Abstract
Background
Surrogate endpoint trials test strategies more efficiently but are accompanied by uncertainty about the relationship between changes in surrogate markers and clinical outcomes.
Methods and Results
We identified cardiovascular trials with primary surrogate endpoints published in the New England Journal of Medicine, Lancet, and JAMA: Journal of the American Medical Association from 1990 to 2011 and determined the trends in publication of surrogate endpoint trials and the success of the trials in meeting their primary endpoints. We tracked for publication of clinical outcome trials on the interventions tested in surrogate trials. We screened 3016 articles and identified 220 surrogate endpoint trials. From the total of 220 surrogate trials, 157 (71.4%) were positive for their primary endpoint. Only 59 (26.8%) surrogate trials had a subsequent clinical outcomes trial. Among these 59 trials, 24 outcomes trial results validated the positive surrogates, whereas 20 subsequent outcome trials were negative following positive results on a surrogate. We identified only 3 examples in which the surrogate trial was negative but a subsequent outcomes trial was conducted and showed benefit. Findings were consistent in a sample cohort of 383 screened articles inclusive of 37 surrogate endpoint trials from 6 other high‐impact journals.
Conclusions
Although cardiovascular surrogate outcomes trials frequently show superiority of the tested intervention, they are infrequently followed by a prominent outcomes trial. When there was a high‐profile clinical outcomes study, nearly half of the positive surrogate trials were not validated. Cardiovascular surrogate outcome trials may be more appropriate for excluding benefit from the patient perspective than for identifying it.
Keywords: clinical trial, outcome, surrogate endpoints
Subject Categories: Quality and Outcomes
Introduction
According to an Institute of Medicine report, a surrogate endpoint is intended to substitute for a clinical endpoint, and expected to predict clinical benefit based on epidemiologic, therapeutic, pathophysiologic, or other evidence.1 Health interventions are expected to impact clinical endpoints through pathways that include intermediary (surrogate) endpoints, and testing of these interventions can be done more quickly using smaller, shorter, and less expensive trials, when focused on surrogates.2, 3 This testing strategy has been particularly adopted in cardiovascular medicine, where many interventions required years to manifest their effects on clinical outcomes.4, 5
For example, dyslipidemia is known to be associated with increased risk of cardiovascular events. Investigators have tested the impacts of health interventions on dyslipidemia, hoping that an intervention that targets dyslipidemia in the short term would likewise lead to better cardiovascular outcomes in the long term. However, torcetrapib, a cholesteryl ester transfer protein inhibitor, improved lipid profile dramatically while clinical outcomes were not improved (and there was suggestion of clinical harm). Similarly, there have been several studies where interventions showed benefits on other surrogate endpoints, while the clinical outcomes were unchanged or worsened.6, 7, 8 Therefore, despite the potential advantages and efficiency, use of surrogate outcomes for testing strategies is accompanied by concern for lack of efficacy on clinical outcomes because of loose (or no) causal relationship between the surrogates and clinically important outcomes, or because of coexisting but unexpected consequences that health interventions may have on pathways other than those of the surrogate outcome.9, 10 However, the extent of this phenomenon has not been characterized.
Our objective was to perform a systematic review of cardiovascular trials published in the highest‐impact journals, characterizing the success of these trials in meeting their primary endpoints. We also examined subsequent publication of clinical outcome trials on interventions identified in our review, determining concordance between the surrogate outcome trials and subsequent clinical outcomes trials.
Methods
Data Source, Inclusion, and Exclusion Criteria
We searched MEDLINE with PubMed interface to screen all publications in the New England Journal of Medicine (NEJM), Lancet, and JAMA: Journal of the American Medical Association (JAMA) (January 1, 1990, to December 31, 2011) based on a search for cardiovascular trials using keywords and Medical Subject Heading terms (Table 1). Studies have shown that trials published in the highest‐impact journals have a higher methodological quality, larger sample size, and lower risk of bias.11 Such journals are more likely to publish important and potentially practice‐changing clinical trials.12, 13
Table 1.
Search Strategy for Included Studies
| 1. (“N Engl J Med”[Journal] OR “Lancet”[Journal] OR “JAMA”[Journal]) AND (Trial*[TIAB] OR random*[TIAB]) AND (cardiovascular*[TIAB] OR cardiac*[TIAB] OR heart*[TIAB] OR coronar*[TIAB] OR vascul*[TIAB] OR cardiov*[TIAB] OR cardiom*[TIAB] OR cardio*[TIAB] OR cardiac*[TIAB] OR myocard*[TIAB] OR pericard*[TIAB] OR epicard*[TIAB] OR endocard*[TIAB] OR stroke*[TIAB] OR cerebrovasc*[TIAB] OR carotid*[TIAB] OR venous*[TIAB] OR vein*[TIAB] OR thrombos*[TIAB] OR thromboembol*[TIAB] OR embolis*[TIAB] OR aort*[TIAB] OR “Acute Coronary Syndrome”[MAJR] OR “Myocardial Infarction”[MAJR] OR “Heart Failure”[MAJR] OR (“Angioplasty”[MAJR] AND coronary) OR “Arrhythmias, Cardiac”[MAJR] OR “Stroke”[MAJR] OR “Arrhythmias, Cardiac”[MAJR] OR “Aorta”[MAJR] OR “Peripheral Vascular Diseases”[MAJR]) |
|
2. (“N Engl J Med”[Journal] OR “Lancet”[Journal] OR “JAMA”[Journal]) AND cardiovascular diseases Filters: Humans; Clinical Trial |
|
3. #1 OR #2 Publication date limit: January 1, 1990, to December 31, 2011. |
We excluded noncardiovascular trials, safety trials, and manuscripts that reported secondary or post hoc analyses. We chose the cutoff date of December 31, 2011, for inclusion of surrogate outcome clinical trials. This decision was made to provide time for publication, in the 3 journals, of pertinent subsequent clinical outcomes trials that followed the included surrogate outcomes clinical trials.
Characterization of Surrogate and Clinical Endpoints and Positive or Negative Results
We first characterized all trials as clinical endpoint trials, or surrogate endpoint trials. Detailed definitions of surrogate endpoints have been provided elsewhere.1 In brief, study endpoints that could not be perceived and directly related to patients but were derived from tests with plausibly important medical information were considered as surrogate endpoints. Common examples included blood tests and various imaging test results. Clinical endpoint trials were those whose primary endpoint was a patient‐important and patient‐perceived outcome, such as mortality, myocardial infarction, stroke, or a composite of such variables. We determined the proportion of surrogate endpoint trials that had positive results (ie, were positive for and met their primary endpoint). Following identification of a surrogate endpoint trial, we searched for clinical endpoint trials on the same tested intervention in the 3 journals published until April 1, 2015. Among surrogate trials that had a subsequent clinical outcomes trial, we determined the concordance rate of the results in surrogate trials and clinical outcomes trials (ie, whether the positive or negative nature of results in a surrogate trial were replicated in the clinical outcome trial). We explored the findings according to the enrolled population (primary prevention, secondary prevention, or hybrid cohorts). We also divided the surrogate outcome trials into 3 subgroups of clinical biomarkers, imaging markers, and others4 (such as blood pressure).
Sample Replication in Another Cohort
To ascertain the robustness of the findings across surrogate endpoint cardiovascular trials published in other journals, we subsequently searched PubMed with the same search strategy, but this time for publications in 6 additional high‐impact journals (Circulation, Journal of the American College of Cardiology, European Heart Journal, JAMA Internal Medicine [formerly Archives of Internal Medicine], British Medical Journal, and Annals of Internal Medicine). Since such a search retrieved an extremely large sample (14 279 hits), we selected a 6‐month period in the middle point of our study (ie, from January 1, 2002, and July 1, 2002) to investigate the broader cohort of journals for publication of surrogate endpoint trials, as well as subsequent publication of clinical outcome trials on those scenarios in any of the aforementioned 6 journals or NEJM, Lancet, or JAMA.
Statistical Analyses
The study was designed by B.B., J.S.R., and H.M.K.. Three additional authors (N.P., Y.A., and I.L.) performed the primary screening and data extraction process. B.B. reviewed all the results for consistency. Disagreements and questions were resolved with J.S.R. and H.M.K. We reported qualitative variables with frequencies and percentages. We used the chi‐square test to report the differences between categorical variables. We used the Mantel–Haenszel test to report the trends. We used a linear regression model to report the R 2 between two variables with a presumably linear relationship. Microsoft Excel (Redmond, WA) and Stata version 12.0 (StataCorp, College Station, TX) were used for data extraction and conducting the statistical analyses. This is a systematic review of the literature, and, hence, no request for institutional review board approval was required.
Results
Between January 1990 and December 2011, the number of articles published in the 3 journals declined, while the number of citations that included “trial*” in the PubMed search remained relatively stable (Table 2). In the study period, we manually screened 3016 articles through the systematic search and identified 220 surrogate endpoint trials. There was an increase in the annual number of surrogate endpoint trials from 1990 to 2007 (P<0.01 for trend) and a decline thereafter (Figure 1).
Table 2.
Number of Publications in the 3 Journals From 1990 to 2011
| Year | All Publicationsa | Publications With “trial*” (Anywhere in the Citation) |
|---|---|---|
| 1990 | 4874 | 392 |
| 1991 | 4924 | 523 |
| 1992 | 5579 | 504 |
| 1993 | 5696 | 471 |
| 1994 | 5511 | 499 |
| 1995 | 5448 | 518 |
| 1996 | 5339 | 503 |
| 1997 | 5153 | 564 |
| 1998 | 5472 | 585 |
| 1999 | 5583 | 656 |
| 2000 | 5478 | 622 |
| 2001 | 5084 | 583 |
| 2002 | 4992 | 619 |
| 2003 | 4913 | 632 |
| 2004 | 4468 | 525 |
| 2005 | 4077 | 503 |
| 2006 | 4013 | 532 |
| 2007 | 3963 | 558 |
| 2008 | 3889 | 560 |
| 2009 | 4004 | 535 |
| 2010 | 3921 | 601 |
| 2011 | 3855 | 550 |
Except for the interval between 1990 and 1991, there was not a major change in the number of clinical trials published in the New England Journal of Medicine, Lancet, and JAMA: Journal of the American Medical Association per year. The number of all publications has had a slight declining trend, whereas the number of publications that had “trial*” anywhere in the article has slightly increased. Data obtained from PubMed search.
P<0.0001 for declining trend.
Figure 1.
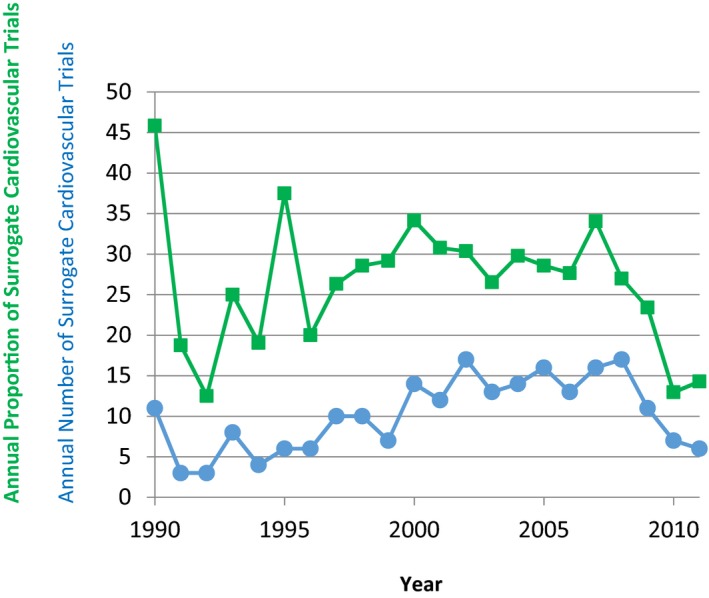
The number and proportion of cardiovascular trials with a primary surrogate endpoint from 1990 to 2011. Circles and blue lines represent the number of trials. Rectangles and green lines represent the proportion of trials with primary surrogate endpoints per 100 cardiovascular trials per year. The y axis represents the number of surrogate endpoint clinical trials or the proportion per 100 cardiovascular trials. Note that there was an increase in publication of surrogate endpoint trials from the 1990s to the mid‐2000s (P<0.01 for trend), and a subsequent decline started.
From the total of 220 surrogate trials, 157 (71.4%) were positive for their primary outcome. Fifty‐nine (26.8%) surrogate endpoint trials were followed by at least 1 clinical endpoint trial. Year of publication had a modest association with presence of subsequent outcome trials (R 2=0.15), with older trials being slightly more frequently followed by a clinical outcomes trial. Among these 59 surrogate endpoint trials that had a subsequent clinical endpoint trial, in 24 cases the clinical endpoint trial results validated the positive surrogate trials, while in 20 the subsequent clinical endpoint trial was negative (Table 3).14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50 A negative surrogate endpoint trial was less likely to be followed by a positive outcome trial and we identified only 3 such examples (P=0.02, Figure 2).
Table 3.
List of Positive Surrogate Endpoint Trials That Had an Associated Negative Clinical Outcomes Trial
| Surrogate Trial | Surrogate Trial Results | Clinical Outcomes Trial | Clinical Outcomes Trials Results |
|---|---|---|---|
| Colucci et al, N Engl J Med (2000)14 | Nesiritide significantly reduced the pulmonary capillary wedge pressure compared with placebo | O'Connor et al, N Engl J Med (2011)15 | Nesiritide was not associated with a decrease in death or heart failure hospitalizations |
| Hambrecht et al, JAMA (2000)16 | Exercise training reduced peripheral resistance and improved stroke volume in patients with heart failure | O'Connor et al, JAMA (2009)17 | Exercise training did not reduce the rate of death or hospitalizations in patients with heart failure |
| VMAC Investigators, JAMA (2002)18 | Intravenous neseritide was associated with decreased pulmonary capillary wedge pressure in patients with decompensated heart failure | O'Connor et al, N Engl J Med (2011)15 | Nesiritide was not associated with a decrease in death or heart failure hospitalizations |
| Lederman et al, Lancet (2002)19 | Intraarterial administration of fibroblast growth factor‐2 improved peak walking time of patients with intermittent claudication | Belch et al, Lancet (2011)20 | Administration of fibroblast growth factor did not reduce the risk of death or time to major amputation in patients with critical limb ischemia |
| Khan et al, N Engl J Med (2004)21 | On‐pump vs off‐pump coronary artery bypass grafting was associated with improved patency rates | Lamy et al, N Engl J Med (2012)22 | There was no significant difference between on‐pump vs off‐pump coronary artery bypass grafting for a composite of death, myocardial infarction, stroke, or need for dialysis |
| Walsh et al, JAMA (1998)23 | Compared with placebo, raloxifene significantly reduced low‐density lipoprotein and fibrinogen | Barrett‐Connor et al, JAMA. (2002)24 | Raloxifene did not reduce cardiovascular events compared with placebo |
| Solomon et al, Lancet (2007)25 | Use of valsartan improved the diastolic relaxation velocity in patients with hypertension and diastolic dysfunction | Massie et al, N Engl J Med (2008)26 | Irbesartan did not improve a composite of death or cardiovascular hospitalizations in patients with heart failure with preserved ejection fraction |
| Howard et al, JAMA (2008)27 | Lower targets for blood pressure and low‐density lipoprotein cholesterol were associated with regression of carotid intima‐media thickness in patients with diabetes | ACCORD Investigators, N Engl J Med (2008)28 | No reduction in cardiovascular events from intensive blood pressure control or combination lipid therapy |
| Taylor et al, N Engl J Med (2009)29 | Niacin was associated with significant regression of carotid intima‐media thickness in patients with coronary artery disease receiving statin therapy | Boden et al, N Engl J Med (2011)30 | In patients with atherosclerotic disease receiving statins, no reduction in major cardiovascular events was noted with niacin |
| Bonaa et al, N Engl J Med (1990)31 | Omega‐3 fatty acids reduced blood pressure in patients with hypertension | Rizos et al, JAMA (2012)32 | Multiple negative secondary prevention trials. Despite modest effects on blood pressure, a meta‐analysis of available randomized trials did not show a decline in stroke risk, the most profoundly influenced cardiovascular outcome by hypertension |
| Coats et al, Lancet (1990)33 | Exercise training improved exercise duration and peak oxygen consumption in patients with heart failure | O'Connor et al, JAMA (2009)17 | Exercise training did not reduce the rate of death or hospitalizations in patients with heart failure |
| Wood et al, N Engl J Med (1991)34 | A low‐fat low‐cholesterol diet was associated with reduced weight and lower cholesterol levels (including in women) | Howard et al, JAMA (2006)35 | Use of a low‐fat diet did not lead to reduced rate of cardiovascular events |
| Pitt et al, Lancet (1997)36 | Losartan compared with captopril was associated with less frequent discontinuation of therapy and a trend towards lower death or hospitalizations in patients with heart failure | Pfeffer et al, N Engl J Med (2003)37 | Valsartan was not superior to captopril for reducing all‐cause death in patients with heart failure |
| Follath et al, Lancet (2002)38 | Compared with dobutamine, levosimendan more frequently led to hemodynamic improvement in patients with heart failure | Mebazaa et al, JAMA (2007)39 | Compared with dobumtamine, levosimendan did not reduce all‐cause mortality |
| Nappo et al, JAMA (1999)40 | Use of vitamin C and vitamin E was associated with improved markers of coagulation and oxidation | Sesso et al, JAMA (2008)41 | Neither vitamin C nor vitamin D reduced cardiovascular events |
| Elam et al, JAMA (2000)42 | Compared with placebo, niacin led to an increase in high‐density lipoprotein and reduction in triglycerides and low‐density lipoprotein | Boden et al, N Engl J Med (2011)30 | In patients with atherosclerotic disease receiving statins, no reduction in major cardiovascular events was noted with niacin |
| Vermeulen et al, Lancet (2000)43 | Folic acid plus vitamin B6 supplementation was associated with lower occurrence of abnormal exercise ECG changes | Bonaa et al, N Engl J Med (2006)44 | No benefits were seen from use of folic acid or vitamin B6 in patients post–myocardial infarction. In the group receiving folate, vitamin B6, and vitamin B12, suggestion for increased rate of major adverse cardiovascular events compared with placebo |
| Masip et al, Lancet (2000)45 | Noninvasive positive pressure ventilation compared with conventional oxygen therapy was associated with better oxygenation in patients with cardiogenic pulmonary edema | Gray et al, N Engl J Med (2008)46 | Compared with conventional oxygen therapy, noninvasive positive pressure ventilation did not reduce the rate of death in patients with cardiogenic pulmonary edema |
| DAIS Investigators, Lancet (2001)47 | Treatment with fenofibrate reduced the angiographic progression of coronary artery disease in patients with diabetes | ACCORD Investigators, N Engl J Med (2010)48 | Adding fenofibrate did not reduce the rate of cardiovascular events in patients with diabetes receiving statin therapy |
| Brown et al, N Engl J Med (2001)49 | Combination therapy with niacin and simvastatin was associated with improvement of lipoproteins, as well as angiographic markers of coronary disease | HPS‐2‐THRIVE Investigators, N Engl J Med (2014)50 | Adding niacin to simvastatin in patients with atherosclerotic disease did not reduce the risk of major vascular events |
ACCORD indicates Action to Control Cardiovascular Risk in Diabetes trial; DAIS, Diabetes Atherosclerosis Intervention Study; HPS2‐THRIVE, Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events trial; VMAC, Vasodilatation in the Management of Acute CHF trial.
Figure 2.
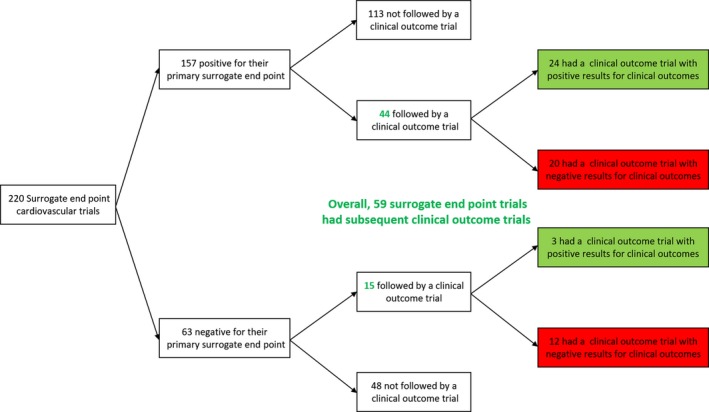
Cardiovascular surrogate endpoint clinical trials published from 1990 to 2011 and subsequent results in clinical outcomes trials. Overall, there were 220 surrogate endpoint trials, of which 157 (71.3%) were positive for their primary endpoint. Overall, 59 of the surrogate endpoint clinical trials were followed by clinical outcome trials. See text for further details.
Among the 220 surrogate endpoint cardiovascular trials, 56 enrolled primary prevention populations, 138 had a secondary prevention population, and 26 had a hybrid cohort (ie, a mix of primary and secondary prevention patients). There was no difference based on the enrolled population in the proportion of studies that had subsequent clinical outcomes trials (P=0.51, Table 4).
Table 4.
Publication of Outcomes Trials Based on the Initial Cohort Used in the Surrogate Trials
| Patient Population | No. of Surrogate Endpoint Trials | Outcomes Trials Published | Proportion of Surrogate Endpoint Trials That Have an Outcomes Trial |
|---|---|---|---|
| Primary prevention | 56 | 12 | 0.21 |
| Secondary prevention | 138 | 40 | 0.28 |
| Hybrid cohort | 26 | 8 | 0.30 |
From the total of 220 surrogate endpoint cardiovascular trials, 101 had an imaging endpoint, 42 had clinical biomarkers, and 77 had other surrogate endpoints. Trials with an imaging‐related primary endpoint were more frequently followed by a clinical outcome trial (37 of 101 versus 22 of 119; P=0.02).
In our robustness search of 6 additional journals (Circulation, Journal of the American College of Cardiology, European Heart Journal, JAMA Internal Medicine, British Medical Journal, and Annals of Internal Medicine) from January 1, 2002, to July 1, 2002, we screened 383 articles and identified 37 eligible surrogate endpoint trials. Most of these trials were small (median sample size: 71 patients) and were positive for their primary surrogate outcome (N=25, 67.5%). Of these 37 trials, the overwhelming majority (N=35, 94.5%) did not have a subsequent clinical outcomes trial.
Discussion
We found that while surrogate endpoint trials published in the highest‐impact journals frequently show superiority of the tested intervention, less than one third of them had a clinical outcomes trial of the intervention for the same purpose published in those same highest‐impact journals. Moreover, when there was a subsequent clinical outcomes trial, nearly half of them failed to validate the positive impact of the intervention on the surrogate marker. The results were fundamentally robust irrespective of enrolled population or type of the surrogate outcome. The findings were also similarly replicated (if not more pronounced) in a sample cohort from other high‐impact journals. Although surrogate markers are intended to ultimately predict benefits for patient‐important outcomes, our findings question this premise. The issue may be not that there are flaws in a surrogate endpoint, but that interventions have a multitude of effects beyond the surrogate, and it is difficult to judge the net result on outcomes based on the surrogate endpoint, even one thought to be central to the mechanism of disease or highly predictive of outcomes.
The suboptimal rate of outcomes trials that accompany a surrogate endpoint trial is concerning and should draw the attention of investigators and policymakers. The increase in publication of surrogate endpoint cardiovascular trails from 1990 until a few years ago is likely reflective of surging enthusiasm and dominance of surrogate endpoints among investigators and academicians.2 The decline in recent years, however, possibly reflective of lessons learned from unexpected results of surrogates on clinical endpoints, is encouraging.
Of the surrogate endpoint trials that were accompanied by a clinical outcomes trial, we noticed several positive surrogate endpoint trials that had a related negative clinical outcomes trials. We hypothesize that the disconnect between the surrogate endpoints and clinical outcomes is multifactorial. Some surrogate endpoints might merely be risk markers but not within the causal pathway, and therefore, intervening on them might have had little impact to improve clinical outcomes.9 Others may have been in the causal pathway but not targeted by the right intervention. Yet, some other surrogate endpoints might have been in the causal pathway but targeted by interventions that had coexisting off‐target effects.6, 51 Trials with positive imaging surrogate endpoints were more frequently followed by clinical outcomes trials. It could be hypothesized that structural changes usually need more time to reflect a change based on an intervention than blood biomarkers and could therefore better predict the ultimate impact of a health intervention. These findings warrant further investigation.
The choice regarding our study cohort is worthy of further discussion. We investigated surrogate endpoint cardiovascular trials published in the NEJM, Lancet, and JAMA in order to focus on those most likely to be the highest‐impact and highest‐quality studies. Although inclusion of all other surrogate endpoint trials for the study could have been ideal, achieving such a task is improbable for our group and many others. A search of merely 6 additional impactful journals, discussed above, retrieved more than 14 000 articles, and expansion to other journals would have made the cohort much larger. Our choice for searching subsequent outcome trials in the top 3 journals should also be discussed. Most often, high‐impact cardiovascular clinical endpoint randomized controlled trials are published in these 3 journals and it would be less frequent, if not rare, that an adequately powered well‐conducted cardiovascular clinical outcome trial gets published outside of these 3 journals. There could be a potential theoretical concern that the negative clinical outcome trials are less likely to get published in those journals. However, negative clinical outcome trials are commonly published in NEJM, Lancet, and JAMA.52 The results in a sample cohort of surrogate endpoint cardiovascular trials published in 6 other high‐impact journals further support the generalizability of our key findings. In our search of a sample of surrogate endpoint trials in other journals, the few associated clinical endpoint trials, all were identified from the 3 highest‐impact journals, with no clinical outcomes trials being found from the other 6 prestigious journals.
Study Strengths
Our study provides real‐world evidence about promises and limitations of surrogate endpoint trials in cardiovascular medicine. Infrequent follow‐up with a clinical outcome trial and poor concordance between positive surrogate endpoint trials and subsequent clinical outcome trials are concerning. We also observed that when a surrogate endpoint trial showed negative results, it was rare that a subsequent clinical endpoint trial proved the benefits of the intervention. We believe that our key findings would be helpful not only for investigators and funders but also for clinicians to recognize the benefits and concerns about clinical decisions based on surrogate endpoint trials. For investigators and funders, if the surrogate endpoint trial shows promise for the tested intervention, subsequent investigation with a clinical outcome trial would be the best next step. However, if the surrogate endpoint trial is well conducted and negative, the available finite resources could be shifted towards more promising health interventions. For practitioners and policymakers, including the Food and Drug Administration, it might be best to focus on clinically important endpoints, unless in scenarios where there is no interim way to obtain clinical outcomes from well‐conducted randomized trials (eg, young patients with familial hypercholesterolemia or those with rare conditions)—and even then, the label should express the limitations of the evidence. These issues are particularly important as the US Congress debates new legislation that directs the Food and Drug Administration to consider being more permissive in its approval process and to depend more heavily on surrogate endpoints.53
Study Limitations
Our study, however, had limitations other than the choice of study cohort discussed above. First, although in many cases the associated clinical outcome trials were negative, a smaller benefit on clinical endpoints could not be excluded. Second, although the surrogate endpoint trials and the identified associated outcome trials were very similar with regards to patient population and interventions, inevitably the subsequent trials may not have been a full replica of the initial surrogate endpoint trials (eg, using the same class of drug, but not necessarily the same agent or the same dose). However, using extremely strict criteria for identicalness of the surrogate endpoint trials and subsequent clinical endpoint trials would mean that an even lower proportion of the 220 surrogate endpoint trials were followed by clinical outcome trials than the 59 that we identified by reasonable clinical similarity. Third, although we believe that our study elucidates some fundamental advantages and challenges of surrogate endpoint trials, the focus was on cardiovascular trials. Therefore, extrapolation to other study fields requires further investigation. Preliminary results from other fields such as oncology concur with our findings.54, 55
Conclusions
Our findings raise concern about the certainty of assuming efficacy based on surrogate endpoints. Even if used for approval of therapies in urgent situations, postmarketing outcome trials are necessary. The good sensitivity of surrogate endpoint trials for detection of possible benefits, however, is encouraging. Based on our findings, cardiovascular surrogate endpoint trials may be more appropriate for excluding benefit from the patient perspective than for identifying it—and all surrogate endpoint trials should be interpreted in light of the possibility that they might not be validated in a clinical outcomes trial.
Disclosures
Drs Ross and Krumholz receive support through Yale University from Johnson and Johnson to develop methods of clinical trial data sharing and from Medtronic, Inc. and the Food and Drug Administration to develop methods for postmarket surveillance of medical devices; and from the Centers of Medicare & Medicaid Services to develop and maintain performance measures that are used for public reporting. Dr Ross receives support through Yale University from the Blue Cross Blue Shield Association to better understand medical technology evaluation, the Laura and John Arnold Foundation to support the Collaboration on Research Integrity and Transparency at Yale, and the Food and Drug Administration as part of the Centers for Excellence in Regulatory Science and Innovation program. Dr Krumholz reported that he chairs a cardiac scientific advisory board for UnitedHealth, is on the advisory board for Element Science, and is a participant/participant representative of the IBM Watson Health Life Sciences Board. Dr Krumholz is also the founder of Hugo, a personal health information platform.
(J Am Heart Assoc. 2017;6:e005285. DOI: 10.1161/JAHA.116.005285.)
References
- 1. Micheel CM, Ball JR, eds. Evaluation of Biomarkers and Surrogate Endpoints in Chronic Disease. Washington, DC: Academies Press; 2010. [PubMed] [Google Scholar]
- 2. Yudkin JS, Lipska KJ, Montori VM. The idolatry of the surrogate. BMJ. 2011;343:d7995. [DOI] [PubMed] [Google Scholar]
- 3. la Cour JL, Brok J, Gotzsche PC. Inconsistent reporting of surrogate outcomes in randomised clinical trials: cohort study. BMJ. 2010;341:c3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tardif JC, Heinonen T, Orloff D, Libby P. Vascular biomarkers and surrogates in cardiovascular disease. Circulation. 2006;113:2936–2942. [DOI] [PubMed] [Google Scholar]
- 5. Califf RM. Biomarkers, putative surrogates, surrogates, and decision making. Circ Cardiovasc Imaging. 2013;6:6–7. [DOI] [PubMed] [Google Scholar]
- 6. Bikdeli B, Barreto‐Filho JA. Reducing the cardiovascular disease burden: justified means for getting to the end. Circ Cardiovasc Qual Outcomes. 2012;5:580–586. [DOI] [PubMed] [Google Scholar]
- 7. Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez‐Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B; Investigators I . Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. [DOI] [PubMed] [Google Scholar]
- 8. Brousseau ME, Schaefer EJ, Wolfe ML, Bloedon LT, Digenio AG, Clark RW, Mancuso JP, Rader DJ. Effects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterol. N Engl J Med. 2004;350:1505–1515. [DOI] [PubMed] [Google Scholar]
- 9. Loscalzo J. Personalized cardiovascular medicine and drug development: time for a new paradigm. Circulation. 2012;125:638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cohn JN. Introduction to surrogate markers. Circulation. 2004;109:IV20–IV21. [DOI] [PubMed] [Google Scholar]
- 11. Bala MM, Akl EA, Sun X, Bassler D, Mertz D, Mejza F, Vandvik PO, Malaga G, Johnston BC, Dahm P, Alonso‐Coello P, Diaz‐Granados N, Srinathan SK, Hassouneh B, Briel M, Busse JW, You JJ, Walter SD, Altman DG, Guyatt GH. Randomized trials published in higher vs. lower impact journals differ in design, conduct, and analysis. J Clin Epidemiol. 2013;66:286–295. [DOI] [PubMed] [Google Scholar]
- 12. Gupta M, Singh N. Impactful clinical trials of 2012: what clinicians need to know. Can J Cardiol. 2013;29:747–750. [DOI] [PubMed] [Google Scholar]
- 13. Singh N, Gupta M. Impactful clinical trials of 2015: what clinicians need to know. Can J Cardiol. 2016;32:1038.e1017–1038.e1020. [DOI] [PubMed] [Google Scholar]
- 14. Colucci WS, Elkayam U, Horton DP, Abraham WT, Bourge RC, Johnson AD, Wagoner LE, Givertz MM, Liang CS, Neibaur M, Haught WH, LeJemtel TH. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. N Engl J Med. 2000;343:246–253. [DOI] [PubMed] [Google Scholar]
- 15. O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. [DOI] [PubMed] [Google Scholar]
- 16. Hambrecht R, Gielen S, Linke A, Fiehn E, Yu J, Walther C, Schoene N, Schuler G. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: a randomized trial. JAMA. 2000;283:3095–3101. [DOI] [PubMed] [Google Scholar]
- 17. O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL; HF‐ACTION Investigators . Efficacy and safety of exercise training in patients with chronic heart failure: HF‐ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF) . Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287:1531–1540. [DOI] [PubMed] [Google Scholar]
- 19. Lederman RJ, Mendelsohn FO, Anderson RD, Saucedo JF, Tenaglia AN, Hermiller JB, Hillegass WB, Rocha‐Singh K, Moon TE, Whitehouse MJ, Annex BH; TRAFFIC Investigators . Therapeutic angiogenesis with recombinant fibroblast growth factor‐2 for intermittent claudication (the TRAFFIC study): a randomised trial. Lancet. 2002;359:2053–2058. [DOI] [PubMed] [Google Scholar]
- 20. Belch J, Hiatt WR, Baumgartner I, Driver IV, Nikol S, Norgren L, Van Belle E; TAMARIS Committees and Investigators . Effect of fibroblast growth factor NV1FGF on amputation and death: a randomised placebo‐controlled trial of gene therapy in critical limb ischaemia. Lancet. 2011;377:1929–1937. [DOI] [PubMed] [Google Scholar]
- 21. Khan NE, De Souza A, Mister R, Flather M, Clague J, Davies S, Collins P, Wang D, Sigwart U, Pepper J. A randomized comparison of off‐pump and on‐pump multivessel coronary‐artery bypass surgery. N Engl J Med. 2004;350:21–28. [DOI] [PubMed] [Google Scholar]
- 22. Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S, Paolasso E, Straka Z, Piegas LS, Akar AR, Jain AR, Noiseux N, Padmanabhan C, Bahamondes JC, Novick RJ, Vaijyanath P, Reddy S, Tao L, Olavegogeascoechea PA, Airan B, Sulling TA, Whitlock RP, Ou Y, Ng J, Chrolavicius S, Yusuf S; CORONARY Investigators . Off‐pump or on‐pump coronary‐artery bypass grafting at 30 days. N Engl J Med. 2012;366:1489–1497. [DOI] [PubMed] [Google Scholar]
- 23. Walsh BW, Kuller LH, Wild RA, Paul S, Farmer M, Lawrence JB, Shah AS, Anderson PW. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA. 1998;279:1445–1451. [DOI] [PubMed] [Google Scholar]
- 24. Barrett‐Connor E, Grady D, Sashegyi A, Anderson PW, Cox DA, Hoszowski K, Rautaharju P, Harper KD. Raloxifene and cardiovascular events in osteoporotic postmenopausal women: four‐year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial. JAMA. 2002;287:847–857. [DOI] [PubMed] [Google Scholar]
- 25. Solomon SD, Janardhanan R, Verma A, Bourgoun M, Daley WL, Purkayastha D, Lacourciere Y, Hippler SE, Fields H, Naqvi TZ, Mulvagh SL, Arnold JM, Thomas JD, Zile MR, Aurigemma GP; Valsartan In Diastolic Dysfunction (VALIDD) Investigators . Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet. 2007;369:2079–2087. [DOI] [PubMed] [Google Scholar]
- 26. Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A; I‐PRESERVE Investigators . Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. [DOI] [PubMed] [Google Scholar]
- 27. Howard BV, Roman MJ, Devereux RB, Fleg JL, Galloway JM, Henderson JA, Howard WJ, Lee ET, Mete M, Poolaw B, Ratner RE, Russell M, Silverman A, Stylianou M, Umans JG, Wang W, Weir MR, Weissman NJ, Wilson C, Yeh F, Zhu J. Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes: the SANDS randomized trial. JAMA. 2008;299:1678–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Action to Control Cardiovascular Risk in Diabetes Study G , Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail‐Beigi F, Grimm RH Jr, Probstfield JL, Simons‐Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taylor AJ, Villines TC, Stanek EJ, Devine PJ, Griffen L, Miller M, Weissman NJ, Turco M. Extended‐release niacin or ezetimibe and carotid intima‐media thickness. N Engl J Med. 2009;361:2113–2122. [DOI] [PubMed] [Google Scholar]
- 30. Investigators A‐H , Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes‐Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. [DOI] [PubMed] [Google Scholar]
- 31. Bonaa KH, Bjerve KS, Straume B, Gram IT, Thelle D. Effect of eicosapentaenoic and docosahexaenoic acids on blood pressure in hypertension. A population‐based intervention trial from the Tromso study. N Engl J Med. 1990;322:795–801. [DOI] [PubMed] [Google Scholar]
- 32. Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega‐3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta‐analysis. JAMA. 2012;308:1024–1033. [DOI] [PubMed] [Google Scholar]
- 33. Coats AJ, Adamopoulos S, Meyer TE, Conway J, Sleight P. Effects of physical training in chronic heart failure. Lancet. 1990;335:63–66. [DOI] [PubMed] [Google Scholar]
- 34. Wood PD, Stefanick ML, Williams PT, Haskell WL. The effects on plasma lipoproteins of a prudent weight‐reducing diet, with or without exercise, in overweight men and women. N Engl J Med. 1991;325:461–466. [DOI] [PubMed] [Google Scholar]
- 35. Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil‐Smoller S, Kuller LH, LaCroix AZ, Langer RD, Lasser NL, Lewis CE, Limacher MC, Margolis KL, Mysiw WJ, Ockene JK, Parker LM, Perri MG, Phillips L, Prentice RL, Robbins J, Rossouw JE, Sarto GE, Schatz IJ, Snetselaar LG, Stevens VJ, Tinker LF, Trevisan M, Vitolins MZ, Anderson GL, Assaf AR, Bassford T, Beresford SA, Black HR, Brunner RL, Brzyski RG, Caan B, Chlebowski RT, Gass M, Granek I, Greenland P, Hays J, Heber D, Heiss G, Hendrix SL, Hubbell FA, Johnson KC, Kotchen JM. Low‐fat dietary pattern and risk of cardiovascular disease: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:655–666. [DOI] [PubMed] [Google Scholar]
- 36. Pitt B, Segal R, Martinez FA, Meurers G, Cowley AJ, Thomas I, Deedwania PC, Ney DE, Snavely DB, Chang PI. Randomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study, ELITE). Lancet. 1997;349:747–752. [DOI] [PubMed] [Google Scholar]
- 37. Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Kober L, Maggioni AP, Solomon SD, Swedberg K, Van de Werf F, White H, Leimberger JD, Henis M, Edwards S, Zelenkofske S, Sellers MA, Califf RM. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–1906. [DOI] [PubMed] [Google Scholar]
- 38. Follath F, Cleland JG, Just H, Papp JG, Scholz H, Peuhkurinen K, Harjola VP, Mitrovic V, Abdalla M, Sandell EP, Lehtonen L; Steering Committee and Investigators of the Levosimendan Infusion versus Dobutamine (LIDO) Study . Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low‐output heart failure (the LIDO study): a randomised double‐blind trial. Lancet. 2002;360:196–202. [DOI] [PubMed] [Google Scholar]
- 39. Mebazaa A, Nieminen MS, Packer M, Cohen‐Solal A, Kleber FX, Pocock SJ, Thakkar R, Padley RJ, Poder P, Kivikko M; SURVIVE Investigators . Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA. 2007;297:1883–1891. [DOI] [PubMed] [Google Scholar]
- 40. Nappo F, De Rosa N, Marfella R, De Lucia D, Ingrosso D, Perna AF, Farzati B, Giugliano D. Impairment of endothelial functions by acute hyperhomocysteinemia and reversal by antioxidant vitamins. JAMA. 1999;281:2113–2118. [DOI] [PubMed] [Google Scholar]
- 41. Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2008;300:2123–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Elam MB, Hunninghake DB, Davis KB, Garg R, Johnson C, Egan D, Kostis JB, Sheps DS, Brinton EA. Effect of niacin on lipid and lipoprotein levels and glycemic control in patients with diabetes and peripheral arterial disease: the ADMIT study: a randomized trial. Arterial Disease Multiple Intervention Trial. JAMA. 2000;284:1263–1270. [DOI] [PubMed] [Google Scholar]
- 43. Vermeulen EG, Stehouwer CD, Twisk JW, van den Berg M, de Jong SC, Mackaay AJ, van Campen CM, Visser FC, Jakobs CA, Bulterjis EJ, Rauwerda JA. Effect of homocysteine‐lowering treatment with folic acid plus vitamin B6 on progression of subclinical atherosclerosis: a randomised, placebo‐controlled trial. Lancet. 2000;355:517–522. [DOI] [PubMed] [Google Scholar]
- 44. Bonaa KH, Njolstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, Wang H, Nordrehaug JE, Arnesen E, Rasmussen K; NORVIT Trial Investigators . Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–1588. [DOI] [PubMed] [Google Scholar]
- 45. Masip J, Betbese AJ, Paez J, Vecilla F, Canizares R, Padro J, Paz MA, de Otero J, Ballus J. Non‐invasive pressure support ventilation versus conventional oxygen therapy in acute cardiogenic pulmonary oedema: a randomised trial. Lancet. 2000;356:2126–2132. [DOI] [PubMed] [Google Scholar]
- 46. Gray A, Goodacre S, Newby DE, Masson M, Sampson F, Nicholl J; 3CPO Trialists . Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med. 2008;359:142–151. [DOI] [PubMed] [Google Scholar]
- 47. Effect of fenofibrate on progression of coronary‐artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomised study. Lancet. 2001;357:905–910. [PubMed] [Google Scholar]
- 48. ACCORD Study Group , Ginsberg HN, Elam MB, Lovato LC, Crouse JR III, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail‐Beigi F, Bigger JT, Goff DC Jr, Cushman WC, Simons‐Morton DG, Byington RP. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P, Frohlich J, Albers JJ. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592. [DOI] [PubMed] [Google Scholar]
- 50. HPS2‐THRIVE Collaborative Group , Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, Armitage J. Effects of extended‐release niacin with laropiprant in high‐risk patients. N Engl J Med. 2014;371:203–212. [DOI] [PubMed] [Google Scholar]
- 51. Bikdeli B. C‐reactive protein, statins and the risk of vascular events: a better understanding. Cardiovasc Drugs Ther. 2011;25:545–549. [DOI] [PubMed] [Google Scholar]
- 52. Chen R, Chauk KH, Bikdeli B, Akram Y, Punnanithinont N, Ross JS, Krumholz HM. A changing landscape: contemporary characteristics of major cardiovascular superiority trials. Circulation. 2013;128:A17159. [Google Scholar]
- 53. Rules Committee Print 114‐67 Text of House Amendment to the Senate Amendment to H.R. 34, Tsunami Warning, Education, and Research Act of 2015. Available at: http://docs.house.gov/billsthisweek/20161128/CPRT-114-HPRT-RU00-SAHR34.pdf. Accessed December 8, 2016.
- 54. Kim C, Prasad V. Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival: an analysis of 5 years of US Food and Drug Administration approvals. JAMA Intern Med. 2015;175:1992–1994. [DOI] [PubMed] [Google Scholar]
- 55. LeBlanc M, Tangen C. Surrogates for survival or other end points in oncology. JAMA Oncol. 2016;2:263–264. [DOI] [PubMed] [Google Scholar]


