |
| a | 84.6±8.0 71.1–97.3 | 55.4±4.6 47–60.8 | 59.3±5.5 53.5–65.9 | 67.6±3.6 62.3–71.9 | 73.6, 67.9, 75.5 |
| b | 9.9 ± 0.6 9.7–11.1 | 4.3±0.2 4.1–4.5 | 5.2±0.3 4.8–5.5 | 6.4±0.5 5.8–7.3 | 6.7, 8.1, 7.1 |
| c | 129.7±13.2 102.4–147.3 | 29.3±2.5 26.4–32.1 | 42.3±4.9 35–45.3 | 61.7±5.7 53.5–69.5 | 77.9, 108.4, 84.7 |
| c’ | 0.97±0.06 0.89–1.10 | 2.8±0.3 2.6–3.2 | 1.9±0.2 1.64–2.15 | 1.45±0.1 1.38–1.58 | 1.23, 0.90, 1.18 |
| V (%) | 49.2±1.2 47.2–51.3 | – | – | – | – |
| G1 (%) | 6.7±0.7 5.8–7.8 | – | – | – | – |
| G2 (%) | 6.1±0.9 5.4–7.5 | – | – | – | – |
| Developing gonad | – | 16.2±1.2 15–17 | 28.3±7.2 20–33 | 33.3±2.1 31.5–37 | –, 48, 45 |
| d | 2.63±0.1 2.45–2.8 | 2.6±0.2 2.5–2.7 | 2.7±0.3 2.5–3.0 | 2.37±1.0 2.5–2.8 | 2.9, 2.8, 2.9 |
| d’ | 2.02±0.1 1.9–2.1 | 1.8±0.1 1.65–2.3 | 1.95±0.25 1.7–2.3 | 1.9±0.1 1.7–1.9 | 2.0, 2.1, 2.1 |
| Odontostyle | 155.5±5.2 147–163 | 95.8±1.2 82–90.3 | 100.7±3.0 97.5–105 | 118.4±3.7 115–125 | 130, 143, 142 |
| Replacement odontostyle | – | 103.7±3.5 99.5–110 | 115.4±6.0 109–123 | 137.8±2.7 134–143 | 151, 153, 154 |
| Odontophore | 77.7±3.4 71–82 | 47.5±1.4 46–50 | 55±4.2 50–60 | 62.9±2.9 60–68 | 75, 73, 73 |
| Anterior end to guide ring | 38.1±1.9 35–41 | 22.0±1.3 22–24 | 26±1.1 25–27 | 29.9±1.7 27–33 | 36, 37, 35 |
| Bulbus length | 118.5±7.9 105–130 | 65.9±4.5 59–69 | 71.8±3.4 75–83 | 91.1±3.6 86–97 | 104, 116, 101 |
| Bulbus width | 23.4±1.8 20–25 | 13.8±1.2 13–14 | 16.6±0.5 16–17 | 19.2±0.6 18–20 | 22, 22, 21 |
| Pharynx | 478.4±29.4 440.5 –528 | 307.6±12.3 290–319 | 352±12.9 338.5–364 | 409.3±22.9 374–447 | 480, 484, 455 |
| Tail | 38.2±1.8 35 – 42 | 45.4±4.2 42–51.5 | 43.5±2.9 40.5–47 | 42.7±4.2 36–48 | 41, 36, 38 |
| Length of hyaline part | 11.7±0.9 10–13 | 9.5±0.6 9–10 | 8.5±0.6 8–9 | 9.3±1.2 8–11 | 9.5, 12, 8 |
| Body diameter at: – lip region | 14.5±0.6 14–16 | 8.6±0.6 8–10 | 9.6±0.6 9–10 | 11.1±0.3 11–12 | 12, 14, – |
| – guide ring | 29.2±1.6 27–32 | 15.3±0.7 14.5–16 | 18.5±1.3 28–31 | 21.1±1.2 19–23 | 25, 29, 26 |
| – base of pharynx | 48.4±3.3 44–55 | 22.8±0.6 23–24 | 29.2±1.3 28–31 | 36.2±2.3 32–40 | 39,47, 39 |
| – mid–body/at vulva | 58.7±5.4 53–71 | 23.8±0.8 23–25 | 30.9±1.8 29–33 | 38.9±2.7 33–41.5 | 44, 58, 43 |
| – anus | 39.7±3.5 35–46 | 16.1±0.6 15.5–17 | 23±1.6 22–25 | 29.5±2.4 25–32 | 34, 37, 30 |
| – hyaline part | 24.9±3.5 18–29 | 7.4±0.6 6.7–8.4 | 10.8±0.3 10.5–11 | 16.1±1.5 14–18 | –, 25, 18 |
d, distance from the anterior end / body diameter at lip region. d’, body diameter at guide ring / body diameter at lip region (Brown et al., 1994).
Table 3.
Measurements of Longidorus piceicola females (f) from Cernica, and juveniles (j) from Bran, Braşov County, Romania showing different anomalies. All measurements in micrometers except body length (mm).
| Character | f | f | j | j | j | j | j | j | j | j |
|---|---|---|---|---|---|---|---|---|---|---|
| No | 1 | 2 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| L | 5.95 | 5.86 | 4.73 | 2.34 | 2.72 | 2.71 | 2.62 | 1.14 | 3.66 | 2.71 |
| a | 99.1 | 97.7 | 93.0 | 63.6 | 77.7 | 61.9 | 67.0 | 32.6 | 75.1 | 63.8 |
| b | 9.5 | 10.3 | 5.6 | 6.0 | 5.9 | 7.1 | 2.9 | 7.9 | 6.2 | |
| c | 220.3 | 172.4 | 98.8 | 60.5 | 65.2 | 61.9 | 61.4 | – | 70.3 | |
| c’ | 0.75 | 0.94 | 1.3 | 1.3 | 1.6 | 1.4 | 1.7 | – | 1.2 | |
| V | 49.2 | 48.9 | – | – | – | – | – | – | – | – |
| Developing gonade | – | – | 65 | – | – | – | 22 | 27 | ||
| d | 2.93 | 2.73 | 2.6 | 2.8 | 2.9 | 2.9 | 2.5 | 2.7 | 2.8 | |
| d’ | 1.79 | 1.80 | 1.4 | 2.0 | 2.0 | 2.0 | 1.8 | 1.9 | 1.8 | |
| Odontostyle | 165 | 158 | 117 | 127 | 122 | 105 | 81 | 106 | 120 | 125 |
| Replacement odontostyle | 175 | 158 | 131 | 165 | 165 | 135 | 108 | 130 | 140 | 156 |
| Odontophore | 80 | 70 | 78.5 | 61 | 65 | 65 | 60 | 60 | 73 | |
| Anterior end to guide ring | 41 | 41 | 35 | 30 | 32 | 33 | 25 | 26 | 32 | 32 |
| Bulbus length | 132 | 130 | 114 | 81 | 87 | 89 | 95 | 93 | 108 | 86 |
| Bulbus width | 23 | 23 | 22 | 19 | 18 | 20 | 17 | |||
| Pharynx | 627 | – | 461 | 420 | 457 | 464 | 369 | 387 | 463 | 441 |
| Tail | 27 | 34 | 48 | 39 | 42 | 44 | 43 | – | 39 | |
| Length of hyaline part | 11 | 8 | 10 | 9 | 9 | 6 | 9 | |||
| Body diameter at: - lip region |
14 | 15 | 14 | 11 | 11 | 11 | 9 | 11.5 | ||
| - guide ring | 25 | 27 | 19.5 | 21 | 22 | 23 | 11 | 19 | 21 | |
| - base of pharynx | 51 | 50 | 42 | 35 | 32 | 38 | 32 | 29 | 40 | 32 |
| - mid-body/at vulva | 60 | 51 | 37 | 35 | 44 | 39 | 35 | 49 | 43 | |
| - anus | 60 | 38 | 29 | 26 | 32 | 24 | – | 32 | ||
| - hyaline part | 25 | 23 | 20 | 16 | 14 | 15 | 11 | 18 |
Females (Figs 1A, B1–4, G2–4, 5E, 6E, J, O, 7) based on the Larix population, Bran, Braşov County.
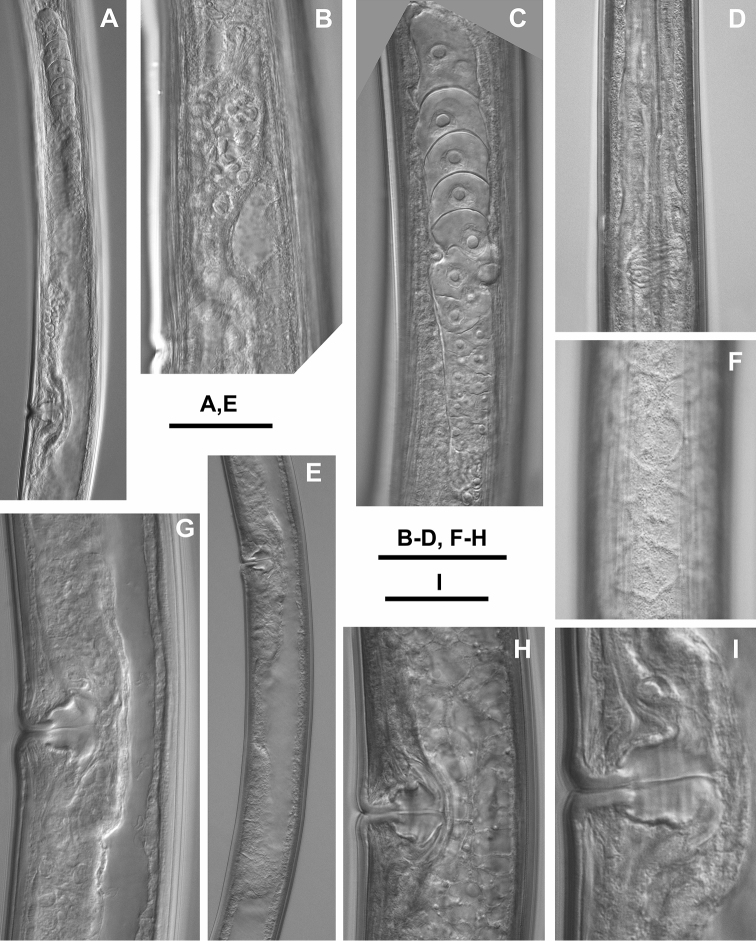
Figure 4.
Longidorus piceicola Female from Bran locality: A Anterior genital branch B Uterus part with sperm C Ovary D Nerve ring E Posterior genital branch F Lateral field and epidermal glans G–I Variations in vagina (different magnifications). Scale bars: A, E 80 μm; B–D, F–H 40 μm; I 20 μm.
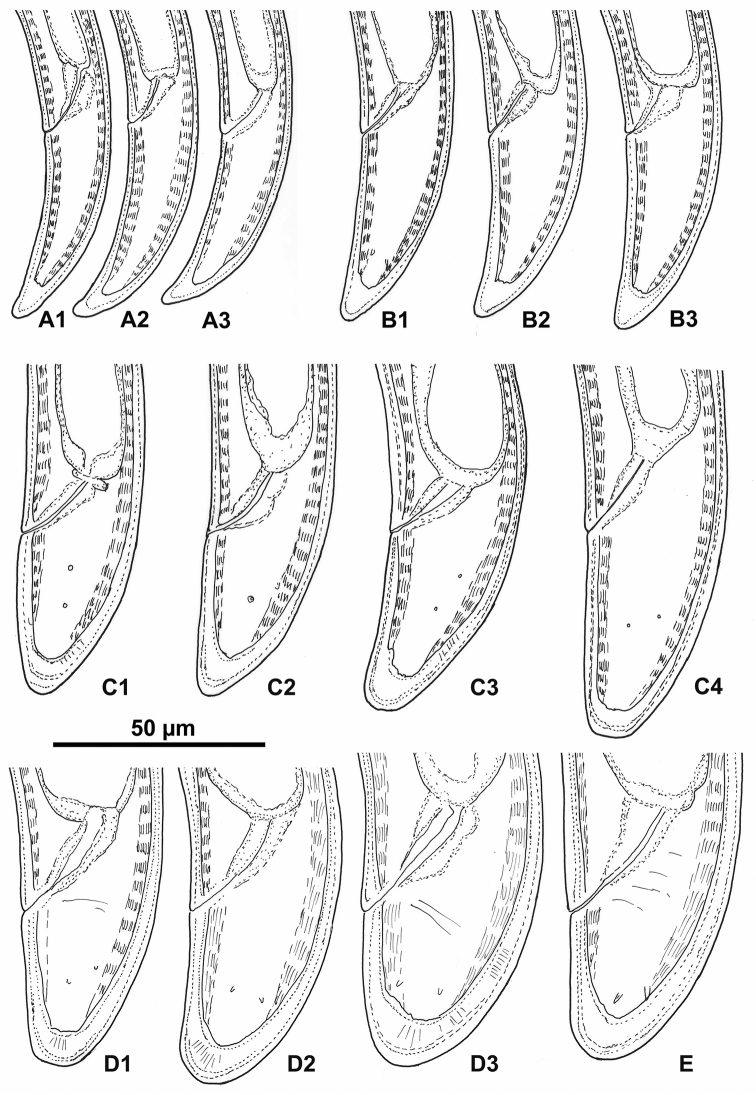
Figure 5.
Longidorus piceicola Juveniles and female from Bran locality: Variations in tail shape of first (A1–A3), second (B1–B3), third (C1–C4), fourth (D1–D3) juvenile stages and female (E).
Figure 6.
Longidorus piceicola Juveniles and female from Bran locality: A–E Anterior ends of first- to fourth-stage juveniles and female F–J Tails of first to fourth juvenile stages and female K–M Genital primordium of first to fourth juvenile stages. O Vagina. Scale bar: 20 μm.
Habitus spiral shaped, more strongly coiled in posterior part of body. Cuticle 3–4 μm thick at guide ring region, ca 3 μm in mid-body, and 5–6 μm on tail posterior to anus. Lip region broadly rounded anteriorly, rounded laterally, almost continuous with rest of body. Amphideal fovea pocket-shaped, varying from not lobed to symmetrically bilobed at base (according to terminology proposed by Decraemer and Coomans 2007) extending to ca half the distance anterior end-guide ring. Left and right fovea of about equal size (12.7 (11–14) μm, n = 5), sensillar pouch (fusus) just posterior the guide ring, the distance from the fovea to fusus 24 (23–29 μm). Pharyngeal bulb occupying 25 (22–29) % of total pharynx length; dorsal nucleus located at 29.5 (27–32) % (n = 7) of bulb length; ventro-sublateral nuclei at 54 (48–57) % (n = 8) (left) and 54 (52–56.5) % (n = 8) (right); opening of the dorsal gland at 9 (7.5–11) % and opening of the ventro-sublateral glands at 84 (80.5–90.5) % of the distance from anterior end of pharyngeal bulb, respectively. In one female, a small vestigium (5 μm) observed in wall of slender pharynx. Two nerve rings observed, the first one at 207.2 ± 8.8 (193–218) μm from anterior end, surrounding about mid-odontophore; the second at 329 ± 11.6 (313–344) μm from anterior end, n = 6, (first at 235.7 ± 12.7 (215–255) and second at 329.3 ± 18.6 (290–343) μm from anterior end, n = 7, Cernica forest). Tail bluntly conical, dorsally convex, flat or shallowly concave ventrally. Two pairs of caudal pores. Reproductive system didelphic, two branches of about equal size. Vagina occupies ca 50 % of corresponding body width; pars distalis vaginae and pars proximalis vaginae 13–15 μm and 15–19 μm long, respectively. Uteri short, anterior uterus 96.3 ± 13.5 (80–120) μm long, posterior 91.0 ± 10.5 (76–107) μm. Uteri shorter in Cernica population – anterior uterus 80.9 ± 7.0 (70–90) μm long and posterior 78.3 ± 8.3 (70–95) μm long. Sphincter between uterus and pars dilatata oviductus well developed. Sperm observed in both uteri of one female.
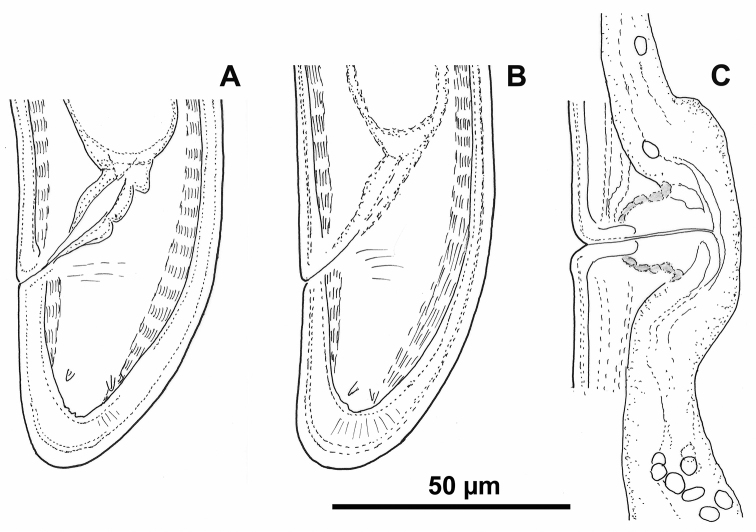
Figure 2.
Longidorus piceicola Female from Bran locality: A, B Variations in tail shape C Vagina.
In the population from Cernica forest two females with reserve odontostyles have been observed (Table 3).
Male. Not found.
Juveniles (Figs 1C–F, H–K; 6A–D, F–I, K–N, 7).
General morphology similar to adult females. Body habitus similar in all stages, open C- to J-shaped. Tail of all juvenile stages conical, but becoming more rounded and c’ decreasing in subsequent stages: tail of first stage juvenile elongate conoid with slightly digitate terminus, in the second stage – elongate conoid, in third – bluntly conoid, variable, with narrow to widely rounded terminus, in fourth – resembling that of female, bluntly conoid (Fig. 5). In several juveniles, the abnormalities in their development did not allow to assign them to a particular stage and the morphometrics are presented separately (Table 3). The lengths of functional and replacement odontostyles used to infer the developmental stages were in contradiction with other measurements such as L, a, b, c etc. which were in correspondence with a different stage, or the functional odontostyle was in the ranges of one stage while the replacement one was not in the ranges of the next stage; in one occasion the length of replacement odontostyle was less than that of the replacement one (Table 3).
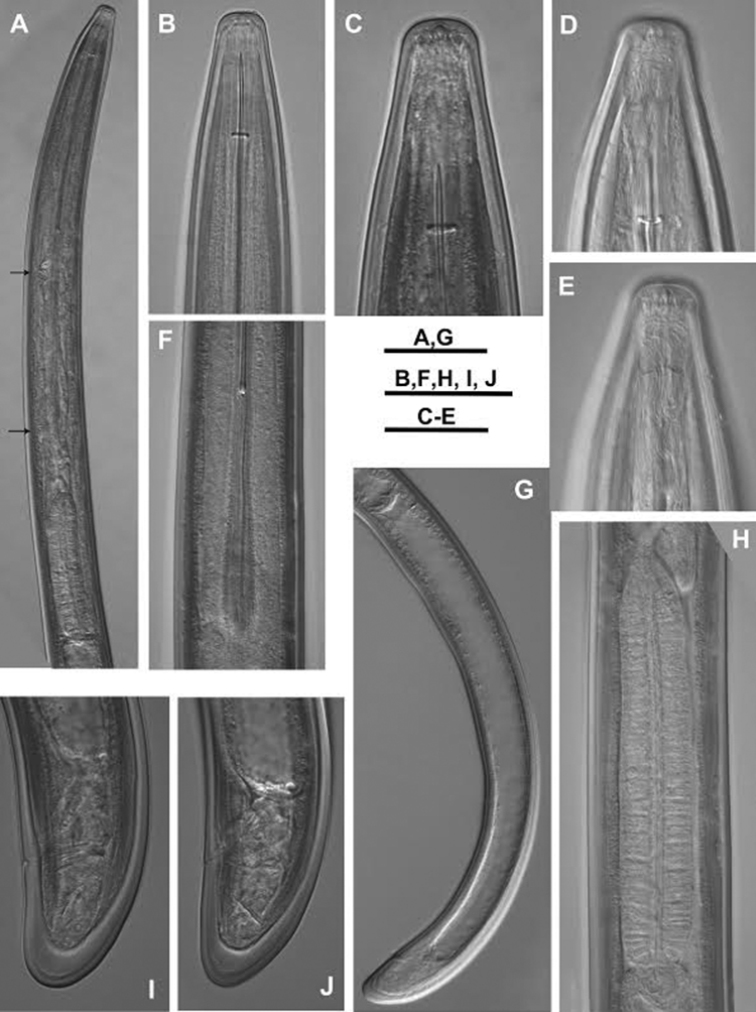
Figure 3.
Longidorus piceicola Female from Bran locality: A Neck region, black arrows indicate nerve rings B, C Head end (different magnifications) D, E Amphideal fovea (right and left) F Odontophore G Prerectum H Pharyngeal bulb I, J Variations in tail shape. Scale bars: A, G 80 μm; B, F, H, I, J 40 μm; C–E 20 μm.
Table 2.
Measurements of females and juvenile stages (J) of Longidorus piceicola from Cernica-Ilfov County, Romania (mean ± standard deviation, with range). All measurements in micrometers except body length (mm).
| Character | Females | J1 | J2 | J3 | J4 |
|---|---|---|---|---|---|
| n | 9 | 11 | 2 | 3 | 5 |
| L | 5.88±0.19 5.17–6.54 | 1.36±0.09 1.21–1.52 | 1.79, 2.16 | 3.29, 3.04, 2.81 | 3.95±0.47 3.6–4.7 |
| a | 95.2±11.5 73.8–105.5 | 58.96±4.9 53–66.8 | 64.1, 67.7 | 68.7, 67.7, 68.7 | 77±8.2 62.5–83.4 |
| b | 10.2±1.2 8.4–12.7 | 4.88±0.8 4.1–6.7 | 8.4, 9, 6.6 | 9±1.5 6.9–10.6 | |
| c | 171.9±28.8 134.4–218.0 | 31.0±1.9 28.3–33.4 | – | 79, 71,64.3 | 102±8.1 89.8–109.8 |
| c’ | 0.85±0.10 0.72–0.99 | 2.9±0.2 2.6–3.1 | – | 1.3, 1, 1.6 | 1±0.1 0.9–1.2 |
| V (%) |
48.1±0.98 47.1–50.6 | – | – | – | |
| G1 (%) | 5.8±0.8 4.7–7.1 | – | – | – | – |
| G2 (%) | 5.4±0.7 4.4–6.4 | – | – | – | – |
| d | 2.9±0.1 2.7–3.1 | 2.9±0.2 2.6–3.3 | 2.78, 2.99 | 3, 3, 3.4 | 3±0.2 2.8–3.2 |
| d’ | 1.8±0.1 1.7–1.9 | 1.9±0.2 1.5–2.2 | 1.8, 1.7 | 2, 2, 2 | 2±0.1 1.7–2 |
| Anterior end to guide ring | 42.2±1.8 40–45 | 22.8±1.4 21–26 | 25, 29 | 34.8, 34, 34 | 37.2±0.9 36–39 |
| Odontostyle | 155.4±5.4 150–165 | 86.9±2.7 82–90 | 97, 102 | 122, 124, 108 | 136.8±3.4 132–141.5 |
| Bulbus length | 135±4.9 126–141 | 72.7±4.3 65–78.5 | 71, 86 | 104, 104, 100 | 113.7±5.9 108–120 |
| Bulbus width | 24.7±2.0 22–29 | 12.5±0.9 11–14 | 15, 14.5 | 18, 21, 19 | 19.9±2.1 17–21 |
| Replacement odontostyle | – | 95.7±3.7 92–102 | 109, 111 | 142, 136, 132 | 157.3±6.7 150–165 |
| Odontophore | 78.1±4.9 70–83 | 52.5±4.8 48–65 | 55, 60 | 72, 60, 65 | 72.2±3.0 69–76 |
| Oesophagus length | 579.3±47.6 514–661 | 284.9±42.4 219–356 | 320, 366 | 393, 321,425 | 460.3±84.3 345–545 |
| Tail | 34.8±4.4 30–41.5 | 43.58±2.3 39–47 | – | 42, 43, 44 | 38.7±3.5 34–43 |
| Length of hyaline part | 12.3±1.1 11–14 | 9.5±0.9 8–11 | 10, 10 | 9, 10, 10 | 10±0.6 9.4–11.1 |
| Body diameter at: – lip region |
14.7±0.4 14–15 | 7.9±0.2 7.5–8 | 9, 10 | 11, 11,10 | 12.4±0.5 12–13 |
| – guide ring | 26.1±1.2 24–27 | 14.7±1.5 12–18 | 16, 16 | 20.5, 21, 20 | 23.16±0.6 22.5–24 |
| – base of pharynx | 53.6±6.1 45–62 | 21.9±0.9 20–23 | 25, 28.6 | 39, 38, 38 | 43.5±3.0 38.9–46.4 |
| – mid–body/at vulva | 63.2±4.5 58–70 | 23.2±1.4 21–26 | 28, 32 | 48, 45, 41 | 51.8±5.8 45–58 |
| – anus | 40.8±2.1 38–44 | 15.2±1.3 14–18 | – | 31, 30, 27 | 36±3.4 33.2–41.2 |
| – hyaline part | 27.8±1.9 25–31.5 | 7.4±0.6 7–8 | 7, 8 | 18, 16, 15 | 22±1.4 20–23 |
Sequences and phylogenetic analyses.
The amplification of the ITS and the D2-D3 expansion domains of the 28S rRNA gene yielded fragments of 1646 and 756 bps, respectively, based on sequencing. The ITS sequences of L. piceicola from Romania were obtained for the first time in the present study. They showed 98 % similarity (962/984 identities, 9 gaps) when compared with the corresponding sequence of L. intermedius (KT308890) and 86 % with the ITS sequence of L. elongatus Hooper, 1961 (AJ549986, AJ549987). Intraspecific variation for the ITS sequences was low, with only two nucleotides difference and no indels.
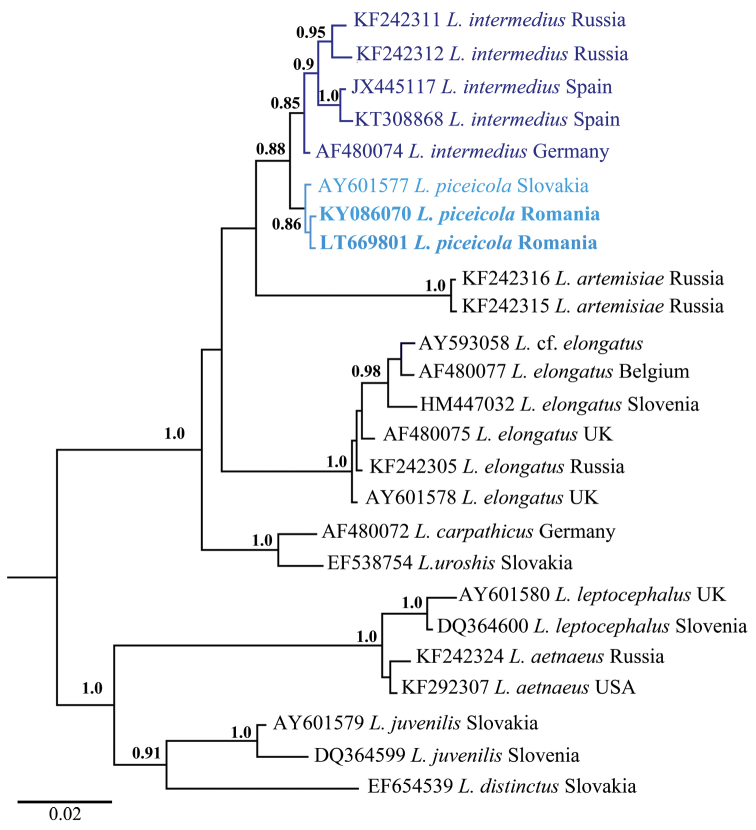
D2-D3 rDNA sequences obtained from both Romanian populations were identical to each other and to the sequence of L. piceicola from Slovakia (AY601577, He et al. 2005). The phylogenetic relationships of L. piceicola with several related species is presented in Figure 8. Longidorus intermedius revealed sister relationships with L. piceicola and the sequences from both species formed a well-supported clade. In addition, five sequences of L. intermedius from Germany (AF480074, Rubtsova et al. 2001), Russia (KF242311 and KF242312, Subbotin et al. 2014), Spain (KT308868, Gutiérrez-Gutiérrez et al 2013 and JX445117, Archidona-Yuste et al. 2016), and the L. piceicola sequence were realigned separately and pairwise distances estimated. A total of 737 positions was included in the dataset. The between species dissimilarities (p-distances) were 0.3–0.9 % (or 2–6 bp differences). Similarly, the intraspecific p-distances of L. intermedius from the three European countries were 0.4-0.9 % (i.e. 3–6 bp).
Figure 8.
Phylogenetic tree using D2-D3 28S rDNA and inferred from a Bayesian analysis with GTR+G model and midpoint rooting. Posterior probabilities ≥ than 0.8 are presented.
The SNPs analysis comparing all D2-D3 sequences of L. piceicola and L. intermedius revealed three parsimony-informative sites (i.e. nucleotide positions with transitions 89T/C, 134T/C and 297A/G) when compared to the reference sequence of L. piceicola (AY601577) (Table 4). The most similar sequence to the L. piceicola sequence was that of L. intermedius from Germany, revealing the highest similarity and only two interspecies differentiating nucleotides at positions 89 and 134 compared to the reference sequence (Table 4).
Table 4.
The variable positions in D2-D3 28S rDNA control region sequences of Longidorus piceicola and L. intermedius. The L. piceicola sequence from Slovakia (Acc. no AY601577) was used as a reference.
| SNPositions | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 89 | 129 | 134 | 197 | 255 | 285+1gap | 285+2gap | 297 | 310 | 413 | 514 | 584 | |
| AY601577 reference sequence | T | C | T | A | C | – | – | A | T | G | G | C |
| AY601577 L. piceicola Slovakia | . | . | . | . | . | – | – | . | . | . | . | . |
| KY086070 L. piceicola Romania 1 | . | . | . | . | . | – | – | . | . | . | . | . |
| LT669801 L. piceicola Romania 2 | . | . | . | . | . | – | – | . | . | . | . | . |
| AF480074 L. intermedius Germany | C | . | C | . | . | – | – | . | . | . | . | . |
| JX445117 L. intermedius Spain | C | . | C | . | T | A | T | G | G | . | S | . |
| KT308868 L. intermedius Spain | C | . | C | . | T | A | T | G | G | . | T | . |
| KF242312 L. intermedius Russia | C | T | C | . | . | – | – | G | . | T | . | T |
| KF242311 L. intermedius Russia | C | . | C | C | . | – | – | G | . | T | . | . |
Discussion
Morphologically, the specimens of L. piceicola from Romania are similar to the type-population from Slovakia (Lišková et al. 1997), except for the slightly longer body (av. 5.88 vs 5.19 mm) and shorter tail (av. 34.5 vs 42 µm, av. c = 172 vs c = 125) in the population from Cernica forest. Barsi and Lamberti (2001) described several L. piceicola populations from Bosnia and Herzegovina, Serbia and Montenegro. In comparison with those populations, the nematodes from Romania have a narrower lip region (avs. 14.5, 14.7 vs avs. 16–17 µm), a shorter odontostyle (avs. 155.4, 155.5 vs avs. 167–188 µm) and tail (av. 35 vs avs. 39–46 μm) in specimens from Bran population. Compared to subsequently recorded L. piceicola population from Poland, specimens from Romania have, again, a much shorter body (avs. 4.9, 5.2 vs av. 6.5 mm) and tail (avs. 34, 38 vs av. 47.4 μm).
The observed abnormalities (presence of reserve odontostyle) in females have been reported for other longidorids (Ferris et al. 2012) whereas atypical development in juveniles has not been recorded previously to such a great extent (ca 30 % of all juveniles studied from L. decidua forest were atypical). Ferris et al. (2012) hypothesized that “anatomical aberrations possibly are results from accidents in transcription of the genetic code or mutations which may or may not be mechanistically limiting to reproduction and therefore may or may not be maintained in the genome through either apomixis or amphimixis”.
Longidorus piceicola was previously recovered in association with P. abies, Abies alba L., Fagus sylvatica L., Carpinus betulus L. and Vitis vinifera L. in Slovakia, West Balkans and Poland (Lišková et al. 1997, Barsi and Lamberti 2001, Kornobis and Peneva 2011, Skwiercz et al. 2015), and our findings in coniferous forest dominated by larch and mixed deciduous forest (Fraxinus, Quercus and Tilia) in Romania extend the geographical and plant association ranges further southeast.
Based on the molecular and morphological characterization L. piceicola is closely related to L. intermedius: however, it differs in having a much longer odontostyle (151–169 μm in the type population and reported range of 144–183 μm vs 105–118 μm and 97–121 μm, respectively), generally longer body (4.22–5.97 mm in the type population and reported range of 4.42–7.99 mm vs 3.6–4.5 mm and 3.11–5.4 mm, respectively) and bigger anterior end – guide ring distance (37–45 μm in the type population and a range of 34–46 μm vs 25–34 μm and 27–36 μm, respectively); a wider lip region (14–18 vs 11–12.5 μm), more ventromedian supplements (11 vs 5–7) in the males, and four vs three juvenile stages (Lišková et al. 1997, Peneva et al. 2001, Barsi and Lamberti 2001, Kumari et al. 2006, Kornobis and Peneva 2011, Gutiérrez-Gutiérrez et al. 2013). Sequence and SNPs analyses of the D2-D3 rDNA region of L. piceicola and L. intermedius populations showed three transitions and four transversions that can be used to s differentiate between both species. Furthermore, L. piceicola was more frequently found in association with conifers, while L. intermedius occurred mainly in oak forests.
Supplementary Material
Acknowledgements
This work was supported by the BAS project ANIDIV 2. The authors are thankful to Dr Nathalie Yonow from Swansea University, Wales, UK for critical reading of the manuscript and helpful suggestions.
Citation
Groza M, Lazarova S, De Luca F, Fanelli E, Elshishka M, Radoslavov G, Hristov P, Coman M, Peneva V (2017) The morphological and molecular identity of Longidorus piceicola Lišková, Robbins & Brown, 1997 from Romania (Nematoda, Dorylaimida). ZooKeys 667: 1–19. https://doi.org/10.3897/zookeys.667.12011
References
- Archidona-Yuste A, Navas-Cortés JA, Cantalapiedra-Navarrete C, Palomares-Rius JE. (2016) Unraveling the biodiversity and molecular phylogeny of needle nematodes of the genus Longidorus (Nematoda: Longidoridae) in olive and a description of six new species. PLoS ONE 11(1): e0147689. https://doi.org/10.1371/journal.pone.0147689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsi L, Lamberti F. (2001) Morphometric variation and juvenile stages of Longidorus piceicola Liskova et al., 1997 (Nematoda: Longidoridae) from the former territory of Yugoslavia. Russian Journal of Nematology 9: 77–83. [Google Scholar]
- Brown DJF, Grunder J, Hooper DJ, Kuntz P. (1994) Longidorus arthensis sp. n. (Nematoda: Longidoridae) a vector of cherry rosette disease caused by a new nepovirus in cherry trees in Switzerland. Nematologica 40: 133–140. https://doi.org/10.1163/003525994X00094 [Google Scholar]
- Cobb NA. (1918) Estimating the nema population of the soil. Agricultural Technology Circular I. Bureau of Plant Industry, United States Department of Agriculture, 48 pp. [Google Scholar]
- Decraemer W, Coomans A. (2007) Revision of some species of the genus Paralongidorus sensu Siddiqi et al. (1993), with a discussion on the relationships within the family Longidoridae (Nematoda: Dorylaimida). Nematology 9: 643–62. https://doi.org/10.1163/156854107782024776 [Google Scholar]
- De Ley P, Félix MA, Frisse LM, Nadler SA, Sternberg PW, Thomas WK. (1999) Molecular and morphological characterisation of two reproductively isolated species with mirror-image anatomy (Nematoda: Cephalobidae). Nematology 1: 591–612. https://doi.org/10.1163/156854199508559 [Google Scholar]
- De Luca F, Reyes A, Grunder J, Kunz P, Agostinelli A, De Giorgi C, Lamberti F. (2004) Characterization and sequence variation in the rDNA region of six nematode species of the genus Longidorus (Nematoda). Journal of Nematology 36: 147–152. [PMC free article] [PubMed] [Google Scholar]
- Ferris H, Robbins R, Yeates G. (2012) Atypical development in plant and soil nematodes. Journal of Nematology 44: 1–6. [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez‐Gutiérrez C, Cantalapiedra‐Navarrete C, Montes‐Borrego M, Palomares‐Rius JE, Castillo P. (2013) Molecular phylogeny of the nematode genus Longidorus (Nematoda: Longidoridae) with description of three new species. Zoological Journal of the Linnean Society 167(4): 473–500. https://doi.org/10.1111/zoj.12019 [Google Scholar]
- He Y, Subbotin S, Rubtsova TV, Lamberti F, Brown DJF, Moens M. (2005) A molecular phylogenetic approach to Longidoridae (Nematoda: Dorylaimida). Nematology 7: 111–124. https://doi.org/10.1163/1568541054192108 [Google Scholar]
- Holterman M, Wurff AVD, Elsen SVD, Megen HV, Bongers T, Holovachov O, Bakker J, Helder J. (2006) Phylum-wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown clades. Molecular Biology and Evolution 23: 1792–1800. https://doi.org/10.1093/molbev/msl044 [DOI] [PubMed] [Google Scholar]
- Hooper DJ. (1961) A redescription of Longidorus elongatus (De Man, 1876) Thorne & Swanger, 1936, (Nematoda, Dorylaimidae) and descriptions of five new species of Longidorus from Great Britain. Nematologica 6(3): 237–257. https://doi.org/10.1163/187529261X00072 [Google Scholar]
- Huelsenbeck JP, Ronquist F. (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. https://doi.org/10.1093/bioinformatics/17.8.754 [DOI] [PubMed] [Google Scholar]
- Kornobis FW, Peneva V. (2011) Longidorus piceicola Lišková et al., 1997 and L. poessneckensis Altherr, 1974 (Nematoda: Longidoridae) – new records from Poland with first description of L. poessneckensis male and a bivulval female. Systematic Parasitology 80(3): 205–216. https://doi.org/10.1007/s11230-011-9325-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowska J, Seinhorst JW. (1979) Longidorus elongatus and closely related species in The Netherlands and Lower Saxony (Germany), with the description of two new species, L. cylindricaudatus and L. intermedius (Nematoda: Dorylaimida). Nematologica 25: 42–53. https://doi.org/10.1163/187529279X00361 [Google Scholar]
- Kumar S, Stecher G, Tamura K. (2016) MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution (online). https://doi.org/10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed]
- Kumari S, Chaloupková M, Jokeš M. (2006) First record of Longidorus intermedius Kozlowska and Seinhorst, 1979 (Nematoda: Longidoridae) from the Czech Republic. Helminthologia 43: 122–124. https://doi.org/10.2478/s11687-006-0023-z [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. (2007) “ClustalW and ClustalX version 2”. Bioinformatics 23(21): 2947–2948. https://doi.org/10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- Lazarova S, Peneva V, Kumari S. (2016) Morphological and molecular characterisation, and phylogenetic position of X. browni sp. n., X. penevi sp. n. and two known species of Xiphinema americanum-group (Nematoda, Longidoridae). ZooKeys 574: 1–42. https://doi.org/10.3897/zookeys.574.8037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lišková M, Robbins RT, Brown DJF. (1997) Descriptions of three new Longidorus species from Slovakia (Nemata: Longidoridae). Journal of Nematology 29: 336–348. [PMC free article] [PubMed] [Google Scholar]
- Nedelchev S, Elshishka M, Lazarova S, Radoslavov G, Hristov P, Peneva V. (2014) Calcaridorylaimus castaneae sp. n. (Nematoda: Dorylaimidae) from Bulgaria with an identification key to the species of the genus. ZooKeys 410: 41–61. https://doi.org/10.3897/zookeys.410.6955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peneva V, Loof PAA, Penev LD, Brown DJF. (2001) Description of the male and first stage juvenile of Longidorus intermedius Kozlowska & Seinhorst, 1979 (Nematoda: Dorylaimida), and notes on morphology and distribution. Systematic Parasitology 49: 127–137. https://doi.org/10.1023/A:1010608418412 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Systematic Biology 61: 539–542. https://doi.org/10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsova TV, Subbotin SA, Brown DJF, Moens M. (2001) Description of Longidorus sturhani sp. n. (Nematoda: Longidoridae) and molecular characterisation of several longidorid species from Western Europe. Russian Journal of Nematology 9: 127–136. [Google Scholar]
- Seinhorst JW. (1959) A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica 4: 67–69. https://doi.org/10.1163/187529259X00381 [Google Scholar]
- Sela I, Ashkenazy H, Katoh K, Pupko T. (2015) GUIDANCE2: accurate detection of unreliable alignment regions accounting for the uncertainty of multiple parameters. Nucleic Acids Research 43 (Web Server issue): W7–W14. https://doi.org/10.1093/nar/gkq443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skwiercz AT, Dzięgielewska M, Szelągowska P. (2015) Nematodes in the vineyards in the northwestern part of Poland. Acta Scientiarum Polonorum Hortorum Cultus 14(3): 3–12. [Google Scholar]
- Subbotin SA, Rogozhin EA, Chizhov VN. (2014) Molecular characterisation and diagnostics of some Longidorus species (Nematoda: Longidoridae) from Russia and other countries using rRNA genes. European Journal of Plant Pathology 138: 377–390. https://doi.org/10.1007/s10658-013-0338-9 [Google Scholar]
- Subbotin SA, Vierstraete A, De Ley P, Rowe J, Waeyenberge L, Moens M, Vanfleteren JR. (2001) Phylogenetic relationships within the cyst-forming nematodes (Nematoda, Heteroderidae) based on analysis of sequences from the ITS regions of ribosomal DNA. Molecular Phylogenetics and Evolution 21: 1–16. https://doi.org/10.1006/mpev.2001.0998 [DOI] [PubMed] [Google Scholar]
- Yoder M, De Ley IT, King IW, Mundo-Ocampo M, Mann J, Blaxter M, Poiras L, De Ley P. (2006) DESS: a versatile solution for preserving morphology and extractable DNA of nematodes. Nematology 8(3): 367–76. https://doi.org/10.1163/156854106778493448 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.