Abstract
Background
There are sex-differences in mortality while awaiting heart transplantation and the reason remains unclear.
Methods and Results
We included all adults in the Scientific Registry of Transplant Recipients placed on the heart transplant active waitlist from 2004–2015. The primary endpoint was all-cause mortality. Multivariable Cox proportional hazards models were performed to evaluate survival by UNOS status at time of listing. Random Survival Forest was used to identify sex-interactions for the competing risk of death and transplantation. There were 33,069 patients (25% women) awaiting heart transplantation. This cohort included 7,681 UNOS Status 1A (26% women), 13,027 UNOS Status 1B (25% women), and 12,361 UNOS status 2 (26% women). During a median follow-up of 4.3 mo, 1,351 women and 4,052 men died. After adjusting for over 20 risk factors, female sex was associated with a significant risk of death among UNOS Status 1A (aHR 1.14, 95% CI 1.01 to 1.29) and UNOS Status 1B (aHR 1.17, 95% CI 1.05–1.30). In contrast, female sex was significantly protective for time to death among UNOS Status 2 (aHR 0.85, 95% CI 0.76 to 0.95). Sex-differences in probability of transplantation were present for every UNOS Status and over 20 sex-interactions were identified for mortality and transplantation.
Conclusions
When stratified by initial UNOS Status, women had a higher mortality then men as UNOS Status 1 and a lower mortality as UNOS Status 2. With over 20 sex-interactions for mortality and transplantation, further evaluation is warranted to form a more equitable allocation system.
Keywords: heart failure, sex, outcome, UNOS and transplantation
Journal Subject Terms: Heart Failure, Transplantation
Women have a higher mortality rate than men while awaiting orthotopic heart transplantation and the reason remains unclear. In one small European study (58 women, 260 men), women had a higher risk of death/deterioration (HR 2.3; 95% CI 1.04–5.12; p=0.04) even after adjusting for age, Heart Failure Survival Score, serum creatinine, inpatient status, cardiac index, low vocational level, smoking, and low emotional support at time of transplant listing.1 In the United States the higher risk of death in women occurred despite shorter waiting times for heart transplantation according to Scientific Registry of Transplant Recipients (SRTR) data from 1999–2008.2
Timing of advanced heart failure care is important yet there are many uncertainties when making decisions to list a woman for heart transplantation. How long will she wait until transplantation or will she die on the waiting list? Does the initial UNOS Status influence the outcome and did this change after FDA approval of smaller left ventricular assist devices that fit in women? Finally, what pre-listing characteristics affect waitlist survival more in women than men, and do these also influence time to transplantation? These questions and more need further investigation.
Therefore, to better advise women who are contemplating heart transplantation, the aims of this study are to 1) evaluate sex-differences in waitlist survival and time to transplantation based on initial UNOS Status using patient characteristics at time of listing, 2) determine if sex-differences in waitlist survival or time to transplantation have changed over the years, and 3) identify factors associated with waitlist mortality and timing of transplantation that are different for women than for men.
Methods
Scientific Registry of Transplant Recipients (SRTR)
This study used data from the SRTR. The SRTR database includes data on all donors, wait-listed candidates, and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere. The Health Resources and Services Administration (HRSA) provides oversight to the activities of the OPTN and SRTR contractors. Human error collecting data is minimized by edit checks, validation of data at time of entry and internal verification when there are outliers.3 The study was approved by the Institutional Review Board at the Cleveland Clinic, and informed consent was waived because all data was obtained for routine care and de-identified by SRTR prior to submission to the investigators.
Patient Population and UNOS Status
We included all adult patients in the SRTR database placed on the active waiting list for heart transplantation from January 1, 2004–August 31, 2015. We excluded inactive adult candidates (UNOS Status 7 patients at time of initial listing: 375 women and 1053 men) and patients < 18 years of age since UNOS criteria for pediatrics differs from adults and the donor pools are distinguished by age.4
Data were evaluated by UNOS status at time of initial wait listing. UNOS status 1A (high priority) included patients requiring total artificial heart (TAH), extracorporeal membrane oxygenation (ECMO), ventricular assist device (VAD) with device complications, VADs without complications for a total of 30 days, intra-aortic balloon pump (IABP), mechanical ventilation, multiple inotropes with hemodynamic monitoring, single high dose inotrope with hemodynamic monitoring or an exemption for critical illness such as ventricular tachycardia. UNOS status 1B (next highest status) included patients on continuous intravenous dose inotrope support and stable VADs. UNOS Status 2 included all other patients and is the least urgent status for patients actively waiting heart transplantation.
Outcome Measures
The aims of this study were to 1) evaluate sex-differences in waitlist survival and time to transplantation based on initial UNOS Status using patient characteristics at time of listing, 2) determine if sex-differences in waitlist survival or time to transplantation have changed over the years, and 3) identify factors associated with waitlist mortality and timing of transplantation that are different for women than for men.
Statistical Analysis
All-cause mortality was assessed as a right-censored time to death with follow-up censored at the time of heart transplantation. This analysis was based on Intent-to-Treat such that deaths following removal from the waiting list were included in the primary analysis. SRTR mortality data were maintained by transplant centers and verified with the complete Social Security Death Master File recently available through a specific waiver granted to the SRTR.
Sex-specific baseline characteristics were reported according to UNOS Status at time of listing for heart transplantation. Continuous variables were expressed as medians with interquartile ranges. Categorical variables were expressed as number of patients with frequency except if patient number <10 absolute values were not provided in order to protect the identity of the cohort as per SRTR policy. Sex-specific survival analysis was performed for UNOS Status 1A, 1B, and 2 patients in 3 eras (2004–2008, 2009–2011, 2012–2015) using the Kaplan-Meier method with censoring for heart transplantation. These eras were chosen for historical consideration. FDA approval of HeartMate II, a small device that could be implanted in most women, occurred in April 2008.5 Prior to this time, there were limited devices available for bridging smaller women to transplantation. The era between 2009–2011 included the usage of HeartMate II. The era 2012–2015 included data granted to SRTR with a special waiver for the complete updated Death Master File. This era also included FDA approval on November 20, 2012 of HeartWare,6 another device that could be implanted in smaller patients.
Cox proportional hazard models were created for each UNOS Status to explore the association between female sex and time to death. Each model was adjusted for continuous and categorical baseline characteristics at time of listing. Continuous variables included year, age, body mass index (BMI), estimated glomerular filtration rate (GFR) calculated by the MDRD (Modification of Diet in Renal Disease) equation, pulmonary artery mean, pulmonary capillary wedge pressure (PCWP) mean, total albumin, and cardiac index. Categorical variables included type of ventricular assist device (TAH, LVAD, RVAD +/− LVAD, unspecified mechanical circulatory device), extracorporeal membrane oxygenation (ECMO), intra-aortic balloon pump (IABP), inotropes, mechanical ventilator, diabetes mellitus, dialysis, race (White, Black, Hispanic, Asian, Other), cerebral vascular accident, history of tobacco, inotropes, insurance (private, medicare, medicaid, other), cardiac diagnosis (dilated cardiomyopathy, ischemic cardiomyopathy, congenital heart disease, hypertrophic cardiomyopathy, restrictive cardiomyopathy, valvular cardiomyopathy, and other), ABO blood type, antiarrhythmic, hypertension, malignancy, peripheral vascular disease, and implantable cardioverter defibrillator (ICD). Variables with a high proportion of missing data were excluded from the analysis. These included peak oxygen consumption (>30 % missingness), peripheral vascular disease (21% missingness), and antiarrythmic (25% missing). Multiple imputation was used for the remaining missing data and performed with SAS (v.9.4., Cary, N.C.) using Proc MI in order to generate 5 imputed data sets to utilize in the Cox proportional hazard models. We did not include outcomes, time or cumulative hazard in the imputation assuming these were not systematically different for the primary explanatory variables. However, estimates were similar when log survival and death were added to the imputation. The MI procedure assumes data are from a multivariable normal distribution using the MCMC method. Proc MIANALYZE was then used to generate parameter estimates and standard errors for statistical inference.
Random Survival Forest analysis machine learning methodology7, 8 was employed for a competing risk analysis for the competing risk of death on the waitlist and transplantation. Missing data were pre-imputed using missForest9 imputation methodology, a special type of random forest (RF)7 strategy. No outcome information was utilized in the imputation. This pre-imputed data, comprised of the variables mentioned above and peak oxygen consumption were used for the RSF analysis. However, in order to estimate sex-interactions, the data were expanded to include all pairwise interactions of sex with each of the original 29 independent variables. For categorical variables, one interaction for each level of the variable was created. Contrasts were not utilized (i.e., baseline values were not used). This yielded a total of 97 independent variables, including the original 29 variables.
The data was stratified by UNOS category. For each category, three separate RSF competing risk forests were fit, with each forest compromised of 1000 random competing risk trees. Trees were constructed from independently drawn bootstrap data. On average, each tree was grown from 63% of the data (in-sample bootstrapped data); the remaining unused data (37%), referred to as out-of-bag (OOB) data, was used to calculate OOB cross-validated survival for each patient and variable importance (VIMP) measures for each of the 97 independent variables.10 Different survival splitting rules were employed by the three separate RSF competing risk forests. In the first, trees were grown using a composite (equally weighted) generalized log-rank splitting rule. This yields an analysis most suitable for estimation of the cumulative incidence function (CIF). Trees for the remaining two forests were grown using a modified Gray splitting rule, with each weighted in favor of one of the two competing risks. These are most suitable for the analysis of the cause-specific hazard and for identifying cause specific risk factors.
Variable importance (VIMP) estimates the difference in prediction error for a RSF with and without a variable. Positive values indicate variables that are predictive, adjusting for all other variables. In order to derive valid standard errors and confidence regions for the estimated VIMP, thereby allowing us to identify statistically significant VIMP, each RSF procedure was repeatedly subsampled.
We drew a random sample of size n/10 without replacement. Subsampled data were fit using the same RSF strategy described above and the resulting VIMP stored. The procedure was repeated 1000 times independently. The 1000 values were then used to construct confidence regions using subsampling methodology.10 All RSF calculations were based on randomForestSRC R-software11 under default competing risk settings.
Results
Sex-Differences at Time of Waitlist
Baseline characteristics of 33,069 adult heart failure patients (25% women) on the active heart transplant waiting list are shown in Table 1 (See supplementary Table 1 for expanded list of baseline characteristics). This cohort included 7,681 UNOS Status 1A (26% women), 13,027 UNOS Status 1B (25% women), and 12,361 UNOS status 2 (26% women). The majority of patients were male, white, over 50 years of age, blood type O, and with private insurance. Women compared to men were younger, had slightly lower BMI, and higher frequency of dilated cardiomyopathy. They were less likely than men to have an ischemic cardiomyopathy, antiarrythmic therapy, or an ICD.
Table 1.
Sex-Differences in Baseline Characteristics While Awaiting Heart Transplantation
| UNOS Status 1A | UNOS Status 1B | UNOS Status 2 | ||||
|---|---|---|---|---|---|---|
| Variable | Female | Male | Female | Male | Female | Male |
| N= 1965 | N=5716 | N=3226 | N=9801 | N=3197 | N=9164 | |
| Age(y), median (Q1, Q3) | 51 (37,59) | 55 (45,62 | 53 (40,60) | 56 (46,62) | 51 (39,59) | 57 (48,62) |
| Race, n (%) | ||||||
| White | 1150 (59) | 3802 (67) | 1802 (56) | 6433 (66) | 2156 (67) | 6999 (76) |
| Black | 558 (28) | 1157 (20) | 1069 (33) | 2252 (23) | 674 (21) | 1246 (14) |
| Hispanic | 176 (9) | 467 (8) | 243 (8) | 781 (8) | 253 (8) | 594 (6) |
| Asian | 58 (3) | 234 (4) | 73 (2) | 256 (3) | 71 (2) | 213 (2) |
| Other | 23 (1) | 56 (1) | 39 (1) | 79 (1) | 43 (1) | 112 (1) |
| BMI, median (Q1, Q3) | 25 (22, 29) | 27 (24,30) | 26 (23,31) | 27 (24,31) | 27(23, 31) | 28 (25,31) |
| ABO blood type, n (%) | ||||||
| A | 731 (37) | 2207 (39) | 1162 (36) | 3651 (37) | 1172(37) | 3685 (40) |
| B | 321 (16) | 840 (15) | 441 (14) | 1361 (14) | 401 (13) | 1090 (12) |
| O | 826 (42) | 2361 (41) | 1464 (45) | 4357 (44) | 1498 (47) | 3992 (44) |
| AB | 87 (4) | 308 (5) | 159 (5) | 432 (4) | 126 (4) | 397 (4) |
| Diagnosis, n (%) | ||||||
| Dilated | 1185 (60) | 2634 (46) | 2120 (66) | 4877 (50) | 1655 (52) | 3245(35) |
| Ischemic | 400 (20) | 2459 (43) | 631 (20) | 4058 (41) | 586 (18) | 4325 (47) |
| Congenital | 35 (2) | 91 (2) | 114 (4) | 184 (2) | 268 (8) | 352 (4) |
| Hypertrophic | 48 (2) | 71 (1) | 68 (2) | 128 (1) | 153 (5) | 209 (2) |
| Restrictive | 62 (3) | 104 (2) | 86 (3) | 168 (2) | 162 (5) | 329 (4) |
| Valvular | 50 (3) | 79 (1) | 82 (3) | 132 (1) | 96 (3) | 173 (2) |
| Other | 185 (9) | 278 (5) | 125 (4) | 254 (3) | 277 (9) | 531 (6) |
| ICD, n (%) | 1096 (56) | 3815 (67) | 2356 (73) | 7729 (79) | 2187 (68) | 7028 (77) |
| eGFR mls/min/1.73m2, median (Q1, Q3) | 65 (47,89) | 67 (49,89) | 66 (48,85) | 67 (51,86) | 63 (48,82) | 65 (51,81) |
| Serum Albumin g/dl, median (Q1, Q3) | 3.3 (2.9,3.8) | 3.4 (3.0,3.9) | 3.7 (3.3,4.1) | 3.7 (3.2,4.1) | 4.0 (3.6,4.4) | 4.0 (3.6,4.3) |
| Mean PAP mmHg, median (Q1, Q3) | 30 (23,37) | 32 (24,39) | 29 (23,36) | 31 (24,38) | 26 (20,33) | 28 (21,35) |
| PCWP mmHg, median (Q1, Q3) | 20 (14,26) | 22 (15, 28) | 20 (14,25) | 21 (15,27) | 17 (12,23) | 19 (13,25) |
| CI l/min, median (Q1, Q3) | 2.1 (1.6,2.5) | 2.1 (1.7,2.6) | 2.0(1.7,2.5) | 2.1(1.7,2.5) | 2.2 (1.8,2.6) | 2.1 (1.8,2.5) |
| PVO2 ml/kg/min, median (Q1, Q3) | 10 (8,13) | 11 (9,14) | 11 (9,13) | 12 (9,14) | 11 (9,14) | 12 (10,14) |
| Ventilator, n (%) | 219 (11) | 439 (8) | 41 (1) | 101 (1) | 12 (0) | 59 (1) |
| Inotrope, n (%) | 906 (46) | 2403 (42) | 1691 (52) | 4880 (50) | 157 (5) | 531 (6) |
| LVAD, n (%) | 447 (23) | 1675 (29) | 655 (20) | 2660 (27) | 72 (2) | 241 (3) |
| RVAD +/− LVAD, MCS unspecified, n (%) | 229 (12) | 579 (10) | 114 (4 | 321 (3) | 16 (1) | 79 (1) |
| TAH, n (%) | 18 (1) | 86 (2) | * | 32 (0) | * | 13 (0) |
| ECMO, n (%) | 121 (6) | 200 (4) | * | * | * | * |
| IABP, n (%) | 300 (15) | 966 (17) | 57 (2) | 167 (2) | 33 (1) | 97 (1) |
= Frequency ≤10 patients
y=years, Q1, Q3= 25-th and, 75-th percentiles, CMP=cardiomyopathy, CAD=coronary artery disease, ICD=implantable cardioverter-defibrillator, PVD=peripheral vascular disease, CVA=cerebral vascular accident, eGFR=estimated glomerular filtration rate, PAP=pulmonary arterial pressure, CI=cardiac index, PVO2 =peak oxygen consumption, LVAD=left ventricular assist device, RVAD=right ventricular assist device, TAH=total artificial heart, MCS=mechanical circulatory support, ECMO=extracorporeal membrane oxygenation, IABP=intra-aortic balloon pump,
Amongst UNOS Status 1A patients women were more likely than men to require a ventilator, inotropes or ECMO support and less likely to have a TAH, LVAD support, IABP, or ICD. About 50% of the UNOS Status 1B patients were on inotropes at time of listing with slightly more usage among women compared to men. About 25% of the UNOS Status 1B patients had a LVAD which was more likely in men than women. Among UNOS Status 2 patients, women compared to men had lower peak VO2 values and worse estimated GFR.
Sex-Differences in Waitlist Mortality and Transplantation
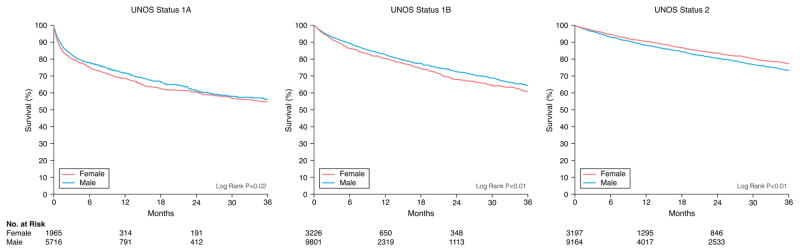
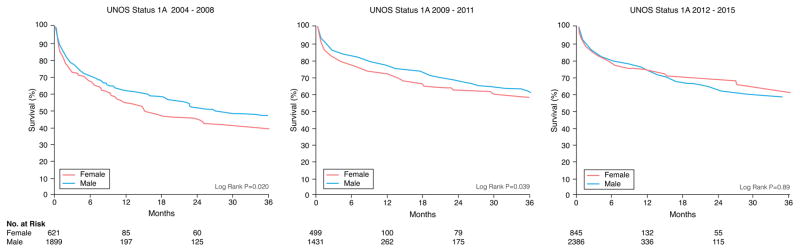
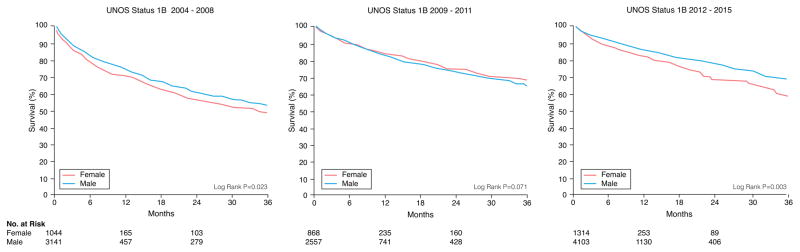
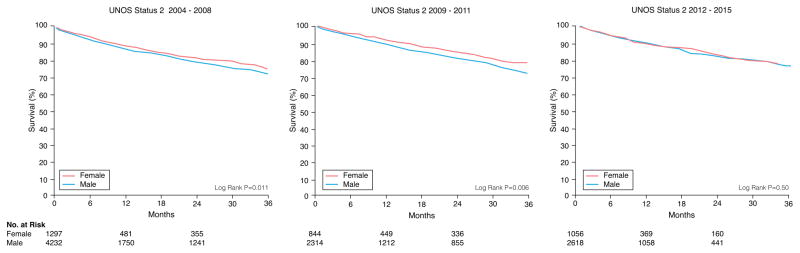
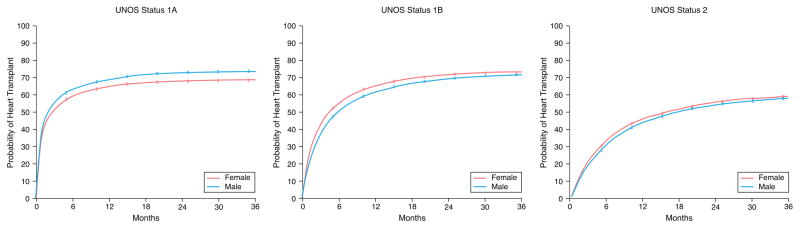
There were 1351 women and 4052 men who died during a median follow-up of 4.3 months. Unadjusted Kaplan-Meier survival revealed women who were initially listed as UNOS Status 1A and 1B were more likely to die on the waitlist than men and less likely if listed as UNOS Status 2 (Figure 1). When looking at different eras, unadjusted Kaplan-Meier waitlist survival for UNOS Status 1A and B candidates was better after 2008. Among UNOS Status 1A candidates, women had a higher mortality than men from 2004–2011. Between 2012–2015, survival was better for women but slightly worse for men resulting in no sex-difference in mortality during this time period (See Figure 2A). Among patients initially listed as UNOS Status 1B, women had a worse survival than men in 2004–2008 and 2012–2015 but no sex-differences in survival between 2009–2011 (Figure 2B). Among UNOS Status 2 patients, men had a higher mortality than women between 2004–2012 and similar survival as women between 2012–2015 (Figure 2C).
Figure 1. Sex-Differences in Survival in Heart Failure Patients Awaiting Transplantation.
Sex-specific Kaplan-Meier survival curves were generated for patients initially listed as (A) UNOS Status 1A, (B) UNOS Status 1B, and (C) UNOS Status 2
Figure 2. Sex-Differences in Survival in Heart Failure Patients Awaiting Transplantation During Three Time Periods.
Sex-specific Kaplan-Meier survival curves were generated for patients initially listed as (A) UNOS Status 1A, (B) UNOS Status 1B, and (C) UNOS Status 2 during three time periods: 2004–2008, 2009–2011, and 2012–2015. All data was censored for heart transplantation.
Cox regression of time until death revealed female sex was associated with a significant risk of death among UNOS Status 1A and1B after multivariable risk adjustment (Table 2). In contrast, female sex was protective for time to death among UNOS Status 2 patients in both unadjusted and risk adjusted models.
Table 2.
Female Sex and Mortality While Awaiting Heart Transplantation: Cox Proportional Hazards Analyses
| UNOS Status 1A HR (95% CI) |
UNOS Status 1B HR (95% CI) |
UNOS Status 2 HR (95% CI) |
|
|---|---|---|---|
| Female deaths (total at risk) | 410 (1965) | 508 (3226) | 473 (3197) |
| Male deaths (total at risk) | 1004 (5716) | 1405 (9801) | 1714 (9164) |
| Unadjusted | 1.15* (1.10–1.21) | 1.20* (1.15–1.26) | 0.83* (0.79–0.87) |
| Multivariable adjusted | 1.14* (1.01–1.29) | 1.17* (1.05–1.30) | 0.85* (0.76–0.95) |
Hazards ratios (HR) represent female:male comparison.
Multivariable adjusted for the following variables: gender, age, race, body mass index, insurance, initial year on waitlist for heart transplantation, ABO blood type, cardiac diagnosis (dilated, ischemic, congenital, hypertrophic, restrictive, valvular, other), defibrillator, dialysis at listing, diabetes mellitus, hypertension, tobacco usage, malignancy, prior cerebral vascular accident, estimated glomerular filtration rate, serum albumin, mean pulmonary arterial pressure, pulmonary capillary wedge pressure, cardiac index, mechanical ventilator, inotrope usage, left ventricular assist device, right ventricular assist device, total artificial heart, intra-aortic balloon pump, extracorporeal membrane oxygenation
RSF competing risk analysis revealed women were less likely than men to be transplanted as UNOS Status 1A and more likely than men as UNOS Status 1B and 2 (Figure 3). When evaluating heart transplantation over time, there were many sex-differences (Supplementary Figure 1A). For UNOS Status 1A, the probability of heart transplantation was higher in men than women until 2011. Between 2012–2015 there no longer was a significant sex-difference. For UNOS Status 1B, there was no substantial sex-difference until 2012–2015 when the probability of transplantation in women significantly exceeded that in men (Supplementary Figure 1B). For UNOS Status 2, the probability of transplantation was higher in women compared to men in 2004–2008 and 2012–2015 (Supplementary Figure 1C).
Figure 3. Sex-Differences in Heart Transplantation Based on UNOS Status.
Sex-specific cumulative incidence curves were generated for patients initially listed as (A) UNOS Status 1A, (B) UNOS Status 1B, and (C) UNOS Status 2. Figures show uncertainty in estimators of plus or minus two standard errors, where standard errors were estimated using subsampling.
Sex-Differences in Waitlist Risk Factors
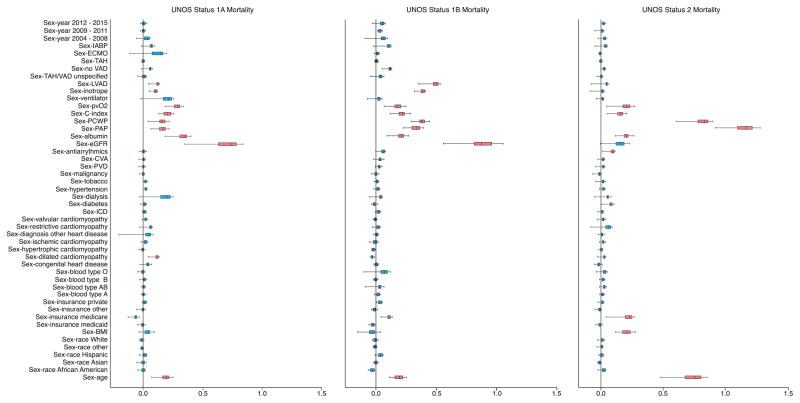
There were many sex-interactions with risk factors for death and heart transplantation (Figure 4 A and B). Among patients initially listed as UNOS Status 1A, the most important sex-interactions associated with death were renal function, serum albumin, age, peak oxygen consumption, cardiac index, pulmonary artery pressure, pulmonary capillary wedge pressure, LVAD, and inotropes. Among patients initially listed as UNOS Status 1B or 2, sex-interactions associated with death were similar to UNOS Status 1A but varied in magnitude and order of importance with a few exceptions. There were no sex-interactions associated with renal function, LVAD or inotrope for UNOS Status 2 but there was a sex-interaction with Medicare and BMI.
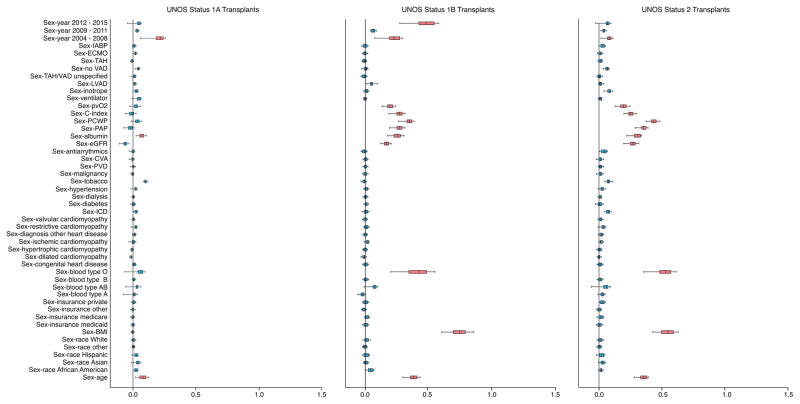
Figure 4. Sex-Interactions for Risk of Death and Heart Transplantation.
Variable importance (VIMP) of sex-interactions for risk of death (A) and heart transplantation (B) are depicted based on initially listing as UNOS Status 1A, 1B or 2. Sex by covariate represents female:male by covariate. Boxes encompass median (line) and 25th and 75th percentile confidence limits, and whiskers 95% confidence limits. Black vertical line at 0.0 VIMP represents the point at which an interaction does not contribute predictive power to the model. Thus, blue boxes indicate interactions non-contributory and red boxes indicate interactions contributing to predictive power.
For transplantation among patients initially listed as UNOS Status 1A, there were three sex-interactions: 2004–2008, serum albumin and age. For UNOS Status 1B there were many sex-interactions that included BMI, 2004–2008, 2012–2015, blood type O, age, peak oxygen consumption, renal function, serum albumin, and hemodynamic variables. Sex-interactions associated with transplantation for patients initially listed as UNOS Status 2 were similar to UNOS Status 1B but varied in magnitude of importance.
Discussion
In a large, national transplant registry we found sex-differences in mortality while awaiting heart transplantation, sex-differences in time to transplantation, and many sex-interactions for risk of death and transplantation when patients were stratified by UNOS Status at time of initial wait listing. Female sex was associated with a higher risk of death among patients initially listed as UNOS Status 1A and 1B and lower risk of death compared to men initially listed as UNOS Status 2 even after adjustment for over 20 possible confounding variables. Rate of heart transplantation was lower in women compared to men listed as UNOS Status 1A and higher in women compared to men listed as UNOS Status 1B or 2. There were many sex-interactions for death and heart transplantation that varied with UNOS Status and had not been previously described. More research is needed to understand the mechanism of these findings in hopes of providing more equitable therapy for both women and men with advanced heart failure.
Sex-differences in survival on the national heart transplant waiting list has been present and known for many years but few studies have addressed this issue.1, 12, 13 Our analysis provides some insight and demonstrates changes in outcome over time. For women and men initially listed as UNOS Status 1A or 2, the sex-disparity in waitlist survival resolved between 2012–2015. During that time period there was no change in advanced heart failure medication but there was FDA approval of small continuous flow devices that could successfully bridge patients to transplantation and be implanted in most women and men (FDA approved HeartMate II in 2008, HeartWare in 2012).5, 6 For patients initially listed as UNOS Status 1B, the sex disparity in survival increased after 2011 with men surviving better than women despite a faster rate of heart transplantation in women compared to men. The reason remains unclear and is concerning since UNOS Status 1B patients are deemed in urgent need of transplantation because they failed conventional therapy and required either inotropes or mechanical circulatory support to survive. Although it is tempting to consider sex differences in the bioavailability of inotropes, this would not explain any changes in mortality over time since inotrope support did not change over this study period. Could sex differences in survival among UNOS Status 1B simply reflect a higher percentage of women bridged to transplantation on inotropes than men after 2011? Possible but in 2012–2015 the percent of women listed as UNOS Status 1B with inotropes was similar to men (45% women, 40% men). What about mechanical circulatory support? We do not know if there were any sex differences in survival or in complications with the most recent generation of devices since the last INTERMACS analysis assessing sex differences with LVADs involved patients with devices implanted between 2006–2010.14 Therefore, the reason why sex differences in outcome still exist remains unknown but likely lies within the complex interplay of sex with many variables related to waitlist mortality and transplantation.
We have identified over 20 factors that importantly interact with sex to affect waitlist prognosis. This is astonishing when you consider that women and men are listed based on criteria that are not sex-specific and factors affecting transplantation are not supposed to favor one sex over another. Although some sex-interactions were unique for a given UNOS Status, urgent heart transplantation (UNOS Status 1A and 1B) shared many sex-interactions for death not previously known such as renal function, serum albumin, hemodynamics parameters, LVAD, and inotropes. Renal function was the most important sex-interaction and is concerning given its significance for predicting survival in heart failure.15, 16 Serum albumin is a biomarker for liver function as well as nutritional state. It has not been included in most heart failure risk models,17, 18 but has been found to be a predictor of death in heart failure,19–22 congenital heart disease,23 and in patients undergoing LVAD implantation.24, 25 Hemodynamic parameters are often used to determine candidacy for inotrope or mechanical circulatory support and not known to have any sex-interaction. They are deemed valuable despite the lack of added prognostic significance to the non-invasive Heart Failure Survival Score,17 an advanced heart failure survival model limited by a small derivation cohort (N=268) and the inclusion of ambulatory patients prior to LVADs. Sex-interactions for survival with LVADs and inotropes are worrisome since these variables are used to define urgent transplantation. They have not been previously found and prior literature suggested a similar survival for women and men with LVAD although a higher risk of stroke in women.14 Finally, sex-interactions with peak oxygen consumption26 and age27 have been previously published yet have not changed criteria for listing patients for transplantation.
There were many sex-interactions for transplantation. Limited data exist regarding variables associated with higher rate of heart transplantation. However, sex is known to be a factor necessary to optimize matching of donor and recipient 28 along with body mass index, blood type, and other immune factors (allosensitization). In our analysis, we showed a lower rate of heart transplantation in women compared to men initially listed as UNOS Status 1A and a higher rate of transplantation in women compared to men initially listed as UNOS Status 1B or 2. Among UNOS 1A patients, there were few sex-interactions for heart transplantation. However, many sex-interactions for heart transplantation were identified among patients initially listed as UNOS Status 1B and 2. Although the importance of the sex-interactions varied, UNOS Status 1B and 2 shared sex-interactions for blood type O, age, body mass index, hemodynamics, serum albumin, peak oxygen consumption and renal function. None of these, to the best of our knowledge, have been previously described and all raise concern regarding inequality with transplantation.
The United Network for Organ Sharing (UNOS) Thoracic Organ Transplantation Committee proposed in 2016 a new heart transplant allocation system with more tiers to define urgency given known disparity in survival among certain subgroups.29, 30 How will these changes affect women? It remains unclear since the additional tiers mainly define urgency for existing criteria and do not include sex-differences. What should we do to reduce sex differences in waitlist mortality? More research is needed before we can include sex differences in the UNOS allocation system or guideline therapy. For instance, which woman listed for urgent transplantation is at highest risk of death? This is important, since not all women can be given priority over men who are critically ill. The answer remains unknown and likely is complex. Further research is needed and should focus on how devices and other therapy affect women differently than men and what co-morbidities further modify the risk of death and the rate of transplantation. Only with knowledge regarding population differences are we able to improve our allocation system and provide more equitable distribution of organs.
Limitations
The SRTR database is a large national database that is subject to human error during data entry. This remains a potential problem for all databases, but is minimized in SRTR by edit checks, validation of data at time of entry, and internal verification when there are outliers. Data quality specialists resolve these potential problems by reviewing the data and verifying discrepant data with the involved transplant center. Transplant centers are routinely audited by UNOS and CMS, which further improve the quality of the database. Despite these attempts, there are data that cannot be used due to lack of standardization until recently (i.e. panel of reactive antibody), missing data and possible database errors. Missing data can be imputed if percent is low and will not alter significantly the analysis. Data with high level of missingness is often removed from a standard multi-regression analysis. Therefore, we could not use peak oxygen consumption (>30% missing) with the Cox proportional hazard analysis but was able to use it with Random Survival Forest analysis, a machine learning statistical methods that performs excellently even with heavy missingness (up to 75%) and when missing data are not missing completely at random. Other limitations worthy of discussion are possible database errors in entry of clinical information. The baseline characteristics for UNOS Status 2 included a small percent of patients on mechanical ventilation, inotropes or mechanical circulatory support. These appear to be errors since the level of medical support does not match the severity of illness for an ambulatory UNOS Status 2 candidate. However, centers may list at a lower UNOS Status than clinically indicated so it remains unknown if these are actual database errors or patients intentionally labeled at lower status to prevent heart transplantation while ill. Nonetheless, the low percent of “possible errors” in UNOS Status would not be expected to alter our analysis significantly. Finally, despite the fact that the SRTR database is the best database available to study patients awaiting transplantation it only captures information at given time points and does not require updating data unless the change affects the UNOS status of the patient. A full set of characteristics is obtained at time of listing and at time of transplantation. If time varying co-variables were known and available while patients remained on a waitlist, more data could be used to predict events and better understand the reasons for an event.
Conclusions
In a large national registry we found sex-differences in survival while awaiting heart transplantation, sex-differences in transplantation, and many sex-interactions for risk of death and transplantation when data were evaluated by UNOS Status at time of initial transplant wait listing. Outcomes have changed over time with resolution of sex disparities in waitlist survival among patients initially listed as UNOS Status 1A and 2 but have increased since 2011 among patients initially listed as UNOS Status 1B. The reasons remain unknown but are concerning because women initially listed as UNOS Status 1B had a higher risk of death despite a faster rate of transplantation.
Supplementary Material
Clinical Summary.
For almost a decade women have had a higher mortality rate on the national heart transplant waitlist and the cause remains unclear. To further evaluate, we utilized the scientific registry of transplant recipients with over 30,000 patients (25% women) and stratified the cohort based on sex and UNOS Status at time of listing. Women had a higher mortality than men at the most urgent UNOS Status (1A and 1B) even after adjusting for over 20 risk factors including mechanical circulatory support and inotropes. These sex-differences resolved over time for UNOS Status 1A but worsened for UNOS Status 1B despite a higher rate of transplantation for women than men listed as UNOS Status 1B. The reasons remain unclear but likely are due to the complex interplay of sex with many variables related to waitlist mortality and transplantation. If fact, with machine learning statistics we identified over 20 sex interactions for mortality and transplantation that have not been previously described.
Acknowledgments
Sources of Funding: Supported by the National Heart, Lung and Blood Institute of the National Institute of Health under Award Number R56HL125420-01A1
Footnotes
Disclosures: None
References
- 1.Weidner G, Zahn D, Mendell NR, Smits JM, Deng MC, Zittermann A, Spaderna H. Patients’ sex and emotional support as predictors of death and clinical deterioration in the waiting for a new heart study: results from the 1-year follow-up. Prog Transplant. 2011;21:106–14. doi: 10.1177/152692481102100204. [DOI] [PubMed] [Google Scholar]
- 2. [Accessed on January 6, 2016];OPTN/SRTR Annual Report. Available at http://www.srtr.org/annual_Reports/archives/2009/2009_Annual_Report/waitlist_outcomes.htm.
- 3.Leppke S, Leighton T, Zaun D, Chen SC, Skeans M, Israni AK, Snyder JJ, Kasiske BL. Scientific Registry of Transplant Recipients: collecting, analyzing, and reporting data on transplantation in the United States. Transplant Rev (Orlando) 2013;27:50–6. doi: 10.1016/j.trre.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 4. [Last accessed on January 29, 2017];Organ Procurement and Transplantation Network: Policies. Available at: http://optn.transplant.hrsa.gov/PoliciesandBylaws2/policies/pdfs/policy_9.pdf.
- 5. [Accessed on January 7, 2016];FDA Approval HeartMate II. Available at http://www.fda.gov/newsevents/newsroom/pressannouncements/2008/ucm116881.htm.
- 6. [Accessed on January 7, 2016];FDA Approval of HeartWare. Available at http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm330838.htm.
- 7.Breiman L. Random forests. Mach Learn. 2001;45:5–32. [Google Scholar]
- 8.Ishwaran H, Kogalur UB, Blackstone EH, Lauer MS. Random survival forests. The annals of applied statistics. 2008:841–860. [Google Scholar]
- 9.Stekhoven DJ, Buhlmann P. MissForest--non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012;28:112–8. doi: 10.1093/bioinformatics/btr597. [DOI] [PubMed] [Google Scholar]
- 10.Politis DN, Romano JP, Wolf M. Subsampling. Springer-Verlag; New York: 1999. [Google Scholar]
- 11.Ishwaran H, Kogalur UB. RandomForestSRC: Random Forest for Survival R, and Classification (RF-SRC) R package version 2.20. 2016:2, 3, 8. http://cran.r-progject.org.
- 12.Hsich EM, Starling RC, Blackstone EH, Singh TP, Young JB, Gorodeski EZ, Taylor DO, Schold JD. Does the UNOS heart transplant allocation system favor men over women? JACC Heart Fail. 2014;2:347–55. doi: 10.1016/j.jchf.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Morris AA, Cole RT, Laskar SR, Kalogeropoulos A, Vega JD, Smith A, Butler J. Improved Outcomes for Women on the Heart Transplant Wait List in the Modern Era. J Card Fail. 2015;21:555–60. doi: 10.1016/j.cardfail.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Hsich EM, Naftel DC, Myers SL, Gorodeski EZ, Grady KL, Schmuhl D, Ulisney KL, Young JB. Should women receive left ventricular assist device support?: findings from INTERMACS. Circ Heart Fail. 2012;5:234–40. doi: 10.1161/CIRCHEARTFAILURE.111.963272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fonarow GC, Adams KF, Jr, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–80. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 16.Rahimi K, Bennett D, Conrad N, Williams TM, Basu J, Dwight J, Woodward M, Patel A, McMurray J, MacMahon S. Risk prediction in patients with heart failure: a systematic review and analysis. JACC Heart Fail. 2014;2:440–6. doi: 10.1016/j.jchf.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation. 1997;95:2660–7. doi: 10.1161/01.cir.95.12.2660. [DOI] [PubMed] [Google Scholar]
- 18.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–33. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 19.Horwich TB, Kalantar-Zadeh K, MacLellan RW, Fonarow GC. Albumin levels predict survival in patients with systolic heart failure. Am Heart J. 2008;155:883–9. doi: 10.1016/j.ahj.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 20.Biegus J, Hillege HL, Postmus D, Valente MA, Bloomfield DM, Cleland JG, Cotter G, Davison BA, Dittrich HC, Fiuzat M, Givertz MM, Massie BM, Metra M, Teerlink JR, Voors AA, O’Connor CM, Ponikowski P. Abnormal liver function tests in acute heart failure: relationship with clinical characteristics and outcome in the PROTECT study. Eur J Heart Fail. 2016 doi: 10.1002/ejhf.532. [DOI] [PubMed] [Google Scholar]
- 21.Kato TS, Stevens GR, Jiang J, Schulze PC, Gukasyan N, Lippel M, Levin A, Homma S, Mancini D, Farr M. Risk stratification of ambulatory patients with advanced heart failure undergoing evaluation for heart transplantation. J Heart Lung Transplant. 2013;32:333–40. doi: 10.1016/j.healun.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uthamalingam S, Kandala J, Daley M, Patvardhan E, Capodilupo R, Moore SA, Januzzi JL., Jr Serum albumin and mortality in acutely decompensated heart failure. Am Heart J. 2010;160:1149–55. doi: 10.1016/j.ahj.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Kempny A, Diller GP, Alonso-Gonzalez R, Uebing A, Rafiq I, Li W, Swan L, Hooper J, Donovan J, Wort SJ, Gatzoulis MA, Dimopoulos K. Hypoalbuminaemia predicts outcome in adult patients with congenital heart disease. Heart. 2015;101:699–705. doi: 10.1136/heartjnl-2014-306970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cowger J, Sundareswaran K, Rogers JG, Park SJ, Pagani FD, Bhat G, Jaski B, Farrar DJ, Slaughter MS. Predicting survival in patients receiving continuous flow left ventricular assist devices: the HeartMate II risk score. J Am Coll Cardiol. 2013;61:313–21. doi: 10.1016/j.jacc.2012.09.055. [DOI] [PubMed] [Google Scholar]
- 25.Kato TS, Kitada S, Yang J, Wu C, Takayama H, Naka Y, Farr M, Mancini DM, Schulze PC. Relation of preoperative serum albumin levels to survival in patients undergoing left ventricular assist device implantation. Am J Cardiol. 2013;112:1484–8. doi: 10.1016/j.amjcard.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsich E, Chadalavada S, Krishnaswamy G, Starling RC, Pothier CE, Blackstone EH, Lauer MS. Long-term prognostic value of peak oxygen consumption in women versus men with heart failure and severely impaired left ventricular systolic function. Am J Cardiol. 2007;100:291–5. doi: 10.1016/j.amjcard.2007.02.096. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez F, Wang Y, Johnson CE, Foody JM. National patterns of heart failure hospitalizations and mortality by sex and age. J Card Fail. 2013;19:542–9. doi: 10.1016/j.cardfail.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Khush KK, Kubo JT, Desai M. Influence of donor and recipient sex mismatch on heart transplant outcomes: analysis of the International Society for Heart and Lung Transplantation Registry. J Heart Lung Transplant. 2012;31:459–66. doi: 10.1016/j.healun.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Organ Procurement and Transplantation Network. [Accessed on May 14, 2016];Adult Heart Allocation Changes. 2016 Available at https://optn.transplant.hrsa.gov/governance/public-comment/adult-heart-allocation-changes-2016/
- 30.OPTN/UNOS Thoracic Organ Transplantation Committee. [Last accessed on January 2017];Public Comment Proposal to Modify the Adult Heart Allocation System. Available at https://optn.transplant.hrsa.gov/media/1921/thoracic_adult_heart_allocation_modification_20160815.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.