Abstract
Background
Thoracic aortic disease has a high mortality. We sought to establish the contribution of unwarranted variation in care to regional differences in outcomes observed in patients with thoracic aortic disease in England.
Methods and Results
Data from the Hospital Episode Statistics (HES) and the National Adult Cardiac Surgery Audit (NACSA) were extracted. A parallel systematic review/meta‐analysis through December 2015, and structure and process questionnaire of English cardiac surgery units were also accomplished. Treatment and mortality rates were investigated. A total of 24 548 adult patients in the HES study, 8058 in the NACSA study, and 103 543 from a total of 33 studies in the systematic review were obtained. Treatment rates for thoracic aortic disease within 6 months of index admission ranged from 7.6% to 31.5% between English counties. Risk‐adjusted 6‐month mortality in untreated patients ranged from 19.4% to 36.3%. Regional variation persisted after adjustment for disease or patient factors. Regional cardiac units with higher case volumes treated more‐complex patients and had significantly lower risk‐adjusted mortality relative to low‐volume units. The results of the systematic review indicated that the delivery of care by multidisciplinary teams in high‐volume units resulted in better outcomes. The observational analyses and the online survey indicated that this is not how services are configured in most units in England.
Conclusions
Changes in the organization of services that address unwarranted variation in the provision of care for patients with thoracic aortic disease in England may result in more‐equitable access to treatment and improved outcomes.
Keywords: aortic disease, aortic dissection, cardiac surgery, quality of care
Subject Categories: Health Services, Mortality/Survival, Quality and Outcomes
Introduction
Diseases of the thoracic aorta are increasing in prevalence worldwide.1, 2 In the United Kingdom (UK), between 1999 and 2010, hospital admissions for thoracic aortic dissection increased from 7.2 to 8.8 and for thoracic aortic aneurysm from 4.4 to 9.0 per 100 000 inhabitants.3 These diseases have a high mortality; in the UK, mortality rates for thoracic aortic dissection and aneurysm are 3.2 and 7.5 per 100 000 inhabitants, respectively.3 There is evidence of regional variation in clinical outcomes for patients with thoracic aortic disease (TAD)4, 5, 6, 7, 8, 9, 10, 11; for example, operative mortality rates for acute type A dissection, the most common acute presentation of TAD, range from 2.8% to 47.6% between centers.4, 9, 10, 11, 12, 13, 14, 15, 16 This may reflect differences in socioeconomic, ethnic, and other demographic characteristics of local populations, but there is also evidence of variation in the provision of aortic services in the UK and elsewhere.7, 10, 16 Targeting unwarranted variation is a key objective for health services as a means of improving the quality and equity of access to care.17
The aim of the current study was to evaluate the contribution of unwarranted variations in care to regional differences in outcome observed in TAD patients in England and identify areas of structure and process for quality improvement.
Methods
Study Design
We measured mortality (primary outcome) along with a range of other important measures of quality, such as equity of access, timeliness of surgery, and the effect of treatment on longer‐term patient outcomes in national databases used to monitor quality of care by National Health Service (NHS) England; the National Adult Cardiac Surgery Audit (NACSA), used by the National Institute for Comparative Outcomes Research (NICOR) to monitor cardiac surgeon and unit specific hospital mortality, and the administrative NHS database Hospital Episode Statistics (HES), used by Dr Foster to measure hospital performance. We also asked cardiac surgical units in England to complete a questionnaire on the structure and organization of TAD services. Because there is no agreed service specification for TAD services in England and elsewhere,7, 10, 16, 18, 19 and current recommendations for service organization are not evidence based,20, 21, 22, 23, 24 we also conducted a parallel systematic review of existing studies that have considered quality standards for TAD service delivery. Data were extracted from the HES and the NICOR NACSA registry, according to The REporting of studies Conducted using Observational Routinely collected health Data (RECORD) statement (Table S1).25 The need to obtain informed consent from patients was waived by the University of Leicester Research Governance Office because the identifiable information was either removed or pseudonymized. The study was approved by the NICOR NACSA Research Board (study reference 14‐ACS‐25). A systematic review and meta‐analysis on the standard of care for the management of TAD was also performed and adhered to MOOSE and PRISMA guidelines (Tables S2 and S3).26, 27
Data Sources and Study Populations
Data, outcomes, and study populations obtained from HES and NICOR NACSA registries are fully reported in Data S1. Briefly, HES is the national hospital administrative database for England and covers all admissions to public (NHS) hospitals in the country.28 The data contain demographic, administrative, and clinical information, including procedures and operations (Table S4). The NICOR NACSA registry (version 4.1.2) contains prospectively data for all English adult patients undergoing major thoracic aortic surgery and undergoes robust validation and checking procedures to maintain data quality.16, 29, 30, 31
To complement the NACSA study, we also contacted the Society for Cardiothoracic Surgery Unit Representatives for every cardiac surgery unit in England assessing their current service organization for TAD. Surgeons were queried on the presence of a dedicated aortic team, a specific on‐call rota for thoracic aortic disease, a hybrid theater, and an aortic multidisciplinary team (MDT) recognized in the consultant job plan.
The study was approved by the NICOR NACSA Research Board (study reference 14‐ACS‐25). The need to obtain informed consent from patients was waived by the University of Leicester Research Governance Office because the identifiable information was either removed or pseudonymized.
Systematic Review and Meta‐Analysis
Electronic search strategy, objectives, criteria for study selection, eligibility, data collection, and assessment of study quality were published online and registered in the PROSPERO International Prospective Register of Systematic Reviews (PROSPERO registry—CRD42015024137).32
Briefly, 3 reviewers systematically searched electronic databases (MEDLINE [PubMed and Ovid], Embase, SCOPUS, and Cochrane Library) without date or language restriction from inception to the end of December 2015. Our keywords and MeSH terms pertinent to the exposure of interest were used in relevant combinations and included: “aorta”, “aorta, thoracic”, “aorta, thoracoabdominal”, “aortic aneurysm”, “aortic dissection”, “standard of care”, “health care”, “treatment outcome”, “hospital mortality”, “hospital volume”, “surgeon volume”, “volume outcome relationship”, “teaching hospital”, and “urban hospital”. In addition, the reference lists of all retrieved articles were reviewed for further identification of potentially relevant studies that were not previously identified.
All adult major thoracic aortic procedures were considered. Exposures of interest included hospital volume activity, generally defined as yearly number of major aortic operations performed, subdivided in low‐ or high‐volume, surgeon volume, presence of multidisciplinary thoracic aortic surgery program, and teaching/urban hospital status. The primary outcome of interest was all‐cause mortality in hospital or within 30 days from index admission or procedure. Secondary outcomes included postoperative stroke, re‐exploration for bleeding/tamponade, postoperative renal failure, and total length of hospital stay. Inclusion and exclusion criteria for qualitative/quantitative analyses were summarized according to PICOS approach (Table S5).
Year of publication, study design, country, sample size, recruitment period, inclusion and exclusion criteria, measured outcomes, aortic center configuration, and definition of low‐ and high‐volume threshold, baseline patient demographics (age, sex), and outcomes among low‐ and high‐volume groups were extracted. Quality assessment was performed using the Newcastle‐Ottawa Scale (NOS).33
Statistical Analysis
HES cohort
Outcomes were calculated as crude proportions and adjusted for a number of patient factors as listed in Tables S6 and S7. Comorbidity information was taken from the index admission or any admission for any reason in the previous year. All outcomes were binary, so logistic regression was used. These models were hierarchical with 2 levels, with random effects for each county. Predicted probabilities for each patient were derived from the fixed‐effects part of the model.34 Hierarchical models adjust for the clustering of patients within county and allow the estimation of the proportion of variation in the outcome that is attributable to each level of the model (ie, to patient factors and to county). To obtain adjusted outcome rates by county, observed and predicted probabilities were summed by county, with the former divided by the latter to obtain relative risks. These were then multiplied by the national crude outcome rate to obtain adjusted rates. Rates were put onto funnel plots with 95% and 99.8% control limits, the latter to determine how many counties were statistical outliers. To assess model fit, deviance residuals were plotted and the Hosmer‐Lemeshow risk deciles and chi‐squared value also inspected. Discrimination was assessed using the area under the receiver‐operating characteristic curve (c statistic). This was done for logistic regression without adjustment for clustering, because there is no consensus over how to calculate the equivalent measures for hierarchical models.35 As well as random effects for each geographical area, random slopes were tried in the model for receiving surgery for several binary patient factors: age over 75 versus younger, 1 or more versus none of 5 major comorbidities, dissection versus none, and aneurysm versus none. This was to see whether any of these factors had different effects on the outcome depending on the area. We did not find such effects and therefore report only results from using fixed slopes. Finally, obtained rates for TAD treatment by county as well as 6‐month mortality for treated and untreated patients were mapped using ArcMap version 10.3 (ESRI, Redlands, CA).
NACSA cohort
Categorical and dichotomous variables were summarized as absolute number and percentage. Non‐normally distributed continuous data were summarized as medians and interquartile ranges (IQRs). The effects of operational and institutional characteristics on in‐hospital mortality were assessed using multiple logistic regression models. Relevant patient‐level variables were offered to the models to adjust for any potential confounding factors. Results of the regression analyses were expressed as odds ratios (ORs) and 95% CIs. Box and whisker plots were used to present case mix distributions by center: These plots show the 25th percentile, median and 75th percentile of a given distribution at the bottom, middle and top of the boxes, respectively, then the mean is then plotted as a dot, and the lower and upper whiskers then represent the 5th and 95th percentiles, respectively. Scatter plots were generated to assess the relationship between observed in‐hospital mortality and volume, and ordinary least squares (OLS) regression lines were included for visual inspection. Statistical analyses were performed with SAS software (version 9.3; SAS Institute Inc., Cary, NC). In all cases, P<0.05 was considered statistically significant.
Systematic review and meta‐analysis
Treatment effect on operative outcomes is reported as ORs with a 95% CI. Yates correction was implemented if a cell contained a zero in the 2×2 contingency table.36 Individual ORs (OR <1: high volume centers better) and variance were computed by using number of events and sample size and pooled by using Mantel–Haenszel method and random‐effects model.37 A fixed‐effects model was also computed as sensitivity analysis. A subgroup analysis according to the primary aortic pathology (aneurysm vs aortic dissection) was performed, being a possible significant effect modifier. Finally, to account for inherent patient selection bias related with an observational study design, individual risk‐adjusted ORs for the primary endpoint were obtained when reported, and pooled adjusted risk estimates were computed by using log transformation and a generic inverse‐variance weighting method. I2 statistic was used to estimate the percentage of total variation across studies attributed to heterogeneity rather than chance. Suggested thresholds for heterogeneity were used, with I2 values of 25% to 49%, 50% to 74%, and ≥75%, indicative of low, moderate, and high heterogeneity.38 Publication bias was evaluated using visual inspection of funnel plot asymmetry and by Egger's test.39 P<0.05 was used as the level of significance and 95% CIs were reported where appropriate. Statistical analysis was conducted using meta package for R (version 4.3‐2; R Foundation for Statistical Computing, Vienna, Austria).40, 41
Results
HES Cohort
Of 26 551 patients with a TAD admission in England between 2004–2005 and 2010–2011, 25 282 had not had such an admission in the previous 5 years and were defined as index admissions. Seven hundred thirty‐four (2.8%) were excluded because of lack of area identifiers, leaving a final population of 24 548 adult patients coded as having a new diagnosis of TAD. Of these, 16 448 (67%) were affected by aneurysms, 6345 (25.9%) by dissections, and 1665 (6.8%) by unspecified TAD. A total of 5445 (22%) underwent treatment (surgical and/or endovascular) within 6 months of diagnosis. The 6‐month mortality in treated patients was 17.7% and in untreated patients was 30%. Patient characteristics are summarized in Table 1.
Table 1.
National Crude Outcome Rates Split by Age, Major Comorbidity, and TAD Subtype (HES Cohort)
| Patient Factora | Receiving Operation (%) | Nonemergent Operation (%) | Postoperative Mortality (%) | Mortality in Patients With No Operation (%) |
|---|---|---|---|---|
| Age <75, y | 31.6 | 54.4 | 14.0 | 18.4 |
| Age 75+, y | 11.6 | 52.6 | 24.3 | 40.7 |
| TAD: dissection | 19.4 | 10.9 | 21.5 | 39.9 |
| TAD: aneurysm | 15.8 | 69.3 | 12.5 | 26.4 |
| No major comorbidity | 27.6 | 50.1 | 12.2 | 27.6 |
| 1+ major comorbidities | 18.8 | 58.2 | 21.0 | 31.7 |
| All patients combined | 22.2 | 53.7 | 16.7 | 30.5 |
HES indicates Hospital Episodes Statistics; TAD, thoracic aortic disease.
Patients with neither dissection nor aneurysm recorded have been omitted from the rows for TAD subtype. Major comorbidities covered ischemic heart disease, cerebrovascular disease, chronic obstructive pulmonary disease, renal disease, and cancer. Mortality is defined as death in or out of hospital within 6 months of diagnosis or operation.
Variation attributable to patient‐related factors
Predictive variables for receiving treatment, or an emergent procedure, are reported in Table S6. Briefly, increasing age, pre‐existing diabetes mellitus, comorbidities, and a diagnosis of cancer were associated with a conservative approach. The greater the deprivation status, the lower were the odds of being treated. Patients affected by aortic dissection and comorbid conditions, including ischemic heart disease and congenital and other vascular disorders, were more prone to be treated on an emergent basis. Predictors of 6‐month mortality in treated and untreated patients are summarized in Table S7. In patients receiving treatment, mortality was associated with increasing age, comorbidity presence, and nonelective admission. Emergent/urgent admission, increasing age, severe comorbidity, and the presence of aortic dissection were associated with mortality in untreated patients.
Variation attributable to non‐patient‐related factors
Significant variance by county of residence was observed in terms of the percentages of patients receiving treatment within 6 months of their index admission, ranging from 7.6% in Leicestershire to 31.5% in the West Midlands (Figure 1). The percentage of subjects treated emergently ranged from 29.6% in Merseyside to 67.9% in Durham (Figure 2). Multilevel modeling confirmed the statistically significant differences in treatment rates by county of residence. A null model containing only random effects for the counties estimated the variance between counties in log odds of treatment as 0.096 (SE, 0.024); adding patient factors to this null model actually increased the estimated between‐county variance (to 0.105; SE, 0.026). To adjust for regional differences in detection rates, the TAD admission rates per 100 000 resident populations for each county were entered into the model. This reduced the between‐county variance in treatment rates by 40% to 0.060 (SE, 0.016). Despite this, the overall number of counties flagged as high or low outliers on funnel plots using 99.8% control limits were very similar to those observed using crude TAD admission rates.
Figure 1.
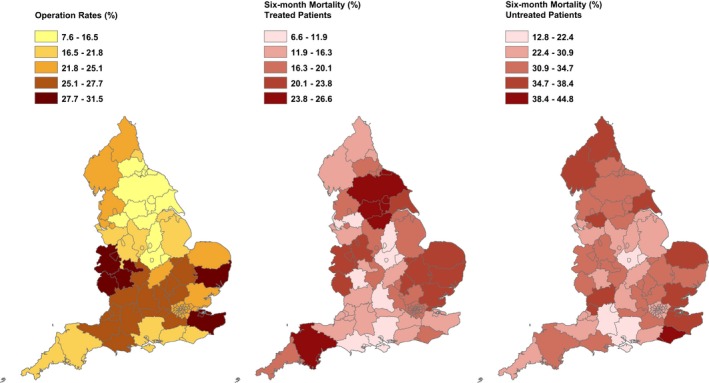
Geographical variation by county across England with reference to treatment rates in patients diagnosed with thoracic aortic disease (left panel), 6‐month mortality in treated (mid panel) and untreated (right panel) patients. From HES (Hospital Episodes Statistics) cohort data.
Figure 2.
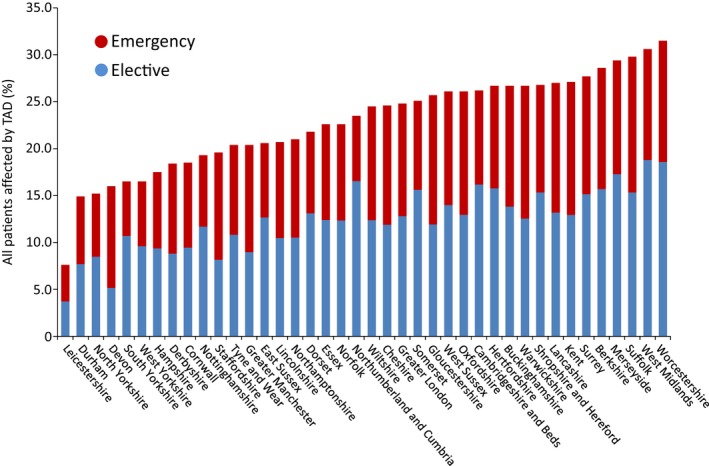
Percentage of patients affected by thoracic aortic disease (TAD) by county and urgency of the operation received (elective vs emergent). From HES (Hospital Episodes Statistics) cohort data.
Regional differences by county were also observed for 6‐month mortality (Figure 1 and Figure S1). For treated patients, risk‐adjusted mortality rates ranged from 6.5% in Oxfordshire to 23.3% in North Yorkshire. Risk‐adjusted mortality rates in untreated patients ranged from 19.4% in Leicestershire to 36.3% in East Sussex. Adding patient factors to the null model reduced the variation in mortality in treated patients attributable to county of origin by 27%, therefore becoming nonsignificant (variance estimate, 0.037; SE, 0.023). A 62% fall in the variation in mortality was observed in untreated patients, although the between‐county variance remained statistically significantly greater than zero, but modest in size (0.027; SE, 0.009).
Using Pearson correlation with counties weighted by their total number of index admissions, we compared the sets of county‐level adjusted outcome rates. The proportion of patients receiving treatment showed positive, but nonsignificant, correlations with the proportion of treatments done nonemergently (ρ=+0.20; P=0.209) and the postoperative mortality rate (ρ=+0.25; P=0.114). Conversely, the proportion of treated patients demonstrated a positive and statistically significant correlation with the mortality rate in untreated patients (ρ=+0.47; P=0.002). This latter relation was driven by patients without dissection: for these patients alone, the correlation was +0.68 (P<0.001), whereas for those with dissection it was −0.28 (P=0.079). In order to verify whether the positive correlation between treatment rates and 6‐month mortality in both treated and untreated patients may reflect an underlying difference in access to care, patients' risk profiles were analyzed. No correlation between the proportion of high‐risk patients treated by county, defined as those in the highest tertile for predicted probability, and mortality rate in the untreated was observed (ρ=+0.17; P=0.294).
NACSA Cohort
We considered that confounders not contained within HES data might also contribute to unwarranted variance. We therefore evaluated regional variance in treatment of TAD using the NACSA registry, which contains validated data on the severity and complexity as well as the treatment of TAD by regional cardiac centers.
Study cohort
Of the 219 741 patients that underwent surgery in cardiac surgery centers in England, complete case data were available on 8058 major aortic surgery cases from 29 hospitals that comprised the analysis data set. Patient characteristics and operative details are summarized in Table 2 and Table S8. All centers provided data on patients operated on root/ascending aorta and aortic arch segments, 28 (96.6%) centers on the descending thoracic aorta, and 17 (58.6%) on the thoracoabdominal aorta (Figure 3).
Table 2.
Baseline, Operative, and Mortality Details by Center Volume (Tertiles of Latest 3‐Year Activity) (NACSA Cohort)
| Patient Factora | Low‐Volume Center (n=1308) | Medium‐Volume Center (n=2159) | High‐Volume Center (n=4591) |
|---|---|---|---|
| Demographics | |||
| Age at operation, y | 64 (52, 72) | 64 (52, 73) | 64 (51, 73) |
| BMI, kg/m2 | 27.2 (24.4, 30.2) | 27.0 (24.1, 30.4) | 26.7 (23.9, 29.9) |
| Female sex | 450 (34.4) | 715 (33.1) | 1526 (33.2) |
| Comorbidities | |||
| Unstable angina | 77 (5.9) | 114 (5.3) | 181 (3.9) |
| NYHA ≥III | 411 (31.4) | 721 (33.4) | 1184 (25.8) |
| MI within 90 days of operation | 58 (4.4) | 72 (3.3) | 138 (3.0) |
| Previous cardiac surgery | 150 (11.5) | 307 (14.2) | 795 (17.3) |
| Previous aortic surgery | 27 (2.1) | 71 (3.3) | 237 (5.2) |
| Diabetes mellitus | 102 (7.8) | 175 (8.1) | 276 (6.0) |
| Current smoker | 166 (12.7) | 236 (12.2) | 478 (10.4) |
| Hypertension | 838 (64.1) | 1419 (65.7) | 2779 (60.5) |
| Creatinine >200 μmol/L | 39 (3.0) | 62 (2.9) | 123 (2.7) |
| History of renal dysfunction | 16 (1.2) | 37 (1.7) | 81 (1.8) |
| History of pulmonary disease | 153 (11.7) | 267 (12.4) | 554 (12.1) |
| History of stroke | 119 (9.1) | 192 (8.9) | 350 (7.6) |
| Neurological dysfunction | 55 (4.2) | 91 (4.2) | 160 (3.5) |
| Peripheral vascular disease | 213 (16.3) | 452 (20.9) | 647 (14.1) |
| Preoperative nonsinus heart rhythm | 182 (13.9) | 278 (12.9) | 478 (10.4) |
| Triple vessel disease | 68 (5.2) | 134 (6.2) | 169 (3.7) |
| Left main stem disease | 30 (2.3) | 45 (2.1) | 77 (1.7) |
| Moderate ejection fraction (30–50%) | 308 (23.6) | 419 (19.4) | 857 (18.7) |
| Poor ejection fraction (<30%) | 57 (4.4) | 92 (4.3) | 179 (3.9) |
| PA systolic >60 mm Hg | 26 (2.0) | 26 (1.2) | 42 (0.9) |
| Preoperative IV nitrates | 62 (4.7) | 118 (5.5) | 231 (5.0) |
| Preoperative IV inotropes | 35 (2.7) | 58 (2.7) | 133 (2.9) |
| Preoperative ventilation | 22 (1.7) | 42 (2.0) | 102 (2.2) |
| Preoperative cardiogenic shock | 88 (6.7) | 100 (4.6) | 154 (3.4) |
| Nonelective priority | 497 (38.0) | 801 (37.1) | 1643 (35.8) |
| Urgent priority | 202 (15.4) | 396 (18.3) | 697 (15.2) |
| Emergency priority | 267 (20.4) | 355 (16.4) | 888 (19.3) |
| Salvage priority | 28 (2.1) | 50 (2.3) | 58 (1.3) |
| Dominant pathology | |||
| Aneurysm | 697 (53.3) | 1248 (57.8) | 2477 (54.0) |
| Dissection | 326 (24.9) | 481 (22.3) | 1012 (22.0) |
| Trauma | 7 (0.5) | 7 (0.3) | 36 (0.8) |
| “Other” | 166 (12.7) | 366 (17.0) | 702 (15.3) |
| Data N/A | 112 (8.6) | 57 (2.6) | 364 (7.9) |
| Aortic segment | |||
| Root/ascending aorta | 1211 (92.6) | 1798 (83.3) | 3839 (83.6) |
| Aortic arch | 75 (5.7) | 275 (12.7) | 412 (9.0) |
| Descending aorta | 17 (1.3) | 58 (2.7) | 245 (5.3) |
| Thoracoabdominal aorta | 5 (0.4) | 28 (1.3) | 95 (2.1) |
| Surgical data | |||
| Concomitant valve operation | 948 (72.5) | 1339 (62.0) | 3032 (66.0) |
| Concomitant CABG operation | 237 (18.1) | 399 (18.5) | 846 (18.4) |
| Concomitant “other” cardiac operation | 510 (39.0) | 728 (33.7) | 1408 (30.7) |
| CPB time, min | 178 (129, 240) | 152 (114, 208) | 162 (116, 229) |
| ACC time, min | 114 (82, 154) | 104 (74, 136) | 105 (75, 143) |
| Circulatory arrest time, min | 26 (18, 37) | 25 (17, 34) | 27 (18, 39) |
| Outcome | |||
| In‐hospital mortality | 138 (10.6) | 206 (9.5) | 404 (8.8) |
ACC indicates aortic cross‐clamp; BMI, body mass index; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; IV, intravenous; NACSA, National Adult Cardiac Surgery audit; N/A, not available; NYHA, New York Heart Association; PA, pulmonary artery.
Numerical data are expressed as median and interquartile range (IQR); categorical data as absolute number (percentage).
Figure 3.
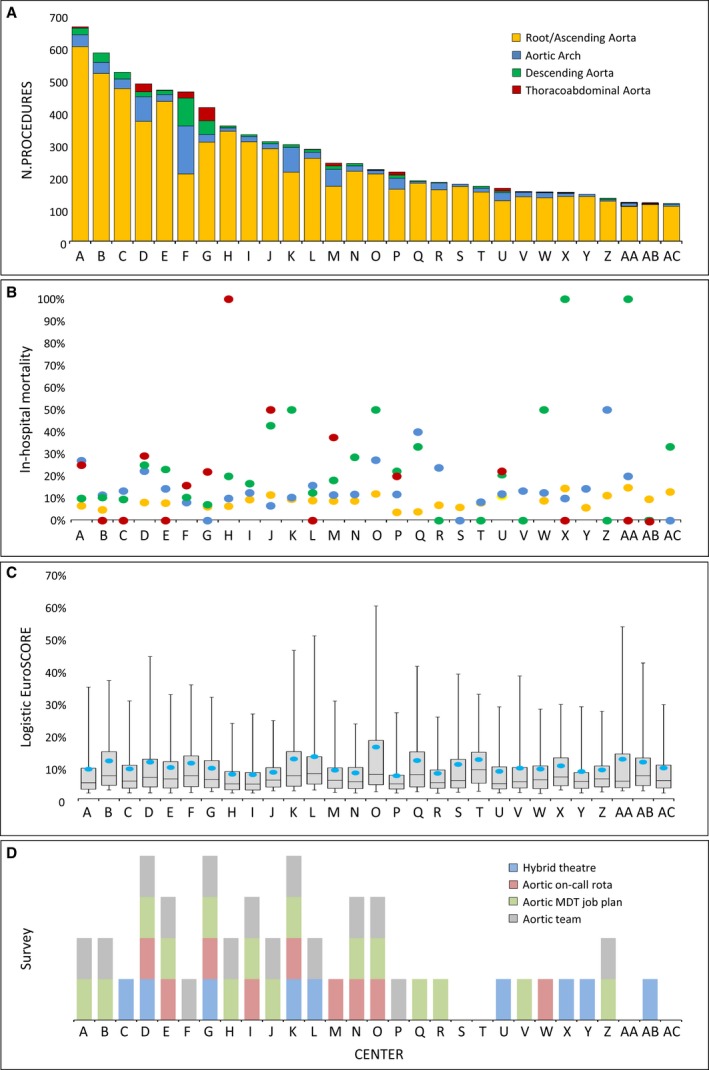
Activity (total number of procedures) (A) and in‐hospital mortality rate (B) by center, by most distal aortic segment; patient risk profile by center expressed by EuroSCORE II (C). From NACSA (National Adult Cardiac Surgery Audit) cohort data. Results of the national survey assessing current service organization for thoracic aortic disease in cardiac surgery centers across England; surgeons were queried on the presence of a dedicated aortic team, a specific on‐call rota for thoracic aortic disease, a hybrid theater, and an aortic multidisciplinary team (MDT) recognized in the consultant job plan (D): The presence of a vertical bar for a given center means that that center had the particular feature given in the chart key.
Variation attributable to patient‐related factors
There were differences between centers with respect to predicted operative risk (Figure 3C); the median calculated logistic EuroSCORE of in‐hospital mortality ranged from 4.6% to 9.1%. Pathology, emergent treatment, and the most distal aortic surgery segment treated (case complexity) were important determinants of hospital mortality (Table 3).
Table 3.
Unadjusted and Risk‐Adjusted In‐Hospital Mortality Effects (NACSA Cohort)
| Frequency | Observed Mortality (%) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|---|---|
| Aortic segmenta | ||||
| Root/ascending aorta | 6848 | 8.3 | Reference | Reference |
| Aortic arch | 762 | 13.3 | 1.75 (1.42, 2.16) | 1.86 (1.47, 2.35) |
| Descending aorta | 320 | 15.3 | 1.86 (1.38, 2.51) | 2.30 (1.66, 3.18) |
| Thoracoabdominal aorta | 128 | 22.7 | 1.91 (1.18, 3.09) | 2.75 (1.67, 4.56) |
| Activity tertileb | ||||
| Low‐volume (latest 3 years' activity) | 1308 | 10.6 | Reference | Reference |
| Medium‐volume (latest 3 years' activity) | 2159 | 9.5 | 0.89 (0.71, 1.12) | 0.80 (0.62, 1.02) |
| High‐volume (latest 3 years' activity) | 4591 | 8.8 | 0.82 (0.67, 1.00) | 0.76 (0.60, 0.95) |
| Dominant pathologyc | ||||
| Aneurysm | 4422 | 4.9 | Reference | Reference |
| Dissection | 1819 | 17.2 | 4.07 (3.39, 4.89) | 2.27 (1.82, 2.82) |
| Other | 1284 | 13.2 | 2.97 (2.40, 3.67) | 2.05 (1.61, 2.61) |
| Data N/A | 533 | 9.6 | 2.07 (1.50, 2.85) | 1.78 (1.26, 2.52) |
| Priorityb | ||||
| Elective | 5117 | 4.8 | Reference | Reference |
| Nonelective | 2941 | 17.1 | 4.08 (3.47, 4.78) | 2.54 (2.09, 3.08) |
Adjusted for preoperative comorbidities, operative risk factors, and activity tertile.
Adjusted for preoperative comorbidities, operative risk factors, and most distal aortic segment.
Adjusted for preoperative comorbidities, operative risk factors, most distal aortic segment, and activity tertile.
Variation attributable to non‐patient‐related factors
There were differences between centers with respect to the complexity and volume of cases performed. The largest volume of cases by a single center was 662 and the smallest 117 (Figure 3A). The percentage of root/ascending aortic operations as a share of total aortic operations ranged from 45.1% to 96.0%, for aortic arch procedures the range was from 1.7% to 32.1%, for descending thoracic aortic procedures from 0.7% to 18.7%, and for thoracoabdominal aortic procedures from 0.2% to 9.9% (Table S9 and Figure S2). More‐complex surgery was more common in high‐volume centers. The results of the survey of service organization are shown alongside details of case volume, complexity, and outcome by unit in Figure 3D. All the units responded to the questionnaire. This demonstrated regional variation in care delivery in terms of the presence of dedicated aortic teams, multidisciplinary aortic team meetings, specific on‐call rotas for aortic emergencies, or use of hybrid operating theaters.
Table 3 and Table S10 show unadjusted and fully risk‐adjusted in‐hospital mortality effects by operation category, tertile of volume activity, dominant pathology, and priority. Case complexity was a principal determinant of in‐hospital mortality. Relative to proximal segments (root/ascending) the adjusted ORs for aortic arch procedures as well as descending thoracic and thoracoabdominal aortic procedures were 1.88 (95% CI, 1.48–2.37), 2.47 (95% CI, 1.77–3.44), and 3.05 (95% CI, 1.82–5.11), respectively. For the increasing volume activity tertiles, the corresponding adjusted risk of in‐hospital mortality relative to low‐volume centers was 0.84 (95% CI, 0.66–1.07) for medium‐volume centers and 0.72 (95% CI, 0.57–0.89) for high‐volume centers (Table 4). Similar results were observed when cases where stratified by most distal segment, as well for the OLS regression analyses that demonstrated lower mortality in centers with high‐volume activity (Figure S3).
Table 4.
Mortality Rates by Volume Center Activity (NACSA Cohort)
| Frequency | Observed Mortality (%) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|---|---|
| Activity tertilea | ||||
| Low‐volume (latest 3 years' activity) | 1308 | 10.6 | Reference | Reference |
| Medium‐volume (latest 3 years' activity) | 2159 | 9.5 | 0.89 (0.71, 1.12) | 0.80 (0.62, 1.02) |
| High‐volume (latest 3 years' activity) | 4591 | 8.8 | 0.82 (0.67, 1.00) | 0.76 (0.60, 0.95) |
| Activity tertilea | ||||
| Low‐volume (6 years' activity) | 1424 | 11.1 | Reference | Reference |
| Medium‐volume (6 years' activity) | 2353 | 9.5 | 0.84 (0.68, 1.05) | 0.83 (0.66, 1.05) |
| High‐volume (6 years' activity) | 4281 | 8.6 | 0.75 (0.62, 0.91) | 0.71 (0.57, 0.88) |
NACSA indicates National Adult Cardiac Surgery audit; OR, odds ratio.
Adjusted for preoperative comorbidities, operative risk factors, and most distal aortic segment.
Systematic Review and Meta‐Analysis
Of the 12 804 records identified, 33 eligible observational cohort studies were included in the systematic review, comprising a total of 103 543 patients (Figure S4).6, 7, 8, 9, 10, 11, 12, 13, 14, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67 The identified studies (20 multicenter and 13 single‐center) were published between 1994 and 2015. Study characteristics and collected study outcomes are summarized in Tables S11 through S13. Quality assessment indicated that 20 of 33 studies were at significant risk of bias (NOS, <8; Table S14). Twelve observational cohort studies analyzing impact of hospital volume on in‐hospital mortality were identified for the primary analysis, including a total of 14 562 and 16 036 patients who underwent surgery in high‐ and low‐volume centers, respectively. Pooled unadjusted ORs showed that high‐volume centers were associated with a 50% relative risk reduction in mortality when compared with low‐volume centers (Figure 4, upper panel),1 with a moderate heterogeneity among studies (I2=53.4%). No publication bias was found (P=0.19; Figure S5). Overall, 9 studies reported on adjusted effect size of hospital volume on mortality (Table S15). Pooled adjusted estimates of individual log ORs confirmed that high‐volume centers were independently associated with a significantly reduced incidence of in‐hospital/30‐day mortality (adjusted OR, 0.56; 95% CI, 0.45–0.70; I2=70.2%; Figure 4 lower panel).7, 8, 10, 13, 50, 58, 59, 60, 62 Subgroup analysis showed similar effects for high‐volume centers with respect to both aneurysms and aortic dissection (Figure S6). Pooled estimates did not reveal any significant differences between high‐ and low‐volume centers with reference to postoperative stroke (OR, 1.29; 95% CI, 0.85–1.95; I2=58.8%), re‐exploration for bleeding/tamponade (OR, 0.91; 95% CI, 0.72–1.15; I2=68.5%), and postoperative renal failure (OR, 0.82; 95% CI, 0.65–1.04; I2=77.6%; Figure S7). Centers that introduced a specific multidisciplinary TAD program also reported a significant reduction in mortality (OR, 0.35; 95% CI, 0.13–0.96) although with significant heterogeneity (I2=75.7%; Figure S8). Surgeon volume (high‐ vs low‐volume surgeon) and hospital status (teaching vs nonteaching hospitals and urban vs rural hospitals) had no effect on hospital outcomes (Figures S9 and S10).
Figure 4.
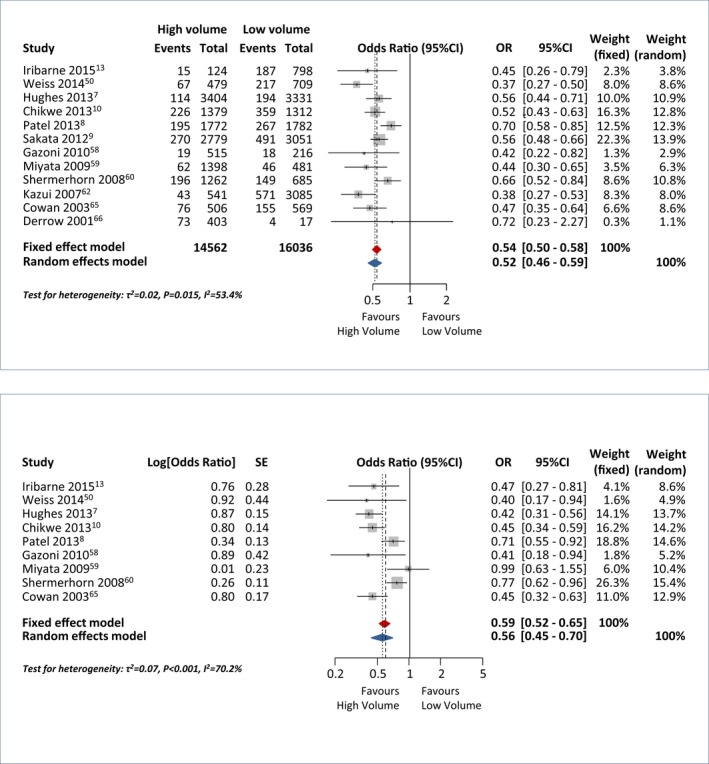
Forest plot with unadjusted (top) and adjusted (bottom) risk estimates for in‐hospital/30‐day mortality in high‐ versus low‐volume hospitals. OR indicates odds ratio.
Discussion
The current study has demonstrated significant regional variation in access to treatment, the organization of clinical services, and mortality for patients with TAD in England. An analysis of HES data demonstrated that the variation in the proportion of TAD patients treated within 6 months of diagnosis ranged from 7.6% to 31.5% among counties and remained statistically significant after adjustment for potential confounders, including comorbidity, deprivation, disease severity, and population density. Regional variation was not associated with differences in mortality rates for patients that received treatment, but was associated with differences in mortality in those that did not receive treatment, implying that inequity in access to care has important effects on outcome. The analysis of NASCA data indicated wide regional variation in the volume and complexity of TAD cases undertaken in English cardiac centers. Centers undertaking higher volumes were more likely to treat more‐complex disease and had lower risk‐adjusted mortality. A systematic review that attempted to benchmark service specifications for TAD indicated that patients treated by multidisciplinary teams in high‐volume centers have better clinical outcomes. A survey of structure and processes indicated that this standard of care is not consistently available to patients in England. In addition, our systematic literature search confirmed a world‐wide knowledge gap with respect to the safest, most effective referral model/organization of services for the management of TAD. Neither was identified the minimum service specification for centers that undertake interventions on the thoracic aorta.
To our knowledge, this is the first nation‐wide analysis of the quantity and quality of care for patients with TAD. The study used prospectively collected data from 2 large independent national databases used by the NHS to monitor quality. These contain data on every patient presenting to hospital with TAD or undergoing surgery for TAD in English hospitals. The limitations are those of all registry analyses, notably the risks of confounding and other sources of bias, including variable data quality. We attempted to minimize confounding by adjusting for a large number of baseline patient‐related factors, including demographics, social deprivation, comorbidity, and presentation. Detection bias was mitigated by using objective measures of outcome and exposures of interest. The NACSA database uses consistent, well‐defined definitions of exposures and outcomes and undergoes regular internal and external quality assurance processes. Being an administrative database, HES is more likely to have variations in data quality by hospital; under‐recording of comorbidities, for instance, is a well‐known limitation. For the TAD diagnosis date, we used the date of first recording of TAD in 1 of the diagnosis fields or the date of the TAD procedure if the patient had no earlier TAD admission, implying some uncertainty in the actual diagnosis date. However, certain fields, such as for the primary diagnosis and procedure, have been shown to be reliable by a recent systematic review.68 A further limitation is that it was not possible to link the HES and NACSA analyses to further explore potential reasons for variability in care. This is because the geographical regions served by individual cardiac centers often overlap. The 2 cohorts also considered different time periods; this was attributed to the availability of complete, cleaned HES data to March 2011, and the availability of data that used consistent definitions for aortic disease in the NACSA database between 2007 and 2013. Our analyses did not specifically consider the role of thoracic endovascular aortic repair (TEVAR). It has been suggested that this has important effects on the mortality of aortic disease, and almost all studies have shown excellent operative mortality rates post‐TEVAR compared to open repair, with even higher survival rates after emergent aortic procedures.57, 69 The total numbers of TEVARs listed in the HES database to 2011 were small (n=532), however, preventing useful and detailed analysis.
In addition, our analysis did not account for the potential impact of surgical techniques adopted across English units in the outcomes of TAD patients undergoing surgery. Rates and modality of circulatory arrest used during complex TAD operation, arterial cannulation, and cerebral protection strategies per center were not collected in HES and NACSA databases. This precluded further analyses and the possibility to evaluate these surgical strategies as an additional measure of the quality of care in TAD patients, although arterial cannulation strategies and cerebral protection strategies have been proved to influence operative outcomes in aortic surgery.70, 71
The systematic review was limited in that it relied on the reported information on confounding variables that were controlled for; consistent analyses of all studies can be done only when data on individual patients are combined. Many of the included studies were at risk of bias, and there was substantial heterogeneity in many of the effect estimates. We speculate that this reflected differences in the definitions of exposures of interest, including the definition of a high case volume or what constituted an aortic multidisciplinary team. For example, the definition of high versus low volume was defined, in some studies, as annualized activity versus study period activity, with the numbers of cases expressed varyingly as tertiles or medians.2
These limitations notwithstanding, this study has demonstrated variability in the quality of care for patients with TAD that appears to be unwarranted. This is a common finding in studies of variation in access to care for patients with cardiovascular disease in England and elsewhere.17, 72 Some of the contributory factors to variation identified in this report, such as social deprivation, require complex and difficult solutions; however, variations in structure and processes of care are more readily addressed. For example, service specifications for the provision of vascular surgery in England have led to substantive reorganization of care pathways, the concentration of multidisciplinary expertise in teams, and significant improvements in key markers of quality, such as mortality following elective aneurysm repair.73, 74 In the current study, higher‐volume units and those undertaking significant numbers of more‐complex procedures were more likely to have structures in place that were identified in the systematic review as being associated with better outcomes, specifically hybrid operating theatres, and adequately resourced MDT aortic teams. On the basis of these results, we suggest that these structures should be included in any future service specification for thoracic aortic disease. The definition of an adequate unit volume is more difficult; the NASCA data identified units in the lowest tertile of total cases (<32 cases per year) as having a higher mortality, often with a denominator that included a less‐complex caseload. However, the current study did not specifically address whether this reflects outcomes following the treatment of emergent patients. This is important: Patients with acute type A dissection who do not receive treatment die at a rate of 1% to 2% per hour during the first day and almost half die by 1 week.75 A reduction in the numbers of units providing emergency services should be balanced by the increase in risk posed by delays in treatment. However, both HES and NACSA databases do not account for information regarding the referral time, interhospital coordination, and transport of patient affected by TAD, especially in the emergent setting, and we were unable to investigate this important aspect of quality of care.56
The results of the NASCA analysis also indicated uncertainty as to whether the volume outcome relationship applied to all segments of the aorta, although this may be attributable to a smaller sample size for more‐distal segments resulting in less precision in the estimates. The systematic review did not indicate that surgeon volume was associated with outcome. This may also reflect the limits of precision when evaluating small numbers of surgeons, the majority of whom undertake low numbers of cases. Alternatively, as suggested by the systematic review, it may be the structures and process beyond that surgeon that are critical determinants of outcome.
The present analyses also identified potential sources of unwarranted variation that will not be addressed solely by reconfiguration of specialist teams, for example, with respect to differences in treatment rates for aneurysms versus nonaneurysms, or for emergent versus nonemergent surgery. We speculate that additional barriers to treatment exist before hospital treatment. This variation can be addressed by guidelines for screening, for example, in first‐degree relatives of patients with acute aortic syndromes or bicuspid aortic valves, the use of appropriate imaging, and referral to the TAD service. However, a barrier to the development of these processes is the absence of evidence from randomized trials as to how TAD should be diagnosed and treated. Recent guidelines were based exclusively on evidence from observational analyses and expert opinion.20, 21, 22
A final comment is that the variation in TAD services that were observed in this study were not apparent when comparing mortality rates in treated patients by center, the current methods used by Dr Foster (HES), and the National Institute for Comparative Outcomes Research (NASCA) for measuring quality in English cardiac uits.28, 29, 30 It is also noteworthy that there was no evidence from the HES analysis that cardiac surgery units preferentially select patients that will have an acceptable outcome following surgery; treatment rates were not determined by patient mortality risk. This refutes a common criticism that the publication of mortality rates in treated patients, as has occurred in England since 2004, contributes to unwarranted variation.76
In conclusion, evidence of unwarranted variation in the quality of care supports a reorganization of TAD services in England, with greater emphasis on care delivered by multidisciplinary teams in specialist centers. Similar service specifications and recommendations for standards of care and service delivery for TAD patients have also been commonly observed in other countries, mainly in North America and Europe. However, the safest, most effective referral model/organization of services for the management of TAD has not been identified. Further research must focus on the identification of barriers to early diagnosis and referral for treatment, and comparative trials of treatment options for patients with TAD.
Appendix
Collaborators
UK Aortic Forum (Chairman: Mr Geoff Tsang): Mr Alan J. Bryan, Bristol Heart Institute, University Hospital Bristol NHS Trust; Mr Graham Cooper, Sheffield Teaching Hospitals; Mr Andrew Duncan, Biomedical Research Unit, The Royal Brompton Hospital; Miss Deborah Harrington and Mr Manoj Kuduvalli, Liverpool Heart and Chest Hospital; Mr Jorge Mascaro, Queen Elizabeth Hospital, University Hospitals Birmingham; Mr Ulrich Rosendahl, Aortic Centre, Royal Brompton and Harefield NHS Trust; Mr Geoff Tsang, University Hospital Southampton NHS Foundation Trust; Mr Jonathan Unsworth‐White, Southwest Cardiothoracic Centre, Derriford Hospital, Plymouth.
Author Contributions
Bottle, Mariscalco, Murphy, and Shaw had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Bashir, Benedetto, Bottle, Mariscalco, Murphy, and Oo. Acquisition of data: Aylin, Bottle, Mariani, Mariscalco, Saratzis, and Shaw. Analysis and interpretation of data: Benedetto, Bottle, Mariscalco, Murphy, and Oo. Drafting of the manuscript: Bottle, Mariscalco and Murphy. Critical revision of the manuscript for important intellectual content: Aylin, Bashir, Bottle, Jenkins, Mariani, Mariscalco, Murphy, and Oo. Paper supervision: Aylin, Mariscalco, Murphy, and Oo. Statistical analysis: Benedetto, Bottle, and Shaw.
Sources of Funding
This study was supported by Leicester NIHR Cardiovascular Biomedical Research Units and British Heart Foundation. The Dr Foster Unit at Imperial College London (Bottle and Aylin) is partially funded by a grant from Dr Foster, a private health care information company. The Unit is also partly funded by research grants from the National Institute for Health Research (NIHR) Health Services Research and is affiliated with the NIHR Imperial Patient Safety Translational Research Centre. The NIHR Imperial Patient Safety Translational Centre is a partnership between the Imperial College Healthcare NHS Trust and Imperial College London. The Unit is grateful for support from the NIHR Biomedical Research Centre funding scheme.
Disclosures
Mariscalco, Oo and Murphy declare that they have received support from Vascutek, an aortic prosthesis manufacturer, to attend scientific meetings. Oo has received fees for acting as a proctor for Vascutek. Benedetto has received support to attend scientific meetings from Maquet, who also manufacture aortic prostheses. These authors declare that they have no other conflicts of interest. Members of the UK Aortic forum have also declared competing interests: Debora Harrington, Manoj Kuduvalli, Jorge Mascaro, Geoff Tsang, Graham Cooper, and Jonathan Unsworth‐White declare that they have received support from Vascutek for attending scientific meetings. These members of the UK Aortic Forum declare that they have no other conflicts of interest. The remaining authors and members of the UK Aortic Forum declare that they have no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years, no other relationships or activities that could appear to have influenced the submitted work, and no other relevant relationships with industry or other disclosures.
Supporting information
Data S1. Supplemental methods.
Table S1. The RECORD Statement—Checklist of Items, Extended From the STROBE Statement
Table S2. MOOSE Checklist for Meta‐Analyses of Observational Studies10
Table S3. PRISMA Checklist of Items to Include When Reporting a Systematic Review or Meta‐Analysis11
Table S4. List of ICD‐10 Codes for the Comorbidities Used in the HES Analysis
Table S5. PICOS Criteria for Inclusion and Exclusion of Studies Into Meta‐Analysis
Table S6. Risk Factors for Patients Affected by Thoracic Aortic Disease Who Received Treatment and for Patients Who Received Nonemergent Rather Than Emergent Treatment (HES Cohort)
Table S7. Risk Factors for 6‐Month Mortality in Patients Receiving Treatment for Thoracic Aortic Disease and in Those Not Receiving Any Thoracic Aortic Treatment (HES Cohort)
Table S8. Baseline, Operative, and Mortality Details by Most Distal Aortic Segment (NACSA Cohort)
Table S9. Hospital Volume Tertiles by Most Distal Aortic Segment (Calculated by Mean 3‐Year Annual Activity) (NACSA Cohort)
Tables S10. Unadjusted and Adjusted In‐Hospital Mortality Rates by Aortic Procedure and Hospital Volume (NACSA Cohort)*
Table S11. Characteristics of the Studies Included in the Systematic Review
Table S12. Study Outcomes Stratified by Hospital and Surgeon Volume
Table S13. Study Outcomes for Study With Defined a Specific Thoracic Aortic Program
Table S14. Quality Assessment of Observational Studies According to the Newcastle‐Ottawa Scale
Table S15. List of Variables Included in the Final Multivariable Model
Figure S1. Adjusted 6‐month mortality in patients affected by TAD receiving an operation (treated) and in those who did not (untreated) by county (HES cohort).
Figure S2. Center activity by the most distal aortic segment (NACSA data set).
Figure S3. Correlation between the hospital activity (number of cases) and in‐hospital mortality (NACSA data set).
Figure S4. PRISMA flow chart of search strategy.11
Figure S5. Funnel plots showing the absence of publication bias.
Figure S6. Forest plot for high‐ versus low‐volume hospitals on operative mortality according to the primary aortic pathology (upper panel), and forest plot reporting risk adjusted estimates for high‐ vs low‐volume hospitals on operative mortality according to the primary aortic pathology (lower panel).
Figure S7. Forest plots comparing the effect of hospital volume for secondary outcomes.
Figure S8. Forest plots comparing the effect of a multidisciplinary TAD program presence on outcomes.
Figure S9. Forest plots comparing the effect of surgeon volume for hospital mortality and secondary outcomes.
Figure S10. Forest plots comparing the effect of hospital status on hospital mortality.
(J Am Heart Assoc. 2017;6:e004913. DOI: 10.1161/JAHA.116.004913.)
Notes
References
- 1. Sampson UK, Norman PE, Fowkes FG, Aboyans V, Yanna Song, Harrell FE Jr, Forouzanfar MH, Naghavi M, Denenberg JO, McDermott MM, Criqui MH, Mensah GA, Ezzati M, Murray C. Global and regional burden of aortic dissection and aneurysms: mortality trends in 21 world regions, 1990 to 2010. Glob Heart. 2014;9:171–180. [DOI] [PubMed] [Google Scholar]
- 2. Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population‐based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114:2611–2618. [DOI] [PubMed] [Google Scholar]
- 3. von Allmen RS, Anjum A, Powell JT. Incidence of descending aortic pathology and evaluation of the impact of thoracic endovascular aortic repair: a population‐based study in England and Wales from 1999 to 2010. Eur J Vasc Endovasc Surg. 2013;45:154–159. [DOI] [PubMed] [Google Scholar]
- 4. Raghupathy A, Nienaber CA, Harris KM, Myrmel T, Fattori R, Sechtem U, Oh J, Trimarchi S, Cooper JV, Booher A, Eagle K, Isselbacher E, Bossone E; International Registry of Acute Aortic Dissection (IRAD) Investigators . Geographic differences in clinical presentation, treatment, and outcomes in type A acute aortic dissection (from the International Registry of Acute Aortic Dissection). Am J Cardiol. 2008;102:1562–1566. [DOI] [PubMed] [Google Scholar]
- 5. Liao JM, Bakaeen FG, Cornwell LD, Simpson K, Lemaire SA, Coselli JS, Chu D. Nationwide trends and regional/hospital variations in open versus endovascular repair of thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg. 2012;144:612–616. [DOI] [PubMed] [Google Scholar]
- 6. Goodney PP, Brooke BS, Wallaert J, Travis L, Lucas FL, Goodman DC, Cronenwett JL, Stone DH. Thoracic endovascular aneurysm repair, race, and volume in thoracic aneurysm repair. J Vasc Surg. 2013;57:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hughes GC, Zhao Y, Rankin JS, Scarborough JE, O'Brien S, Bavaria JE, Wolfe WG, Gaca JG, Gammie JS, Shahian DM, Smith PK. Effects of institutional volumes on operative outcomes for aortic root replacement in North America. J Thorac Cardiovasc Surg. 2013;145:166–170. [DOI] [PubMed] [Google Scholar]
- 8. Patel VI, Mukhopadhyay S, Ergul E, Aranson N, Conrad MF, Lamuraglia GM, Kwolek CJ, Cambria RP. Impact of hospital volume and type on outcomes of open and endovascular repair of descending thoracic aneurysms in the United States Medicare population. J Vasc Surg. 2013;58:346–354. [DOI] [PubMed] [Google Scholar]
- 9. Sakata R, Kuwano H, Yokomise H. Hospital volume and outcomes of cardiothoracic surgery in Japan: 2005–2009 national survey. Gen Thorac Cardiovasc Surg. 2012;60:625–638. [DOI] [PubMed] [Google Scholar]
- 10. Chikwe J, Cavallaro P, Itagaki S, Seigerman M, Diluozzo G, Adams DH. National outcomes in acute aortic dissection: influence of surgeon and institutional volume on operative mortality. Ann Thorac Surg. 2013;95:1563–1569. [DOI] [PubMed] [Google Scholar]
- 11. Wang W, Duan W, Xue Y, Wang L, Liu J, Yu S, Yi D; Registry of Aortic Dissection in China Sino‐RAD Investigators . Clinical features of acute aortic dissection from the Registry of Aortic Dissection in China. J Thorac Cardiovasc Surg. 2014;148:2995–3000. [DOI] [PubMed] [Google Scholar]
- 12. Russo CF, Mariscalco G, Colli A, Santè P, Nicolini F, Miceli A, De Chiara B, Beghi C, Gerosa G, Glauber M, Gherli T, Nappi G, Murzi M, Molardi A, Merlanti B, Vizzardi E, Bonadei I, Coletti G, Carrozzini M, Gelsomino S, Caiazzo A, Lorusso R. Italian multicentre study on type A acute aortic dissection: a 33‐year follow‐up. Eur J Cardiothorac Surg. 2016;49:125–131. [DOI] [PubMed] [Google Scholar]
- 13. Iribarne A, Milner R, Merlo AE, Singh A, Saunders CR, Russo MJ. Outcomes following emergent open repair for thoracic aortic dissection are improved at higher volume centers. J Card Surg. 2015;30:74–79. [DOI] [PubMed] [Google Scholar]
- 14. Andersen ND, Ganapathi AM, Hanna JM, Williams JB, Gaca JG, Hughes GC. Outcomes of acute type a dissection repair before and after implementation of a multidisciplinary thoracic aortic surgery program. J Am Coll Cardiol. 2014;63:1796–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pompilio G, Spirito R, Alamanni F, Agrifoglio M, Polvani G, Porqueddu M, Reali M, Biglioli P. Determinants of early and late outcome after surgery for type A aortic dissection. World J Surg. 2001;25:1500–1506. [DOI] [PubMed] [Google Scholar]
- 16. Bridgewater B, Keogh B, Kinsman R, Walton P. The Society for Cardiothoracic Surgery in Great Britain & Ireland: The Sixth National Adult Cardiac Surgical Database Report. Henley‐on‐Thames, UK: Dendrite Clinical Systems Ltd; 2009. Available at: http://www.scts.org/_userfiles/resources/SixthNACSDreport2008withcovers.pdf. Accessed June 30, 2015. [Google Scholar]
- 17. https://www.england.nhs.uk/rightcare/intel/cfv/atlas/. Accessed May 31, 2016.
- 18. http://www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2015/01/a10-spec-adlt-cardiac-surgry.pdf. Accessed June 30, 2016.
- 19. http://www.vascularsociety.org.uk/wp-content/uploads/2013/06/Service-Specification.pdf. Accessed June 30, 2016.
- 20. Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA, Kouchoukos NT, Lytle BW, Milewicz DM, Reich DL, Sen S, Shinn JA, Svensson LG, Williams DM; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American College of Radiology; American Stroke Association; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology; Society of Thoracic Surgeons; Society for Vascular Medicine . 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol. 2010;55:e27–e129. [DOI] [PubMed] [Google Scholar]
- 21. Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, Grabenwöger M, Haverich A, Iung B, Manolis AJ, Meijboom F, Nienaber CA, Roffi M, Rousseau H, Sechtem U, Sirnes PA, Allmen RS, Vrints CJ; ESC Committee for Practice Guidelines . 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873–2926. [DOI] [PubMed] [Google Scholar]
- 22. Svensson LG, Adams DH, Bonow RO, Kouchoukos NT, Miller DC, O'Gara PT, Shahian DM, Schaff HV, Akins CW, Bavaria JE, Blackstone EH, David TE, Desai ND, Dewey TM, D'Agostino RS, Gleason TG, Harrington KB, Kodali S, Kapadia S, Leon MB, Lima B, Lytle BW, Mack MJ, Reardon M, Reece TB, Reiss GR, Roselli EE, Smith CR, Thourani VH, Tuzcu EM, Webb J, Williams MR. Aortic valve and ascending aorta guidelines for management and quality measures: executive summary. Ann Thorac Surg. 2013;95:1491–1505. [DOI] [PubMed] [Google Scholar]
- 23. Yan TD, Tian DH, LeMaire SA, Hughes GC, Chen EP, Misfeld M, Griepp RB, Kazui T, Bannon PG, Coselli JS, Elefteriades JA, Kouchoukos NT, Underwood MJ, Mathew JP, Mohr FW, Oo A, Sundt TM, Bavaria JE, Di Bartolomeo R, Di Eusanio M, Trimarchi S; International Aortic Arch Surgery Study Group . Standardizing clinical end points in aortic arch surgery: a consensus statement from the International Aortic Arch Surgery Study Group. Circulation. 2014;129:1610–1616. [DOI] [PubMed] [Google Scholar]
- 24. Boodhwani M, Andelfinger G, Leipsic J, Lindsay T, McMurtry MS, Therrien J, Siu SC; Canadian Cardiovascular Society . Canadian Cardiovascular Society position statement on the management of thoracic aortic disease. Can J Cardiol. 2014;30:577–589. [DOI] [PubMed] [Google Scholar]
- 25. Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, Sørensen HT, von Elm E, Langan SM; RECORD Working Committee . The REporting of studies Conducted using Observational Routinely‐collected health Data (RECORD) statement. PLoS Med. 2015;12:e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. http://www.hscic.gov.uk/hes. Accessed June 30, 2015.
- 29. http://www.ucl.ac.uk/nicor/audits/adultcardiac/documents/datasets/NACSAdatasetV4.1.2. Accessed April 30, 2015.
- 30. http://www.ucl.ac.uk/nicor/audits/adultcardiac/documents/datasets/nacsacleaning10.3. Accessed April 30, 2015.
- 31. Hickey GL, Grant SW, Cosgriff R, Dimarakis I, Pagano D, Kappetein AP, Bridgewater B. Clinical registries: governance, management, analysis and applications. Eur J Cardiothorac Surg. 2013;44:605–614. [DOI] [PubMed] [Google Scholar]
- 32. Mariscalco G, Murphy GJ, Mariani S, Saratzis A. Standard of care for the management of thoracic aortic disease: a systematic review and meta‐analysis. PROSPERO 2015:CRD42015024137.
- 33. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed June 30, 2016.
- 34. Cohen ME, Dimick JB, Bilimoria KY, Ko CY, Richards K, Hall BL. Risk adjustment in the American College of Surgeons National Surgical Quality Improvement Program: a comparison of logistic versus hierarchical modeling. J Am Coll Surg. 2009;209:687–693. [DOI] [PubMed] [Google Scholar]
- 35. van Klaveren D, Steyerberg EW, Perel P, Vergouwe Y. Assessing discriminative ability of risk models in clustered data. BMC Med Res Methodol. 2014;14:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yates F. Contingency tables involving small numbers and the χ2 test. J R Stat Soc. 1934;suppl 1:217–235. [Google Scholar]
- 37. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed‐effect and random‐effects models for meta‐analysis. Res Synth Methods. 2010;1:97–111. DOI: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 38. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 39. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. Available at: http://www.R-project.org/. Accessed March 1, 2016. [Google Scholar]
- 41. https://cran.r-project.org/web/packages/meta/meta.pdf. Accessed March 1, 2016.
- 42. Schaffer JM, Lingala B, Fischbein MP, Dake MD, Woo YJ, Mitchell RS, Miller DC. Midterm outcomes of open descending thoracic aortic repair in more than 5,000 Medicare patients. Ann Thorac Surg. 2015;100:2087–2094. [DOI] [PubMed] [Google Scholar]
- 43. Schaffer JM, Lingala B, Miller DC, Woo YJ, Mitchell RS, Dake MD. Midterm survival after thoracic endovascular aortic repair in more than 10,000 Medicare patients. J Thorac Cardiovasc Surg. 2015;149:808–820. [DOI] [PubMed] [Google Scholar]
- 44. Bhatt P, Patel NJ, Patel A, Sonani R, Patel A, Panaich SS, Thakkar B, Savani C, Jhamnani S, Patel N, Patel N, Pant S, Patel S, Arora S, Dave A, Singh V, Chothani A, Patel J, Ansari M, Deshmukh A, Bhimani R, Grines C, Cleman M, Mangi A, Forrest JK, Badheka AO. Impact of hospital volume on outcomes of endovascular stenting for adult aortic coarctation. Am J Cardiol. 2015;116:1418–1424. [DOI] [PubMed] [Google Scholar]
- 45. Brat R, Gaj J, Barta J. Early and mid‐term outcomes of the aortic arch surgery: experience from the low‐volume centre. J Cardiothorac Surg. 2015;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grau JB, Kuschner CE, Ferrari G, Wilson SR, Brizzio ME, Zapolanski A, Yallowitz J, Shaw RE. Effects of a protocol‐based management of type A aortic dissections. J Surg Res. 2015;197:265–269. [DOI] [PubMed] [Google Scholar]
- 47. Lenos A, Bougioukakis P, Irimie V, Zacher M, Diegeler A, Urbanski PP. Impact of surgical experience on outcome in surgery of acute type A aortic dissection. Eur J Cardiothorac Surg. 2015;48:491–496. [DOI] [PubMed] [Google Scholar]
- 48. Murzi M, Miceli A, Di Stefano G, Cerillo AG, Kallushi E, Farneti P, Solinas M, Glauber M. Enhancing quality control and performance monitoring in thoracic aortic surgery: a 10‐year single institutional experience. Eur J Cardiothorac Surg. 2015;47:608–615. [DOI] [PubMed] [Google Scholar]
- 49. Sales Mda C, Frota Filho JD, Aguzzoli C, Souza LD, Rösler AM, Lucio EA, Leães PE, Pontes MR, Lucchese FA. Aortic Center: specialized care improves outcomes and decreases mortality. Rev Bras Cir Cardiovasc. 2014;29:494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weiss A, Anderson JA, Green A, Chang DC, Kansal N. Hospital volume of thoracoabdominal aneurysm repair does not affect mortality in California. Vasc Endovascular Surg. 2014;48:378–382. [DOI] [PubMed] [Google Scholar]
- 51. Arnaoutakis DJ, Propper BW, Black JH III, Schneider EB, Lum YW, Freischlag JA, Perler BA, Abularrage CJ. Racial and ethnic disparities in the treatment of unruptured thoracoabdominal aortic aneurysms in the United States. J Surg Res. 2013;184:651–657. [DOI] [PubMed] [Google Scholar]
- 52. Soppa G, Abdulkareem N, Smelt J, Van Besouw JP, Jahangiri M. High‐volume practice by a single specialized team reduces mortality and morbidity of elective and urgent aortic root replacement. Aorta (Stamford). 2013;1:40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tsagakis K, Konorza T, Dohle DS, Kottenberg E, Buck T, Thielmann M, Erbel R, Jakob H. Hybrid operating room concept for combined diagnostics, intervention and surgery in acute type A dissection. Eur J Cardiothorac Surg. 2013;43:397–404. [DOI] [PubMed] [Google Scholar]
- 54. Chavanon O, Baguet JP, Albaladéjo P, Blin D, Vanzetto G. Direct admission to the operating room: an efficient strategy for patients with diagnosed or highly suspected acute type A aortic dissection. Can J Cardiol. 2011;27:685–691. [DOI] [PubMed] [Google Scholar]
- 55. Gopaldas RR, Dao TK, LeMaire SA, Huh J, Coselli JS. Endovascular versus open repair of ruptured descending thoracic aortic aneurysms: a nationwide risk‐adjusted study of 923 patients. J Thorac Cardiovasc Surg. 2011;142:1010–1018. [DOI] [PubMed] [Google Scholar]
- 56. Harris KM, Strauss CE, Duval S, Unger BT, Kroshus TJ, Inampudi S, Cohen JD, Kapsner C, Boland LL, Eales F, Rohman E, Orlandi QG, Flavin TF, Kshettry VR, Graham KJ, Hirsch AT, Henry TD. Multidisciplinary standardized care for acute aortic dissection: design and initial outcomes of a regional care model. Circ Cardiovasc Qual Outcomes. 2010;3:424–430. [DOI] [PubMed] [Google Scholar]
- 57. Davies MG, Younes HK, Harris PW, Masud F, Croft BA, Reardon MJ, Lumsden AB. Outcomes before and after initiation of an acute aortic treatment center. J Vasc Surg. 2010;52:1478–1485. [DOI] [PubMed] [Google Scholar]
- 58. Gazoni LM, Speir AM, Kron IL, Fonner E, Crosby IK. Elective thoracic aortic aneurysm surgery: better outcomes from high‐volume centers. J Am Coll Surg. 2010;210:855–859. [DOI] [PubMed] [Google Scholar]
- 59. Miyata H, Motomura N, Ueda Y, Tsukihara H, Tabayashi K, Takamoto S. Toward quality improvement of thoracic aortic surgery: estimating volume‐outcome effect from nationwide survey. Eur J Cardiothorac Surg. 2009;36:517–521. [DOI] [PubMed] [Google Scholar]
- 60. Schermerhorn ML, Giles KA, Hamdan AD, Dalhberg SE, Hagberg R, Pomposelli F. Population‐based outcomes of open descending thoracic aortic aneurysm repair. J Vasc Surg. 2008;48:821–827. [DOI] [PubMed] [Google Scholar]
- 61. Knipp BS, Deeb GM, Prager RL, Williams CY, Upchurch GR Jr, Patel HJ. A contemporary analysis of outcomes for operative repair of type A aortic dissection in the United States. Surgery. 2007;142:524–528. [DOI] [PubMed] [Google Scholar]
- 62. Committee for Scientific Affairs , Kazui T, Osada H, Fujita H. An attempt to analyze the relation between hospital surgical volume and clinical outcome. Gen Thorac Cardiovasc Surg. 2007;55:483–492. [DOI] [PubMed] [Google Scholar]
- 63. Rigberg DA, McGory ML, Zingmond DS, Maggard MA, Agustin M, Lawrence PF, Ko CY. Thirty‐day mortality statistics underestimate the risk of repair of thoracoabdominal aortic aneurysms: a statewide experience. J Vasc Surg. 2006;43:217–222. [DOI] [PubMed] [Google Scholar]
- 64. Narayan P, Caputo M, Rogers CA, Alwair H, Mahesh B, Angelini GD, Bryan AJ. Early and mid‐term outcomes of surgery of the ascending aorta/arch: is there a relationship with caseload? Eur J Cardiothorac Surg. 2004;25:676–682. [DOI] [PubMed] [Google Scholar]
- 65. Cowan JA Jr, Dimick JB, Henke PK, Huber TS, Stanley JC, Upchurch GR Jr. Surgical treatment of intact thoracoabdominal aortic aneurysms in the United States: hospital and surgeon volume‐related outcomes. J Vasc Surg. 2003;37:1169–1174. [DOI] [PubMed] [Google Scholar]
- 66. Derrow AE, Seeger JM, Dame DA, Carter RL, Ozaki CK, Flynn TC, Huber TS. The outcome in the United States after thoracoabdominal aortic aneurysm repair, renal artery bypass, and mesenteric revascularization. J Vasc Surg. 2001;34:54–61. [DOI] [PubMed] [Google Scholar]
- 67. Albrink MH, Rodriguez E, England GJ, McKeown PP, Hurst JM, Rosemurgy AS II. Importance of designated thoracic trauma surgeons in the management of traumatic aortic transection. South Med J. 1994;87:497–501. [DOI] [PubMed] [Google Scholar]
- 68. Burns EM, Rigby E, Mamidanna R, Bottle A, Aylin P, Ziprin P, Faiz OD. Systematic review of discharge coding accuracy. J Public Health (Oxf). 2012;34:138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. von Allmen RS, Anjum A, Powell JT. Outcomes after endovascular or open repair for degenerative descending thoracic aortic aneurysm using linked hospital data. Br J Surg. 2014;101:1244–1251. [DOI] [PubMed] [Google Scholar]
- 70. Benedetto U, Mohamed H, Vitulli P, Petrou M. Axillary versus femoral arterial cannulation in type A acute aortic dissection: evidence from a meta‐analysis of comparative studies and adjusted risk estimates. Eur J Cardiothorac Surg. 2015;48:953–959. [DOI] [PubMed] [Google Scholar]
- 71. Angeloni E, Melina G, Refice SK, Roscitano A, Capuano F, Comito C, Sinatra R. Unilateral versus bilateral antegrade cerebral protection during aortic surgery: an updated meta‐analysis. Ann Thorac Surg. 2015;99:2024–2031. [DOI] [PubMed] [Google Scholar]
- 72. https://www.bhf.org.uk/publications/statistics/cvd-stats-2015. Accessed May 31, 2016.
- 73. Karthikesalingam A, Hinchliffe RJ, Loftus IM, Thompson MM, Holt PJ. Volume‐outcome relationships in vascular surgery: the current status. J Endovasc Ther. 2010;17:356–365. [DOI] [PubMed] [Google Scholar]
- 74. Holt PJ, Poloniecki JD, Khalid U, Hinchliffe RJ, Loftus IM, Thompson MM. Effect of endovascular aneurysm repair on the volume‐outcome relationship in aneurysm repair. Circ Cardiovasc Qual Outcomes. 2009;2:624–632. [DOI] [PubMed] [Google Scholar]
- 75. Booher AM, Isselbacher EM, Nienaber CA, Trimarchi S, Evangelista A, Montgomery DG, Froehlich JB, Ehrlich MP, Oh JK, Januzzi JL, O'Gara P, Sundt TM, Harris KM, Bossone E, Pyeritz RE, Eagle KA; IRAD Investigators . The IRAD classification system for characterizing survival after aortic dissection. Am J Med. 2013;126:730.e19‐24. [DOI] [PubMed] [Google Scholar]
- 76. Moffatt‐Bruce SD, Nguyen MC, Fann JI, Westaby S. Our new reality of public reporting: shame rather than blame? Ann Thorac Surg. 2016;101:1255–1261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental methods.
Table S1. The RECORD Statement—Checklist of Items, Extended From the STROBE Statement
Table S2. MOOSE Checklist for Meta‐Analyses of Observational Studies10
Table S3. PRISMA Checklist of Items to Include When Reporting a Systematic Review or Meta‐Analysis11
Table S4. List of ICD‐10 Codes for the Comorbidities Used in the HES Analysis
Table S5. PICOS Criteria for Inclusion and Exclusion of Studies Into Meta‐Analysis
Table S6. Risk Factors for Patients Affected by Thoracic Aortic Disease Who Received Treatment and for Patients Who Received Nonemergent Rather Than Emergent Treatment (HES Cohort)
Table S7. Risk Factors for 6‐Month Mortality in Patients Receiving Treatment for Thoracic Aortic Disease and in Those Not Receiving Any Thoracic Aortic Treatment (HES Cohort)
Table S8. Baseline, Operative, and Mortality Details by Most Distal Aortic Segment (NACSA Cohort)
Table S9. Hospital Volume Tertiles by Most Distal Aortic Segment (Calculated by Mean 3‐Year Annual Activity) (NACSA Cohort)
Tables S10. Unadjusted and Adjusted In‐Hospital Mortality Rates by Aortic Procedure and Hospital Volume (NACSA Cohort)*
Table S11. Characteristics of the Studies Included in the Systematic Review
Table S12. Study Outcomes Stratified by Hospital and Surgeon Volume
Table S13. Study Outcomes for Study With Defined a Specific Thoracic Aortic Program
Table S14. Quality Assessment of Observational Studies According to the Newcastle‐Ottawa Scale
Table S15. List of Variables Included in the Final Multivariable Model
Figure S1. Adjusted 6‐month mortality in patients affected by TAD receiving an operation (treated) and in those who did not (untreated) by county (HES cohort).
Figure S2. Center activity by the most distal aortic segment (NACSA data set).
Figure S3. Correlation between the hospital activity (number of cases) and in‐hospital mortality (NACSA data set).
Figure S4. PRISMA flow chart of search strategy.11
Figure S5. Funnel plots showing the absence of publication bias.
Figure S6. Forest plot for high‐ versus low‐volume hospitals on operative mortality according to the primary aortic pathology (upper panel), and forest plot reporting risk adjusted estimates for high‐ vs low‐volume hospitals on operative mortality according to the primary aortic pathology (lower panel).
Figure S7. Forest plots comparing the effect of hospital volume for secondary outcomes.
Figure S8. Forest plots comparing the effect of a multidisciplinary TAD program presence on outcomes.
Figure S9. Forest plots comparing the effect of surgeon volume for hospital mortality and secondary outcomes.
Figure S10. Forest plots comparing the effect of hospital status on hospital mortality.


