Abstract
p53 deletion prevents the embryonic lethality of normal tissues lacking Mdm2, suggesting that cells can survive without Mdm2 if p53 is also absent. Here we report evidence challenging this view, with implications for therapeutically targeting Mdm2. Deletion of Mdm2 in T cell lymphomas or sarcomas lacking p53 induced apoptosis and G2 cell cycle arrest, prolonging survival of mice with these tumors. p53−/− fibroblasts showed similar results, indicating that the effects of Mdm2 loss extend to pre-malignant cells. Mdm2 deletion in p53−/− cells upregulated p53 transcriptional target genes that induce apoptosis and cell cycle arrest. Mdm2 deletion also increased levels of p73, a p53 family member. RNAi-mediated attenuation of p73 rescued the transcriptional and biological effects of Mdm2 loss, indicating that p73 mediates the consequences of Mdm2 deletion. Additionally, Mdm2 deletion differed from blocking Mdm2 interaction with p53 family members, as Nutlin-3 induced G1 arrest but did not activate apoptosis in p53−/− sarcoma cells. Our results indicate that, in contrast to current dogma, Mdm2 expression is required for cell survival even in the absence of p53. Moreover, our results suggest that p73 compensates for loss of p53 and that targeting Mdm2 in p53-deficient cancers has therapeutic potential.
Keywords: Mdm2, p53, p73, apoptosis, cancer
Introduction
Mdm2 negatively regulates the p53 tumor suppressor by inhibiting its transcriptional activity and targeting it for degradation via ubiquitination (1). During development in mice, global Mdm2 deletion results in embryonic lethality, which is rescued with accompanying p53 deletion (2,3), genetically establishing Mdm2 regulation of p53. Deletion of Mdm2 in specific tissues (e.g., cardiomyocytes, central nervous system, and hepatocytes) induces apoptosis that is rescued with p53 deletion (4–6). Induced Mdm2 deletion in adult mice resulted in abnormalities in multiple tissues, (e.g., spleen, liver, and kidney), which were not evident when performed on a p53-null background (7). Fibroblasts from Mdm2/p53-double null mice were viable and grew at similar rates as fibroblasts from p53-null only mice (8,9). Furthermore, the rate of tumor development was analogous between Mdm2−/−p53−/− and p53−/− mice (8). These data led to the conclusion that the primary function of Mdm2 was to regulate p53 and that the deleterious effects of Mdm2 loss are p53-dependent.
MDM2 is frequently overexpressed in human malignancies (10), making MDM2 an attractive therapeutic target. Recently, drugs such as Nutlin-3 have been developed that interfere with Mdm2:p53 binding, thereby activating p53 and killing cancer cells (11). However, p53 is mutated or deleted in half of human cancers, making compounds that disrupt Mdm2:p53 binding not viable for these malignancies (12). Additionally, resistance to these compounds develops through p53 inactivation (13–15). p73, a p53 family member, is rarely mutated in human cancers (16). Both p53 and p73 activation upregulate transcriptional targets that induce cell cycle arrest and/or apoptosis (17). Mdm2 can bind and regulate p73 (18–21); yet, the circumstances under which this takes place remains incompletely resolved. Insight into their interaction may be exploited therapeutically in tumors with inactivated p53 (16). For example, high concentrations of Nutlin-3 induced apoptosis of p53-null HCT116 colon carcinoma cells partially through activation of p73 (22). Therefore, identifying alternative approaches to activate p73-induced apoptosis in cells lacking functional p53 may be beneficial.
Since Mdm2 loss in the context of p53 inactivation could hold therapeutic promise and has not been thoroughly examined outside of development, we utilized a conditional Mdm2 deletion mouse model to determine the effect of Mdm2 loss on p53−/− cells. Unexpectedly, both T-cell lymphoma and sarcoma cells lacking p53 underwent apoptosis when Mdm2 was deleted, resulting in significantly diminished cancer cell growth, reduced tumor burden, and extended survival. Immortalized adult mouse fibroblasts were similarly affected by Mdm2 deletion. Mechanistically, we determined p73 mediated the effects of Mdm2 deletion. Thus, Mdm2 is critical for cell survival independent of p53. Therefore, targeting Mdm2 directly may offer therapeutic potential for cancers that have deleted p53 by activating p73.
Materials and Methods
Mice, cells, and tumor development
C57Bl/6 Mdm2fl/fl and p53−/− mice (2), provided by Dr. Guillermina Lozano (MD Anderson), were mated and offspring intercrossed to generate Mdm2fl/flp53−/− mice. Ear punches to derive fibroblasts or tumors (T-cell lymphoma and sarcoma) that developed in Mdm2fl/flp53−/− mice were harvested and placed in short-term culture (see Supplementary Information). The cultured cells were confirmed mycoplasma negative (MycoSensor Mycoplasma Detection PCR Assay Kit; Agilent Technologies). Female nude mice (6–7 weeks old; Envigo) were injected subcutaneously with 1×106 Mdm2fl/flp53−/− T-cell lymphoma or sarcoma cells expressing CreERT2 (23). Tamoxifen (2mg) or corn oil (vehicle) was injected (intraperitoneal) once daily for three days after lymphomas became palpable and once daily for four days beginning the day of sarcoma cell injection. Tumor volumes calculated from caliper measurements. Mice were sacrificed at humane endpoints and tumors harvested for analyses. In a second cohort of mice, when tumors were palpable, tamoxifen or corn oil was injected into mice with size-matched tumors and the tumors harvested 48hrs (lymphoma) or 72hrs (sarcoma) later. All studies complied with state and federal guidelines and were approved by the Vanderbilt Institutional Animal Care and Use Committee.
Proliferation
T-cell lymphoma, sarcoma, and fibroblast cells expressing CreERT2 and GFP or GFP alone (retrovirus) were plated and 1μM 4-hydroxytamoxifen (4-OHT) or EtOH vehicle or Nutlin-3 (10, 20 or 30μM; Sigma) or DMSO vehicle added. Cell number and viability were determined by Trypan Blue Dye exclusion (triplicate). Proliferation determined by MTS (492nm; Promega) or MTT (562nm; Sigma) assays (quadruplicate), as we previously reported (24), (25).
Cell cycle and apoptosis
Cell cycle and fragmented (sub-G1) DNA was analyzed by flow cytometry following propidium iodide staining as we described (24). The percentage of cells in each phase of the cell cycle was determined using the Dean-Jett-Fox model on FlowJo (TreeStar). Phosphorylated histone H3 (phospho-S10, Abcam) was detected according to manufacturer’s protocol; colcemid (4hrs, 0.05μg/mL, Gibco) treated cells were a positive control for cells in M phase. Annexin-V/7-AAD (BD-Pharmingen) staining was performed as we described (24). All assays (triplicate) were conducted following addition of 1μM 4-OHT, 30μM Nutlin-3, or vehicle control (EtOH or DMSO, respectively).
Western blot
Whole-cell protein lysates were Western blotted as previously described (26). Antibodies used include: Mdm2 (2A10, Calbiochem), cleaved Caspase-3 (D175) and cleaved PARP (Asp214) from Cell Signaling, p73 (EP436Y, Abcam), and β-actin (Sigma).
PCR, qRT-PCR, and RNA-sequencing
PCR was performed on genomic DNA with primers specific for unrearranged and rearranged Mdm2 as published (27). PCR genotyping was also used to confirm the T-cell lymphoma, sarcoma, and fibroblasts evaluated were p53-null (Supplementary Fig. S1). Total RNA was extracted using TRIzol (Invitrogen) according to the manufacturer’s protocol. qRT-PCR (triplicate) for mRNA analysis was performed as we described (28) (primers in Supplementary Information). mRNA levels were normalized to β-actin levels and then made relative to vehicle control and presented as 2−ΔΔCT. Following RNA isolation, samples were subjected to RNA-sequencing using the Illumia NextSeq500 platform; GEO accession number is GSE98705.
Bioinformatic Analysis
RNA-sequencing data were analyzed by Kallisto v0.43.0 (29). Murine transcript definitions (Ensembl release 85) were used for transcriptome quantification. Tximport (30) was used to summarize transcript-level estimates for gene-level analysis. Differential gene expression analysis was performed using the R package edgeR (31) as indicated by Tximport (30). Details are in Supplementary Information.
shRNA knockdown
Lentiviral vectors for two p73 shRNA and their respective control non-targeting shRNA were provided by Jennifer Pietenpol (Vanderbilt University). Infected sarcoma cells were selected with puromycin (2.5μg/mL) for 3 days prior to CreERT2 activation.
Statistics
Means ± SEM are plotted. Log-rank tests used for Kaplan-Meier survival analyses. All other statistical analyses used the Student’s t-test, except for RNA-sequencing analysis (see Supplementary Information).
Results
Mdm2 is required for p53-null T-cell lymphoma survival
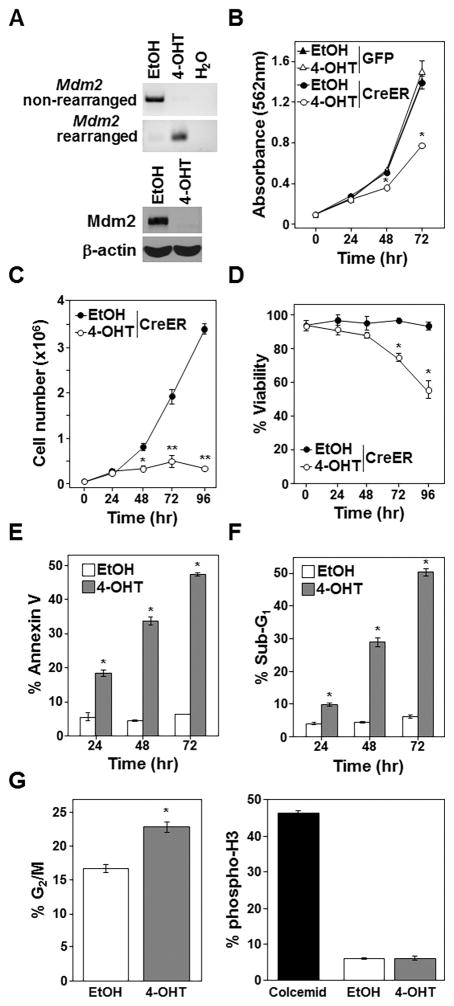
Previous studies showed cell death from loss of Mdm2 during development is rescued by p53 deletion (2,3), indicating cells can survive without Mdm2 if p53 is also absent. We questioned whether there would be a consequence to deleting Mdm2 in mature, fully developed cells that lacked p53. Additionally, since many human cancers delete p53 (12), we sought to determine whether Mdm2 loss in p53-null malignant cells would affect their growth and/or survival. We generated p53-null mice homozygous for the conditional Mdm2 knockout (27). Because p53−/− mice develop T-cell lymphoma (32), we isolated and cultured a T-cell lymphoma that arose in an Mdm2fl/flp53−/− mouse. CreERT2, a 4-OHT inducible form of Cre, was expressed (retrovirally) in the lymphoma cells (23). Rearrangement of the Mdm2 loci occurred within 24 hours after 4-OHT addition (Fig. 1A).
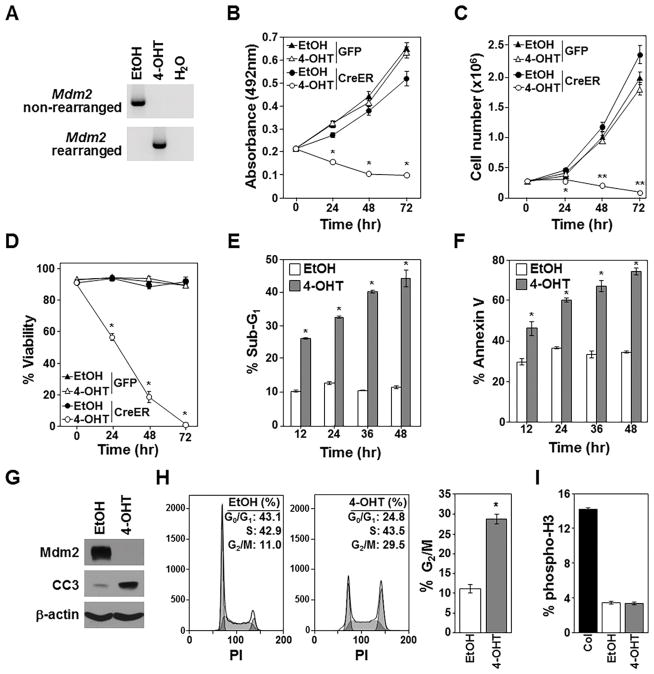
Figure 1. Deletion of Mdm2 inhibits growth and survival of p53-null lymphomas.
Vehicle control (EtOH) or 4-OHT or was added to Mdm2fl/flp53−/− lymphomas expressing CreERT2 and GFP or GFP alone. A–F) PCR Mdm2 gene rearrangement (A), proliferation (MTS assay, quadruplicate; B), cell number (C), viability (D), sub-G1 DNA (E), and Annexin-V (F) measured (C–F, triplicates). G) Western blotting 16hrs after EtOH or 4-OHT addition; cleaved Caspase-3 (CC3). H, I) Cell cycle (H; representative histograms; values in inset, left; G2/M mean values, right) and phospho-histone H3 (I) 12hrs after EtOH or 4-OHT (triplicate); colcemid (Col). B, *p<0.0002; C, *p=0.011, **p<0.0002; D, *p<0.0002; E, *p<0.00009; F, *p<0.006; H, *p=0.00102.
To determine whether Mdm2 loss affects lymphoma cell growth, proliferation was assessed by MTS assay. Surprisingly, p53-null lymphoma cells with CreERT2-mediated Mdm2 deletion showed reduced proliferation compared to vehicle control treated lymphoma cells (Fig. 1B). Lymphoma cell numbers and viability significantly declined following CreERT2 activation with half the cells dead by 24 hours and <5% alive by 72 hours, whereas numbers of vehicle control treated lymphoma cells increased (Fig. 1C and 1D). Within 12 hours of CreERT2 activation, the percentage of cells with fragmented (sub-G1) DNA and that were Annexin-V positive was significantly higher in those that deleted Mdm2, and these differences increased over 48 hours (Fig. 1E and 1F). Mdm2-deleted lymphoma cells also showed the appearance of cleaved Caspase 3 (Fig. 1G). Previously, we reported activation of CreERT2 in lymphoma cells lacking p53 had no effect (24), indicating the apoptosis in the CreERT2 activated Mdm2fl/flp53−/− lymphoma cells was specific to Mdm2 loss. Interestingly, there were significantly more cells in the G2/M phase of the cell cycle 12 hours following CreERT2 activation in the Mdm2fl/flp53−/− lymphoma cells, indicating cell cycle arrest may be occurring (Fig. 1H). To differentiate G2 from M phase, phosphorylated histone H3, a marker of M phase, was measured after Mdm2 deletion. No difference in the percentage of cells in M phase between control and CreERT2 activated lymphomas was detected (Fig. 1I), indicating the cells were likely arresting in G2 and not M phase. Our data indicate Mdm2 deletion in p53−/− T-cell lymphoma inhibits growth and survival by initiating a G2 cell cycle arrest and apoptosis.
p53-null sarcoma cells require Mdm2 for growth and survival
To test the reproducibility of our results and determine whether the apoptosis and cell cycle arrest observed following Mdm2 deletion was specific to p53−/− T-cell lymphoma, we evaluated a different p53-null cancer. Because p53−/− mice also develop sarcomas, we isolated and cultured a spindle-cell sarcoma from an Mdm2fl/flp53−/− mouse. Following retroviral expression of CreERT2 in the sarcoma cells, Mdm2 was deleted within 24 hours of 4-OHT addition (Fig. 2A). There was significantly reduced proliferation by 72 hours (Fig. 2B). Consistent with this, the Mdm2-deleted sarcoma cells showed reduced cell number (Fig. 2C) and viability (Fig. 2D) compared to controls. Within 24 hours following CreERT2 activation, there were significantly elevated levels of Annexin-V positive sarcoma cells (Fig. 2E) and within 36 hours, increased sub-G1 DNA content (Fig. 2F). Cleaved PARP was also evident within 36 hours of 4-OHT addition (Fig. 2G). Thus, apoptosis of the sarcoma cells was occurring upon Mdm2 deletion.
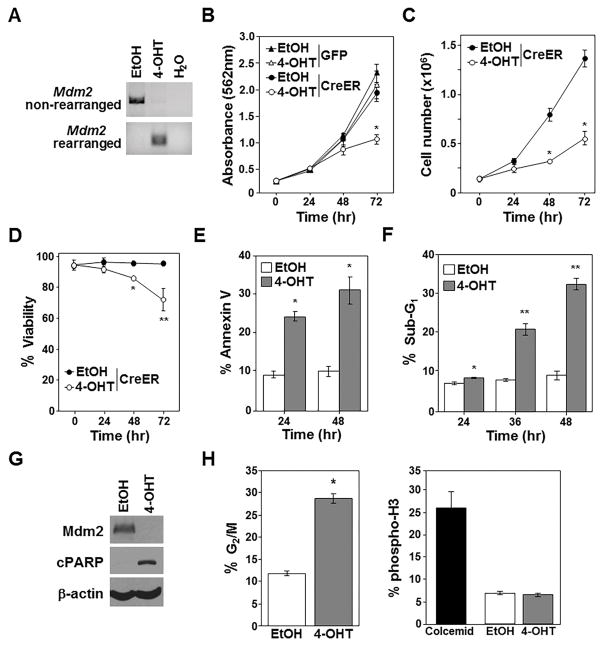
Figure 2. Mdm2 loss significantly impedes growth and survival of p53-null sarcomas.
Vehicle control (EtOH) or 4-OHT or was added to Mdm2fl/flp53−/− sarcoma cells expressing CreERT2 and GFP or GFP alone. A–F) PCR Mdm2 gene rearrangement (A), proliferation (MTT assay, quadruplicate; B), cell number (C), viability (D), Annexin-V (E), and sub-G1 DNA (F) measured (C–F, triplicates). G, H) Following 36hrs of EtOH or 4-OHT addition, Western blotting (G, cleaved-PARP, cPARP) performed, and cells in G2/M (left) and phospho-histone H3 (H, triplicate) determined. B and C, *p<0.002; D, *p<0.005, **p<0.02; E, *p<0.0003; F, *p<0.02, **p<0.0067; H, *p=0.00086.
Similar to the lymphoma cells, there were more sarcoma cells in the G2/M phase of the cell cycle 36 hours after CreERT2 activation compared to cells with vehicle control (Fig. 2H; Supplementary Fig. S2). Analysis of phosphorylated histone H3 showed no difference in the percentage of cells in M phase between control and CreERT2 activated sarcomas, indicating the increase of cells in G2/M is likely due to a G2 cell cycle arrest. Therefore, when Mdm2 was deleted in either p53−/− sarcoma or lymphoma cells, growth and survival are greatly diminished by a combination of apoptosis and G2 cell cycle arrest.
Mdm2 deletion inhibits p53−/− T-cell lymphoma growth in vivo
To test whether Mdm2 deletion would impact p53-null tumor growth in vivo, we injected CreERT2 expressing Mdm2fl/flp53−/− T-cell lymphoma cells subcutaneously into nude mice. Following detection of palpable tumors at day 15 (Fig. 3A), tamoxifen or corn oil vehicle control was administered to all mice once daily for three days. Lymphomas in the vehicle control group continued to increase in volume, whereas the lymphomas in the mice administered tamoxifen regressed and were still undetectable at day 21 when the first vehicle control treated mouse was sacrificed (Fig. 3A and 3B). This reduced lymphoma tumor burden in mice receiving tamoxifen resulted in a significant increase in survival compared to mice receiving vehicle control (Fig. 3B).
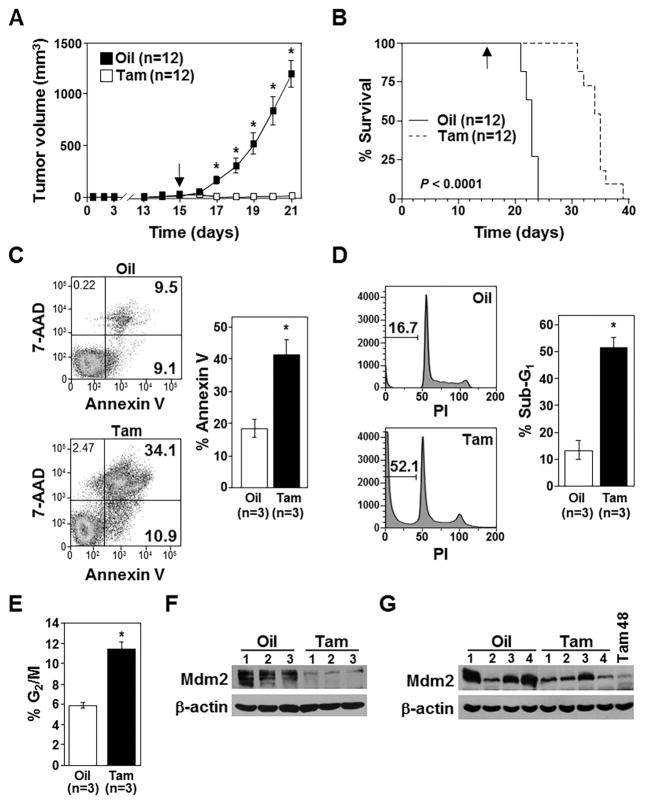
Figure 3. Loss of Mdm2 inhibits lymphoma growth in vivo.
A) Tumor volumes in mice (number indicated) injected (subcutaneously) with CreERT2 expressing Mdm2fl/flp53-null lymphoma cells and administered tamoxifen (Tam) or vehicle control (corn oil, Oil) starting day 15 were measured (*p<0.0006). B) Kaplan-Meier survival curves of the mice in A (p<0.0001). Arrow indicates the day tamoxifen or oil administration began. C–E) Annexin-V (C), sub-G1 DNA (D), and cells in G2/M (E) measured in extracted lymphoma cells 48hrs after tamoxifen or vehicle control administration to mice. For C and D, representative data, left; mean values, right. C, *p=0.006; D, *p=0.0008; E, *p=0.013. F, G) Western blots of lymphomas harvested after 48hrs (F) or at humane endpoints (G) following tamoxifen (Tam) or vehicle control (Oil) administration. For G, a lymphoma harvested 48hrs after tamoxifen administration (Tam 48) included for comparison.
In a separate cohort of mice, T-cell lymphomas were excised and evaluated 48 hours after the first injection of tamoxifen to activate CreERT2 or vehicle control. In mice that received tamoxifen, there were significantly more lymphoma cells that were Annexin-V positive (Fig. 3C), that had increased sub-G1 DNA (Fig. 3D), and that were in G2/M of the cell cycle (Fig. 3E; Supplementary Fig. S3). Only trace amounts of Mdm2 protein were detectable in the lymphomas harvested from mice 48 hours after receiving tamoxifen compared to controls (Fig. 3F). However, the lymphomas that ultimately grew in mice receiving tamoxifen in Figure 3B showed Mdm2 protein was present (Fig. 3G), indicating the tumors formed from cells that had not deleted Mdm2. Therefore, deletion of Mdm2 in p53−/− lymphoma in vivo causes an increase in cells in G2/M and apoptosis, dramatically diminishing their growth and prolonging survival of the mice.
Mdm2 deletion in p53−/− sarcomas inhibits growth in vivo
We next evaluated Mdm2 deletion in Mdm2fl/flp53−/− sarcoma cells in vivo. CreERT2 expressing Mdm2fl/flp53−/− sarcoma cells were injected subcutaneously into nude mice and tamoxifen or corn oil vehicle control was administered. Survival was significantly extended in mice administered tamoxifen to induce Mdm2 deletion (Fig. 4A), as vehicle-treated sarcoma tumors grew significantly larger more quickly than those that had deleted Mdm2 (Fig. 4B).
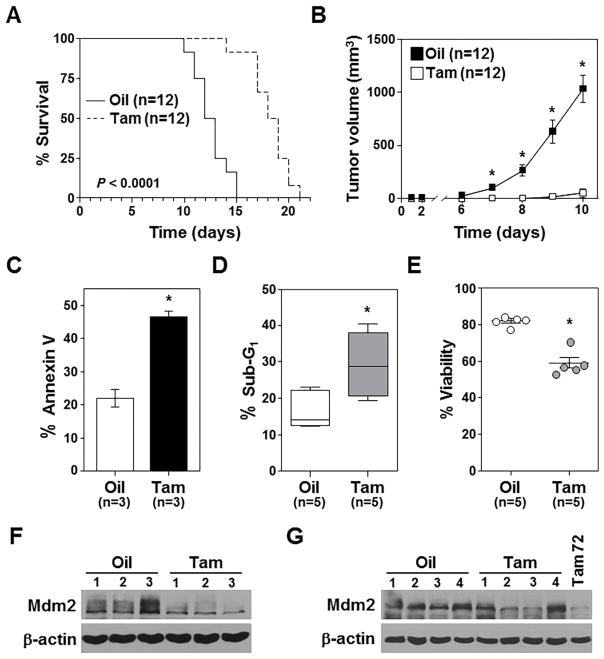
Figure 4. Mdm2 loss inhibits sarcoma growth in vivo.
A) Kaplan-Meier survival curves of mice (number indicated) injected (subcutaneously) with CreERT2 expressing Mdm2fl/flp53−/− sarcoma cells and administered tamoxifen (Tam) or vehicle (corn oil, Oil) control starting the day of cell injection (p<0.0001). B) Tumor volumes in mice in A measured at intervals (*p<0.00003). C–E) 72hrs following tamoxifen or vehicle control administration to mice, Annexin-V (C), sub-G1 DNA (D), and viability (E) were measured in extracted sarcoma cells. For D, whiskers represent the minimum and maximum, the line the median, and the box the 25th to 75th percentiles. C, *p=0.001; D, *p=0.012; E, *p<0.0001. F, G) Western blots of lymphomas harvested after 72hrs (F) or at humane endpoints (G) after tamoxifen (Tam) or vehicle control (Oil) administration. For G, a sarcoma harvested 72hrs after tamoxifen administration (Tam 72) included for comparison.
In a separate cohort of mice, 72 hours following the first administration of tamoxifen or vehicle control, sarcomas were excised and evaluated. In mice that received tamoxifen to delete Mdm2, there were significantly more sarcoma cells that were Annexin-V positive (Fig. 4C; Supplementary Fig. S4), contained sub-G1 DNA (Fig. 4D), and had decreased viability (Fig. 4E). There was little Mdm2 protein in the tumors 72 hours after tamoxifen (Fig. 4F). However, the sarcoma tumors that developed in the mice receiving tamoxifen in Figure 4A retained Mdm2 (Fig. 4G). Our data show that similar to p53-null lymphoma, loss of Mdm2 in p53−/− sarcoma cells induces apoptosis and significantly inhibits tumor cell growth in vitro and in vivo.
Mature p53-null immortalized fibroblasts also require Mdm2
Our data with hematopoietic and non-hematopoietic cancer cells indicate Mdm2 expression is required for malignant cell survival even when p53 is absent. To determine whether the effects of Mdm2 loss extended to non-cancerous cells, we evaluated deletion of Mdm2 in p53−/− adult mouse fibroblasts. Activation of CreERT2 deleted Mdm2 in Mdm2fl/flp53−/− fibroblasts resulting in reduced Mdm2 protein (Fig. 5A). Mdm2 loss significantly decreased proliferation (Fig. 5B), cell number (Fig. 5C), and viability (Fig. 5D) within 48 hours. Following 24 hours of CreERT2 activation, Annexin-V positivity and sub-G1 DNA content were significantly elevated and this increased over 72 hours (Fig. 5E and 5F, respectively). Interestingly, as with the cancer cells, the immortalized p53-null fibroblasts had increased cell numbers in G2 after Mdm2 deletion (Fig. 5G). Therefore, loss of Mdm2 results in reduced cell survival characterized by cell cycle arrest and apoptosis in both malignant and immortalized cells lacking p53.
Figure 5. Mdm2 deletion in non-transformed p53-null fibroblasts inhibits growth and survival.
Vehicle control (EtOH) or 4-OHT was added to Mdm2fl/flp53−/− fibroblasts expressing CreERT2 and GFP or GFP alone. A, B) PCR Mdm2 gene rearrangement (A, top) and Western blotting (A, bottom) performed. C–F) Proliferation (MTT assay, quadruplicate; B), cell number (C), viability (D), Annexin-V (E), and sub-G1 DNA (F) measured. G) Cells in G2/M and phospho-histone H3 evaluated 24hrs following 4-OHT or EtOH administration. C–G, triplicates. B, *p<0.009; C, *p<0.003, **p<0.0009; D, *p<0.006; E, *p<0.0004; F, *p<0.00009; G, *p=0.0042.
Mdm2 deletion activates p53/p73 transcriptional target genes, inducing apoptosis and cell cycle arrest
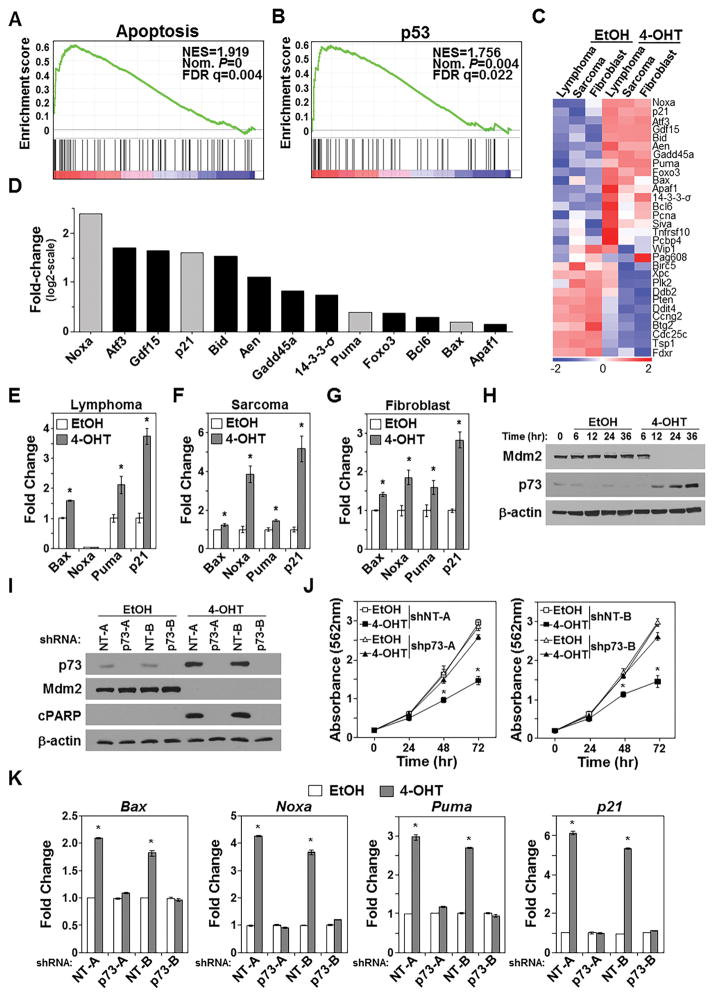
Because it was unexpected for loss of Mdm2 to kill p53-null cells, to gain insight into the mechanism responsible for the apoptosis induced by Mdm2 loss, we performed RNA-sequencing analysis following CreERT2 activation to delete Mdm2 or vehicle control to leave Mdm2 intact in all three p53-null cell types. Gene ontology analysis showed 52 of the 312 significantly upregulated genes (>2 fold) following Mdm2 deletion were linked to apoptotic processes (p=2.63×10−15). Additionally, gene set enrichment analysis showed genes linked to apoptosis (Fig. 6A; Supplementary Fig. S5), and pathway analysis of the genes significantly upregulated indicated genes involved in apoptosis (p=1.19×10−7). Unexpectedly, pathway analysis also showed genes significantly upregulated in the Mdm2-deleted p53-null cells were in the p53 pathway (p=2.62×10−8). Gene set enrichment analysis revealed p53-regulated gene sets in all three cell types after Mdm2 deletion (Fig. 6B; Supplementary Fig. S5). Although the cells were p53-null, p53 target genes showed differential expression in cells that deleted Mdm2 (Fig. 6C). Many of the upregulated genes are linked to apoptosis and cell cycle arrest (Fig. 6D). In independent experiments, qRT-PCR was used to verify the RNA-sequencing results and evaluate a subset of the upregulated p53 target genes that mediate apoptosis and cell cycle arrest that spanned the range of expression observed (gray bars in Fig. 6D). Following CreERT2 activation to delete Mdm2 in the lymphoma cells, the pro-apoptotic Bcl-2 family members, Bax and Puma, were significantly elevated, whereas Noxa, also a pro-apoptotic Bcl-2 family member remained undetectable (Fig. 6E). Levels of the cell cycle inhibitor, p21 also significantly increased (Fig. 6E). Mdm2 deletion in p53−/− sarcoma significantly elevated levels of Bax, Noxa, Puma, and p21 (Fig. 6F), and similar results were obtained from Mdm2 loss in fibroblasts (Fig. 6G). Our data show Mdm2 deletion in p53−/− lymphoma, sarcoma, and pre-cancerous fibroblasts decreased growth and survival, consistent with the upregulation of pro-apoptotic and cell cycle arrest genes known to be transcriptionally regulated by p53.
Figure 6. Mdm2 loss leads to activation of p73.
Mdm2fl/flp53−/− cells expressing CreERT2 received 4-OHT or vehicle control (EtOH). A–D) RNA-sequencing was performed after 6hrs (lymphoma) or 48hrs (sarcoma and fibroblasts) of treatment. Gene set enrichment analysis of pooled samples (A, B); normalized enrichment score (NES), nominal (Nom.) P-value, false discovery rate (FDR). Heat-map of individual samples (C) and fold-change of significantly increased p53/p73 target genes in 4-OHT-treated pooled samples relative to vehicle-treated pooled samples (D). Genes verified with qRT-PCR are in gray. E–G) qRT-PCR (triplicate) assessed specific mRNA levels. E, Bax *p=0.00008, Puma *p=0.045, p21 *p=0.0009; F, Bax *p=0.035, Noxa *p=0.001, Puma *p=0.003, p21 *p=0.0005; G, Bax *p=0.0007, Noxa *p=0.009, Puma *p=0.03, p21 *p=0.0001. H) Western blotting CreERT2 expressing Mdm2fl/flp53−/− sarcoma cells after 4-OHT or EtOH. I–K) CreERT2 expressing Mdm2fl/flp53−/− sarcoma cells expressing p73 shRNA (shp73-A or shp73-B) or non-targeting controls (shNT-A or shNT-B). Western blotting following 48hrs of 4-OHT or EtOH (I; cleaved PARP, cPARP). Proliferation (J, MTT assay, quadruplicate); *p<0.002. qRT-PCR (triplicate) for mRNA following 48hrs of 4-OHT or EtOH (K). Bax *p<0.002; Noxa *p<0.0009; Puma *p<0.0007; p21 *p<0.0002.
p73, a p53 family member, can bind Mdm2 and is capable of mediating apoptosis and cell cycle arrest by transactivating p53 target genes (18–21). Because we observed upregulation of genes transcriptionally regulated by p53 following Mdm2 deletion in cells lacking p53, we investigated whether p73 was involved in transcriptionally activating these genes. We first evaluated whether p73 levels were altered upon Mdm2 deletion. Loss of Mdm2 increased p73 protein (Fig. 6H). Next, to test whether p73 was mediating the negative effects of Mdm2 deletion, we used two different shRNA to knockdown p73 (Fig. 6I). Following CreERT2 activation to delete Mdm2 in the sarcoma cells (Fig. 6I), decreased cell growth was only observed in cells expressing the non-targeting control shRNA (Fig. 6J). In contrast, cells with p73 knockdown grew at the same rate as vehicle control cells (Fig. 6J). Additionally, after CreERT2 activation, cleaved PARP was absent in the sarcoma cells with p73 knockdown, but present in the non-targeting shRNA controls (Fig. 6I). Evaluation of Bax, Noxa, Puma, and p21 mRNA levels showed that following p73 knockdown, Mdm2 deletion did not increase these mRNA, whereas the cells with non-targeting shRNA were upregulated (Fig. 6K). Our data indicate the apoptosis and cell cycle arrest induced by loss of Mdm2 is transcriptionally mediated by p73 in cells lacking p53.
Mdm2 deletion is not the same as blocking Mdm2 interaction with p53 family members
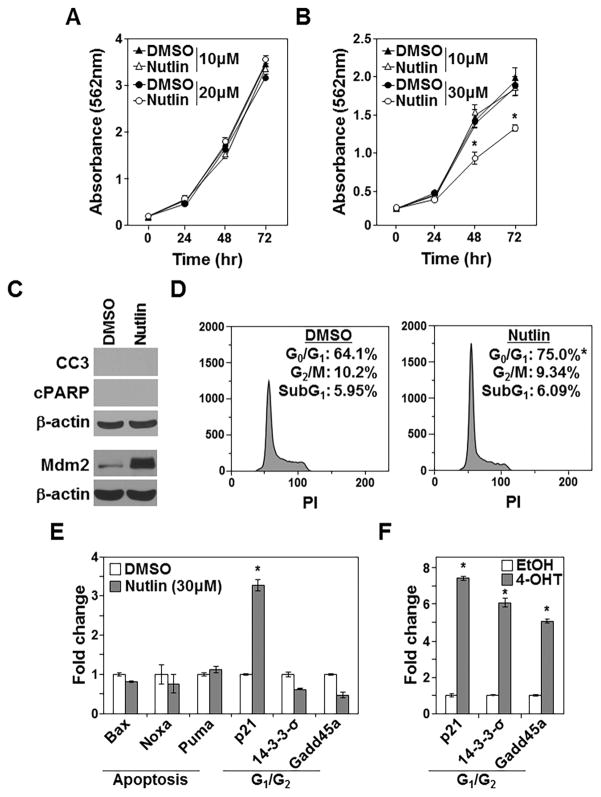
High concentrations (e.g., 30μM) of Nutlin-3 inhibit the growth and survival of p53−/− colon cancer cells, in part by activating p73 (22). We evaluated Nutlin-3 treatment of Mdm2fl/fp53−/− sarcoma cells to determine whether the effects of inhibiting Mdm2 interaction with p53 family members pharmacologically were analogous to deleting Mdm2. While neither 10μM nor 20μM of Nutlin-3 affected sarcoma proliferation over 72 hours (Fig. 7A), 30μM of Nutlin-3 significantly inhibited proliferation by 48 hours (Fig. 7B). The decrease in proliferation was not due to apoptosis, as neither cleaved Caspase 3 nor cleaved PARP were detected (Fig. 7C), and there was no change in sub-G1 DNA content after 30μM Nutlin-3 treatment (Fig. 7D). Mdm2 protein levels were increased in p53-null sarcoma cells treated with Nutlin-3 (Fig. 7C), as we and others previously reported in other cell types (11,33,34). Notably, sarcoma cells treated with 30μM Nutlin-3 showed no change in cell number in G2/M over 48 hours relative to control; however, there was a significant increase of cells in G1 of the cell cycle (Fig. 7D). Therefore, the increase of cells in G1 and not apoptosis accounted for the reduction in proliferation caused by Nutlin-3.
Figure 7. Inhibition of Mdm2 is not the same as Mdm2 deletion.
A–E) Mdm2fl/flp53−/− sarcomas treated with the indicated concentrations or 30μm of Nutlin-3 or DMSO control. A, B) Proliferation (MTT assays, quadruplicate) measured; *p<0.003. C) Western blots performed following 48hrs of treatment (same lysates run into two different gels); cleaved Caspase-3 (CC3), cleaved PARP (cPARP). D) Cell cycle and sub-G1 DNA were measured (triplicate) at 48hrs. Representative histograms with mean values inset; G0/G1, *p<0.0009; G2/M, p=0.401. E) qRT-PCR (triplicate) was performed. F) CreERT2 expressing Mdm2fl/flp53−/− sarcomas received 4-OHT or EtOH control and qRT-PCR (triplicate) was performed. E, *p<0.0003; F, *p<0.0006.
It was previously reported that p53 family target genes, Noxa, Puma, and p21 were upregulated upon high dose Nutlin-3 exposure in p53-null colon cancer cells (22). We assessed these mRNA as well as Bax following 30μM Nutlin-3 treatment in the sarcoma cells. Consistent with the increase of cells in G1 of the cell cycle and no induction of apoptosis, p21 expression was elevated 3.3-fold relative to control, but there were no significant changes in the pro-apoptotic Bcl-2 family genes Bax, Noxa, or Puma mRNA (Fig. 7E). Transcriptional targets of p53/p73 involved in G2 cell cycle arrest, including 14-3-3-σ and Gadd45a (35), also remained unchanged following addition of 30μM Nutlin-3 (Fig. 7E). In contrast, 14-3-3-σ, Gadd45a, and p21 were significantly increased following deletion of Mdm2 (Fig. 7F). Our results indicate Mdm2 deletion in p53−/− cells inhibits proliferation and induces apoptosis, which is distinct from blocking Mdm2 from binding p53 family members with Nutlin-3, leading to increased levels of Mdm2 and cell cycle arrest. Thus, loss of Mdm2 and elevated levels of Mdm2 unable to interact with p53 family members produce different intracellular responses, with loss of Mdm2 being cytotoxic in a p53-null background.
Discussion
Prior to this current study, it was thought that Mdm2 was unnecessary for survival of cells lacking p53. However, our unanticipated results that deletion of Mdm2 in p53-null cells induces apoptosis is a significant, unexpected finding with important clinical ramifications. While inhibiting Mdm2 interaction with p53 family members in p53 wild-type containing tumors is being clinically pursued (36), evidence indicates that resistance develops through p53 inactivation (13–15). Additionally, many human cancers present with p53 deletions (12). Therefore, better treatment options are needed for patients with p53-null cancers. We describe a mechanism through which Mdm2 loss in hematopoietic and non-hematopoietic malignancies lacking p53 results in activation of p73 and upregulation of its target genes that induce apoptosis and G2 cell cycle arrest, causing p53-null cancer cells to die. In vivo, when Mdm2 was deleted, p53-null lymphoma and sarcoma had dramatically reduced tumor growth, extending survival of the mice. Given this surprising requirement of Mdm2 for p53-null cancer cell survival, our results demonstrate that targeting Mdm2, leading to its reduced expression in cancer cells, is likely to be therapeutic in cancers that have deleted p53. Furthermore, analogous results were obtained in immortalized p53-null cells, suggesting sensitivity for pre-cancerous cells to Mdm2 loss as well.
Numerous studies show that when wild-type p53 is present, loss of Mdm2 induces a p53-dependent death (2,3), leading to the belief that Mdm2 is unnecessary if p53 is inactivated. Therefore, our data showing Mdm2 deletion in p53-null cells led to apoptosis was not predicted. This was not just evident in primary cell culture, as our in vivo experiments showed that while Mdm2 protein was lost initially after receiving tamoxifen to activate CreERT2 and delete Mdm2, Mdm2 was detectable at levels similar to vehicle treated controls in the tumors that ultimately emerged. Therefore, the tumors developed from a population of cells that retained Mdm2, indicating Mdm2 expression is required for the growth and survival of p53-null lymphoma and sarcoma in vivo. Consistent with this is that MDM2 is not deleted in any human cancers lacking p53 (37). We similarly demonstrated that mature fibroblasts lacking p53 are also dependent on Mdm2 for survival. These results are in contrast to an in vivo study showing induced deletion of Mdm2 in multiple tissues of p53−/− mice did not cause tissue defects or lethality of the mice (7). Our data suggest a possible explanation for the discrepancy between these studies. Both the lymphoma and sarcoma cells that survived CreERT2 activation in vivo had retained Mdm2. Therefore, we postulate in the Zhang et al. study that any cells that died from loss of Mdm2 in the p53-null mice were replaced in a timely manner with cells that contained Mdm2 so that no overt tissue damage occurred and mice survived. Another possibility is that proliferating immortalized or malignant cells may be more reliant on Mdm2 than less proliferative, non-transformed cells in vivo and therefore, be more sensitive to Mdm2 loss. Future studies are warranted to further investigate the cell types and conditions that confer sensitivity to Mdm2 loss in the absence of p53.
The p53 family member p73 shares significant homology to p53 and many of the same target genes (16,17). p73 is rarely inactivated in human cancers, in contrast with the high frequency of p53 mutation or deletion (16). Our data show that Mdm2 loss in p53-null cancer cells upregulates p53/p73 transcriptional targets that mediate cell cycle arrest (p21, Gadd45a, and 14-3-3-σ) and apoptosis (Bax, Puma, and Noxa), both of which occurred. p21 is known to exert its influence at the G1 phase of the cell cycle, but can also mediate progression through G2 (38–41). Gadd45a and 14-3-3-σ are established mediators of the p53-induced G2 checkpoint (35). The pro-apoptotic Bcl-2 family members, Bax, Puma, and Noxa, are effective at inducing apoptosis (42). Here, we show that Mdm2 loss induces apoptosis and G2 cell cycle arrest in p53-null cancer cells and that loss of p73 was sufficient to rescue these effects, indicating that p73 mediates the negative consequences of Mdm2 deletion when p53 is absent. Mdm2 can regulate p73 stability and transcription (18–21), but the conditions in which this occurs remained incompletely resolved. Most importantly, what was not known or predicted that our data revealed was that cells lacking p53 would die when Mdm2 was deleted and that this was mediated by p73. Our data indicate p73 functionally replaced p53 in p53-null cancer cells and mature fibroblasts and consequently, p73 was stabilized and activated upon Mdm2 deletion. Our results also imply that during embryogenesis, p73 does not replace p53, since Mdm2−/−p53−/− embryos survive; however, additional investigations will be needed to test Mdm2 and p73 interaction during embryogenesis.
Since the discovery of Nutlin-3, a compound that blocks Mdm2:p53 interactions, there has been increased effort examining the therapeutic utility of activating the p53 pathway in tumor cells by blocking Mdm2 binding (36). Additionally, compounds that bind the p53-binding pocket of Mdm2 can cause apoptosis and/or cell cycle arrest in p53-null cancer cells through other mechanisms, such as activation of p73 and inhibition of DNA break repair (22,33). Nutlin-3 can activate p73 in malignant cells lacking p53, but this required high concentrations of Nutlin-3 (22). Consistent with this report, we observed the standard 10μM of Nutlin-3 as well as 20μM had no effect, but 30μM induced a G1 cell cycle arrest of p53-null sarcoma cells. In contrast to blocking p53 family members from binding Mdm2, Mdm2 deletion induced a G2 arrest and apoptosis in p53-null cancer cells, indicating different mechanisms or different target genes involved. We detected differences in the target genes induced by Nutlin-3 and Mdm2 deletion that explain these different outcomes. In addition, we previously reported a side effect of Nutlin-3 binding to Mdm2 is Mdm2 stabilization, leading to increased Mdm2 levels (33), which we also observed in this study. Elevated Mdm2 levels can inhibit DNA break repair (33); thus, stabilization of Mdm2 with compounds, such as Nutlin-3, can have consequences other than p53/p73 activation that can induce different cellular responses. Our data indicate that inhibiting Mdm2 from binding p53 family members and Mdm2 deletion have distinctly different effects, but that both are deleterious for the cell. Collectively, our results demonstrate that p53-null cancer cells require Mdm2 for growth and survival, and consequently, this could be exploited therapeutically.
Supplementary Material
Acknowledgments
The authors thank Maria Pia Arrate, Robert Duszynski, and Cathy Alford for technical assistance, Dr. Gigi Lozano for fruitful discussions, Drs. Paolo Fortina and Adam Ertel for RNA-sequencing and alignment, and members of the Eischen laboratory for their feedback.
Funding: Funding provided by NCI with R01CA181204 (CME), T32CA093240 (KPF), and the cancer center support grant P30CA056036 that supports the Flow Cytometry and MetaOmics core facilities.
Footnotes
Conflict of interest: The authors declare no conflict of interest
References
- 1.Eischen CM, Lozano G. The Mdm network and its regulation of p53 activities: a rheostat of cancer risk. Hum Mutat. 2014;35:728–37. doi: 10.1002/humu.22524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montes de Oca Luna R, Wagner DS, Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature. 1995;378:203–6. doi: 10.1038/378203a0. [DOI] [PubMed] [Google Scholar]
- 3.Jones SN, Roe AE, Donehower LA, Bradley A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature. 1995;378:206–8. doi: 10.1038/378206a0. [DOI] [PubMed] [Google Scholar]
- 4.Francoz S, Froment P, Bogaerts S, De Clercq S, Maetens M, Doumont G, et al. Mdm4 and Mdm2 cooperate to inhibit p53 activity in proliferating and quiescent cells in vivo. Proc Natl Acad Sci U S A. 2006;103:3232–7. doi: 10.1073/pnas.0508476103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grier JD, Xiong S, Elizondo-Fraire AC, Parant JM, Lozano G. Tissue-specific differences of p53 inhibition by Mdm2 and Mdm4. Mol Cell Biol. 2006;26:192–8. doi: 10.1128/MCB.26.1.192-198.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kodama T, Takehara T, Hikita H, Shimizu S, Shigekawa M, Tsunematsu H, et al. Increases in p53 expression induce CTGF synthesis by mouse and human hepatocytes and result in liver fibrosis in mice. J Clin Invest. 2011;121:3343–56. doi: 10.1172/JCI44957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Xiong S, Li Q, Hu S, Tashakori M, Van Pelt C, et al. Tissue-specific and age-dependent effects of global Mdm2 loss. J Pathol. 2014;233:380–91. doi: 10.1002/path.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones SN, Sands AT, Hancock AR, Vogel H, Donehower LA, Linke SP, et al. The tumorigenic potential and cell growth characteristics of p53-deficient cells are equivalent in the presence or absence of Mdm2. Proc Natl Acad Sci U S A. 1996;93:14106–11. doi: 10.1073/pnas.93.24.14106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMasters KM, Montes de Oca Luna R, Pena JR, Lozano G. mdm2 deletion does not alter growth characteristics of p53-deficient embryo fibroblasts. Oncogene. 1996;13:1731–6. [PubMed] [Google Scholar]
- 10.Rayburn E, Zhang R, He J, Wang H. MDM2 and human malignancies: expression, clinical pathology, prognostic markers, and implications for chemotherapy. Curr Cancer Drug Targets. 2005;5:27–41. doi: 10.2174/1568009053332636. [DOI] [PubMed] [Google Scholar]
- 11.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–8. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 12.Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2010;2:a001008. doi: 10.1101/cshperspect.a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aziz MH, Shen H, Maki CG. Acquisition of p53 mutations in response to the non-genotoxic p53 activator Nutlin-3. Oncogene. 2011;30:4678–86. doi: 10.1038/onc.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michaelis M, Rothweiler F, Barth S, Cinatl J, van Rikxoort M, Loschmann N, et al. Adaptation of cancer cells from different entities to the MDM2 inhibitor nutlin-3 results in the emergence of p53-mutated multi-drug-resistant cancer cells. Cell Death Dis. 2011;2:e243. doi: 10.1038/cddis.2011.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung J, Lee JS, Dickson MA, Schwartz GK, Le Cesne A, Varga A, et al. TP53 mutations emerge with HDM2 inhibitor SAR405838 treatment in de-differentiated liposarcoma. Nat Commun. 2016;7:12609. doi: 10.1038/ncomms12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dotsch V, Bernassola F, Coutandin D, Candi E, Melino G. p63 and p73, the ancestors of p53. Cold Spring Harb Perspect Biol. 2010;2:a004887. doi: 10.1101/cshperspect.a004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pflaum J, Schlosser S, Muller M. p53 Family and Cellular Stress Responses in Cancer. Front Oncol. 2014;4:285. doi: 10.3389/fonc.2014.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ongkeko WM, Wang XQ, Siu WY, Lau AW, Yamashita K, Harris AL, et al. MDM2 and MDMX bind and stabilize the p53-related protein p73. Curr Biol. 1999;9:829–32. doi: 10.1016/s0960-9822(99)80367-4. [DOI] [PubMed] [Google Scholar]
- 19.Zeng X, Chen L, Jost CA, Maya R, Keller D, Wang X, et al. MDM2 suppresses p73 function without promoting p73 degradation. Mol Cell Biol. 1999;19:3257–66. doi: 10.1128/mcb.19.5.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu H, Leng RP. MDM2 mediates p73 ubiquitination: a new molecular mechanism for suppression of p73 function. Oncotarget. 2015;6:21479–92. doi: 10.18632/oncotarget.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou X, Hao Q, Zhang Q, Liao JM, Ke JW, Liao P, et al. Ribosomal proteins L11 and L5 activate TAp73 by overcoming MDM2 inhibition. Cell Death Differ. 2015;22:755–66. doi: 10.1038/cdd.2014.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau LM, Nugent JK, Zhao X, Irwin MS. HDM2 antagonist Nutlin-3 disrupts p73-HDM2 binding and enhances p73 function. Oncogene. 2008;27:997–1003. doi: 10.1038/sj.onc.1210707. [DOI] [PubMed] [Google Scholar]
- 23.Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237:752–7. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- 24.Adams CM, Eischen CM. Inactivation of p53 is insufficient to allow B cells and B-cell lymphomas to survive without Dicer. Cancer Res. 2014;74:3923–34. doi: 10.1158/0008-5472.CAN-13-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grieb BC, Gramling MW, Arrate MP, Chen X, Beauparlant SL, Haines DS, et al. Oncogenic protein MTBP interacts with MYC to promote tumorigenesis. Cancer Res. 2014;74:3591–602. doi: 10.1158/0008-5472.CAN-13-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alt JR, Bouska A, Fernandez MR, Cerny RL, Xiao H, Eischen CM. Mdm2 binds to Nbs1 at sites of DNA damage and regulates double strand break repair. J Biol Chem. 2005;280:18771–81. doi: 10.1074/jbc.M413387200. [DOI] [PubMed] [Google Scholar]
- 27.Grier JD, Yan W, Lozano G. Conditional allele of mdm2 which encodes a p53 inhibitor. Genesis. 2002;32:145–7. doi: 10.1002/gene.10066. [DOI] [PubMed] [Google Scholar]
- 28.Wang P, Lushnikova T, Odvody J, Greiner TC, Jones SN, Eischen CM. Elevated Mdm2 expression induces chromosomal instability and confers a survival and growth advantage to B cells. Oncogene. 2008;27:1590–8. doi: 10.1038/sj.onc.1210788. [DOI] [PubMed] [Google Scholar]
- 29.Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol. 2016;34:525–7. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- 30.Soneson C, Love MI, Robinson MD. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res. 2015;4:1521. doi: 10.12688/f1000research.7563.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–40. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–21. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 33.Carrillo AM, Hicks M, Khabele D, Eischen CM. Pharmacologically Increasing Mdm2 Inhibits DNA Repair and Cooperates with Genotoxic Agents to Kill p53-Inactivated Ovarian Cancer Cells. Mol Cancer Res. 2015;13:1197–205. doi: 10.1158/1541-7786.MCR-15-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Gilkes D, Li B, Cheng Q, Pernazza D, Lawrence H, et al. Abnormal MDMX degradation in tumor cells due to ARF deficiency. Oncogene. 2012;31:3721–32. doi: 10.1038/onc.2011.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harb Perspect Med. 2016;6:a026104. doi: 10.1101/cshperspect.a026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khoo KH, Verma CS, Lane DP. Drugging the p53 pathway: understanding the route to clinical efficacy. Nat Rev Drug Discov. 2014;13:217–36. doi: 10.1038/nrd4236. [DOI] [PubMed] [Google Scholar]
- 37.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–4. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agarwal ML, Agarwal A, Taylor WR, Stark GR. p53 controls both the G2/M and the G1 cell cycle checkpoints and mediates reversible growth arrest in human fibroblasts. Proc Natl Acad Sci U S A. 1995;92:8493–7. doi: 10.1073/pnas.92.18.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 40.Hoeferlin LA, Oleinik NV, Krupenko NI, Krupenko SA. Activation of p21-Dependent G1/G2 Arrest in the Absence of DNA Damage as an Antiapoptotic Response to Metabolic Stress. Genes Cancer. 2011;2:889–99. doi: 10.1177/1947601911432495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waldman T, Kinzler KW, Vogelstein B. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 1995;55:5187–90. [PubMed] [Google Scholar]
- 42.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.