Abstract
Background
Better cardiovascular health is associated with lower cardiovascular disease risk.
Methods and Results
We determined the association between cardiovascular health and healthcare utilization and expenditures in the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. We included 6262 participants ≥65 years with Medicare fee‐for‐service coverage for the year after their baseline study visit in 2003‐2007. Cardiovascular health at baseline was assessed using the American Heart Association's Life's Simple 7 (LS7) metric, which includes 7 factors: cigarette smoking, physical activity, diet, body mass index, blood pressure, cholesterol, and glucose. Healthcare utilization and expenditures were ascertained using Medicare claims in the year following baseline. Overall, 17.2%, 31.1%, 29.0%, 16.4% and 6.4% of participants had 0 to 1, 2, 3, 4, and 5 to 7 ideal LS7 factors, respectively. The multivariable‐adjusted relative risk (95% confidence interval [CI]) for having any inpatient and outpatient encounters comparing participants with 5 to 7 versus 0 to 1 ideal LS7 factors were 0.55 (0.39, 0.76) and 1.00 (0.98, 1.02), respectively. Among participants with 0 to 1 and 5 to 7 ideal LS7 factors, mean inpatient expenditures were $3995 and $1250, respectively, mean outpatient expenditures were $5166 and $2853, respectively, and mean total expenditures were $9147 and $4111, respectively. After multivariable adjustment, the mean (95% CI) cost difference comparing participants with 5 to 7 versus 0 to 1 ideal LS7 factors was −$2551 (−$3667, −$1435) for inpatient, −$2410 (−$3089, −$1731) for outpatient, and −$5016 (−$6577, −$3454) for total expenditures.
Conclusions
Better cardiovascular health is associated with lower risk for inpatient encounters and lower inpatient and outpatient healthcare expenditures.
Keywords: cost, health services research, Life's Simple 7, Medicare, prevention, risk factor
Subject Categories: Cardiovascular Disease, Risk Factors, Cost-Effectiveness, Health Services, Statements and Guidelines
Introduction
Despite several decades of decline, cardiovascular disease (CVD) remains the leading cause of death and disability in the United States.1 The American Heart Association's (AHA) 2020 Strategic Goals include improving the cardiovascular health of the US population by 20% while reducing deaths from CVD and stroke by 20%.2 To assess progress in reaching this goal, the AHA developed the Life's Simple 7 (LS7) metric. LS7 is a composite measure of cardiovascular health based on cigarette smoking, physical activity, diet, body mass index (BMI), blood pressure (BP), cholesterol, and glucose.2
Most of the growth in healthcare spending over the past 2 decades has been linked to modifiable risk factors including several components of LS7.3, 4 CVD is a major contributor to healthcare utilization and expenditures, particularly among older adults.5, 6 The medical expenditures associated with CVD in the United States were estimated to be $320 billion in 2011 and are projected to increase almost 3‐fold by 2030.1 Given these projections, it is important to identify modifiable risk factors that contribute to healthcare utilization and expenditures. We hypothesized that a better cardiovascular health profile would be associated with lower rates of health service utilization and healthcare expenditures. To test this hypothesis, we analyzed the association between LS7 and healthcare utilization and expenditures using data from REasons for Geographic And Racial Differences in Stroke (REGARDS) study participants with Medicare coverage.
Methods
Study Participants and Data Collection
The REGARDS study enrolled a population‐based sample of community‐dwelling US adults to examine reasons for higher risk for stroke mortality among blacks compared with whites and residents of the southeastern United States compared with the rest of the contiguous United States.7 Overall, 30 239 black and white adults were enrolled between January 2003 and October 2007. By design, REGARDS oversampled blacks and residents from the Southeastern United States, commonly referred to as the stroke belt. REGARDS study participants’ data were linked to Medicare claims using social security number with matches confirmed using sex and date of birth.8
We restricted the current analysis to REGARDS study participants ≥65 years of age at the time of their baseline in‐home study visit who did not have electrocardiogram evidence of a previous myocardial infarction (MI) and did not self‐report a previous stroke, MI, or coronary revascularization procedure. The analyses were further restricted to participants who were alive with continuous Medicare fee‐for‐service coverage including Parts A (inpatient acute care) and B (outpatient) for at least 1 year after their baseline REGARDS study visit. Medicare is a federally administered program in the United States that provides health insurance for adults 65 years of age or older and those under 65 years who are permanently disabled or have end‐stage renal disease (ESRD).9 We restricted the analyses to participants 65 years of age or older because Medicare‐eligible adults under 65 years of age differ from the general population by socioeconomic status, medical comorbidities, and types and amounts of healthcare services utilized.10 Complete claims data are not available for beneficiaries enrolled in Medicare Advantage plans (Medicare Part C). Therefore, we excluded participants with Medicare Part C coverage at any time during the year following their baseline study visit. After these criteria were applied, 6262 participants were included in the analyses (Figure). All participants provided written informed consent, and the REGARDS study was approved by institutional review boards of all participating centers and included permission to link data with Medicare claims.
Figure 1.
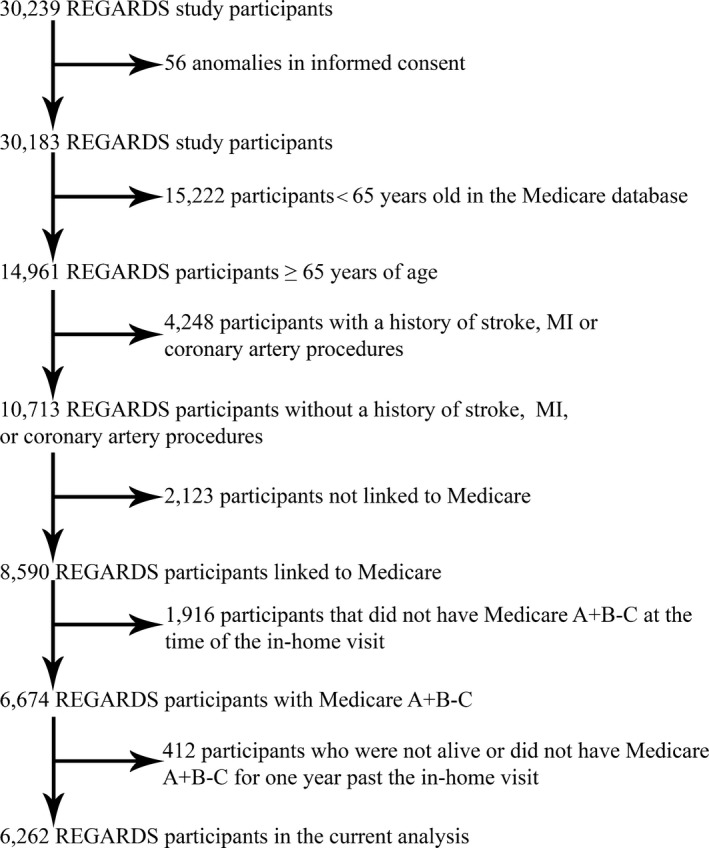
Flowchart showing the inclusion and exclusion criteria for the current analysis of Life's Simple 7 and healthcare utilization and expenditures among REGARDS study participants. BMI indicates body mass index; BP, blood pressure; MI, myocardial infarction; REGARDS, REasons for Geographic And Racial Differences in Stroke. See Xie et al8 for additional details on the linkage of REGARDS study participant data with Medicare claims.
REGARDS study data were collected at baseline through a computer‐assisted telephone interview (CATI), an in‐home examination, and self‐administered questionnaires. The CATI was conducted by trained staff and used to obtain information on demographics (age, race, sex), socioeconomic factors (household income, education, and marital status), and medical history. After the telephone interview, trained health professionals conducted an in‐home study visit that included a physical examination, medication inventory, the collection of blood and urine samples, and an electrocardiogram. Self‐administered questionnaires were left with the participants to complete and return by mail.
Life's Simple 7
Cardiovascular health at baseline was assessed using the American Heart Association's Life's Simple 7 (LS7) metric, which includes 7 factors: cigarette smoking, physical activity, diet, BMI, BP, cholesterol, and glucose. Current and former smoking status and time since smoking cessation for former smokers were assessed during the CATI. Physical activity was assessed through a single question administered during the CATI “How many times per week do you engage in intense physical activity, enough to work up a sweat?” with response options of none, 1 to 3 times per week, and 4 or more times per week. Using a self‐administered Block 98 Food Frequency Questionnaire,11 each participant recorded food intake in the year prior to his in‐home visit. Dietary analysis was conducted by NutritionQuest. Using data from the Block 98 Food Frequency Questionnaire, we defined the diet score for the LS7 based on fish, fruit, and vegetable consumption and sodium, sugar, and fiber/carbohydrate ratio intake. BMI was calculated using height and weight measured with calibrated equipment during the in‐home study visit. The average systolic and diastolic BP, based on 2 measurements taken during the in‐home study visit, was used for all analyses. Total cholesterol and serum glucose were measured by colorimetric reflectance spectrophotometry using blood samples collected during the in‐home study visit. The use of antihypertensive, glucose‐lowering, and lipid‐lowering medication was determined by self‐report during the CATI. Table 1 provides the definitions of poor, intermediate, and ideal status for each of the LS7 factors.
Table 1.
Definitions of Life's Simple 7 Poor, Intermediate, and Ideal Health Factors for Adults
| Metric | Definition | ||
|---|---|---|---|
| Poor Health | Intermediate Health | Ideal Health | |
| Smoking | Yes | Former ≤12 months | Never or quit >12 months |
| BMI | ≥30 kg/m2 | 25 to 29.9 kg/m2 | <25 kg/m2 |
| Physical activitya | None | 1 to 149 min/week moderate intensity or 1 to 74 min/week vigorous intensity or 1 to 149 min/week moderate+vigorous intensity | ≥150 min/week moderate intensity or ≥75 min/week vigorous intensity or ≥150 min/week moderate+vigorous intensity |
| Healthy diet scoreb | 0 to 1 Components | 2 to 3 Components | 4 to 5 Components |
| Total cholesterol | ≥240 mg/dL | 200 to 239 mg/dL or treated to the goal of <200 mg/dL | <200 mg/dL |
| Blood pressure | SBP ≥140 or DBP ≥90 mm Hg | SBP 120 to 139 or DBP 80 to 89 mm Hg or treated to the goal of a SBP <120 mm Hg and a DBP <80 mm Hg | SBP <120 mm Hg and DBP <80 mm Hg |
| Fasting glucose | ≥126 mg/dL | 100 to 125 mg/dL or treated to the goal of <100 mg/dL | <100 mg/dL |
BMI indicates body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Modified for the REasons for Geographic And Racial Differences in Stroke (REGARDS) Study. Participants in REGARDS were asked “How many times per week do you engage in intense physical activity, enough to work up a sweat?” We defined ideal physical activity as a frequency of 4 or more times per week, intermediate as 1 to 3 times per week, and poor as none.
Modified for REGARDS. Responses to the Block Food Frequency Questionnaire were used for the “healthy diet score” that is based on how many components of the 5 diet goals are met. Fruits and vegetables ≥4.5 cups/day; fish 3.5 ounces ≥2 servings/week; sodium <1500 mg/day; sweets/sugar‐sweetened beverages ≤450 kcal/week; whole grains (1.1 g of fiber in 10 g of carbohydrates), 1‐oz equivalent servings ≥3 servings/day.
Medicare Service Utilization and Expenditures
For the primary analysis, we used Medicare claims data for 1 year after each participant's REGARDS in‐home study visit. Acute inpatient encounters and expenditures (ie, hospital expenses) were identified using claims in Medicare inpatient files. We used claims in the Medicare outpatient and carrier files to identify outpatient encounters and expenditures. CVD‐related encounters (ie, inpatient and outpatient) and expenditures were defined as claims with the International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) primary diagnosis codes 390 to 459 and 745 to 747.1 We did not include skilled nursing facilities, home health, and hospice care expenditures, as these data have not been obtained for all REGARDS study participants. Also, we did not analyze medication expenditures because most REGARDS study participants did not have Medicare Part D prescription drug coverage at baseline, as this program did not start until 2006.
Statistical Analyses
We calculated the total number of ideal LS7 factors for each participant. Because only a small number of participants had 0 (N=77), 6 (N=48), or 7 (N=0) ideal LS7 factors, we grouped participants with 0 or 1 and, separately, 5, 6, or 7 ideal LS7 factors. Baseline characteristics were calculated by number of ideal LS7 factors. Trends in baseline characteristics across number of ideal LS7 factors were analyzed using logistic regression for binary variables and linear regression for continuous variables.
We calculated the percentage of participants with inpatient and outpatient encounters in the year following their baseline in‐home visit by number of ideal LS7 factors. Calculations were performed separately for all‐cause and CVD‐related encounters. Poisson regression models with robust standard errors were used to estimate the relative risk (RR) and 95% CI for having any inpatient and outpatient encounters, and CVD‐related inpatient and outpatient encounters, in the year following baseline associated with 2, 3, 4, and 5 to 7 versus 0 to 1 ideal LS7 factors.12 In addition to the unadjusted model, we constructed a model including adjustment for age, race, sex, education, income, and marital status. RRs were estimated for the overall population and in analyses stratified by race and, separately, by sex. P‐trends for any inpatient and outpatient encounters were calculated by modeling the number of ideal LS7 factors as an ordinal variable.
We calculated the mean all‐cause and CVD‐related inpatient, outpatient, and total (ie, inpatient plus outpatient) healthcare expenditures and 95% CI in the year following baseline among REGARDS study participants with 0 to 1, 2, 3, 4, and 5 to 7 ideal LS7 factors. Two‐part regression models were used to estimate the mean cost difference for all‐cause and CVD‐related inpatient, outpatient, and total healthcare expenditures among participants with 2, 3, 4, and 5 to 7 versus 0 to 1 ideal LS7 factors. Specifically, part 1 incorporated a logistic regression determining the participant's probability of inpatient, outpatient, or both inpatient and outpatient expenditures; and in part 2, we ran a generalized linear model with a Γ distribution and log link to account for the skewed distribution of the expenditure data.13, 14 In addition to the unadjusted model, we conducted a model including multivariable adjustment for age, race, sex, education, income, and marital status. Mean cost differences were calculated for the overall population and stratified by race and, separately, by sex. P‐trends for mean cost differences were calculated by modeling the number of ideal LS7 factors as an ordinal variable. In a sensitivity analysis, annualized all‐cause and CVD‐related inpatient, outpatient, and total healthcare expenditures were calculated using all available claims from baseline through participants’ death, loss of Medicare fee‐for‐service coverage, or December 31, 2013, whichever occurred first. Next, we estimated the reduction in inpatient, outpatient, and total expenditures if all fee‐for‐service Medicare beneficiaries ≥65 years of age had 5 to 7 ideal LS7 factors. First, we calculated inpatient and outpatient expenditures attributable to participants having 0 to 1, 2, 3, and 4 versus 5 to 7 ideal LS7 factors in the REGARDS study. For example, inpatient expenditures attributable to participants having 0 to 1 versus 5 to 7 ideal LS7 factors was calculated as the mean inpatient cost among participants with 0 to 1 ideal LS7 factors minus the mean inpatient cost among participants with 5 to 7 ideal LS7 factors, multiplied by the number of participants with 0 to 1 ideal LS7 factors. Second, we divided expenditures attributable to participants having 0 to 1, 2, 3, and 4 versus 5 to 7 ideal LS7 factors by the sum of all expenditures to calculate the attributable inpatient and outpatient cost percentage. Third, we calculated inpatient, outpatient, and total expenditures in 2014 for all Medicare beneficiaries ≥65 years of age without a history of CVD who had fee‐for‐service coverage for the entire calendar year using a 5% random sample. These costs were multiplied by 20 to estimate costs for 100% versus 5% of Medicare beneficiaries. Fourth, we multiplied 2014 Medicare inpatient and outpatient expenditures by the attributable inpatient and outpatient cost percentage from REGARDS to estimate the potential reduction in these expenditures if all fee‐for‐service beneficiaries ≥65 years of age without a history of CVD had 5 to 7 ideal LS7 factors. Potential reductions in Medicare inpatient and outpatient expenditures were summed to estimate the potential reduction in total Medicare expenditures in 2014. Bootstrapping was used to calculate 95% CIs. To account for inflation, all healthcare expenditures were adjusted to third quarter 2015 US dollars (USD) using price indices for the gross domestic product.15
All analyses were conducted using multiple imputation in order to include REGARDS participants with missing data on income (N=874), education (N=3), and LS7 factors, including BMI (N=33), physical activity (N=114), diet (N=1403), total cholesterol (N=236), blood pressure (N=15), and fasting glucose (N=1075). Multiple imputation was conducted using chained equations to obtain 15 imputed data sets for each outcome of interest, separately.16, 17 All analyses were performed in Stata 13 (Stata Corp, College Station, TX) using a 2‐sided level of significance of α<0.05.
Results
Participant Characteristics
Overall, 17.2%, 31.1%, 29.0%, 16.4%, and 6.4% of participants had 0 to 1, 2, 3, 4, and 5 to 7 ideal LS7 factors, respectively. Participants with more ideal LS7 factors were older and less likely to be women, black, have an annual income less than $20 000, less than a high school education, and be unmarried (Table 2).
Table 2.
Characteristics of REGARDS Participants ≥65 Years of Age Included in the Current Analysis by Number of Ideal Life's Simple 7 Factors
| Characteristics of Participants With Dietary Data | Number of Ideal Life's Simple 7 Factorsa | P Trendb | ||||
|---|---|---|---|---|---|---|
| 0 to 1 N=1079 (17.2%) | 2 N=1947 (31.1%) | 3 N=1814 (29.0%) | 4 N=1024 (16.4%) | 5 to 7 N=398 (6.4%) | ||
| Age, y (SE) | 71.8 (0.17) | 72.0 (0.13) | 72.6 (0.14) | 73.0 (0.20) | 72.6 (0.30) | <0.001 |
| Women | 64.2% | 57.8% | 52.3% | 51.9% | 47.7% | <0.001 |
| Black race | 44.9% | 36.2% | 29.2% | 20.9% | 12.8% | <0.001 |
| Annual income <$20 000 | 31.7% | 22.6% | 20.8% | 18.0% | 14.0% | <0.001 |
| Less than a high school education | 21.2% | 15.2% | 10.6% | 8.9% | 5.3% | <0.001 |
| Unmarried | 50.7% | 44.4% | 42.7% | 38.4% | 39.3% | <0.001 |
Values are expressed as percentage or mean (SE). The absolute number of participants in each category of Life's Simple 7 factors was calculated as the average across multiple imputations and rounded to the closest integer number. REGARDS indicates REasons for Geographic And Racial Differences in Stroke; SE, standard error.
Life's Simple 7 factors include cigarette smoking, physical activity, diet, body mass index, blood pressure, cholesterol, and glucose, and ideal levels are defined in Table 1.
P‐trends on baseline characteristics across the number of ideal Life's Simple 7 factors were calculated using logistic regression for binary variables and linear regression for continuous variables.
Health Service Utilization
Participants with more ideal LS7 factors were less likely to have all‐cause and CVD‐related inpatient encounters, and CVD‐related outpatient encounters in the year following their in‐home study visit (Table 3). The vast majority (>95%) of participants had an all‐cause outpatient encounter regardless of the number of ideal LS7 factors. After multivariable adjustment, having more ideal LS7 factors was associated with a lower risk for having all‐cause and CVD‐related inpatient encounters and CVD‐related outpatient encounters. There was no association between the number of ideal LS7 factors and having an all‐cause outpatient encounter after multivariable adjustment. Results were similar for whites and blacks and men and women analyzed separately (Table S1). For example, the RR (95% CI) for an inpatient encounter associated with 5 to 7 versus 0 to 1 LS7 factors was 0.58 (0.41, 0.83) for whites, 0.32 (0.08, 1.36) for blacks, 0.53 (0.32, 0.86) for men, and 0.57 (0.36, 0.91) for women.
Table 3.
Relative Risks and 95% Confidence Intervals for All‐Cause and Cardiovascular Disease–Related Inpatient and Outpatient Encounters Over 1 Year of Follow‐Up by Number of Ideal Life's Simple 7 Factors
| Number of Ideal Life's Simple 7 Factorsa | P Trend | |||||
|---|---|---|---|---|---|---|
| 0 to 1 (N=1079) | 2 (N=1947) | 3 (N=1814) | 4 (N=1024) | 5 to 7 (N=398) | ||
| All‐cause encounters | ||||||
| Inpatient, N (%) | 197 (18.3%) | 308 (15.8%) | 234 (12.9%) | 132 (12.9%) | 41 (10.3%) | — |
| Unadjusted, RR (95% CI) | 1.00 (Ref) | 0.86 (0.71, 1.03) | 0.70 (0.58, 0.83) | 0.69 (0.56, 0.86) | 0.55 (0.40, 0.77) | <0.001 |
| Adjusted, RR (95% CI) | 1.00 (Ref) | 0.86 (0.72, 1.03) | 0.69 (0.57, 0.82) | 0.67 (0.54, 0.83) | 0.55 (0.39, 0.76) | <0.001 |
| Outpatient, N (%) | 1045 (96.8%) | 1876 (96.4%) | 1760 (97.0%) | 982 (95.9%) | 390 (97.8%) | — |
| Unadjusted, RR (95% CI) | 1.00 (Ref) | 1.00 (0.98, 1.01) | 1.00 (0.99, 1.02) | 0.99 (0.97, 1.01) | 1.01 (0.99, 1.03) | 0.871 |
| Adjusted, RR (95% CI) | 1.00 (Ref) | 0.99 (0.98, 1.01) | 1.00 (0.98, 1.01) | 0.98 (0.96, 1.00) | 1.00 (0.98, 1.02) | 0.407 |
| CVD‐related encountersb | ||||||
| Inpatient, N (%) | 56 (5.2%) | 96 (4.9%) | 74 (4.1%) | 46 (4.5%) | 6 (1.6%) | — |
| Unadjusted, RR (95% CI) | 1.00 (Ref) | 0.94 (0.67, 1.32) | 0.78 (0.55, 1.11) | 0.85 (0.57, 1.26) | 0.29 (0.12, 0.70) | 0.007 |
| Adjusted, RR (95% CI) | 1.00 (Ref) | 0.96 (0.68, 1.35) | 0.79 (0.55, 1.13) | 0.85 (0.57, 1.28) | 0.31 (0.13, 0.73) | 0.010 |
| Outpatient, N (%) | 791 (73.3%) | 1314 (67.5%) | 1164 (64.2%) | 592 (57.8%) | 167 (41.9%) | — |
| Unadjusted, RR (95% CI) | 1.00 (Ref) | 0.92 (0.87, 0.97) | 0.87 (0.83, 0.92) | 0.79 (0.73, 0.84) | 0.57 (0.50, 0.65) | <0.001 |
| Adjusted, RR (95% CI) | 1.00 (Ref) | 0.93 (0.89, 0.98) | 0.90 (0.85, 0.94) | 0.81 (0.75, 0.87) | 0.60 (0.53, 0.68) | <0.001 |
Raw values were calculated for the unadjusted model; the estimated adjusted values were based on inclusion of age, race, sex, education, income, and marital status. The absolute number of participants and the number of participants with inpatient and outpatient encounters in each category of Life's Simple 7 factors was calculated as the average across multiple imputations and rounded to the closest integer number. CI indicates confidence interval; CVD, cardiovascular disease; RR, relative risk.
Life's Simple 7 factors include cigarette smoking, physical activity, diet, body mass index, blood pressure, cholesterol, and glucose and ideal levels are defined in Table 1.
CVD‐related healthcare encounters were defined as claims with the International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) primary diagnosis codes 390 to 459 and 745 to 747.
Healthcare Expenditures
Participants with more ideal LS7 factors had lower mean all‐cause inpatient, outpatient, and total expenditures in the year following baseline (Table 4). These associations remained present after multivariable adjustment. CVD‐related inpatient, outpatient, and total expenditures were lower among participants with more ideal LS7 factors in unadjusted and multivariable‐adjusted analyses (Table 5). Having more LS7 factors was associated with lower overall and CVD inpatient and outpatient expenditures among blacks and whites and men and women (Tables S2 and S3). For example, when participants with 5 to 7 are compared to those with 0 to 1 LS7 factors, adjusted mean cost differences (95% CI) for inpatient expenditures were −$2067 (−$3135, −$999) for whites and −$4114 (−$6382, −$1846) for blacks, −$2014 (−$3590, −$438) for men and −$2666 (−$4189, −$1143) for women.
Table 4.
Mean Cost and Cost Differences for All‐Cause Expenditures Over 1 Year of Follow‐Up by Number of Ideal Life's Simple 7 Factors
| Expenditures | Number of Ideal Life's Simple 7 Factorsa | P Trend | ||||
|---|---|---|---|---|---|---|
| 0 to 1 (N=1079) | 2 (N=1947) | 3 (N=1814) | 4 (N=1024) | 5 to 7 (N=398) | ||
| Inpatient, N (%) | 197 (18.3%) | 308 (15.8%) | 234 (12.9%) | 132 (12.9%) | 41 (10.3%) | — |
| Mean cost (95% CI) | $3995 ($2944, $5047) | $2799 ($2237, $3361) | $2402 ($1805, $2998) | $2140 ($1623, $2656) | $1250 ($760, $1740) | — |
| Unadjusted mean cost difference (95% CI) | $0 (Ref) | −$1197 (−$2302, −$91) | −$1594 (−$2702, −$486) | −$1856 (−$3001, −$711) | −$2745 (−$3894, −$1596) | <0.001 |
| Adjusted mean cost difference (95% CI) | $0 (Ref) | −$1119 (−$2152, −$87) | −$1471 (−$2518, −$424) | −$1730 (−$2840, −$621) | −$2551 (−$3667, −$1435) | <0.001 |
| Outpatient, N (%) | 1045 (96.8%) | 1876 (96.4%) | 1760 (97.0%) | 982 (95.9%) | 390 (97.8%) | — |
| Mean cost (95% CI) | $5166 ($4626, $5706) | $4310 ($3972, $4648) | $3737 ($3479, $3996) | $3652 ($3313, $3990) | $2853 ($2423, $3282) | — |
| Unadjusted mean cost difference (95% CI) | $0 (Ref) | −$856 (−$1449, −$264) | −$1428 (−$1998, −$859) | −$1514 (−$2137, −$891) | −$2313 (−$2982, −$1645) | <0.001 |
| Adjusted mean cost difference (95% CI) | $0 (Ref) | −$891 (−$1495, −$287) | −$1479 (−$2060, −$898) | −$1639 (−$2276, −$1002) | −$2410 (−$3089, −$1731) | <0.001 |
| Total, N (%) | 1045 (96.8%) | 1876 (96.4%) | 1760 (97.0%) | 982 (95.9%) | 390 (97.8%) | — |
| Mean cost (95% CI) | $9147 ($7752, $10 542) | $7117 ($6338, $7896) | $6149 ($5402, $6897) | $5771 ($5031, $6511) | $4111 ($3328, $4894) | — |
| Unadjusted mean cost difference (95% CI) | $0 (Ref) | −$2030 (−$3527, −$533) | −$2998 (−$4463, −$1532) | −$3376 (−$4896, −$1856) | −$5036 (−$6599, −$3474) | <0.001 |
| Adjusted mean cost difference (95% CI) | $0 (Ref) | −$2002 (−$3483, −$520) | −$2919 (−$4384, −$1454) | −$3504 (−$5028, −$1980) | −$5016 (−$6577, −$3454) | <0.001 |
Expenditures were adjusted to third quarter 2015 US dollars using price indices for the GDP. Adjusted mean cost differences include adjustment for age, race, sex, education, income, and marital status. The absolute number of participants and the number of participants with inpatient, outpatient, and total encounters in each category of Life's Simple 7 factors was calculated as the average across multiple imputations and rounded to the closest integer number. CI indicates confidence interval; GDP, gross domestic product; SE, standard error. Separate imputations were conducted for inpatient, outpatient and total expenditures. Therefore, inpatient and outpatient expenditures do not sum exactly to the total expenditures.
Life's Simple 7 factors include cigarette smoking, physical activity, diet, body mass index, blood pressure, cholesterol, and glucose, and ideal levels are listed in Table 1.
Table 5.
Mean Cost and Cost Differences for Cardiovascular Disease Expenditures Over 1 Year of Follow‐Up by Number of Ideal Life's Simple 7 Factors
| Expenditures | Number of Ideal Life's Simple 7 Factorsa | P Trend | ||||
|---|---|---|---|---|---|---|
| 0 to 1 (N=1079) | 2 (N=1947) | 3 (N=1814) | 4 (N=1024) | 5 to 7 (N=398) | ||
| Inpatient, N (%) | 56 (5.2%) | 96 (4.9%) | 74 (4.1%) | 46 (4.5%) | 6 (1.6%) | — |
| Mean cost (95% CI) | $988 ($640, $1337) | $890 ($632, $1148) | $688 ($467, $910) | $627 ($393, $861) | $174 ($6, $343) | — |
| Unadjusted mean cost difference (95% CI) | $0 (Ref) | −$98 (−$539, $342) | −$300 (−$717, $117) | −$362 (−$798, $75) | −$814 (−$1220, −$409) | 0.003 |
| Adjusted mean cost difference (95% CI) | $0 (Ref) | −$140 (−$583, $303) | −$349 (−$776, $79) | −$397 (−$848, $53) | −$827 (−$1253, −$400) | 0.002 |
| Outpatient, N (%) | 791 (73.3%) | 1314 (67.5%) | 1164 (64.2%) | 592 (57.8%) | 167 (41.9%) | — |
| Mean cost (95% CI) | $617 ($516, $718) | $509 ($447, $570) | $482 ($415, $549) | $398 ($332, $464) | $249 ($163, $335) | — |
| Unadjusted mean cost difference (95% CI) | $0 (Ref) | −$108 (−$226, $10) | −$135 (−$251, −$19) | −$219 (−$342, −$96) | −$368 (−$497, −$239) | <0.001 |
| Adjusted mean cost difference (95% CI) | $0 (Ref) | −$116 (−$241, $9) | −$145 (−$267, −$22) | −$238 (−$368, −$107) | −$368 (−$506, −$229) | <0.001 |
| Total,b N (%) | 791 (73.3%) | 1314 (67.5%) | 1164 (64.2%) | 592 (57.8%) | 167 (41.9%) | — |
| Mean cost (95% CI) | $1602 ($1184, $2020) | $1403 ($1102, $1703) | $1168 ($908, $1429) | $1024 ($741, $1307) | $423 ($210, $636) | — |
| Unadjusted mean cost difference (95% CI) | $0 (Ref) | −$199 (−$714, $316) | −$433 (−$922, $55) | −$578 (−$1103, −$53) | −$1179 (−$1664, −$694) | <0.001 |
| Adjusted mean cost difference (95% CI) | $0 (Ref) | −$298 (−$847, $251) | −$553 (−$1081, −$25) | −$709 (−$1271, −$147) | −$1234 (−$1771, −$697) | <0.001 |
Expenditures were adjusted to third quarter 2015 US dollars using price indices for the GDP. Adjusted mean cost differences include adjustment for age, race, sex, education, income, and marital status. The absolute number participants and the number of participants with inpatient, outpatient, and total encounters in each category of Life's Simple 7 factors was calculated as the average across multiple imputations and rounded to the closest integer number. CI indicates confidence interval; GDP, gross domestic product. Separate imputations were conducted for inpatient, outpatient and total expenditures. Therefore, inpatient and outpatient expenditures do not sum exactly to the total expenditures.
Life's Simple 7 factors include cigarette smoking, physical activity, diet, body mass index, blood pressure, cholesterol, and glucose, and ideal levels are listed in Table 1.
All participants included for calculation of total healthcare expenditures had an outpatient encounter.
Sensitivity Analysis
On the basis of all available Medicare claims after baseline (median follow‐up 6.9 years; maximum follow‐up 9.9 years), having more ideal LS7 factors was associated with lower all‐cause and CVD‐related inpatient, outpatient, and total healthcare expenditures (Tables S4 and S5). Having more ideal LS7 factors was associated with lower all‐cause and CVD‐related inpatient, outpatient, and total healthcare expenditures among blacks and whites and men and women (Tables S6 and S7).
Potential Cost Reduction Associated With Population‐Wide Achievement of 5 to 7 LS7 Factors
Inpatient, outpatient, and total expenditures for Medicare beneficiaries ≥65 years old without CVD and with fee‐for‐service coverage in 2014 Medicare were 35.9, 73.9, and 109.8 billion USD, respectively (Table S8). The percentage of these expenditures attributable to not having 5 to 7 ideal LS7 factors for inpatient, outpatient, and total annual expenditures was 53.4%, 29.7%, and 37.5%, respectively. The potential annualized cost reductions were 19.2, 22.0, and 41.2 billion USD for inpatient, outpatient, and total expenditures, respectively, if all Medicare beneficiaries had 5 to 7 LS7 factors.
Discussion
In the current study of older, community‐dwelling US adults, having a higher number of ideal LS7 factors was associated with lower risk for all‐cause and CVD‐related inpatient encounters. Having more ideal LS7 factors was not associated with all‐cause outpatient encounters but was associated with lower risk for CVD‐related outpatient encounters. Additionally, participants with more ideal LS7 factors had lower all‐cause and CVD‐related inpatient, outpatient, and total healthcare expenditures. Better cardiovascular health defined by the LS7 score was associated with lower risk for all‐cause and CVD‐related inpatient encounters, CVD‐related outpatient encounters, and lower all‐cause and CVD‐related expenditures. Extension of estimates from the REGARDS study to all Medicare beneficiaries with fee‐for‐service coverage and no previous history of CVD demonstrated that having fewer than 5 to 7 ideal LS7 factors accounted for more than half of inpatient costs and ~30% of outpatient costs. Furthermore, we estimated that the achievement of ideal levels for 5 to 7 LS7 factors for the entire Medicare population could result in a total potential annualized cost reduction of 41.2 billion USD. The potential cost reduction associated with achieving 5 to 7 ideal LS7 factors is likely to be much greater, as we only considered inpatient and outpatient expenditures and restricted this analysis to beneficiaries with Medicare fee‐for‐service for the entire 2014 calendar year.
Cardiovascular health extends the concept of CVD beyond clinically evident disease and provides a framework for primordial prevention, including population‐level and high‐risk prevention approaches. Prior studies highlight the importance of cardiovascular health for disease prevention.18, 19 For example, in the Atherosclerosis Risk in Communities study, there was a graded association with lower risk for incident CVD among participants with progressively more ideal LS7 factors. The hazard ratio for incident CVD comparing participants with 5 and 6 versus 0 ideal LS7 factors was 0.18 (95% CI 0.14‐0.23) and 0.11 (95% CI 0.07‐0.17), respectively.20 Better cardiovascular health assessed by LS7 has also been associated with lower risks for several other outcomes including mortality,21, 22, 23 ESRD,24 stroke,25 cognitive impairment,26 diabetes,27 heart failure,18 and cancer.28 The current study extends these prior findings and demonstrates lower healthcare utilization and expenditures associated with having more ideal LS7 factors in a population free of CVD.
LS7 is being used by the AHA to track the cardiovascular health of the US population. When LS7 was introduced, the AHA statistics committee estimated a low prevalence of ideal factors including 73% for smoking, 45% for physical activity, less than 1% for diet, 33% for BMI, 42% for BP, 45% for total cholesterol, and 58% for glucose.29 Additionally, in 2005‐2006, only 18% of US adults had 6 or 7 ideal LS7 factors.1 Prior studies have estimated that 70% of CVD can be averted through the prevention of risk factors, including those that comprise LS7.23 Given the low prevalence of many LS7 factors, there is a tremendous opportunity to reduce not only CVD but also healthcare utilization and expenditures through population‐wide improvements aimed at improving cardiovascular health.
The association of individual CVD risk factors with healthcare expenditures has been evaluated in prior studies.30, 31, 32, 33, 34, 35, 36 Having low CVD risk in middle age was associated with Medicare expenditures later in life among 279 men and 298 women in the Chicago Heart Association Detection Project.36 Both men and women with low CVD risk (defined by systolic BP/diastolic BP <120/80 mm Hg, serum cholesterol <200 mg/dL, not currently smoking, no electrocardiographic abnormalities, no history of diabetes, and no history of MI) had lower all‐cause and CVD expenditures compared with their counterparts with higher CVD risk. In the Framingham Heart Study the association of cigarette smoking, systolic BP, and serum cholesterol with Medicare expenditures was examined among 1053 participants who attended the Exam 17 cycle in 1984‐1985. A graded association was present between having more CVD risk factors and higher Medicare expenditures in the 2 years following the examination. These studies were conducted using risk factor data collected in the 1960s and 1980s with cost data available from the 1980s and 1990s.
A few studies have examined cardiovascular health profiles and healthcare costs and resource utilization.37, 38 In the Cooper Center Longitudinal Study, investigators evaluated the association between midlife cardiovascular health and later‐life health care costs. In that single‐center cohort, which consisted predominantly of well‐educated whites, having more ideal cardiovascular health components in middle age was associated with lower non‐CVD and CVD healthcare costs in later life.38 Valero‐Elizondo and colleagues used the 2012 Medical Expenditure Panel Survey to examine cardiovascular risk profiles and associated healthcare expenditures and resource utilization.37 In that cohort of US adults with a mean age of 58.5 years, they found that a favorable cardiovascular risk profile was associated with lower healthcare expenditures and utilization.
The current analysis was restricted to older adults, a population that accounts for a disproportionate amount of healthcare resources.39 With the population over 65 years of age in the United States projected to double over the next 25 years,40 there is an emphasis on meeting population health goals and reducing the economic impact of healthcare expenditures, particularly in the Medicare program. An array of strategies have emerged to control the growth of healthcare costs and improve quality, including public reporting, pay for performance, accountable care organizations, bundled payments, and value‐based insurance design.41, 42
Alongside these strategies, the data from the current study highlight potential economic benefits of improving cardiovascular health among older adults. Participants in the current study had similar insurance and access to health services. This demonstrates that even when access to health services is present, there are large differences in healthcare expenditures that appear to be related to behavioral and behavior‐related factors such as those that compose LS7. This finding is similar to that of Chetty and colleagues, who examined the association between income and life expectancy in the United States. They found that major explanations of differences in mortality by income were lifestyle behaviors, not health care access or environmental factors.43 Moreover, randomized controlled trials have demonstrated the benefits of multifaceted interventions (eg, smoking cessation, diet, and exercise) on improvements in risk factors and CVD risk.44, 45 Given the substantially higher healthcare utilization and costs among participants with worse cardiovascular health, the benefits of these interventions may extend to reduced healthcare utilization and costs. These results have broader implications for stakeholders to focus on population behaviors for improving health and reducing costs rather than changes in access to health services and insurance plan design.
The current study has several strengths. The REGARDS study enrolled a large sample of white and black adults from across the United States and included broad data collection allowing the assessment of cardiovascular health. Additionally, the linkage with Medicare claims allowed for the analysis of data on healthcare utilization and expenditures.8 However, the current analysis has several known and potential limitations. The current analysis was restricted to adults ≥65 years of age who live in community settings and not nursing homes. Although REGARDS participants ≥65 years have been shown to be representative of older community‐dwelling Medicare beneficiaries, the results of the current study may have limited generalizability to younger adults and nursing home residents.8 Data on diet were missing for a substantial percentage of study participants. Also, there is the possible misclassification of participants as LS7 factors were assessed on a single occasion. It is possible that better cardiovascular health is associated with other health behaviors not measured in the REGARDS study, and causal inferences should be made with caution.
In conclusion, more favorable cardiovascular health was associated with lower overall and CVD‐related inpatient encounters and CVD‐related outpatient encounters in this large national sample of black and white older adults. Additionally, better cardiovascular health was associated with lower inpatient, outpatient, and total healthcare expenditures. Improving cardiovascular health has the potential to reduce healthcare utilization and expenditures among US adults.
Sources of Funding
This research project is supported by a cooperative agreement U01‐NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Services. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis, or interpretation of the data. Additional support was provided by grants R01‐HL080477 and K24‐HL111154 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Dr Locher is supported by a National Institute on Aging, National Institutes of Health, Department of Health and Human Services grant, K07AG043588, for a Translational Nutrition and Aging Research Academic Career Leadership Award.
Disclosures
None.
Supporting information
Table S1. Adjusted Relative Risks for All‐Cause and Cardiovascular Disease–Related Inpatient and Outpatient Encounters Over 1 Year of Follow‐Up by Number of Ideal Life's Simple 7 Factors, Stratified by Race and by Sex
Table S2. Adjusted Mean Cost Differences for All‐Cause Expenditures Over 1 Year of Follow‐Up by Number of Ideal Life's Simple 7 Factors, Stratified by Race and by Sex
Table S3. Adjusted Mean Cost Differences for Cardiovascular Disease Expenditures Over 1 Year of Follow‐Up by Number of Ideal Life's Simple 7 Factors, Stratified by Race and by Sex
Table S4. Mean Annualized Cost and Annualized Cost Differences for All‐Cause Expenditures Over the Entire Follow‐Up by Number of Ideal Life's Simple 7 Factors
Table S5. Mean Annualized Cost and Cost Differences for Cardiovascular Disease Expenditures Over the Entire Follow‐Up by Number of Ideal Life's Simple 7 Factors
Table S6. Mean Annualized Cost Differences for All‐Cause Expenditures Over the Entire Follow‐Up by Number of Ideal Life's Simple 7 Factors, Stratified by Race and by Sex
Table S7. Mean Annualized Cost Differences for Cardiovascular Disease Expenditures Over the Entire Follow‐Up Period Available by Number of Ideal Life's Simple 7 Factors, Stratified by Race and by Sex
Table S8. Potential Reduction in Medicare Expenditures Associated With the Entire Population Achieving 5 to 7 Ideal Factors of the Life's Simple 7
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions and further information about the study can be found at http://www.regardsstudy.org.
(J Am Heart Assoc. 2017;6:e005106. DOI: 10.1161/JAHA.116.005106.)
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 2. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD; on behalf of the American Heart Association Strategic Planning Task Force and Statistics Committee . Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's Strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 3. O'Donnell MP, Schultz AB, Yen L. The portion of health care costs associated with lifestyle‐related modifiable health risks based on a sample of 223,461 employees in seven industries: the UM‐HMRC Study. J Occup Environ Med. 2015;57:1284–1290. [DOI] [PubMed] [Google Scholar]
- 4. Thorpe K, Allen L, Joski P. The role of chronic disease, obesity, and improved treatment and detection in accounting for the rise in healthcare spending between 1987 and 2011. Appl Health Econ Health Policy. 2015;13:381–387. [DOI] [PubMed] [Google Scholar]
- 5. Odden MC, Coxson PG, Moran A, Lightwood JM, Goldman L, Bibbins‐Domingo K. The impact of the aging population on coronary heart disease in the United States. Am J Med. 2011;124:827–833.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Azhar G, Wei JY. The demographics of aging and its impact on the cardiovascular health. Curr Cardiovasc Risk Rep. 2015;9:1–6. [Google Scholar]
- 7. Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. [DOI] [PubMed] [Google Scholar]
- 8. Xie F, Colantonio LD, Curtis JR, Safford MM, Levitan EB, Howard G, Muntner P. Linkage of a population‐based cohort with primary data collection to Medicare claims: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Am J Epidemiol. 2016;184:532–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Medicare Program—General Information: Department of Health and Human Services Centers for Medicare & Medicaid Services. Available at: http://www.cms.gov/Medicare/Medicare-General-Information/MedicareGenInfo/index.html. Accessed March 16, 2015.
- 10. Pumkam C, Probst JC, Bennett KJ, Hardin J, Xirasagar S. Health care expenditures among working‐age adults with physical disabilities: variations by disability spans. Disabil Health J. 2013;6:287–296. [DOI] [PubMed] [Google Scholar]
- 11. Block G, Woods M, Potosky A, Clifford C. Validation of a self‐administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–1335. [DOI] [PubMed] [Google Scholar]
- 12. Zhao K. Proper estimation of relative risk using PROC GENMOD in population studies. Paper presented at: Western Users of SAS Software 2013; Las Vegas, NV. [Google Scholar]
- 13. Mullahy J. Econometric modeling of health care costs and expenditures: a survey of analytical issues and related policy considerations. Med Care. 2009;47:S104–S108. [DOI] [PubMed] [Google Scholar]
- 14. Diehr P, Yanez D, Ash A, Hornbrook M, Lin DY. Methods for analyzing health care utilization and costs. Annu Rev Public Health. 1999;20:125–144. [DOI] [PubMed] [Google Scholar]
- 15. U.S. Bureau of Economic Analysis, Table 1.1.4. Price indexes for gross domestic product. Available at: http://www.bea.gov. Accessed January 11, 2016.
- 16. Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 18. Shah AM, Claggett B, Folsom AR, Lutsey PL, Ballantyne CM, Heiss G, Solomon SD. Ideal cardiovascular health during adult life and cardiovascular structure and function among the elderly. Circulation. 2015;132:1979–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Folsom AR, Shah AM, Lutsey PL, Roetker NS, Alonso A, Avery CL, Miedema MD, Konety S, Chang PP, Solomon SD. American Heart Association's Life's Simple 7: avoiding heart failure and preserving cardiac structure and function. Am J Med. 2015;128:970–976.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD; ARIC Study Investigators . Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin MP, Ovbiagele B, Markovic D, Towfighi A. “Life's Simple 7” and long‐term mortality after stroke. J Am Heart Assoc. 2015;4:e001470 doi: 10.1161/jaha.114.001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ford ES, Greenlund KJ, Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation. 2012;125:987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB. Trends in cardiovascular health metrics and associations with all‐cause and CVD mortality among US adults. JAMA. 2012;307:1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muntner P, Judd SE, Gao L, Gutiérrez OM, Rizk DV, McClellan W, Cushman M, Warnock DG. Cardiovascular risk factors in CKD associate with both ESRD and mortality. J Am Soc Nephrol. 2013;24:1159–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kulshreshtha A, Vaccarino V, Judd SE, Howard VJ, McClellan WM, Muntner P, Hong Y, Safford MM, Goyal A, Cushman M. Life's Simple 7 and risk of incident stroke: the Reasons for Geographic and Racial Differences in Stroke study. Stroke. 2013;44:1909–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thacker EL, Gillett SR, Wadley VG, Unverzagt FW, Judd SE, McClure LA, Howard VJ, Cushman M. The American Heart Association Life's Simple 7 and incident cognitive impairment: the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. J Am Heart Assoc. 2014;3:e000635 doi: 10.1161/JAHA.113.000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fretts AM, Howard BV, McKnight B, Duncan GE, Beresford SA, Mete M, Zhang Y, Siscovick DS. Life's Simple 7 and incidence of diabetes among American Indians: the Strong Heart Family Study. Diabetes Care. 2014;37:2240–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rasmussen‐Torvik LJ, Shay CM, Abramson JG, Friedrich CA, Nettleton JA, Prizment AE, Folsom AR. Ideal cardiovascular health is inversely associated with incident cancer: the Atherosclerosis Risk in Communities study. Circulation. 2013;127:1270–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lloyd‐Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel‐Smoller S, Wong N, Wylie‐Rosett J, Hong Y; on behalf of the American Heart Association Statistics Committee Stroke Statistics Subcommittee . Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. [DOI] [PubMed] [Google Scholar]
- 30. Hill RK, Thompson JW, Shaw JL, Pinidiya SD, Card‐Higginson P. Self‐reported health risks linked to health plan cost and age group. Am J Prev Med. 2009;36:468–474. [DOI] [PubMed] [Google Scholar]
- 31. Goetzel RZ, Anderson DR, Whitmer RW, Ozminkowski RJ, Dunn RL, Wasserman J; Health Enhancement Research Organization (HERO) Research Committee . The relationship between modifiable health risks and health care expenditures. An analysis of the multi‐employer HERO health risk and cost database. J Occup Environ Med. 1998;40:843–854. [DOI] [PubMed] [Google Scholar]
- 32. Bertera RL. The effects of behavioral risks on absenteeism and health‐care costs in the workplace. J Occup Med. 1991;33:1119–1124. [DOI] [PubMed] [Google Scholar]
- 33. Pronk NP, Goodman MJ, O'Connor PJ, Martinson BC. Relationship between modifiable health risks and short‐term health care charges. JAMA. 1999;282:2235. [DOI] [PubMed] [Google Scholar]
- 34. Goetzel RZ, Pei X, Tabrizi MJ, Henke RM, Kowlessar N, Nelson CF, Metz RD. Ten modifiable health risk factors are linked to more than one‐fifth of employer‐employee health care spending. Health Aff. 2012;31:2474–2484. [DOI] [PubMed] [Google Scholar]
- 35. Leigh JP, Fries JF. Health habits, health care use and costs in a sample of retirees. Inquiry. 1992;29:44–54. [PubMed] [Google Scholar]
- 36. Daviglus ML, Liu K, Greenland P, Dyer AR, Garside DB, Manheim L, Lowe LP, Rodin M, Lubitz J, Stamler J. Benefit of a favorable cardiovascular risk‐factor profile in middle age with respect to Medicare costs. N Engl J Med. 1998;339:1122–1129. [DOI] [PubMed] [Google Scholar]
- 37. Valero‐Elizondo J, Salami JA, Ogunmoroti O, Osondu CU, Aneni EC, Malik R, Spatz ES, Rana JS, Virani SS, Blankstein R. Favorable cardiovascular risk profile is associated with lower healthcare costs and resource utilization: the 2012 Medical Expenditure Panel Survey. Circ Cardiovasc Qual Outcomes. 2016;9:143–153. [DOI] [PubMed] [Google Scholar]
- 38. Willis BL, DeFina LF, Bachmann JM, Franzini L, Shay CM, Gao A, Leonard D, Berry JD. Association of ideal cardiovascular health and long‐term healthcare costs. Am J Prev Med. 2015;49:678–685. [DOI] [PubMed] [Google Scholar]
- 39. The High Concentration of U.S. Health Care Expenditures: Research in Action, Issue 19. Rockville, MD: Agency for Healthcare Research and Quality; June 2006. Available at: http://archive.ahrq.gov/research/findings/factsheets/costs/expriach/index.html. Accessed March 10, 2016. [Google Scholar]
- 40. Centers for Disease Control and Prevention . The State of Aging and Health in America. Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2013. Available at: http://www.cdc.gov/aging/help/dph-aging/state-aging-health.html. Accessed March 5, 2016. [Google Scholar]
- 41. Joynt KE. Health policy and cardiovascular medicine: rapid changes, immense opportunities. Circulation. 2015;131:1098–1105. [DOI] [PubMed] [Google Scholar]
- 42. Huang X, Rosenthal MB. Overuse of cardiovascular services: evidence, causes, and opportunities for reform. Circulation. 2015;132:205–214. [DOI] [PubMed] [Google Scholar]
- 43. Chetty R, Stepner M, Abraham S, Lin S, Scuderi B, Turner N, Bergeron A, Cutler D. The association between income and life expectancy in the United States, 2001‐2014. JAMA. 2016;315:1750–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maruthur NM, Wang N‐Y, Appel LJ. Lifestyle interventions reduce coronary heart disease risk: results from the PREMIER Trial. Circulation. 2009;119:2026–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stamler J, Neaton JD, Cohen JD, Cutler J, Eberly L, Grandits G, Kuller LH, Ockene J, Prineas R; MRFIT Research Group . Multiple Risk Factor Intervention Trial revisited: a new perspective based on nonfatal and fatal composite endpoints, coronary and cardiovascular, during the trial. J Am Heart Assoc. 2012;1:e003640 doi: 10.1161/JAHA.112.003640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Adjusted Relative Risks for All‐Cause and Cardiovascular Disease–Related Inpatient and Outpatient Encounters Over 1 Year of Follow‐Up by Number of Ideal Life's Simple 7 Factors, Stratified by Race and by Sex
Table S2. Adjusted Mean Cost Differences for All‐Cause Expenditures Over 1 Year of Follow‐Up by Number of Ideal Life's Simple 7 Factors, Stratified by Race and by Sex
Table S3. Adjusted Mean Cost Differences for Cardiovascular Disease Expenditures Over 1 Year of Follow‐Up by Number of Ideal Life's Simple 7 Factors, Stratified by Race and by Sex
Table S4. Mean Annualized Cost and Annualized Cost Differences for All‐Cause Expenditures Over the Entire Follow‐Up by Number of Ideal Life's Simple 7 Factors
Table S5. Mean Annualized Cost and Cost Differences for Cardiovascular Disease Expenditures Over the Entire Follow‐Up by Number of Ideal Life's Simple 7 Factors
Table S6. Mean Annualized Cost Differences for All‐Cause Expenditures Over the Entire Follow‐Up by Number of Ideal Life's Simple 7 Factors, Stratified by Race and by Sex
Table S7. Mean Annualized Cost Differences for Cardiovascular Disease Expenditures Over the Entire Follow‐Up Period Available by Number of Ideal Life's Simple 7 Factors, Stratified by Race and by Sex
Table S8. Potential Reduction in Medicare Expenditures Associated With the Entire Population Achieving 5 to 7 Ideal Factors of the Life's Simple 7


