Abstract
PURPOSE OF REVIEW
Scavenger receptor BI (SR-BI) is classically known for its role in anti-atherogenic reverse cholesterol transport as it selectively takes up cholesterol esters from high-density lipoprotein (HDL). Here we have highlighted recent literature that describes novel functions for SR-BI in physiology and disease.
RECENT FINDINGS
A large population based study has revealed that subjects heterozygous for the P376L mutant form of SR-BI showed significantly increased levels of plasma HDL-cholesterol and had increased risk of cardiovascular disease, demonstrating that SR-BI in humans is a significant determinant of cardiovascular disease. Furthermore, SR-BI has been shown to modulate the susceptibility to LPS-induced tissue injury and the ability of sphingosine 1 phosphate to interact with its receptor, linking SR-BI to the regulation of inflammation. In addition, important domains within the molecule (Trp-415) as well as novel regulators (PCEP2) of SR-BI’s selective uptake function have recently been identified. Moreover, relatively high expression levels of the SR-BI protein have been observed in a variety of cancer tissues which is associated with a reduced overall survival rate.
SUMMARY
The HDL receptor SR-BI is a potential therapeutic target not only in the cardiovascular disease setting, but also in inflammatory conditions as well as in cancer.
Keywords: SR-BI, high-density lipoprotein, cardiovascular disease, inflammation, cancer
INTRODUCTION
Shortly after the discovery of the SCARBI gene encoding the scavenger receptor type BI or SR-BI in 1994 [1], Acton et al. published a study demonstrating the ability of SR-BI to selectively remove cholesterol esters from high-density lipoprotein (HDL) [2], finally identifying the cholesterol ester receptor previously characterized in 1983 [3]. Subsequently, it was reported, paradoxically, that deletion of SR-BI in mice was associated with increased atherosclerosis despite a significant increase in HDL-cholesterol concentrations [4,5]. The increased atherosclerosis susceptibility observed in total body SR-BI knockout mice is paralleled by marked changes in lymphocyte homeostasis as evidenced by splenomegaly, exacerbated lymphocyte proliferation and systemic autoimmunity [6]. The increased atherosclerosis susceptibility in the context of high plasma HDL-cholesterol levels in SR-BI knockout mice contrasted the epidemiological data in humans showing that an inverse correlation between plasma HDL-cholesterol concentration and coronary heart disease was a general and reliable prognostic tool [4]. Further illuminating the complex relationship between HDL, SR-BI and atherosclerosis, a study comparing two lines of transgenic mice expressing high or moderate levels of SR-BI was reported. In this study, high levels of SR-BI was found to be nearly as detrimental as no SR-BI with barely detectable HDL levels in plasma [7]. Although fascinating, these finding were mostly classified as rodent specific effects with scant evidence of human relevance. Finally, genetic studies of human SR-BI or SCARB1 variants were reported to be associated with plasma HDL-cholesterol levels [8] as well as with protein levels in peripheral cells [9], but still no evidence that impaired SR-BI function affected human physiology. In 2011, an elegant report [10] showed that human carriers of mutated SR-BI had increased HDL-cholesterol levels, reduced cholesterol efflux, altered platelet function and decreased adrenal steroidogenesis, but still no significant increase in atherosclerosis. Finally, in 2016, a large population based study revealed that subjects heterozygous for the P376L mutant form of SR-BI showed significantly increased levels of plasma HDL-cholesterol and had increased risk of cardiovascular disease [11**], now demonstrating that SR-BI in humans was a significant determinant of cardiovascular disease. In further support, the rs10846744 SNP which resides within the enhancer region of SCARBI was recently shown to be significantly associated with atherosclerotic disease [12]. In light of these new findings, some 23 years since SR-BI’s discovery, it appears now that SR-BI has been “re-discovered” as relevant to human disease in a variety of new pathways and mechanisms, as will be covered in this review.
SR-BI: Relevance to Inflammatory States
SR-BI’s role in inflammation has been well studied and now also shown to be relevant in humans [12,13]. Using RNA-sequencing data, lymphocyte activating 3 (LAG3) RNA was identified as a major target driving the effect of SR-BI on inflammation, since its expression was significantly lower in homozygous carriers of the SR-BI rs10846744 risk allele [12]. LAG3 competes with CD4 in T cells by binding higher affinity MHC class II to negatively regulate T cell activation. LAG3 protein is one of the few important immune checkpoint inhibitors in humans, including programmed death-1 (PD-1) and cytotoxic T-lymphocyte associated protein 4 (CTLA4), highlighting that T cell activation, SR-BI and HDL-cholesterol concentrations are physiologically linked to each other and cardiovascular disease. In mice, SR-BI’s role in regulating lymphocyte activation was demonstrated recently at the level of hematopoietic stem/progenitor cell proliferation [14].
SR-BI interacts with a number of ligands, one of the most important is lipopolysaccharide [15]. Under experimental conditions using global SR-BI knockouts, lipopolysaccharide can induce acute organ damage. In a recent report [16*], a gain of function mouse model was created to avoid changes in glucocorticoid levels associated with this model. In this study pLiv-11 expression vector was used to generate high liver levels of SR-BI expression. Both human SR-BI or SR-BII were overexpressed and the authors concluded that SR-BII and to a lesser extent SR-BI increased lipopolysaccharide-induced inflammation, contributing to lipopolysaccharide-induced tissue injury in liver and kidney.
How SR-BI levels are linked to inflammatory conditions was recently investigated by 2 independent articles. In one, increased levels of T cell heat shock protein 65 was shown to upregulate Src family kinase lymphocyte-specific protein tyrosine kinase (Lck) levels, while coordinately reducing SR-BI and cholesterol efflux in CD4 T cells in vitro [17]. In another study, 3T3 adipocytes incubated with oxidized LDL showed a large increase in SR-BI protein expression, in the presence of HDL but not in its absence [18]. These authors concluded that upregulation of SR-BI prevented oxidized LDL induced endoplasmic reticulum stress and adipocyte inflammation. Insights into how SR-BI might influence inflammation was further shown by an in vitro study in which the interaction between SR-BI and the sphingosine 1 phosphate (S1P) receptor 1 was demonstrated [19**]. In these studies recombinant HDL containing S1P stimulated S1P receptor 1 internalization and intracellular calcium flux. Since plasma HDL acts as a prominent carrier of S1P these studies have far reaching implications linking SR-BI, cholesterol and the regulation of inflammation. Furthermore, it is highly likely given SR-BI’s presence in cholesterol enriched lipid rafts or microdomains, that the ability of SR-BI to mediate bi-directional cholesterol transport underlies its unique role in regulating cholesterol metabolism in inflammatory states [20**].
SR-BI: Bi-directional Cholesterol Transport in Adipocytes - Role of Binding Partners
Inflammation can be initiated by different mechanism in different tissues, although one emerging area is the study of SR-BI in adipose tissue. In the landmark study which first described SR-BI, its expression levels in differentiated 3T3 adipocytes was noted to be very high and increased with length of differentiation [1]. Since then only a handful of investigators have studied the role of SR-BI in adipose, or differentiated 3T3 cells cultured in vitro [21–23], acting as a surrogate for adipocytes. Adipose tissue harbors a large depot of free cholesterol [24*]. However, little is known as to how adipose cholesterol influx and efflux is regulated. Since adipose inflammation is a hallmark of central obesity and type 2 diabetes, loss of adipocyte efflux may directly contribute to inflammation. One important study specifically examined the bi-directional transport of cholesterol in differentiated 3T3 cells in vitro and concluded that cholesterol was mainly mobilized by ATP-binding cassette transport A1 (ABCA1) and SR-BI but not ATP-binding cassette transporter G1 (ABCG1) and that overall cholesterol mobilization was impaired during inflammatory states [25]. The precise role of SR-BI in adipose will require further investigation, but it is possible that due to structural considerations of SR-BI [26**] the receptor requires other molecules for support of bi-directional cholesterol transport. Recently, in vivo studies using genetically engineered mice by Pollard et al. [27**] have suggested that the extracellular matrix protein procollagen C-endopeptidase enhancer protein 2 (PCPE2) indirectly or directly interacts with SR-BI enhancing cholesterol transport function [28].
SR-BI: Biomarker of and therapeutic target in the pathogenesis of cancer
The primary cholesterol metabolite 27-hydroxycholesterol is essential to facilitate the growth of estrogen receptor-positive breast cancers in mice [29]. In support of an important contribution of 27-hydroxycholesterol to breast cancer pathology, high protein levels of the 27-hydroxycholesterol generating enzyme sterol 27-hydroxylase (CYP27A1) within tumors correlate with an increased severity of the disease in humans [29]. Although an effect on tissue 27-hydroxycholesterol levels remains to be investigated in SR-BI knockout mice, it appears that the delivery of 27-hydroxycholesterol to tissues does seem to depend highly on the presence of SR-BI since these mice accumulate 27-hydroxycholesterol in their plasma compartment to an extent that cannot be explained by the general increase in total cholesterol levels only [30]. Theoretically, tumors may also acquire the cholesterol substrate that is needed to synthesize 27-hydroxycholesterol through SR-BI-mediated cholesterol ester uptake, thereby facilitating tumor growth. As such, one can envision that a change in cellular SR-BI expression and/or function may affect the susceptibility to the development of cancer. Interestingly, in the past year several papers have appeared that show the importance of SR-BI in cancer pathology. Yuan et al. detected, using immunohistochemistry, an extensive protein expression level of SR-BI in >90% of breast cancer tissue samples, whilst normal breast tissue is virtually free of SR-BI protein [31**]. In accordance with an overexpression of SR-BI in breast cancer tissue, Li et al. measured a relatively high protein expression using Western blotting in invasive ductal carcinomas and, to a minor extent, in ductal carcinoma in situ specimens [32**]. The relative expression level of SR-BI in cancerous tissue correlates significantly with pathological measures. In the study by Yuan et al., high SR-BI expression was predominantly present in larger breast tumors that were estrogen receptor negative [31**]. Li et al. did also observe an increased SR-BI expression primarily in larger tumors [32**]. In contrast, in the study by Li et al., SR-BI overexpression tended to be more prominent in estrogen receptor positive tissue [32**]. Although multiple studies have suggested that SR-BI expression can be modified by estrogen exposure [33–35], a change in estrogen receptor expression thus does not seem the underlying regulatory mechanism driving the SR-BI overexpression in aggressive tumors. Importantly, the survival rate of female subjects with SR-BIhigh tumors was markedly lower than that of women that displayed a SR-BIlow breast cancer phenotype in both studies [31**,32**]. The carcinogenic function of SR-BI does not appear to be limited to breast cancer as a similar relations between SR-BI expression, disease phenotype, and disease-free survival rates have been observed in a clinical prostate cancer cohort [36]. Of note, SR-BI expression levels in metastasizing tumors are higher than those in primary prostate cancer tissues [36]. Given that metastases are the primary cause of death in cancer patients, this latter finding can possibly explain the relative high mortality rate observed in subjects carrying SR-BIhigh tumors. Furthermore, reducing SR-BI expression and/or function in tumors can potentially serve as novel therapeutic approach to overcome metastasis in breast cancer and prostate cancer patients.
When considering inhibition of SR-BI function as novel treatment option for breast and prostate cancer, it is important to acknowledge that the search for inhibitors of SR-BI function is already ongoing, since SR-BI inhibitors have long been considered of high therapeutic interest in the protection against infection with hepatitis C viruses [37]. In this context, the recent high-throughput screening efforts from the research group of Dr. Monty Krieger clearly deserve attention. These identified three new SR-BI inhibitors, indolinyl-thiazole 17-11 (ML278), bisamide tetrazole 5e (ML279) and benzo-fused lactam 2p (ML312) [38–40], that (1) effectively block the uptake of cholesterol esters from HDL, (2) are not toxic to cells in culture, and (3) show an improved solubility in water as compared to the established SR-BI function inhibitors BLT-1 and BLT-3 that were originally also discovered by the Krieger group [41]. Application of ML278, ML279 and ML312 in mouse models for breast and prostate cancer may aid in showing the actual potential of SR-BI as therapeutic target for the treatment of these respective pathologies in humans.
Although SR-BI may thus play a novel role in the pathogenesis of cancer, it is good to acknowledge that SR-BI is predominantly of high therapeutic interest to the cancer field due to its potential to improve site-specific delivery of anti-cancer drugs. More specifically, SR-BI located on tumor cells can efficiently take up nanoparticles carrying agents that affect the angiogenic potential of tumors. Ouyang et al. [42] showed that reconstituted HDL (rHDL) particles loaded with a p53 gene plasmid and the angiotensin II type 1 receptor blocker candesartan were able to reduce the expression of vascular endothelial growth factor (VEGF) in MBT-2 murine bladder cells in vitro. Importantly, in vivo treatment with these HDL-like nanoparticles virtually eliminated the growth potential of MBT-2-based tumors in mice [42]. Similarly, rHDL-mediated delivery of small interfering RNAs targeting VEGF could diminish the growth of breast tumors in BALB/c nude mice, resulting in a significantly increased overall survival [43]. Furthermore, incorporation of the anti-angiogenic drug gambogic acid in apolipoprotein A1-containing HDL-like nanoparticles enhanced the cytotoxic potential of the drug in HepG2 hepatoma cells in vitro and improved the ability to suppress tumor growth in vivo [44]. It is noteworthy that the absence of off-target effects of the gambogic acid-loaded particles on all major organs and the associated improved survival observed by Ding et al. [44] seems to be related to the EPR effect, e.g. the unique anatomical–pathophysiological nature of tumor blood vessels that facilitates transport of macromolecules into tumor tissues [45]. A recent pre-clinical study by Rui et al. has further highlighted the application potential of rHDL in the context of metastatic breast cancer [46*]. Packaging of the widely used chemotherapeutics paclitaxel and doxorubicin in rHDL particles improved their antitumor efficiency whilst overcoming common side effects on heart and kidneys of established paclitaxel / doxorubicin combination treatment.
CONCLUSION
The therapeutic potential of cardiovascular disease interventions that impact on HDL metabolism has recently been questioned in light of the fact that therapies aimed at increasing plasma HDL-cholesterol levels thus far have not proven to be beneficial of patients at risk of cardiovascular disease [47–49]. However, it is clear from the recent findings reviewed in this paper that a bright future for the HDL receptor SR-BI as therapeutic target can be foreseen not only in the cardiovascular disease setting, but certainly also in inflammatory conditions as well as in cancer (Figure 1). Given the potentially opposite directions in the effect of the presence of SR-BI on the overall disease outcome, i.e. a supposed negative effect in cancer versus a positive effect in inflammation and cardiovascular disease, it is of major importance to evaluate the effect of novel SR-BI expression/activity modifying agents in a variety of disease settings. As such, the development of a combined cancer / cardiovascular disease mouse model is regarded of high interest.
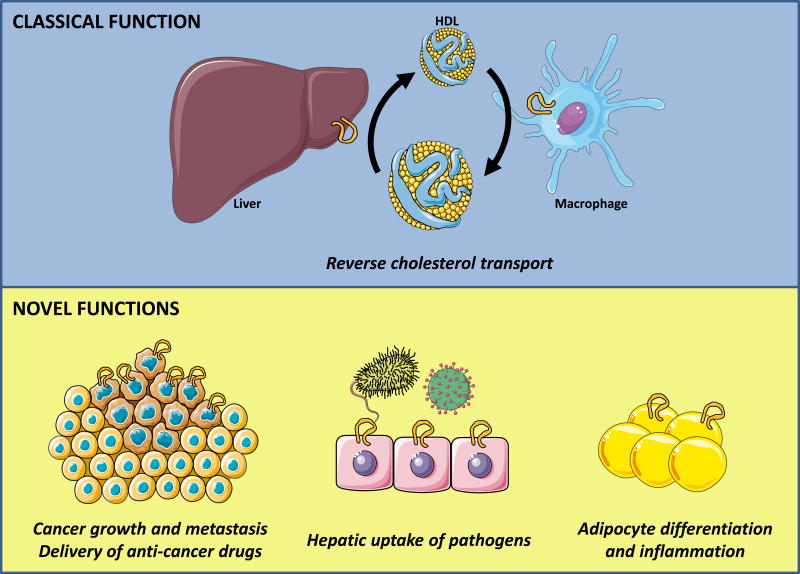
Figure 1. Classical and novel functions of SR-BI in physiology and disease.
Top, shows classical function of liver SR-BI which is responsible for the uptake of HDL-cholesterol for elimination into bile; macrophage SR-BI can transport cholesterol bi-directionally but is believed to unload or efflux excess peripheral cholesterol to HDL which can then go to the liver for completion of reverse cholesterol transport. Bottom, shows newly described novel functions of SR-BI in cancer growth and metastasis, hepatic uptake of pathogens and adipocyte differentiation and inflammation. High SR-BI expression in tumors potentially increases the ability to metastasize, but also provides an opportunity to selectively deliver anti-cancer drugs through packaging in recombinant HDL particles (bottom left). Hepatic SR-BI mediates the uptake of bacteria as well as viruses thereby contributing to the host response to infection (bottom middle). SR-BI fluxes cholesterol from and into adipocytes and thereby modulates the inflammatory state of adipose tissue (bottom right).
KEY POINTS.
SR-BI deficiency predisposes humans to cardiovascular disease.
Hepatic SR-BI/II contributes to lipopolysaccharide-induced inflammation.
SR-BI has multiple binding partners including S1P receptor and the extracellular matrix protein PCPE2.
SR-BI plays an important role in adipose tissue expansion and inflammation.
High SR-BI expression in cancer tissue is associated with reduced survival.
Acknowledgments
We acknowledge Servier for making their powerpoint image bank (http://servier.com/Powerpoint-image-bank) freely available as some elements from this image collection were used to compose our Figure.
Funding
The work was supported by National Institutes of Health grants R01HL112270 and R01HL127649 to Mary Sorci-Thomas.
Footnotes
Conflicts of interest
None.
Contributor Information
Menno Hoekstra, Division of Biopharmaceutics, Cluster BioTherapeutics, Leiden Academic Centre for Drug Research, Leiden, The Netherlands, Hoekstra@lacdr.leidenuniv.nl, Tel: +31-71-5276582.
Mary Sorci-Thomas, Division of Endocrinology, Associate in Pharmacology and Toxicology, Medical College of Wisconsin, Senior Adjunct Investigator at the Blood Research Institute, Blood Center of Wisconsin, msthomas@mcw.edu, Tel: 414-955-5728.
References
- 1.Acton SL, Scherer PE, Lodish HF, Krieger M. Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J Biol Chem. 1994;269:21003–21009. [PubMed] [Google Scholar]
- 2.Acton S, Rigotti A, Landschulz KT, et al. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 3.Glass C, Pittman RC, Weinstein DB, Steinberg D. Dissociation of tissue uptake of cholesterol ester from that of apoprotein A-I of rat plasma high density lipoprotein: selective delivery of cholesterol ester to liver, adrenal, and gonad. Proc Natl Acad Sci U S A. 1983;80:5435–5439. doi: 10.1073/pnas.80.17.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rigotti A, Trigatti BL, Penman M, et al. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc Natl Acad Sci U S A. 1997;94:12610–12615. doi: 10.1073/pnas.94.23.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Eck M, Twisk J, Hoekstra M, et al. Differential effects of scavenger receptor BI deficiency on lipid metabolism in cells of the arterial wall and in the liver. J Biol Chem. 2003;278:23699–23705. doi: 10.1074/jbc.M211233200. [DOI] [PubMed] [Google Scholar]
- 6.Feng H, Guo L, Wang D, et al. Deficiency of scavenger receptor BI leads to impaired lymphocyte homeostasis and autoimmune disorders in mice. Arterioscler Thromb Vasc Biol. 2011;31:2543–2551. doi: 10.1161/ATVBAHA.111.234716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ueda Y, Gong E, Royer L, et al. Relationship between expression levels and atherogenesis in scavenger receptor class B, type I transgenics. J Biol Chem. 2000;275:20368–20373. doi: 10.1074/jbc.M000730200. [DOI] [PubMed] [Google Scholar]
- 8.Acton S, Osgood D, Donoghue M, et al. Association of polymorphisms at the SR-BI gene locus with plasma lipid levels and body mass index in a white population. Arterioscler Thromb Vasc Biol. 1999;19:1734–1743. doi: 10.1161/01.atv.19.7.1734. [DOI] [PubMed] [Google Scholar]
- 9.West M, Greason E, Kolmakova A, et al. Scavenger receptor class B type I protein as an independent predictor of high-density lipoprotein cholesterol levels in subjects with hyperalphalipoproteinemia. J Clin Endocrinol Metab. 2009;94:1451–1457. doi: 10.1210/jc.2008-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vergeer M, Korporaal SJ, Franssen R, et al. Genetic variant of the scavenger receptor BI in humans. N Engl J Med. 2011;364:136–145. doi: 10.1056/NEJMoa0907687. [DOI] [PubMed] [Google Scholar]
- **11.Zanoni P, Khetarpal SA, Larach DB, et al. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science. 2016;351:1166–1171. doi: 10.1126/science.aad3517. This landmark study was the first to show the importance of proper SR-BI function for the protection against cardiovascular disease in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golden D, Kolmakova A, Sura S, et al. Lymphocyte activation gene 3 and coronary artery disease. JCI Insight. 2016;1:e88628. doi: 10.1172/jci.insight.88628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manichaikul A, Wang XQ, Musani SK, et al. Association of the Lipoprotein Receptor SCARB1 Common Missense Variant rs4238001 with Incident Coronary Heart Disease. PLoS One. 2015;10:e0125497. doi: 10.1371/journal.pone.0125497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao M, Zhao D, Schouteden S, et al. Regulation of high-density lipoprotein on hematopoietic stem/progenitor cells in atherosclerosis requires scavenger receptor type BI expression. Arterioscler Thromb Vasc Biol. 2014;34:1900–1909. doi: 10.1161/ATVBAHA.114.304006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morin EE, Guo L, Schwendeman A, Li XA. HDL in sepsis - risk factor and therapeutic approach. Front Pharmacol. 2015;6:244. doi: 10.3389/fphar.2015.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *16.Baranova IN, Souza AC, Bocharov AV, et al. Human SR-BI and SR-BII Potentiate Lipopolysaccharide-Induced Inflammation and Acute Liver and Kidney Injury in Mice. J Immunol. 2016;196:3135–3147. doi: 10.4049/jimmunol.1501709. This article highlights the contribution of hepatic SR-BI/II to inflammation and the associated tissue injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo T, Hu J, Xi D, et al. Lck Inhibits Heat Shock Protein 65-Mediated Reverse Cholesterol Transport in T Cells. J Immunol. 2016;197:3861–3870. doi: 10.4049/jimmunol.1502710. [DOI] [PubMed] [Google Scholar]
- 18.Song G, Wu X, Zhang P, et al. High-density lipoprotein inhibits ox-LDL-induced adipokine secretion by upregulating SR-BI expression and suppressing ER Stress pathway. Sci Rep. 2016;6:30889. doi: 10.1038/srep30889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **19.Lee MH, Appleton KM, El-Shewy HM, et al. S1P in HDL Promotes Interaction Between SR-BI and S1PR1 and Activates S1PR1-Mediated Biological Functions: Calcium Flux and S1PR1 Internalization. J Lipid Res. 2017;58:325–338. doi: 10.1194/jlr.M070706. This study describes that SR-BI and S1PR1 interact to facilitate the cellular response to S1P and has generated the hypothesis that the presence of other/additional HDL receptors and binding proteins might also mediate signaling by HDL-associated S1P at sites where HDL engages these receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **20.Sorci-Thomas MG, Thomas MJ. Microdomains, Inflammation, and Atherosclerosis. Circ Res. 2016;118:679–691. doi: 10.1161/CIRCRESAHA.115.306246. This review highlights studies demonstrating how the cellular balance between free and ester cholesterol is essential for proper immune cell function and prevention of chronic immune cell overstimulation and proliferation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yvan-Charvet L, Bobard A, Bossard P, et al. In vivo evidence for a role of adipose tissue SR-BI in the nutritional and hormonal regulation of adiposity and cholesterol homeostasis. Arterioscler Thromb Vasc Biol. 2007;27:1340–1345. doi: 10.1161/ATVBAHA.106.136382. [DOI] [PubMed] [Google Scholar]
- 22.Tondu AL, Robichon C, Yvan-Charvet L, et al. Insulin and angiotensin II induce the translocation of scavenger receptor class B, type I from intracellular sites to the plasma membrane of adipocytes. J Biol Chem. 2005;280:33536–33540. doi: 10.1074/jbc.M502392200. [DOI] [PubMed] [Google Scholar]
- 23.Umemoto T, Han CY, Mitra P, et al. Apolipoprotein AI and high-density lipoprotein have anti-inflammatory effects on adipocytes via cholesterol transporters: ATP-binding cassette A-1, ATP-binding cassette G-1, and scavenger receptor B-1. Circ Res. 2013;112:1345–1354. doi: 10.1161/CIRCRESAHA.111.300581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *24.Chung S, Parks JS. Dietary cholesterol effects on adipose tissue inflammation. Curr Opin Lipidol. 2016;27:19–25. doi: 10.1097/MOL.0000000000000260. This review highlights the role of cholesterol in adipose tissue proliferation, inflammation and dysfunction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, McGillicuddy FC, Hinkle CC, et al. Adipocyte modulation of high-density lipoprotein cholesterol. Circulation. 2010;121:1347–1355. doi: 10.1161/CIRCULATIONAHA.109.897330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **26.Holme RL, Miller JJ, Nicholson K, Sahoo D. Tryptophan 415 Is Critical for the Cholesterol Transport Functions of Scavenger Receptor BI. Biochemistry. 2016;55:103–113. doi: 10.1021/acs.biochem.5b00804. This study provides new insight into the domains within the SR-BI molecule that are essential for selective cholesterol ester uptake from HDL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **27.Pollard RD, Blesso CN, Zabalawi M, et al. Procollagen C-endopeptidase Enhancer Protein 2 (PCPE2) Reduces Atherosclerosis in Mice by Enhancing Scavenger Receptor Class B1 (SR-BI)-mediated High-density Lipoprotein (HDL)-Cholesteryl Ester Uptake. J Biol Chem. 2015;290:15496–15511. doi: 10.1074/jbc.M115.646240. This study shows that the extracellular matrix protein procollagen endopeptidase enhancer 2 regulates both SR-BI function and expression levels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorci-Thomas MG, Pollard RD, Thomas MJ. What does procollagen C-endopeptidase enhancer protein 2 have to do with HDL-cholesteryl ester uptake? Or how I learned to stop worrying and love reverse cholesterol transport? Curr Opin Lipidol. 2015;26:420–425. doi: 10.1097/MOL.0000000000000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson ER, Wardell SE, Jasper JS, et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342:1094–1098. doi: 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karuna R, Holleboom AG, Motazacker MM, et al. Plasma levels of 27-hydroxycholesterol in humans and mice with monogenic disturbances of high density lipoprotein metabolism. Atherosclerosis. 2011;214:448–455. doi: 10.1016/j.atherosclerosis.2010.10.042. [DOI] [PubMed] [Google Scholar]
- **31.Yuan B, Wu C, Wang X, et al. High scavenger receptor class B type I expression is related to tumor aggressiveness and poor prognosis in breast cancer. Tumour Biol. 2016;37:3581–3588. doi: 10.1007/s13277-015-4141-4. This clinical study shows that relatively high SR-BI expression in breast cancer tissue predicts a low survival and highlights the use of SR-BI as a biomarker for disease. [DOI] [PubMed] [Google Scholar]
- **32.Li J, Wang J, Li M, et al. Up-regulated expression of scavenger receptor class B type 1 (SR-B1) is associated with malignant behaviors and poor prognosis of breast cancer. Pathol Res Pract. 2016;212:555–559. doi: 10.1016/j.prp.2016.03.011. This clinical study shows that relatively high SR-BI expression in breast cancer tissue predicts a low survival and highlights the use of SR-BI as a biomarker for disease. [DOI] [PubMed] [Google Scholar]
- 33.Lopez D, Sanchez MD, Shea-Eaton W, McLean MP. Estrogen activates the high-density lipoprotein receptor gene via binding to estrogen response elements and interaction with sterol regulatory element binding protein-1A. Endocrinology. 2002;143:2155–168. doi: 10.1210/endo.143.6.8855. [DOI] [PubMed] [Google Scholar]
- 34.Landschulz KT, Pathak RK, Rigotti A, et al. Regulation of scavenger receptor, class B, type I, a high density lipoprotein receptor, in liver and steroidogenic tissues of the rat. J Clin Invest. 1996;98:984–995. doi: 10.1172/JCI118883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukata Y, Yu X, Imachi H, et al. 17β-Estradiol regulates scavenger receptor class BI gene expression via protein kinase C in vascular endothelial cells. Endocrine. 2014;46:644–650. doi: 10.1007/s12020-013-0134-5. [DOI] [PubMed] [Google Scholar]
- 36.Schorghofer D, Kinslechner K, Preitschopf A, et al. The HDL receptor SR-BI is associated with human prostate cancer progression and plays a possible role in establishing androgen independence. Reprod Biol Endocrinol. 2015;13:88. doi: 10.1186/s12958-015-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dorner M, Horwitz JA, Robbins JB, et al. A genetically humanized mouse model for hepatitis C virus infection. Nature. 2011;474:208–211. doi: 10.1038/nature10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dockendorff C, Faloon PW, Yu M, et al. Indolinyl-Thiazole Based Inhibitors of Scavenger Receptor-BI (SR-BI)-Mediated Lipid Transport. ACS Med Chem Lett. 2015;6:375–380. doi: 10.1021/ml500154q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dockendorff C, Faloon PW, Germain A, et al. Discovery of bisamide-heterocycles as inhibitors of scavenger receptor BI (SR-BI)-mediated lipid uptake. Bioorg Med Chem Lett. 2015;25:2594–2598. doi: 10.1016/j.bmcl.2015.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dockendorff C, Faloon PW, Pu J, et al. Benzo-fused lactams from a diversity-oriented synthesis (DOS) library as inhibitors of scavenger receptor BI (SR-BI)-mediated lipid uptake. Bioorg Med Chem Lett. 2015;25:2100–2105. doi: 10.1016/j.bmcl.2015.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nieland TJ, Shaw JT, Jaipuri FA, et al. Identification of the molecular target of small molecule inhibitors of HDL receptor SR-BI activity. Biochemistry. 2008;47:460–472. doi: 10.1021/bi701277x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ouyang Q, Duan Z, Jiao G, Lei J. A Biomimic Reconstituted High-Density-Lipoprotein-Based Drug and p53 Gene Co-delivery System for Effective Antiangiogenesis Therapy of Bladder Cancer. Nanoscale Res Lett. 2015;10:965. doi: 10.1186/s11671-015-0965-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding Y, Wang Y, Zhou J, et al. Direct cytosolic siRNA delivery by reconstituted high density lipoprotein for target-specific therapy of tumor angiogenesis. Biomaterials. 2014;35:7214–7227. doi: 10.1016/j.biomaterials.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Ding Y, Wang Y, Opoku-Damoah Y, et al. Dual-functional bio-derived nanoparticulates for apoptotic antitumor therapy. Biomaterials. 2015;72:90–103. doi: 10.1016/j.biomaterials.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 45.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- *46.Rui M, Xin Y, Li R, et al. Targeted Biomimetic Nanoparticles for Synergistic Combination Chemotherapy of Paclitaxel and Doxorubicin. Mol Pharm. 2017;14:107–123. doi: 10.1021/acs.molpharmaceut.6b00732. This preclinical study highlights that packaging of anti-cancer drugs in HDL for selective delivery by SR-BI improves their clinical application potential. [DOI] [PubMed] [Google Scholar]
- 47.Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 48.HPS2-THRIVE Collaborative Group. Landray MJ, Haynes R, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]