Abstract
Background
Aortic stiffness impairs optimal ventricular–vascular coupling and left ventricular systolic function, particularly in the long axis. Left ventricular global longitudinal strain (GLS) has recently emerged as a sensitive measure of early cardiac dysfunction. In this study, we investigated the relation between aortic stiffness and GLS in a large community‐based sample.
Methods and Results
In 2495 participants (age 39–90 years, 57% women) of the Framingham Offspring and Omni cohorts, free of cardiovascular disease, we performed tonometry to measure arterial hemodynamics and echocardiography to assess cardiac function. Aortic stiffness was evaluated as carotid–femoral pulse wave velocity and as characteristic impedance, and GLS was calculated using speckle tracking–based measurements. In multivariable analyses adjusting for age, sex, height, systolic blood pressure, augmentation index, left ventricular structure, and additional cardiovascular risk factors, increased carotid–femoral pulse wave velocity (B±SE: 0.122±0.030% strain per SD, P<0.0001) and characteristic impedance (0.090±0.029, P=0.002) were both associated with worse GLS. We observed effect modification by sex on the relation between characteristic impedance and GLS (P=0.004); in sex‐stratified multivariable analyses, the relation between greater characteristic impedance and worse GLS persisted in women (0.145±0.039, P=0.0003) but not in men (P=0.73).
Conclusions
Multiple measures of increased aortic stiffness were cross‐sectionally associated with worse GLS after adjusting for hemodynamic variables. Parallel reductions in left ventricular long axis shortening and proximal aortic longitudinal strain in individuals with a stiffened proximal aorta, from direct mechanical ventricular‐vascular coupling, offers an alternative explanation for the observed relations.
Keywords: aortic stiffness, characteristic impedance, global longitudinal strain, left ventricle function, pulse wave velocity, ventricular/vascular coupling hemodynamics
Subject Categories: Epidemiology, Hemodynamics, Echocardiography
Introduction
Left ventricular (LV) function can be evaluated using directional components of myocardial deformation or strain. Longitudinal LV strain (also referred to as global longitudinal strain, GLS) appears to be a sensitive measure of impaired LV systolic function1, 2, 3 and has been shown in several studies to be better than ejection fraction at predicting cardiovascular disease events and death.4, 5, 6, 7 During systole, longitudinal shortening of the LV produces aortic displacement8, 9, 10 and stretches the ascending aorta.11 The force required to produce longitudinal strain of the aorta represents an often overlooked form of direct mechanical load on the LV that may have important implications for the relation between aortic stiffness and LV systolic function, particularly in the long axis.11, 12
Alterations in both LV and aortic physiology may play an important role in predisposition to heart failure and especially heart failure with preserved ejection fraction (HFpEF). Whereas HFpEF is almost as common as heart failure with reduced ejection fraction, HFpEF has proven relatively refractory to treatment in a number of randomized clinical trials,13, 14, 15 underscoring the importance of efforts to better understand its pathophysiology. Interestingly, HFpEF has been related to both reduced GLS and increased aortic stiffness in a number of prior studies.3, 7, 16, 17, 18, 19, 20 Furthermore, both HFpEF and aortic stiffness are prevalent in older individuals, particularly women,16, 17 suggesting possible pathophysiologic links between aortic stiffness and subclinical alterations in LV systolic function that may promote the development of HFpEF in susceptible individuals. Therefore, to further clarify the interrelations of large artery hemodynamics and alterations in LV systolic function, we investigated the association between aortic stiffness and GLS in a large, community‐based sample free of overt cardiovascular disease.
Methods
Study Sample
Participant selection criteria and the design of the Framingham Offspring and the Framingham Omni studies have been previously described.21, 22, 23 Offspring Study participants who attended their eighth examination (2005–2008) and Omni Study participants who attended their third examination (2007–2008) underwent a standardized medical history and examination (N=3319), and underwent routine echocardiography (N=3185) with digital image acquisition using a Hewlett‐Packard 5500 machine (Philips Healthcare, Andover, MA). A total of 3086 individuals had echocardiographic images deemed appropriate for speckle‐tracking analyses based on the following criteria: ≤1 segment of dropout for any of the predefined views (apical 2‐chamber, apical 4‐chamber, or parasternal short‐axis at the level of the midventricle) and absence of arrhythmia during image capture.24 From this sample, we excluded individuals in hierarchical, non‐exclusive fashion for the following reasons: prior history of cardiovascular disease (N=252), prevalent atrial fibrillation (N=124), valvular heart disease (N=63), missing covariate data (N=12), and missing carotid–femoral pulse wave velocity (CFPWV, N=140), resulting in a final sample of 2495 participants eligible for the present investigation. The Institutional Review Board of Boston University Medical Campus approved the study protocols, and all participants provided written informed consent.
Clinical Evaluation and Definitions
Medical history, physical examination, and electrocardiography were performed routinely at each Framingham Heart Study examination.22 Physician‐acquired blood pressures represent the mean of 2 auscultatory measurements obtained on the left arm of seated participants at the time of the Framingham clinic examination. The physician blood pressures were acquired using a mercury column sphygmomanometer and a standardized protocol with excellent measurement reproducibility. Body mass index was calculated by dividing weight in kilograms by the square of the height in meters. Blood glucose was measured in the morning after participants had fasted overnight. Criteria for diabetes mellitus were a blood glucose level of 126 mg/dL (7.0 mmol/L) or greater, or the use of medications to treat diabetes. Smoking was defined as regular use of cigarettes in within the year preceding the Framingham Heart Study visit.
Assessment of Noninvasive Hemodynamics
Hemodynamic data were acquired as previously described.25 Participants were studied in the supine position after 5 minutes of rest. Supine auscultatory brachial systolic and diastolic blood pressure at the time of tonometry (referred to as tonometric measures) were obtained using a computer‐controlled device. Arterial tonometry with simultaneous electrocardiography was obtained from brachial, radial, femoral, and carotid arteries using a custom tonometer. Next, 2‐dimensional echocardiographic images of the LV outflow tract were obtained from a parasternal long‐axis view followed by pulsed Doppler of the LV outflow tract from an apical 5‐chamber view. Tonometric, electrocardiographic, and echocardiographic data were digitized during the primary acquisition and transferred to the core laboratory (Cardiovascular Engineering, Inc, Norwood, MA) for analysis by trained analyzers blinded to participant characteristics.
Tonometry waveforms were signal‐averaged using the electrocardiographic R‐wave as a fiducial point.25 Since tonometry and echocardiography were performed during the same examination session for each participant, blood pressure measurements recorded as part of the tonometry assessment are also considered contemporary with the echocardiographic assessment. Cuff systolic and diastolic pressures obtained at the time of tonometry were used to calibrate the peak and trough of the signal‐averaged brachial pressure waveform. Mean arterial pressure was calculated by integration of the calibrated brachial pressure waveform.26 Diastolic pressure and mean arterial pressure were then used to calibrate carotid pressure tracings.27 Calibrated carotid pressure was used as a surrogate for central pressure.27 CFPWV was calculated from carotid and femoral pressure waveforms, and body surface measurements were corrected for parallel transmission by subtracting the distance from suprasternal notch to carotid artery, from the distance from suprasternal notch to femoral artery, as previously described.28 Reproducibility of the major tonometric measures in our laboratory has been previously reported.29 In a random sample of 50 cases that were blindly re‐analyzed by a second observer, the correlation coefficient was r=0.972 for CFPWV and r=0.997 for augmentation index (AI). Characteristic impedance (Zc) was calculated from the ratio of change in carotid pressure and the change in flow in the proximal aorta during early systole.26 Early systole was defined as the time interval between the onset of flow and the time that flow reaches 95% of its maximum value.
Assessment of Cardiac Strain
We used an off‐line speckle‐tracking software package (2D Cardiac Performance Analysis v1.1; TomTec Imaging Systems, Unterschleißheim, Germany) to analyze LV cardiac strain in each of the pre‐defined 2‐dimensional views (apical 2‐chamber, apical 4‐chamber, and midventricular parasternal short‐axis) according to a standardized protocol.24 The TomTec 2D software package allows for performing strain measurements according to an established speckle‐tracking algorithm that has been validated with sonomicrometry as well as cardiac magnetic resonance imaging, as reported previously.30, 31, 32 GLS was calculated as the average of longitudinal strains from the 2‐ and 4‐chamber views and global circumferential strain (GCS) was calculated from the short‐axis view. Negative strain values indicate LV myofiber shortening. Excellent intra‐ and interobserver reproducibility for strain measures have been reported previously.24
Statistical Methods
Clinical, tonometric, and echocardiographic characteristics of the study sample were tabulated separately by sex. Negative inverse CFPWV and natural log‐transformed Zc were used in statistical analyses to minimize heteroscedasticity and normalize distributions.
We used multivariable‐adjusted regression models to quantify associations between each of the primary hemodynamic measures (CFPWV and Zc; independent variables) and each LV cardiac strain measure (GLS and GCS; dependent variables). We adjusted for (1) cohort (Omni versus Offspring), age, sex, and height; and (2) additionally for weight, glucose, total/high‐density lipoprotein cholesterol ratio, natural log‐triglycerides, diabetes mellitus, current smoker, antihypertensive medication use, lipid‐lowering medication use, heart rate during tonometry, tonometric systolic blood pressure, and AI. To account for multiple testing performed with 2 primary aortic stiffness independent measures and 2 primary strain dependent measures, we used a conservative Bonferroni‐corrected statistical significance threshold of P<0.0125=(0.05/4). In secondary analyses, we repeated analyses with additional adjustment for LV mass, LV wall thickness, and LV end‐diastolic dimension. We also tested for modification of strain associations by age and sex. Figure models were performed with native units of CFPWV and Zc for easier interpretation of results and were not significantly different from models using transformed values. Figures were created using restricted cubic splines with knots at the 5th, 50th, and 95th percentiles.
All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC). The authors have full access to and take full responsibility for the integrity of the data. All authors read and agreed to the manuscript as written.
Results
Table 1 displays the clinical characteristics of men and women in the study sample. Table 2 presents the clinical characteristics of both included and excluded participants. Individuals excluded tended to be older, have higher body mass index, and a higher prevalence of diabetes mellitus and treatment for hypertension and dyslipidemia. Table 3 displays vascular and cardiac measures of men and women in the study sample. Men had a higher CFPWV and a larger LV structure, whereas women had a higher Zc and larger magnitudes of GLS and GCS.
Table 1.
Sample Characteristics
| Variables | Men (N=1065) | Women (N=1430) |
|---|---|---|
| Age, y | 65±9 | 66±9 |
| Nonwhite race/ethnicity, N (%) | 90 (8) | 147 (10) |
| Height, cm | 175±7 | 161±6 |
| Weight, kg | 87±15 | 71±15 |
| Body mass index, kg/m2 | 28.5±4.5 | 27.4±5.6 |
| Seated blood pressure, mm Hg | ||
| Systolic | 129±16 | 127±18 |
| Diastolic | 76±10 | 73±10 |
| Glucose, mg/dL | 109±24 | 102±20 |
| Total/HDL cholesterol ratio | 3.7±1.1 | 3.3±1.0 |
| Triglycerides, mg/dL a | 101 (73, 144) | 99 (73, 135) |
| Diabetes mellitus, N (%) | 171 (16) | 130 (9) |
| Current smoker, N (%) | 82 (8) | 129 (9) |
| Hypertension treatment, N (%) | 543 (51) | 646 (45) |
| Lipid treatment, N (%) | 517 (49) | 578 (40) |
Values are shown as means±SD or number (percent frequency) unless otherwise indicated. HDL indicates high‐density lipoprotein.
Median (25th, 75th percentile).
Table 2.
Included Versus Excluded Sample Characteristics
| Variables | Included (N=2495) | Excluded (N=824) |
|---|---|---|
| Age, y | 65±9 | 71±10 |
| Women, N (%) | 1430 (57) | 419 (51) |
| Nonwhite race/ethnicity, N (%) | 237 (10) | 61 (7) |
| Height, cm | 167±10 | 167±10 |
| Weight, kg | 78±17 | 82±20 |
| Body mass index, kg/m2 | 27.9±5.2 | 30.0±6.2 |
| Seated blood pressure, mm Hg | ||
| Systolic | 128±17 | 130±18 |
| Diastolic | 74±10 | 71±11 |
| Glucose, mg/dL | 105±22 | 112±30 |
| Total/HDL cholesterol ratio | 3.5±1.0 | 3.5±1.1 |
| Triglycerides, mg/dL a | 100 (73, 138) | 107 (75, 153) |
| Diabetes mellitus, N (%) | 301 (12) | 179 (25) |
| Current smoker, N (%) | 211 (8) | 73 (9) |
| Hypertension treatment, N (%) | 1189 (48) | 619 (75) |
| Lipid treatment, N (%) | 1095 (44) | 490 (60) |
Values are shown as means±SD or number (percent frequency) unless otherwise indicated. HDL indicates high‐density lipoprotein.
Median (25th, 75th percentile).
Table 3.
Vascular and Cardiac Measures
| Variables | Men (N=1065) | Women (N=1430) |
|---|---|---|
| Tonometric measures | ||
| Heart rate, min−1 | 59±10 | 61±10 |
| Supine blood pressure, mm Hg | ||
| Systolic | 140±19 | 140±20 |
| Diastolic | 70±9 | 69±9 |
| Mean | 98±11 | 98±12 |
| Pulse pressure | 70±17 | 71±19 |
| Carotid–femoral pulse wave velocity, m/s | 10.6±3.7 | 9.9±3.4 |
| Negative inverse CFPWV, ms/m | −103±28 | −110±29 |
| Characteristic impedance (Zc), dyne·s/cm5 | 218±74 | 262±104 |
| Log‐characteristic impedance, dyne·s/cm5 | 5.3±0.3 | 5.5±0.4 |
| Augmentation index | 10.5±10.5 | 16.9±12.5 |
| Echocardiographic measures | ||
| LV wall thickness, cm | 2.1±0.2 | 1.8±0.2 |
| LV end‐diastolic dimension, cm | 5.1±0.4 | 4.6±0.4 |
| LV mass, g | 195.2±41.6 | 139.8±30.8 |
| LV ejection fraction, % | 65.7±6.8 | 69.1±6.3 |
| Global longitudinal strain (GLS), % | −19.7±2.9 | −21.5±3.2 |
| Global circumferential strain (GCS), % | −31.0±5.5 | −33.1±5.7 |
Values are shown as means±SD or number (percent frequency) unless otherwise indicated. CFPWV indicates carotid–femoral pulse wave velocity; LV, left ventricular.
Multivariable‐adjusted associations of the aortic stiffness measures (CFPWV and Zc) with LV strain measures (GLS and GCS) are shown in Table 4 and in Figures 1 and 2. Greater global aortic stiffness, as represented by CFPWV, was associated with worse (less negative) GLS in all models, and was associated with worse (less negative) GCS in the age‐, sex‐, and height‐adjusted model only (Table 4 and Figure 1). There was no significant effect modification by age or sex on the relations between CFPWV and either GLS or GCS (Table 5). Greater proximal aortic stiffness, as represented by Zc, was also associated with worse GLS and worse GCS in multivariable‐adjusted models (Table 4). Although there was no significant effect modification by age on these associations, or by sex on the associations of Zc with GCS, there was a significant sex interaction in models relating Zc with GLS (Table 5). In sex‐stratified analyses, Spearman correlation coefficients were calculated for the relations of pulse pressure with CFPWV and Zc: ρ=0.52 and 0.69, respectively, in men; ρ=0.57 and 0.71, respectively, in women. In secondary analyses of CFPWV and Zc with GLS and GCS additionally adjusting for serum creatinine as a measure of renal function, results were unchanged (data not shown).
Table 4.
Multivariable‐Adjusted Associations of Hemodynamic Measures With Indices of LV Strain
| Vascular Measures | Global Longitudinal Strain (GLS) | Global Circumferential Strain (GCS) | ||
|---|---|---|---|---|
| Coefficient (SE)a | P Value | Coefficient (SE)a | P Value | |
| Negative inverse CFPWV | ||||
| Model 1 | 0.211 (0.024) | <0.0001 | 0.077 (0.026) | 0.003 |
| Model 2 | 0.099 (0.029) | 0.0005 | 0.038 (0.031) | 0.22 |
| Model 3 | 0.122 (0.030) | <0.0001 | 0.063 (0.032) | 0.05 |
| Log‐Zcb | ||||
| Model 1 | 0.095 (0.023) | <0.0001 | 0.051 (0.024) | 0.04 |
| Model 2 | 0.082 (0.028) | 0.003 | 0.080 (0.030) | 0.008 |
| Model 3 | 0.090 (0.029) | 0.002 | 0.109 (0.031) | 0.0005 |
Model 1 is adjusted for cohort, age, sex, and height. Model 2 is adjusted for the covariates in Model 1 plus weight, glucose, total/HDL cholesterol ratio, natural log‐triglycerides, diabetes mellitus, current smoker, antihypertensive medication use, lipid‐lowering medication use, heart rate, systolic blood pressure, and augmentation index. Model 3 is adjusted for the covariates in Model 2 plus LV mass, LV wall thickness, and LV end‐diastolic dimension. CFPWV indicates carotid–femoral pulse wave velocity; HDL, high‐density lipoprotein; Zc, characteristic impedance; LV, left ventricular.
Coefficients denote estimated variation in the cardiac strain measure (dependent variable in units of SD) per 1 SD of the hemodynamic measure (independent variable). The sex‐pooled mean±SD of negative inverse CFPWV was −107±29 ms/m; thus, 1 SD higher negative inverse CFPWV corresponds to a reverse‐transformed difference of 3.5 m/s (the difference between −107 and −107+29 transformed back to native units). The sex‐pooled mean±SD for Log‐Zc was 5.4±0.4; thus, 1 SD higher Log‐Zc corresponds to an inverse transformed 1 SD difference of 95 dyne·s/cm5. Thus, Model 3 results indicate that a CFPWV that was 3.5 m/s higher than the mean would be associated with a 0.12 SD higher (worse) GLS.
Relations between Log‐Zc and GLS exhibited a sex‐interaction (Table 5). Analyses were therefore repeated for men and women separately (Model 2: Men −0.012 [0.041] P=0.77, Women 0.146 [0.037] P<0.0001; Model 3: Men 0.015 [0.043] P=0.73, Women: 0.145 [0.039] P=0.0003).
Figure 1.
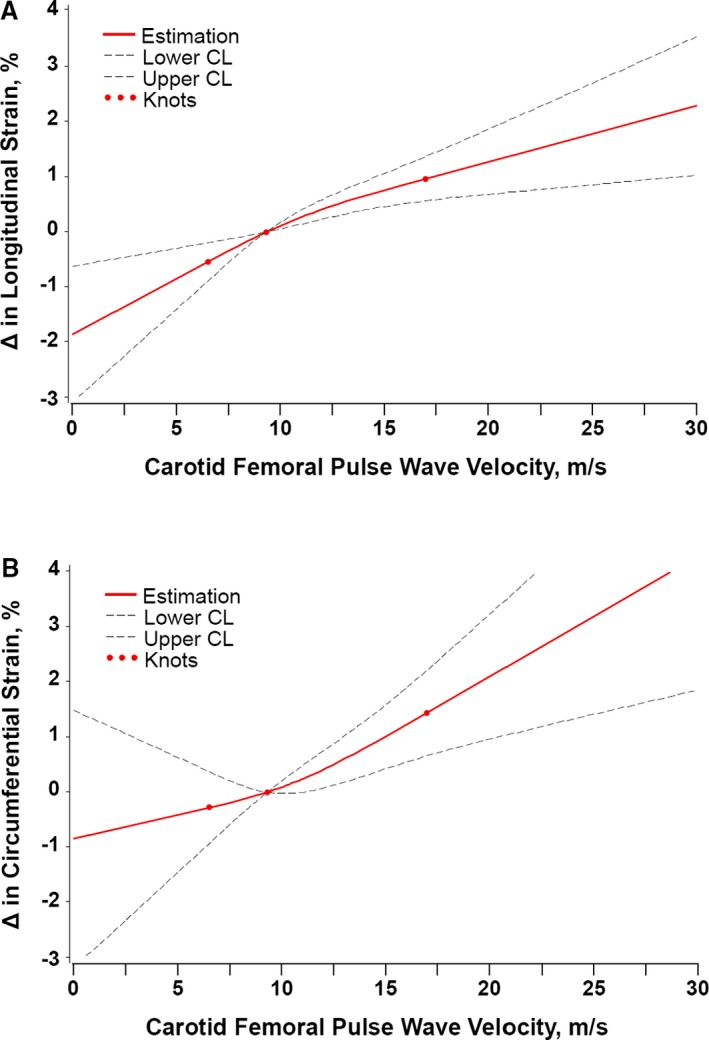
Multivariable adjusted associations between carotid–femoral pulse wave velocity (CFPWV) and (A) global longitudinal strain (GLS) and (B) global circumferential strain (GCS). GLS was significantly worse (less negative) with greater CFPWV, and GCS was significantly worse (less negative) with greater CFPWV. Analyses were adjusted for key covariates: cohort, age, sex, height, weight, glucose, total/HDL cholesterol ratio, natural log‐triglycerides, diabetes mellitus, current smoker, antihypertensive medication use, lipid‐lowering medication use, heart rate, systolic blood pressure, augmentation index, left ventricular mass, left ventricular wall thickness, and left ventricular diastolic dimension. The symbol Δ refers to difference in GLS or GCS compared to the median per CFPWV value. CL indicates confidence limit; HDL, high‐density lipoprotein.
Figure 2.
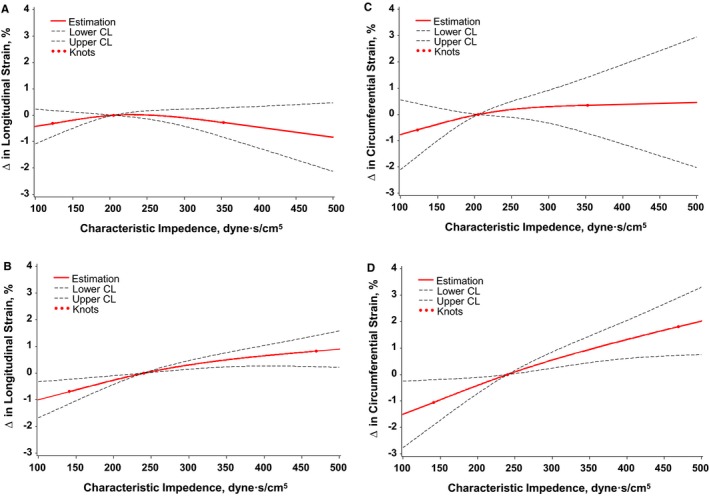
Multivariable adjusted associations between characteristic impedance (Zc) and global longitudinal strain (GLS) in (A) men and (B) women and between Zc and global circumferential strain (GCS) in (C) men and (D) women. Greater Zc was not significantly associated with either GLS or GCS in men; by contrast, Zc was associated with significantly worse (less negative) GLS and GCS. Analyses were adjusted for key covariates: cohort, age, sex, height, weight, glucose, total/HDL cholesterol ratio, natural log‐triglycerides, diabetes mellitus, current smoker, antihypertensive medication use, lipid‐lowering medication use, heart rate, systolic blood pressure, augmentation index, left ventricular mass, left ventricular wall thickness, and left ventricular diastolic dimension. The symbol Δ refers to difference in GLS or GCS compared to the median per Zc value. CL indicates confidence limit; HDL, high‐density lipoprotein.
Table 5.
Age and Sex Interactions in Multivariable‐Adjusted Associations
| Vascular Measures | Longitudinal Strain (GLS) | Circumferential Strain (GCS) | ||
|---|---|---|---|---|
| Coefficient (SE)a | P Value | Coefficient (SE)a | P Value | |
| Age interaction terms | ||||
| Age×(negative inverse CFPWV) | ||||
| Model 2 | 0.024 (0.044) | 0.58 | 0.026 (0.047) | 0.58 |
| Model 3 | 0.065 (0.046) | 0.16 | 0.056 (0.049) | 0.25 |
| Age×Log‐Zc | ||||
| Model 2 | −0.0004 (0.041) | 0.99 | 0.022 (0.044) | 0.62 |
| Model 3 | 0.009 (0.043) | 0.83 | 0.034 (0.045) | 0.45 |
| Sex interaction terms | ||||
| Sex×(negative inverse CFPWV) | ||||
| Model 2 | 0.044 (0.039) | 0.27 | −0.065 (0.042) | 0.12 |
| Model 3 | 0.049 (0.042) | 0.24 | −0.082 (0.044) | 0.06 |
| Sex×Log‐Zc | ||||
| Model 2 | 0.118 (0.040) | 0.004 | 0.016 (0.044) | 0.72 |
| Model 3 | 0.107 (0.042) | 0.01 | 0.021 (0.045) | 0.65 |
Model 2 is adjusted for cohort, age, sex, height, weight, glucose, total/HDL cholesterol ratio, natural log‐triglycerides, diabetes mellitus, current smoker, antihypertensive medication use, lipid‐lowering medication use, heart rate, systolic blood pressure, and augmentation index. Model 3 is adjusted for the covariates in Model 2 plus LV mass, LV wall thickness, and LV end‐diastolic dimension. CFPWV indicates carotid–femoral pulse wave velocity; GCS, global circumferential strain; GLS, global longitudinal strain; HDL, high‐density lipoprotein; LV, left ventricular; Zc, characteristic impedance.
Coefficients are for the age interaction term (older vs younger age was defined by the median age [65 years]) or the sex interaction term (testing for difference in slopes between women vs men) as independent measures in the multivariable models.
Given the significant sex interaction observed for the association between Zc and GLS, we performed sex‐stratified analyses. In analyses adjusting for clinical covariates including systolic blood pressure and AI, greater Zc was associated with worse GLS in women (0.146% [SE 0.037] strain per SD in log‐Zc, P<0.0001) but not in men (Table 4). In multivariable analyses with additional adjustment for conventional measures of LV structure, the association of greater Zc and worse GLS remained significant in women (0.145% [SE 0.039] strain per SD in log‐Zc, P=0.0003) and not in men (Table 4 and Figure 2).
Discussion
In a cross‐sectional community‐based cohort of middle‐aged and older adults, greater aortic wall stiffness, as evaluated by CFPWV, was associated with worse (less negative) GLS. Prior studies have investigated the extent to which hemodynamic load, represented by indices of aortic wave reflection, are related to advanced measures of LV function.33 By contrast, we focused our investigation on the extent to which aortic stiffness possibly confers a non‐hemodynamic effect on LV function, particularly in the long axis. CFPWV was related to GLS, but not GCS, in both men and women, consistent with the hypothesis that aortic stiffening imposes a direct mechanical load on long‐axis LV function. Zc was related to GLS in women only, suggesting that LV long‐axis function may be particularly sensitive to mechanical coupling with the proximal aorta in women. By contrast, short‐axis function was sensitive to Zc in men and women, suggesting that Zc contributes to elements of global hemodynamic load separate from systolic pressure and AI. The association between aortic wall stiffness (evaluated as either CFPWV or Zc) and GLS persisted after adjusting for systolic blood pressure and AI, indicating that the relation between aortic wall stiffness and LV long‐axis function may not be explained fully by potential effects of aortic stiffening on blood pressure and wave reflection.
Increased aortic stiffness has been linked to impaired LV systolic function, particularly along the LV long axis.34, 35, 36, 37, 38, 39 The relation is often attributed to increased hemodynamic load imposed by stiffer arteries.36, 40 However, our observed relations between aortic stiffness measures and GLS persisted after adjusting for traditional measures of hemodynamic load. Direct mechanical ventricular–vascular coupling provides an alternative explanation for the observed relation between aortic stiffness and LV systolic function. Systolic contraction shortens the LV long axis by pulling the aortic annulus and sinotubular junction of the aorta towards the LV apex, which moves minimally during systole.8, 9, 11 The combination of aortic annulus displacement along with minimal movement of the aortic arch implies that there is considerable longitudinal stretch of the ascending aorta during systole.10, 11, 41 Aortic stretch increases from the beginning until the end of systole and imposes a progressive systolic load on the heart.9, 12 If the aorta stiffens, the heart must contract with greater long‐axis force in order to produce the same amount of aortic displacement; for a given LV contraction strength, a stiff aorta would be displaced and stretched less than a compliant aorta would. Therefore, LV long‐axis shortening and GLS may be reduced when pulling against a stiffer aorta because of a potential mechanical ventricular–vascular interaction.
Both increased aortic stiffness and impaired GLS have been associated with impaired LV diastolic function in previous studies12, 19, 34, 35, 38, 42, 43, 44, 45, 46, 47 as well as in our cohort.48 The relation between aortic stiffness and diastolic function is often attributed to hemodynamic effects, while the relation between GLS and diastolic function has been attributed to recoil of contracted LV muscle fibers.49, 50 Recent studies have suggested that diastolic recoil of the aorta and left atrium, which are stretched during systole, facilitates LV filling and ejection.12, 51, 52, 53, 54, 55 The association between increased aortic stiffness and worse GLS observed in our study may relate to both the systolic and diastolic components of direct mechanical ventricular–vascular coupling and requires further study.48
Women tend to have greater aortic longitudinal strain, and better (more negative) GLS and LV ejection fraction than men even in older age.11, 56, 57, 58, 59 Despite having better apparent LV systolic function than men, older women are more likely to develop diastolic dysfunction and HFpEF.16, 17, 38, 60, 61 In our study, both men and women had an association between CFPWV (a global measure of aortic stiffness) and GLS. However, only women had an association between Zc (a local measure of proximal aortic stiffness) and GLS. Previous studies have found that increased proximal aortic stiffness (evaluated as Zc) is associated with impaired diastolic function measures and prevalence of diastolic dysfunction in women only.60 Additionally, the amount of elastic energy stored as a result of systolic proximal aortic stretch has been shown to relate to improved early diastolic filling in older men but not older women,12 indicating that women may fail to recover this energy as a facilitator of longitudinal LV recoil and enhanced early diastolic filling. Volumetric diastolic filling and work stored during aortic stretch were not evaluated in this study, but the observed sex differences in the relation between GLS and Zc may be linked to sex differences in diastolic function.
CFPWV is closely associated with stiffening of the aortic wall, while Zc is more sensitive to differences in aortic diameter.62, 63 Women have genetically smaller aortas than men, which may limit the amount of aortic remodeling possible in response to increased hemodynamic load, for example, as a consequence of midlife weight gain. The disparity in the relations of CFPWV and Zc with GLS for men and women may be attributable in part to the markedly higher Zc values observed in women in our older sample. CFPWV and Zc also differ in their associations with GCS. CFPWV was associated with GLS and not GCS, which may indicate that long‐axis function of the LV is more closely linked with global aortic wall stiffness than is short‐axis function. In prior work conducted in this study sample, we have observed that CFPWV is significantly associated with measures of LV diastolic function but not with conventional measures of LV systolic function.48 In light of these prior results, the lack of association of CFPWV with circumferential strain is consistent with the concept that mechanical (ie, non‐hemodynamic) coupling is selectively related to LV function in the long axis. Zc was associated with both GLS and GCS, which may be related to the strong association between Zc and pressure pulsatility,62 possibly contributing to a secondary association between pressure pulsatility (which imposes an omnidirectional load) and global LV load that includes GCS.64 Additional research is needed to investigate differences in relations of CFPWV and Zc with LV function.
Our study has limitations that should be considered. The study sample included middle‐aged to older men and women of predominantly European ancestry. Thus, additional studies should be performed in other age groups and ethnicities to establish the generalizability of our results. We and others have previously reported on associations of arterial stiffness in relation to adverse cardiovascular events and on the relation of altered myocardial long‐axis strain with all‐cause mortality.4, 65, 66 For the present study sample, in whom both arterial stiffness and myocardial strain measures were performed, the limited number of total events adjudicated to date precludes adequately powered analyses of their combined effects on outcomes. Because repeated measures of both arterial stiffness and myocardial strain were not available in our sample, further studies are needed to conduct longitudinal analyses of these measures also in relation to outcomes. The extent to which certain medications that might confer de‐stiffening properties may have been more or less prevalent among certain subgroups in our study sample could not be precisely ascertained. Further investigations, including prospective studies, are needed to determine the effects of medications with de‐stiffening properties on the observed relationships between arterial stiffness and LV mechanical function. Additionally, although not available in our study sample, measurements of aortic strain and ascending aorta dimension warrant attention in future studies as variation in these measures could account for at least some of our observed major findings, including differences between men and women. The strength of our study is the large community‐based sample of well‐characterized participants with routine ascertainment of comprehensive echocardiography and aortic stiffness measures.
Conclusion
Aortic stiffness is linked to LV function perhaps partially through direct mechanical coupling. During systole, LV long‐axis shortening produces longitudinal aortic stretch. As the aorta stiffens with advancing age or disease, the force required to stretch the proximal aorta will increase, which will increase load on the long axis of the LV and may result in less LV long axis shortening. In our study, both men and women exhibited a relation between increased global aortic wall stiffness, as evaluated by CFPWV, and impaired GLS, whereas only women exhibited a relation between increased proximal aortic stiffness, as evaluated by Zc, and GLS. Future studies should explore the relations between different measures of aortic stiffness and LV function and investigate possible sex differences in these relations.
Sources of Funding
This work was supported by the Ellison Foundation (Cheng), the National Heart, Lung and Blood Institute's Framingham Heart Study (Contract No. N01‐HC‐25195) and HHSN268201500001I (Vasan), and the following grants: T32 GM74905 (McCabe), R00HL107642 (Cheng), R01HL131532 (Cheng), R01HL093328 (Vasan), R01HL107385 (Vasan, Mitchell), and R01HL126136 (Vasan, Mitchell).
Disclosures
Dr Mitchell is the owner of Cardiovascular Engineering, Inc, a company that develops and manufactures devices to measure vascular stiffness, and serves as a consultant to and has received honoraria and grants from Novartis, Merck, Servier, and Philips. Ms Bell is an employee of Cardiovascular Engineering, Inc.
(J Am Heart Assoc. 2017;6:e004903. DOI: 10.1161/JAHA.116.004903.)
Contributor Information
Gary F. Mitchell, Email: garyfmitchell@mindspring.com.
Susan Cheng, Email: scheng@rics.bwh.harvard.edu.
References
- 1. Diller GP, Kempny A, Liodakis E, Alonso‐Gonzalez R, Inuzuka R, Uebing A, Orwat S, Dimopoulos K, Swan L, Li W, Gatzoulis MA, Baumgartner H. Left ventricular longitudinal function predicts life‐threatening ventricular arrhythmia and death in adults with repaired tetralogy of fallot. Circulation. 2012;125:2440–2446. [DOI] [PubMed] [Google Scholar]
- 2. Donal E, Bergerot C, Thibault H, Ernande L, Loufoua J, Augeul L, Ovize M, Derumeaux G. Influence of afterload on left ventricular radial and longitudinal systolic functions: a two‐dimensional strain imaging study. Eur J Echocardiogr. 2009;10:914–921. [DOI] [PubMed] [Google Scholar]
- 3. Wang J, Khoury DS, Yue Y, Torre‐Amione G, Nagueh SF. Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure. Eur Heart J. 2008;29:1283–1289. [DOI] [PubMed] [Google Scholar]
- 4. Cheng S, McCabe EL, Larson MG, Merz AA, Osypiuk E, Lehman BT, Stantchev P, Aragam J, Solomon SD, Benjamin EJ, Vasan RS. Distinct aspects of left ventricular mechanical function are differentially associated with cardiovascular outcomes and all‐cause mortality in the community. J Am Heart Assoc. 2015;4:e002071 doi: 10.1161/JAHA.115.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta‐analysis of global longitudinal strain and ejection fraction. Heart. 2014;100:1673–1680. [DOI] [PubMed] [Google Scholar]
- 6. Russo C, Jin Z, Elkind MS, Rundek T, Homma S, Sacco RL, Di Tullio MR. Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community‐based cohort. Eur J Heart Fail. 2014;16:1301–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vinereanu D, Lim PO, Frenneaux MP, Fraser AG. Reduced myocardial velocities of left ventricular long‐axis contraction identify both systolic and diastolic heart failure—a comparison with brain natriuretic peptide. Eur J Heart Fail. 2005;7:512–519. [DOI] [PubMed] [Google Scholar]
- 8. Beller CJ, Labrosse MR, Thubrikar MJ, Robicsek F. Role of aortic root motion in the pathogenesis of aortic dissection. Circulation. 2004;109:763–769. [DOI] [PubMed] [Google Scholar]
- 9. Kozerke S, Scheidegger MB, Pedersen EM, Boesiger P. Heart motion adapted cine phase‐contrast flow measurements through the aortic valve. Magn Reson Med. 1999;42:970–978. [DOI] [PubMed] [Google Scholar]
- 10. Weber TF, Muller T, Biesdorf A, Worz S, Rengier F, Heye T, Holland‐Letz T, Rohr K, Kauczor HU, von Tengg‐Kobligk H. True four‐dimensional analysis of thoracic aortic displacement and distension using model‐based segmentation of computed tomography angiography. Int J Cardiovasc Imaging. 2014;30:185–194. [DOI] [PubMed] [Google Scholar]
- 11. Bell V, Mitchell WA, Sigurethsson S, Westenberg JJ, Gotal JD, Torjesen AA, Aspelund T, Launer LJ, de Roos A, Gudnason V, Harris TB, Mitchell GF. Longitudinal and circumferential strain of the proximal aorta. J Am Heart Assoc. 2014;3:e001536 doi: 10.1161/JAHA.114.001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bell V, Sigurdsson S, Westenberg JJ, Gotal JD, Torjesen AA, Aspelund T, Launer LJ, Harris TB, Gudnason V, de Roos A, Mitchell GF. Relations between aortic stiffness and left ventricular structure and function in older participants in the Age, Gene/Environment Susceptibility‐Reykjavik Study. Circ Cardiovasc Imaging. 2015;8:e003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Conraads VM, Metra M, Kamp O, De Keulenaer GW, Pieske B, Zamorano J, Vardas PE, Bohm M, Dei CL. Effects of the long‐term administration of nebivolol on the clinical symptoms, exercise capacity, and left ventricular function of patients with diastolic dysfunction: results of the ELANDD study. Eur J Heart Fail. 2012;14:219–225. [DOI] [PubMed] [Google Scholar]
- 14. Edelmann F, Wachter R, Schmidt AG, Kraigher‐Krainer E, Colantonio C, Kamke W, Duvinage A, Stahrenberg R, Durstewitz K, Loffler M, Dungen HD, Tschope C, Herrmann‐Lingen C, Halle M, Hasenfuss G, Gelbrich G, Pieske B. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo‐DHF randomized controlled trial. JAMA. 2013;309:781–791. [DOI] [PubMed] [Google Scholar]
- 15. Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O'Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E. Effect of phosphodiesterase‐5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alagiakrishnan K, Banach M, Jones LG, Datta S, Ahmed A, Aronow WS. Update on diastolic heart failure or heart failure with preserved ejection fraction in the older adults. Ann Med. 2013;45:37–50. [DOI] [PubMed] [Google Scholar]
- 17. Borlaug BA, Kass DA. Ventricular‐vascular interaction in heart failure. Heart Fail Clin. 2008;4:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Desai AS, Mitchell GF, Fang JC, Creager MA. Central aortic stiffness is increased in patients with heart failure and preserved ejection fraction. J Card Fail. 2009;15:658–664. [DOI] [PubMed] [Google Scholar]
- 19. Ibrahim E, Miller AB, White RD. The relationship between aortic stiffness and E/A filling ratio and myocardial strain in the context of left ventricular diastolic dysfunction in heart failure with normal ejection fraction: insights from magnetic resonance imaging. Magn Reson Imaging. 2011;29:1222–1234. [DOI] [PubMed] [Google Scholar]
- 20. Yip G, Wang M, Zhang Y, Fung JW, Ho PY, Sanderson JE. Left ventricular long axis function in diastolic heart failure is reduced in both diastole and systole: time for a redefinition? Heart. 2002;87:121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dawber TR, Meadors GF, Moore FE Jr. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham Offspring Study. Am J Epidemiol. 1979;110:281–290. [DOI] [PubMed] [Google Scholar]
- 23. Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB Sr, Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. [DOI] [PubMed] [Google Scholar]
- 24. Cheng S, Larson MG, McCabe EL, Osypiuk E, Lehman BT, Stanchev P, Aragam J, Benjamin EJ, Solomon SD, Vasan RS. Reproducibility of speckle‐tracking‐based strain measures of left ventricular function in a community‐based study. J Am Soc Echocardiogr. 2013;26:1258–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation. 2010;122:1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kelly R, Fitchett D. Noninvasive determination of aortic input impedance and external left ventricular power output: a validation and repeatability study of a new technique. J Am Coll Cardiol. 1992;20:952–963. [DOI] [PubMed] [Google Scholar]
- 28. Mitchell GF, Tardif JC, Arnold JM, Marchiori G, O'Brien TX, Dunlap ME, Pfeffer MA. Pulsatile hemodynamics in congestive heart failure. Hypertension. 2001;38:1433–1439. [DOI] [PubMed] [Google Scholar]
- 29. Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. [DOI] [PubMed] [Google Scholar]
- 30. Amundsen BH, Helle‐Valle T, Edvardsen T, Torp H, Crosby J, Lyseggen E, Stoylen A, Ihlen H, Lima JA, Smiseth OA, Slordahl SA. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol. 2006;47:789–793. [DOI] [PubMed] [Google Scholar]
- 31. Korinek J, Wang J, Sengupta PP, Miyazaki C, Kjaergaard J, McMahon E, Abraham TP, Belohlavek M. Two‐dimensional strain—a Doppler‐independent ultrasound method for quantitation of regional deformation: validation in vitro and in vivo. J Am Soc Echocardiogr. 2005;18:1247–1253. [DOI] [PubMed] [Google Scholar]
- 32. Langeland S, D'hooge J, Wouters PF, Leather HA, Claus P, Bijnens B, Sutherland GR. Experimental validation of a new ultrasound method for the simultaneous assessment of radial and longitudinal myocardial deformation independent of insonation angle. Circulation. 2005;112:2157–2162. [DOI] [PubMed] [Google Scholar]
- 33. Russo C, Jin Z, Takei Y, Hasegawa T, Koshaka S, Palmieri V, Elkind MS, Homma S, Sacco RL, Di Tullio MR. Arterial wave reflection and subclinical left ventricular systolic dysfunction. J Hypertens. 2011;29:574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsai WC, Lee KT, Kuo HF, Tang WH, Jhuo SJ, Chu CS, Lin TH, Hsu PC, Lin MY, Lin FH, Su HM, Voon WC, Lai WT, Sheu SH. Association of increased arterial stiffness and p wave dispersion with left ventricular diastolic dysfunction. Int J Med Sci. 2013;10:1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Schinkel LD, Auger D, van Elderen SG, Ajmone MN, Delgado V, Lamb HJ, Ng AC, Smit JW, Bax JJ, Westenberg JJ, de Roos A. Aortic stiffness is related to left ventricular diastolic function in patients with diabetes mellitus type 1: assessment with MRI and speckle tracking strain analysis. Int J Cardiovasc Imaging. 2013;29:633–641. [DOI] [PubMed] [Google Scholar]
- 36. Kim HL, Seo JB, Chung WY, Kim SH, Kim MA, Zo JH. Independent association between brachial‐ankle pulse wave velocity and global longitudinal strain of left ventricle. Int J Cardiovasc Imaging. 2015;31:1563–1570. [DOI] [PubMed] [Google Scholar]
- 37. Kiotsekoglou A, Saha SK, Moggridge JC, Kapetanakis V, Bijnens BH, Mullen MJ, Camm J, Sutherland GR, Wilkinson IB, Child AH. Effect of aortic stiffness on left ventricular long‐axis systolic function in adults with Marfan syndrome. Hellenic J Cardiol. 2010;51:501–511. [PubMed] [Google Scholar]
- 38. Russo C, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Arterial stiffness and wave reflection: sex differences and relationship with left ventricular diastolic function. Hypertension. 2012;60:362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ye Z, Coutinho T, Pellikka PA, Villarraga HR, Borlaug BA, Kullo IJ. Associations of alterations in pulsatile arterial load with left ventricular longitudinal strain. Am J Hypertens. 2015;28:1325–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Krishnasamy R, Hawley CM, Stanton T, Pascoe EM, Campbell KL, Rossi M, Petchey W, Tan KS, Beetham KS, Coombes JS, Leano R, Haluska BA, Isbel NM. Left ventricular global longitudinal strain is associated with cardiovascular risk factors and arterial stiffness in chronic kidney disease. BMC Nephrol. 2015;16:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Morrison TM, Choi G, Zarins CK, Taylor CA. Circumferential and longitudinal cyclic strain of the human thoracic aorta: age‐related changes. J Vasc Surg. 2009;49:1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hasselberg NE, Haugaa KH, Sarvari SI, Gullestad L, Andreassen AK, Smiseth OA, Edvardsen T. Left ventricular global longitudinal strain is associated with exercise capacity in failing hearts with preserved and reduced ejection fraction. Eur Heart J Cardiovasc Imaging. 2015;16:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ballo P, Nistri S, Cameli M, Papesso B, Dini FL, Galderisi M, Zuppiroli A, Mondillo S. Association of left ventricular longitudinal and circumferential systolic dysfunction with diastolic function in hypertension: a nonlinear analysis focused on the interplay with left ventricular geometry. J Card Fail. 2014;20:110–120. [DOI] [PubMed] [Google Scholar]
- 44. Hsu PC, Tsai WC, Lin TH, Su HM, Voon WC, Lai WT, Sheu SH. Association of arterial stiffness and electrocardiography‐determined left ventricular hypertrophy with left ventricular diastolic dysfunction. PLoS One. 2012;7:e49100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim HL, Im MS, Seo JB, Chung WY, Kim SH, Kim MA, Zo JH. The association between arterial stiffness and left ventricular filling pressure in an apparently healthy Korean population. Cardiovasc Ultrasound. 2013;11:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Borlaug BA, Melenovsky V, Redfield MM, Kessler K, Chang HJ, Abraham TP, Kass DA. Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. J Am Coll Cardiol. 2007;50:1570–1577. [DOI] [PubMed] [Google Scholar]
- 47. Kim MN, Park SM, Shim WJ, Kim YH, Kim SA, Cho DH. The relationship between aortic stiffness and left ventricular dyssynchrony in hypertensive patients with preserved left ventricular systolic function. Clin Exp Hypertens. 2012;34:410–416. [DOI] [PubMed] [Google Scholar]
- 48. Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Cheng S, Aragam J, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Relations of central hemodynamics and aortic stiffness with left ventricular structure and function: the Framingham Heart Study. J Am Heart Assoc. 2016;5:e002693 doi: 10.1161/JAHA.115.002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Firstenberg MS, Smedira NG, Greenberg NL, Prior DL, McCarthy PM, Garcia MJ, Thomas JD. Relationship between early diastolic intraventricular pressure gradients, an index of elastic recoil, and improvements in systolic and diastolic function. Circulation. 2001;104:I330–I335. [DOI] [PubMed] [Google Scholar]
- 50. Notomi Y, Martin‐Miklovic MG, Oryszak SJ, Shiota T, Deserranno D, Popovic ZB, Garcia MJ, Greenberg NL, Thomas JD. Enhanced ventricular untwisting during exercise: a mechanistic manifestation of elastic recoil described by Doppler tissue imaging. Circulation. 2006;113:2524–2533. [DOI] [PubMed] [Google Scholar]
- 51. Arutunyan A. Atrio‐ventricular plane displacement is the sole mechanism of atrial and ventricular refill. Am J Physiol Heart Circ Physiol. 2015;308:H1317–H1320. [DOI] [PubMed] [Google Scholar]
- 52. Carlhall C, Kindberg K, Wigstrom L, Daughters GT, Miller DC, Karlsson M, Ingels NB Jr. Contribution of mitral annular dynamics to LV diastolic filling with alteration in preload and inotropic state. Am J Physiol Heart Circ Physiol. 2007;293:H1473–H1479. [DOI] [PubMed] [Google Scholar]
- 53. Carlsson M, Ugander M, Mosen H, Buhre T, Arheden H. Atrioventricular plane displacement is the major contributor to left ventricular pumping in healthy adults, athletes, and patients with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2007;292:H1452–H1459. [DOI] [PubMed] [Google Scholar]
- 54. Henein MY, Gibson DG. Long axis function in disease. Heart. 1999;81:229–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Maksuti E, Bjallmark A, Broome M. Modelling the heart with the atrioventricular plane as a piston unit. Med Eng Phys. 2015;37:87–92. [DOI] [PubMed] [Google Scholar]
- 56. Chung AK, Das SR, Leonard D, Peshock RM, Kazi F, Abdullah SM, Canham RM, Levine BD, Drazner MH. Women have higher left ventricular ejection fractions than men independent of differences in left ventricular volume: the Dallas Heart Study. Circulation. 2006;113:1597–1604. [DOI] [PubMed] [Google Scholar]
- 57. Kocabay G, Muraru D, Peluso D, Cucchini U, Mihaila S, Padayattil‐Jose S, Gentian D, Iliceto S, Vinereanu D, Badano LP. Normal left ventricular mechanics by two‐dimensional speckle‐tracking echocardiography. Reference values in healthy adults. Rev Esp Cardiol (Engl Ed). 2014;67:651–658. [DOI] [PubMed] [Google Scholar]
- 58. Puntmann VO, Nagel E, Hughes AD, Gebker R, Gaddum N, Chowienczyk P, Jahnke C, Mirelis J, Schnackenburg B, Paetsch I, Fleck E. Gender‐specific differences in myocardial deformation and aortic stiffness at rest and dobutamine stress. Hypertension. 2012;59:712–718. [DOI] [PubMed] [Google Scholar]
- 59. Raymond I, Pedersen F, Steensgaard‐Hansen F, Green A, Busch‐Sorensen M, Tuxen C, Appel J, Jacobsen J, Atar D, Hildebrandt P. Prevalence of impaired left ventricular systolic function and heart failure in a middle aged and elderly urban population segment of Copenhagen. Heart. 2003;89:1422–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular‐arterial interactions. J Am Coll Cardiol. 2013;61:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shim CY, Park S, Choi EY, Hong GR, Choi D, Jang Y, Chung N. The relationship between ventricular‐vascular uncoupling during exercise and impaired left ventricular longitudinal functional reserve in hypertensive patients. J Am Soc Hypertens. 2013;7:198–205. [DOI] [PubMed] [Google Scholar]
- 62. Mitchell GF, Lacourciere Y, Ouellet JP, Izzo JL Jr, Neutel J, Kerwin LJ, Block AJ, Pfeffer MA. Determinants of elevated pulse pressure in middle‐aged and older subjects with uncomplicated systolic hypertension: the role of proximal aortic diameter and the aortic pressure‐flow relationship. Circulation. 2003;108:1592–1598. [DOI] [PubMed] [Google Scholar]
- 63. Mitchell GF, Gudnason V, Launer LJ, Aspelund T, Harris TB. Hemodynamics of increased pulse pressure in older women in the community‐based Age, Gene/Environment Susceptibility‐Reykjavik Study. Hypertension. 2008;51:1123–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Burns AT, La GA, D'hooge J, MacIsaac AI, Prior DL. Left ventricular strain and strain rate: characterization of the effect of load in human subjects. Eur J Echocardiogr. 2010;11:283–289. [DOI] [PubMed] [Google Scholar]
- 65. Cooper LL, Rong J, Benjamin EJ, Larson MG, Levy D, Vita JA, Hamburg NM, Vasan RS, Mitchell GF. Components of hemodynamic load and cardiovascular events: the Framingham Heart Study. Circulation. 2015;131:354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tsao CW, Lyass A, Larson MG, Levy D, Hamburg NM, Vita JA, Benjamin EJ, Mitchell GF, Vasan RS. Relation of central arterial stiffness to incident heart failure in the community. J Am Heart Assoc. 2015;4:e002189 doi: 10.1161/JAHA.115.002189. [DOI] [PMC free article] [PubMed] [Google Scholar]


