Abstract
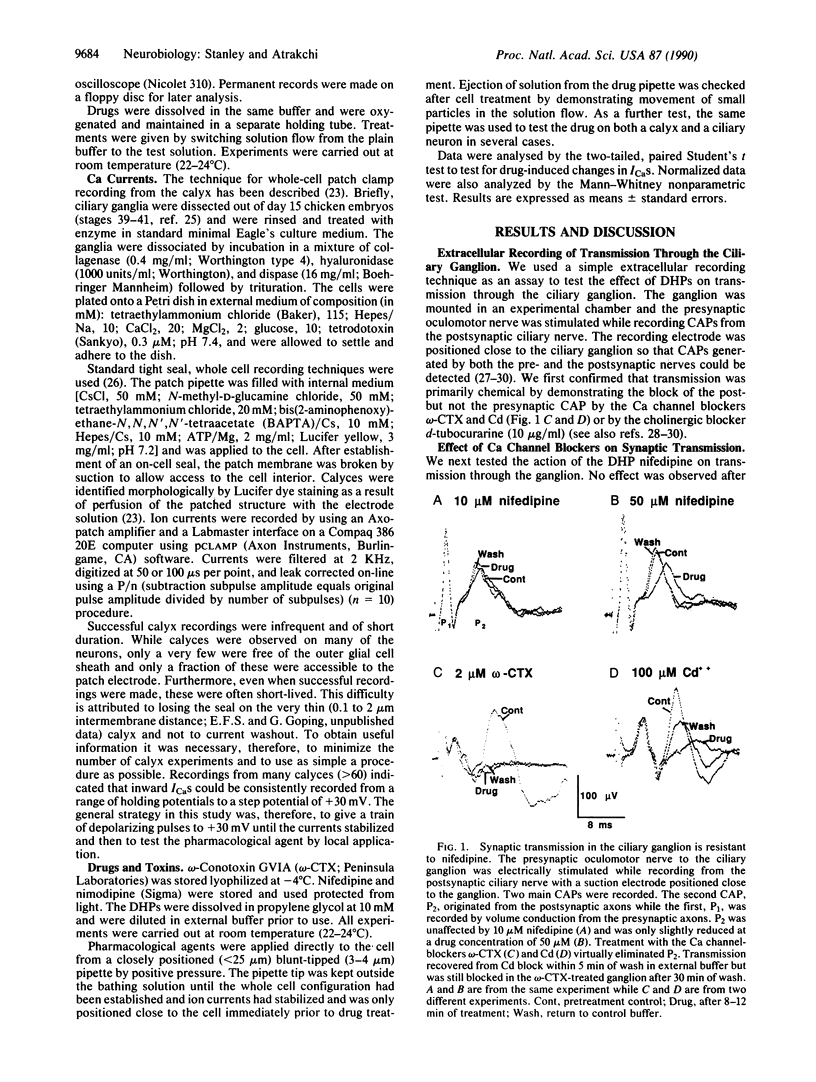
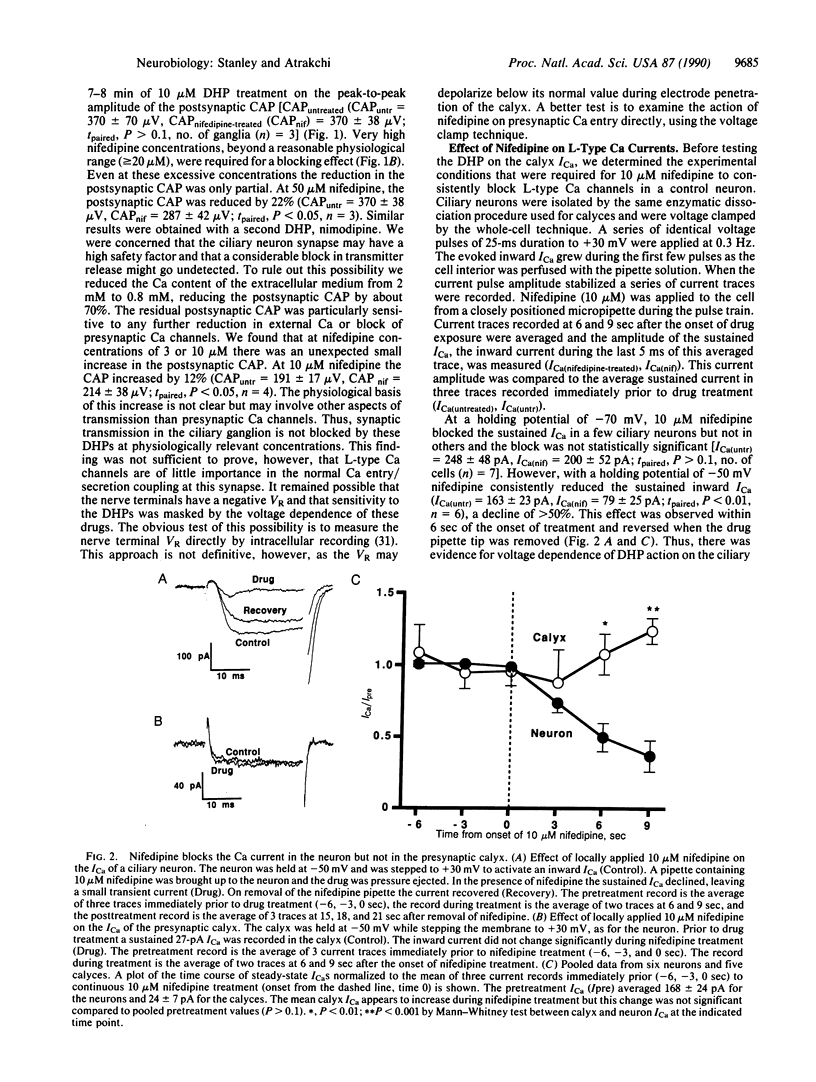
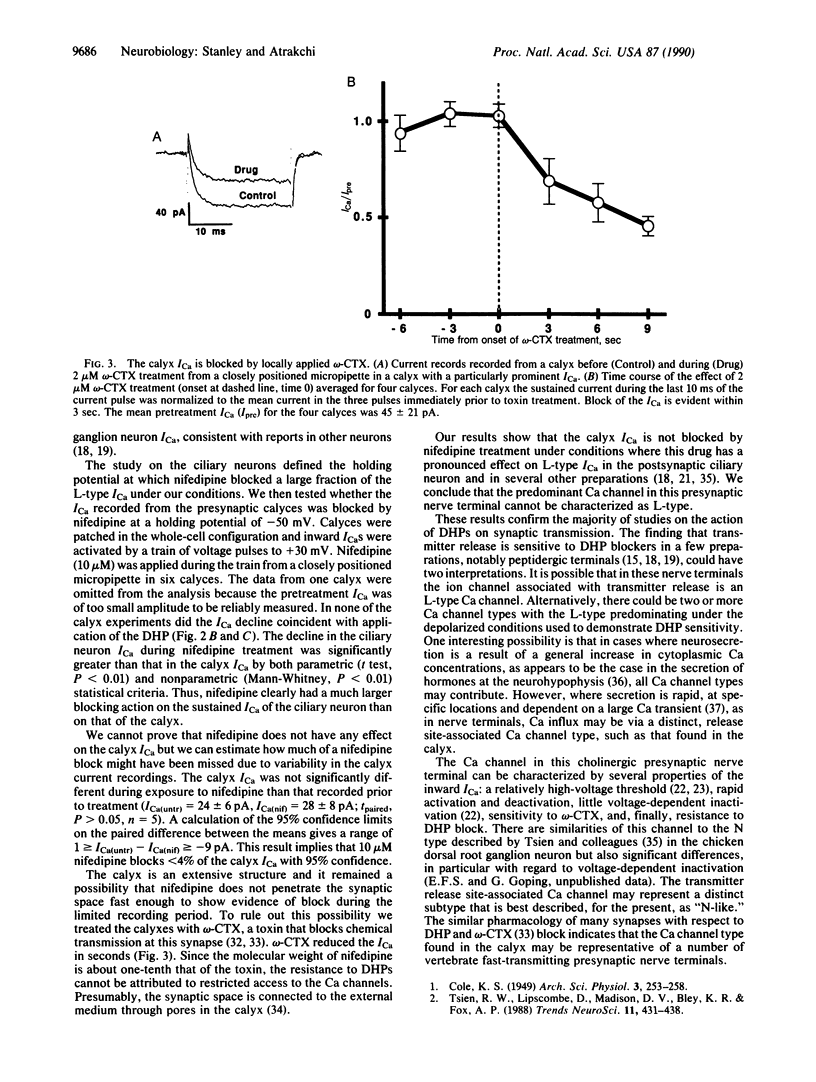
The influx of Ca ions into the presynaptic nerve terminal through ion channels is a key link between the action potential and the release of chemical transmitters. It is not clear, however, which types of Ca channel are involved in neurosecretion at vertebrate synapses. In particular, there is disagreement as to whether these channels are sensitive to dihydropyridine blockers, characteristic of L-type Ca channels. We have used the chicken ciliary ganglion calyx synapse to test the effect of the dihydropyridine nifedipine on Ca current recorded directly from a cholinergic presynaptic nerve terminal. We used a control neuron to define the experimental conditions under which L-type Ca channels are blocked by 10 microM nifedipine. We then tested the effect of the dihydropyridine on Ca currents recorded from the presynaptic terminal using the same conditions. Nifedipine did not reduce the calyx Ca current nor did it block chemical transmission through the ganglion. The lack of effect of the dihydropyridine was not due to restricted access since omega-conotoxin GVIA, a peptide toxin that blocks transmission at this synapse, rapidly blocked the calyx Ca current. Thus, the predominant Ca channel in this presynaptic nerve terminal is not dihydropyridine sensitive and, hence, cannot be characterized as L-type.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atchison W. D. Dihydropyridine-sensitive and -insensitive components of acetylcholine release from rat motor nerve terminals. J Pharmacol Exp Ther. 1989 Nov;251(2):672–678. [PubMed] [Google Scholar]
- Bean B. P. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- Daniell L. C., Barr E. M., Leslie S. W. 45Ca2+ uptake into rat whole brain synaptosomes unaltered by dihydropyridine calcium antagonists. J Neurochem. 1983 Nov;41(5):1455–1459. doi: 10.1111/j.1471-4159.1983.tb00845.x. [DOI] [PubMed] [Google Scholar]
- Dunlap K., Holz G. G., Lindgren C. A., Moore J. W. Calcium channels that regulate neurosecretion. Soc Gen Physiol Ser. 1989;44:239–250. [PubMed] [Google Scholar]
- Ebstein R. P., Daly J. W. Release of norepinephrine and dopamine from brain vesicular preparations: effects of calcium antagonists. Cell Mol Neurobiol. 1982 Sep;2(3):205–213. doi: 10.1007/BF00711148. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Nagakuro C. Three-dimensional structure of the presynaptic nerve ending in the ciliary ganglion of the chick embryo: a scanning electron microscopic study. Neurosci Lett. 1989 Mar 27;98(2):125–128. doi: 10.1016/0304-3940(89)90496-5. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hess A., Pilar G., Weakly J. N. Correlation between transmission and structure in avian ciliary ganglion synapses. J Physiol. 1969 Jun;202(2):339–354. doi: 10.1113/jphysiol.1969.sp008815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirning L. D., Fox A. P., McCleskey E. W., Olivera B. M., Thayer S. A., Miller R. J., Tsien R. W. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988 Jan 1;239(4835):57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- Holz G. G., 4th, Dunlap K., Kream R. M. Characterization of the electrically evoked release of substance P from dorsal root ganglion neurons: methods and dihydropyridine sensitivity. J Neurosci. 1988 Feb;8(2):463–471. doi: 10.1523/JNEUROSCI.08-02-00463.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L., Pilar G. The onset and development of transmission in the chick ciliary ganglion. J Physiol. 1972 May;222(3):691–713. doi: 10.1113/jphysiol.1972.sp009822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim N. F., Nowycky M. C., Bookman R. J. Direct measurement of exocytosis and calcium currents in single vertebrate nerve terminals. Nature. 1990 Mar 29;344(6265):449–451. doi: 10.1038/344449a0. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Simon S. M. Transmission by presynaptic spike-like depolarization in the squid giant synapse. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2415–2419. doi: 10.1073/pnas.79.7.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudes C., Faivre-Bauman A., Patte C., Tixier-Vidal A. Involvement of DHP voltage-sensitive calcium channels and protein kinase C in thyroliberin (TRH) release by developing hypothalamic neurons in culture. Brain Res. 1988 Jul 26;456(2):324–332. doi: 10.1016/0006-8993(88)90235-1. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. DUAL MODE OF SYNAPTIC TRANSMISSION IN THE AVIAN CILIARY GANGLION. J Physiol. 1963 Sep;168:443–463. doi: 10.1113/jphysiol.1963.sp007202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. TRANSMISSION THROUGH THE CILIARY GANGLION OF THE CHICK. J Physiol. 1963 Sep;168:464–475. doi: 10.1113/jphysiol.1963.sp007203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. R., Patel V., Faille L., Mallart A. Presynaptic calcium currents recorded from calyciform nerve terminals in the lizard ciliary ganglion. Neurosci Lett. 1989 Oct 23;105(1-2):14–18. doi: 10.1016/0304-3940(89)90004-9. [DOI] [PubMed] [Google Scholar]
- Marwitt R., Pilar G., Weakly J. N. Characterization of two ganglion cell populations in avian ciliary ganglia. Brain Res. 1971 Jan 22;25(2):317–334. doi: 10.1016/0006-8993(71)90441-0. [DOI] [PubMed] [Google Scholar]
- Middlemiss D. N., Spedding M. A functional correlate for the dihydropyridine binding site in rat brain. Nature. 1985 Mar 7;314(6006):94–96. doi: 10.1038/314094a0. [DOI] [PubMed] [Google Scholar]
- Miller R. J. Multiple calcium channels and neuronal function. Science. 1987 Jan 2;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Perney T. M., Hirning L. D., Leeman S. E., Miller R. J. Multiple calcium channels mediate neurotransmitter release from peripheral neurons. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6656–6659. doi: 10.1073/pnas.83.17.6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane S. G., Holz G. G., 4th, Dunlap K. Dihydropyridine inhibition of neuronal calcium current and substance P release. Pflugers Arch. 1987 Aug;409(4-5):361–366. doi: 10.1007/BF00583789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds I. J., Wagner J. A., Snyder S. H., Thayer S. A., Olivera B. M., Miller R. J. Brain voltage-sensitive calcium channel subtypes differentiated by omega-conotoxin fraction GVIA. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8804–8807. doi: 10.1073/pnas.83.22.8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby I. A., Kongsamut S., Freedman S. B., Miller R. J. The effects of dihydropyridines on neurotransmitter release from cultured neuronal cells. Life Sci. 1984 Sep 17;35(12):1289–1295. doi: 10.1016/0024-3205(84)90100-0. [DOI] [PubMed] [Google Scholar]
- Stanley E. F. Calcium currents in a vertebrate presynaptic nerve terminal: the chick ciliary ganglion calyx. Brain Res. 1989 Dec 29;505(2):341–345. doi: 10.1016/0006-8993(89)91465-0. [DOI] [PubMed] [Google Scholar]
- Suszkiw J. B., Murawsky M. M., Shi M. Further characterization of phasic calcium influx in rat cerebrocortical synaptosomes: inferences regarding calcium channel type(s) in nerve endings. J Neurochem. 1989 Apr;52(4):1260–1269. doi: 10.1111/j.1471-4159.1989.tb01874.x. [DOI] [PubMed] [Google Scholar]
- Suszkiw J. B., O'Leary M. E., Murawsky M. M., Wang T. Presynaptic calcium channels in rat cortical synaptosomes: fast-kinetics of phasic calcium influx, channel inactivation, and relationship to nitrendipine receptors. J Neurosci. 1986 May;6(5):1349–1357. doi: 10.1523/JNEUROSCI.06-05-01349.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W., Lipscombe D., Madison D. V., Bley K. R., Fox A. P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988 Oct;11(10):431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- Woodward J. J., Leslie S. W. Bay K 8644 stimulation of calcium entry and endogenous dopamine release in rat striatal synaptosomes antagonized by nimodipine. Brain Res. 1986 Apr 9;370(2):397–400. doi: 10.1016/0006-8993(86)90502-0. [DOI] [PubMed] [Google Scholar]
- Yoshikami D., Bagabaldo Z., Olivera B. M. The inhibitory effects of omega-conotoxins on Ca channels and synapses. Ann N Y Acad Sci. 1989;560:230–248. doi: 10.1111/j.1749-6632.1989.tb24100.x. [DOI] [PubMed] [Google Scholar]


