Abstract
Background
The atherosclerosis cardiovascular disease (ASCVD) Pooled Cohort risk equations have shown different calibration across US populations with varied levels of social deprivation.
Methods and Results
We analyzed the calibration and discrimination of the Pooled Cohort risk equations by social deprivation status among 9066 REGARDS (REasons for Geographic And Racial Differences in Stroke) study participants not taking statins for whom ASCVD risk may lead to statin initiation. Patients were aged 45 to 79 years, had no ASCVD or diabetes mellitus, and had a low‐density lipoprotein cholesterol level 70 to 189 mg/dL. Social deprivation was defined using 3 indicators: annual household income <$25 000, less than a high school education, and living without a partner. At baseline in 2003–2007, 54.6%, 27.4%, and 18.0% of participants had 0, 1, and 2 or 3 indicators showing deprivation, respectively. From baseline through December 2012, 457 participants developed ASCVD (nonfatal/fatal stroke, myocardial infarction, or coronary heart disease death). Predicted and observed ASCVD incidence per 1000 person‐years were 8.02 and 6.23 (95% CI, 5.31–7.31), respectively, among participants with 0 indicators of deprivation (Hosmer–Lemeshow P=0.01); 8.05 and 6.61 (95% CI, 5.29–8.24), respectively, with 1 indicator (P=0.09); and 9.83 and 11.40 (95% CI, 9.23–14.05), respectively, with 2 or 3 indicators (P=0.12). The C‐index (95% CI) was 0.72 (0.69–0.75), 0.73 (0.69–0.78), and 0.70 (0.65–0.75) among participants with 0, 1, and 2 or 3 indicators of deprivation, respectively. The net reclassification improvement after adding deprivation data to the Pooled Cohort risk equations was modest (0.12; 95% CI, 0.03–0.21).
Conclusions
The Pooled Cohort risk equations have good calibration among individuals with social deprivation but overestimate ASCVD risk among those with less social deprivation.
Keywords: cardiovascular disease, primary prevention, risk assessment, risk factor, socioeconomic position
Subject Categories: Epidemiology, Cardiovascular Disease, Primary Prevention, Risk Factors, Lifestyle
Introduction
In 2013, the American College of Cardiology (ACC) and the American Heart Association (AHA) published a set of equations for estimating 10‐year atherosclerotic cardiovascular disease (ASCVD) risk, the Pooled Cohort risk equations.1 Simultaneously, the ACC/AHA published a cholesterol management guideline that recommended using the Pooled Cohort risk equations to guide the decision to initiate statins.2 After these publications, the ACC/AHA Pooled Cohort risk equations were found to overestimate ASCVD risk in some contemporary US cohorts, including Women's Health Study (WHS), Physicians' Health Study (PHS), and Women's Health Initiative (WHI).3, 4 Participants included in the WHS and PHS were health professionals, and >40% of women enrolled in the WHI had a managerial or professional occupation.5, 6, 7 In contrast, results from REasons for Geographic And Racial Differences in Stroke (REGARDS) study suggest that the Pooled Cohort risk equations have good calibration among white and black adults from the general US population for whom ASCVD‐predicted risk may lead a discussion of statin initiation.8
Social deprivation is associated with increased risk for incident ASCVD.9, 10, 11 Studies from Europe have found that some cardiovascular risk prediction models show substantial differences in calibration across deprivation levels.12, 13 Therefore, it is possible that the calibration of the Pooled Cohort risk equations differs by social deprivation status, which may have contributed to the mismatch between predicted and observed ASCVD risk found in some US cohorts. We used data from the REGARDS study to investigate whether the calibration and discrimination of the ASCVD Pooled Cohort risk equations differ by levels of social deprivation. If the calibration or discrimination of the ASCVD Pooled Cohort risk equations are different across levels of social deprivation, this would support the need to include data on social deprivation for ASCVD risk prediction in the United States. We further investigated whether adding indicators of social deprivation would improve the discrimination of the ASCVD Pooled Cohort risk equations.
Methods
Study Population
The REGARDS study was designed to investigate the reasons underlying the higher rate of stroke mortality among blacks compared with whites, and among residents of the Southeastern United States versus other US regions.14 Coronary heart disease (CHD) events are being identified and adjudicated in an ancillary study.15 A total of 30 239 participants 45 years and older from all 48 contiguous US states and the District of Columbia were enrolled between January 2003 and October 2007. For the present analysis, we included REGARDS study participants for whom ASCVD risk may lead to consideration of statin initiation: those aged 45 to 79 years without a history of ASCVD or diabetes mellitus who were not taking statins and had fasting low‐density lipoprotein cholesterol 70 to 189 mg/dL or non–high‐density lipoprotein cholesterol (non–HDL‐C) 100 to 219 mg/dL (see below).2 We excluded participants with atrial fibrillation or heart failure because they were not included in the population used to develop the ASCVD Pooled Cohort risk equations.1 Participants with no follow‐up to detect incident ASCVD, missing data to calculate 10‐year ASCVD‐predicted risk, or missing data on income, education, or relationship status (married, living with someone in married‐like relationship) were excluded. Overall, 9066 REGARDS study participants were included in the current analysis (Figure S1). The REGARDS study protocol was approved by the institutional review boards governing research in human subjects at the participating centers. All participants provided written informed consent.
Baseline Assessment
A computer‐assisted telephone interview was used to collect self‐reported information on participants' age, race, sex, education, annual household income, place of residence, relationship status, current smoking, history of comorbid conditions (eg, myocardial infarction [MI], stroke, diabetes mellitus, atrial fibrillation) and vascular interventions (eg, coronary and lower extremity revascularization procedures, aortic aneurysm repair surgery), and use of antihypertensive, antidiabetes, and lipid‐lowering medications. Trained health professionals conducted an in‐home examination following standardized protocols. Procedures included 2 blood pressure measurements that were averaged, electrocardiography, collection of blood samples, and an inventory of all medications taken during the 2‐week period prior to the study visit. Serum total cholesterol, HDL‐C, triglyceride, and glucose levels were measured by colorimetric reflectance spectrophotometry using the collected blood samples. For participants with fasting triglycerides <400 mg/dL, low‐density lipoprotein cholesterol was calculated using the Friedewald equation.16 For participants who did not fast (n=1369) or who had triglycerides ≥400 mg/dL (n=91), non–HDL‐C was calculated as total cholesterol minus HDL‐C.
History of ASCVD at baseline was defined as self‐report of a physician diagnosis of MI or stroke; a prior coronary artery bypass, coronary angioplasty or stenting; a lower extremity revascularization procedure or an aortic aneurysm repair surgery; or evidence of a previous MI on the study electrocardiogram. Diabetes mellitus was defined as fasting glucose ≥126 mg/dL or nonfasting glucose ≥200 mg/dL, or self‐report of a prior diagnosis of diabetes mellitus with current use of antidiabetes medications. Atrial fibrillation was defined using the baseline electrocardiogram or self‐report of a physician diagnosis. Information on heart failure was not ascertained at baseline. Therefore, we defined prevalent heart failure as treatment with digoxin based on the study inventory of medications.17 We defined statin use through the medication inventory or by self‐reported use of lipid‐lowering medications.
We studied 3 indicators of social deprivation that can be easily assessed in clinical practice: self‐reported annual household income, education, and relationship status. Reporting an annual household income <$25 000, having less than a high school education, and living without a partner were each defined as deprivation. Living without a partner was defined by reporting a marital status other than married (ie, single, divorced, widowed, or other) and responding “no” to the question “Are you currently living with someone in a marriage‐like relationship?” For secondary analyses, social deprivation was defined using the area deprivation index (ADI) based on participants' census tracts.18 Higher values on the ADI represent more social deprivation.
ASCVD Incidence
Living REGARDS participants or proxy respondents were contacted every 6 months via telephone to assess incident stroke or CHD events. When nonfatal events were reported, medical records were retrieved for adjudication. Stroke events were confirmed by a panel of experts following the World Health Organization definition.19 Nonfatal MIs were adjudicated by trained clinicians following published guidelines.20, 21, 22, 23 When deaths were reported, interviews with next‐of‐kin or proxies, medical records in the last year of life, death certificates, and autopsy reports were used to determine whether a stroke or a CHD event was the main underlying cause. We defined incident ASCVD as an incident nonfatal or fatal stroke, nonfatal MI, or CHD death.2 Stroke and CHD events were adjudicated through December 31, 2012.
Statistical Analysis
We calculated the cumulative number of indicators showing deprivation for each participant, with possible values ranging from 0 (less social deprivation) to 3 (more social deprivation). Baseline characteristics of participants were calculated by the number of indicators showing deprivation (0, 1, and 2 or 3).
Calibration and discrimination of the Pooled Cohort risk equations were assessed in strata defined by the cumulative number of indicators showing deprivation and by categories of each indicator of social deprivation status, separately. For calibration, observed and predicted number of ASCVD events at 5 years were calculated by quintiles of predicted risk and compared using a modified Hosmer‐Lemeshow χ2 statistic.24 A 5‐year observation period was selected because REGARDS participants currently have less than 10 years of follow‐up (median follow‐up 7.0 years; maximum follow‐up 9.9 years). We used the Kaplan–Meier method to calculate the cumulative incidence of ASCVD and the observed number of ASCVD events at 5 years.25 The predicted number of events at 5 years was estimated as shown in Table S1.8 Discrimination was evaluated using the Harrell's C‐index.26 We conducted a sensitivity analysis of the calibration and discrimination of the Pooled Cohort risk equations using multiple imputation to include participants with missing data to calculate 10‐year ASCVD‐predicted risk (n=528) and indicators of social deprivation status (n=1174).
Cox‐proportional hazard models were used to analyze the association of the number of indicators showing deprivation and, separately, each indicator of social deprivation status with ASCVD. In addition to a crude model, 2 levels of adjustment were performed. Model 1 adjusted for age, sex, and race. Model 2 adjusted for variables in model 1 plus the remaining variables in the Pooled Cohort risk equations (ie, total cholesterol, HDL‐C, systolic blood pressure, use of antihypertensive medications, and smoking status).
For each race‐sex group, we developed best‐fit Cox‐proportional hazard regression models for incident ASCVD including variables in the Pooled Cohort risk equations. Next, we added the cumulative number of indicators showing deprivation to the best‐fit models. The C‐index for these models were calculated and change in risk classification across the models was analyzed using the continuous net reclassification improvement method.27 The C‐index and net reclassification improvement were also calculated after adding annual household income, education, and relationship status, separately to the best‐fit models. Proportional hazards assumptions were evaluated by plots (ie, the log (‐log (survival)) plot) and adding interaction terms between social deprivation status and the log of follow‐up time. These assumptions were not violated.
In a secondary analysis, we assessed the calibration and discrimination of the ASCVD Pooled Cohort risk equations in strata defined by quartiles of the ADI. We used chained equations in Stata/I.C. 12.1 (Stata Corporation, College Station, TX) to impute 12 data sets for analyses using multiple imputation.28 All other analyses were conducted in SAS 9.3 (SAS Institute Inc., Cary, NC) using a level of significance α<0.05.
Results
Among participants included in the analysis, 4944 (54.6%), 2487 (27.4%), and 1635 (18.0%) had 0, 1, and 2 or 3 indicators showing deprivation, respectively. Participants with a higher cumulative number of indicators showing deprivation were older, less likely to be male, and more likely to be black, current smokers, and taking antihypertensive medication (Table 1).
Table 1.
Characteristics of REGARDS Study Participants by the Number of Indicators Showing Deprivation (N=9066)
| No. of Indicators Showing Deprivationa | |||
|---|---|---|---|
| 0 (Less Deprivation) | 1 | 2 or 3 (More Deprivation) | |
| (n=4944) | (n=2487) | (n=1635) | |
| ASCVD Pooled Cohort risk equation componentsb | |||
| Age, mean (SD), y | 60.4 (8.0) | 61.8 (8.2) | 64.2 (8.5) |
| Men, No. (%) | 2635 (53.3) | 769 (30.9) | 417 (25.5) |
| Blacks, No. (%) | 1299 (26.3) | 1191 (47.9) | 951 (58.2) |
| Current smoking, No. (%) | 529 (10.7) | 435 (17.5) | 397 (24.3) |
| SBP, mean (SD), mm Hg | 123.3 (15.1) | 125.6 (16.1) | 128.4 (17.9) |
| Antihypertensive medication, No. (%) | 1594 (32.2) | 952 (38.3) | 741 (45.3) |
| Total cholesterol, mean (SD), mg/dL | 200.4 (30.0) | 203.5 (31.5) | 204.4 (31.2) |
| HDL‐C, mean (SD), mg/dL | 52.8 (16.4) | 55.4 (16.6) | 55.3 (16.2) |
| Region of residence, No. (%)c | |||
| Stroke buckle | 1040 (21.0) | 463 (18.6) | 332 (20.3) |
| Stroke belt | 1722 (34.8) | 808 (32.5) | 655 (40.1) |
| Other contiguous US states | 2182 (44.1) | 1216 (48.9) | 648 (39.6) |
| Area deprivation index in participants' census tract,d No. (%) | |||
| 47.0 to <96.7 | 1423 (28.8) | 491 (19.7) | 128 (7.8) |
| 96.7 to <106.5 | 1273 (25.7) | 519 (20.9) | 253 (15.5) |
| 106.5 to <112.8 | 1054 (21.3) | 612 (24.6) | 377 (23.1) |
| 112.8–127.2 | 724 (14.6) | 615 (24.7) | 706 (43.2) |
| Indicators of social deprivation status | |||
| Annual household income, No. (%), US$ | |||
| ≥$50 000 | 3164 (64.0) | 627 (25.2) | 6 (0.4) |
| $25 000 to <$50 000 | 1780 (36.0) | 1293 (52.0) | 42 (2.6) |
| <$25 000a | 0 (0.0)a, e | 567 (22.8)a | 1587 (97.1)a |
| Education, No. (%) | |||
| College graduate and above | 2540 (51.4) | 981 (39.4) | 222 (13.6) |
| High school/some college | 2404 (48.6) | 1322 (53.2) | 909 (55.6) |
| Less than high schoola | 0 (0.0)a, e | 184 (7.4)a | 504 (30.8)a |
| Relationship status, No. (%) | |||
| Living with a partner | 4944 (100.0) | 751 (30.2) | 175 (10.7) |
| Living without a partnera | 0 (0.0)a, e | 1736 (69.8)a | 1460 (89.3)a |
ASCVD indicates atherosclerotic cardiovascular disease; HDL‐C, high‐density lipoprotein cholesterol; REGARDS, REasons for Geographic And Racial Differences in Stroke; SBP, systolic blood pressure.
The cumulative number of indicators showing deprivation is calculated by adding 1 for being in each of the following 3 categories: annual household income <$25 000; less than a high school education; and living without a partner (these categories are followed by an asterisk in the table). Possible values for the cumulative number of indicators showing deprivation range from 0 to 3.
REGARDS study participants with diabetes mellitus were excluded from the analysis.
Stroke buckle includes coastal plains of North Carolina, South Carolina, and Georgia. Stroke belt includes the remaining parts of the stroke buckle states and Tennessee, Mississippi, Alabama, Louisiana, and Arkansas.
Categories of area deprivation index were defined using quartiles of distribution.
By definition, there are no participants with annual household income <$25 000, less than a high school education, or living without a partner among those with 0 indicators showing deprivation.
A total of 457 incident ASCVD events occurred during 59 648 person‐years of follow‐up. Participants with more indicators showing deprivation had a higher incidence of ASCVD (Figure and Table 2, top panel). Among participants with 0 or 1 indicator showing deprivation, the Pooled Cohort risk equations overestimated ASCVD risk (Table S2). However, the Pooled Cohort risk equations underestimated ASCVD risk among participants with 2 or 3 indicators showing deprivation. Discrimination of the Pooled Cohort risk equations was similar among participants with 0, 1, and 2 or 3 indicators showing deprivation.
Figure 1.
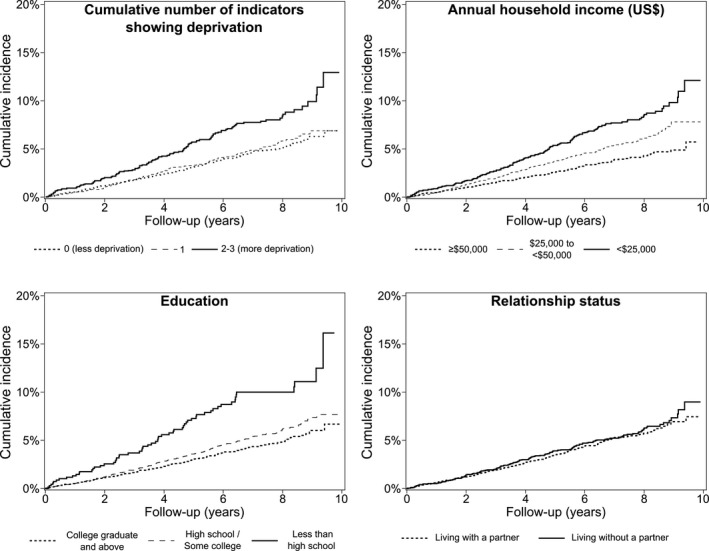
Cumulative incidence of atherosclerotic cardiovascular disease by social deprivation status. US$ indicates US dollars.
Table 2.
Observed and Predicted Incidence Rates of Atherosclerotic Cardiovascular Disease and Calibration and Discrimination of the Pooled Cohort Risk Equations by Social Deprivation Status
| Events/Person‐Years | Events in 5 Years | 5‐Year Incidence Ratea | Calibration | Discrimination | ||||
|---|---|---|---|---|---|---|---|---|
| Observedb | Predictedc | Observed (95% CI)b | Predictedc | Hosmer–Lemeshow χ2 | P Value | C‐Index (95% CI) | ||
| Cumulative number of indicators of showing deprivation | ||||||||
| 0 (Less deprivation) | 223/33 266 | 154 | 198 | 6.23 (5.31–7.31) | 8.02 | 12.43 | 0.01 | 0.718 (0.686–0.751) |
| 1 | 117/16 259 | 82 | 100 | 6.61 (5.29–8.24) | 8.05 | 6.60 | 0.09 | 0.734 (0.687–0.781) |
| 2 or 3 (More deprivation)d | 117/10 122d | 93d | 80d | 11.40 (9.23–14.05)d | 9.83d | 5.77d | 0.12d | 0.695 (0.645–0.746)d |
| Indicators of social deprivation status | ||||||||
| Annual household income, US$ | ||||||||
| ≥$50 000 | 140/25 454 | 98 | 131 | 5.15 (4.21–6.29) | 6.91 | 10.91 | 0.01 | 0.724 (0.683–0.765) |
| $25 000 to <$50 000 | 166/20 791 | 117 | 143 | 7.48 (6.22–9.00) | 9.16 | 8.09 | 0.04 | 0.711 (0.671–0.751) |
| <$25 000d | 151/13 403d | 116d | 105d | 10.73 (8.88–12.95)d | 9.72d | 4.74d | 0.19d | 0.703 (0.660–0.746)d |
| Education | ||||||||
| College graduate and above | 164/25 627 | 113 | 145 | 6.03 (5.01–7.26) | 7.74 | 9.01 | 0.03 | 0.724 (0.685–0.763) |
| High school/some college | 233/29 844 | 166 | 193 | 7.18 (6.15–8.39) | 8.33 | 8.62 | 0.03 | 0.704 (0.671–0.737) |
| Less than high schoold | 60/4 178d | 50d | 41d | 14.56 (10.92–19.35)d | 11.87d | 8.92d | 0.03d | 0.742 (0.676–0.808)d |
| Relationship status | ||||||||
| Living with a partner | 292/39 086 | 203 | 247 | 6.92 (6.02–7.96) | 8.42 | 11.45 | 0.01 | 0.720 (0.692–0.749) |
| Living without a partnerd | 165/20 562d | 124d | 131d | 7.79 (6.50–9.32)d | 8.23d | 7.49d | 0.06d | 0.722 (0.680–0.763)d |
Data used to calculate the Hosmer–Lemeshow χ2 are shown in Tables S2 through S5. The median and maximum follow‐up among participants included in the present analysis were 7.0 and 9.9 years, respectively.
Incidence rates are expressed per 1000 person‐years.
Adjusted using the Kaplan–Meier method.
Determined using the atherosclerotic cardiovascular disease Pooled Cohort risk equations.
Categories used to define deprivation within each indicator of social deprivation status.
Participants with lower income, less education, and living without a partner, separately, had a higher 5‐year incidence of ASCVD (Table 2, bottom panel). The ASCVD Pooled Cohort risk equations had good calibration among participants with an annual household income <$25 000 and living without a partner, but underestimated ASCVD risk among those with less than high a school education (Tables S3 through S5). The ASCVD Pooled Cohort risk equations overestimated ASCVD risk among individuals with annual household income ≥$25 000, with high school education and above, and those living with a partner. Discrimination of the ASCVD Pooled Cohort risk equations was similar across categories of each indicator of social deprivation status. Results were similar in sensitivity analyses using multiple imputation (Table S6).
Having 2 or 3 versus 0 indicators showing deprivation, and, separately, lower annual household income and less education were associated with an increased hazard ratio for ASCVD after adjustment for variables in the ASCVD Pooled Cohort risk equations (Table 3). Relationship status was not associated with ASCVD risk after adjustment for variables in the Pooled Cohort risk equations. Adding the cumulative number of indicators showing deprivation and, separately, annual household income, improved risk classification using the best‐fit Cox regression models (Table 4). Adding education and relationship status did not improve risk classification.
Table 3.
Hazard Ratios for Atherosclerotic Cardiovascular Disease Associated With Social Deprivation Status
| Hazard Ratio (95% CI) | |||
|---|---|---|---|
| Crude | Model 1 | Model 2 | |
| Cumulative number of indicators showing deprivation | |||
| 0 (Less deprivation) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| 1 | 1.08 (0.86–1.35) | 1.25 (0.99–1.58) | 1.13 (0.89–1.43) |
| 2 or 3 (More deprivation)a | 1.73 (1.39–2.17) | 1.87 (1.46–2.39) | 1.52 (1.18–1.95) |
| Indicators of social deprivation status | |||
| Annual household income, US$ | |||
| ≥$50 000 | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| $25 000 to <$50 000 | 1.45 (1.16–1.82) | 1.26 (1.00–1.59) | 1.12 (0.89–1.42) |
| <$25 000a | 2.06 (1.63–2.59) | 1.90 (1.48–2.43) | 1.51 (1.18–1.94) |
| Education | |||
| College graduate and above | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| High school/some college | 1.23 (1.01–1.50) | 1.27 (1.04–1.55) | 1.12 (0.91–1.37) |
| Less than high schoola | 2.26 (1.68–3.04) | 1.92 (1.41–2.61) | 1.50 (1.10–2.04) |
| Relationship status | |||
| Living with a partner | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Living without a partnera | 1.08 (0.89–1.30) | 1.28 (1.04–1.59) | 1.17 (0.94–1.45) |
Model 1 includes adjustment for age, sex, and race. Model 2 includes adjustment for age, sex, race, smoking status, total cholesterol, high‐density lipoprotein cholesterol, systolic blood pressure, and use of antihypertensive medications.
Categories used to define deprivation within each indicator of social deprivation status.
Table 4.
Change in the Discrimination of the Atherosclerotic Cardiovascular Disease Pooled Cohort Risk Equations When Adding Indicators of Social Deprivation Status
| C‐Index Before Adding Indicator (95% CI) | C‐Index After Adding Indicator (95% CI) | Difference in C‐Index (95% CI) | Continuous NRIa (95% CI) | |
|---|---|---|---|---|
| Cumulative number of indicators showing deprivation | 0.739 (0.716–0.762) | 0.742 (0.719–0.765) | 0.003 (−0.001 to 0.007) | 0.12 (0.03–0.21) |
| Indicators of social deprivation status | ||||
| Annual household income | 0.739 (0.716–0.762) | 0.743 (0.720–0.766) | 0.004 (0.000–0.008) | 0.16 (0.06–0.25) |
| Education | 0.739 (0.716–0.762) | 0.739 (0.716–0.763) | 0.001 (−0.002 to 0.003) | 0.07 (−0.02 to 0.15) |
| Relationship status | 0.739 (0.716–0.762) | 0.739 (0.716–0.762) | 0.000 (−0.001 to 0.001) | 0.02 (−0.07 to 0.11) |
The C‐index before adding each indicator of social deprivation status was calculated using the 5‐year predicted risk using best‐fit models for the atherosclerotic cardiovascular Pooled Cohort risk equations in the REGARDS (REasons for Geographic And Racial Differences in Stroke) study. The C‐index after adding indicators of social deprivation status was calculated using the 5‐year predicted risk using best‐fit models for the atherosclerotic cardiovascular Pooled Cohort risk equations which include each indicator in the table, separately. The difference in C‐index was calculated as C‐index after adding each indicator of social deprivation status minus C‐index before adding indicators. All analyses in the current table were conducted using bootstrapping techniques.
Secondary Analysis
Participants with a higher ADI had a higher 5‐year incidence of ASCVD (Table S7). Calibration of the ASCVD Pooled Cohort risk equations was good (each χ2 P>0.15) with the exception of participants with an ADI in the second lowest quartile (ADI range: 96.7 to <106.5; χ2 P=0.03; Table S8). ADI was not associated with incident ASCVD after adjustment for variables in the ASCVD Pooled Cohort risk equations (Table S9).
Discussion
In the current analysis of a large population‐based cohort of black and white US adults, the Pooled Cohort risk equations had good calibration or underestimated ASCVD risk among participants with more social deprivation defined using information on annual household income, education, and relationship status. However, the equations overestimated ASCVD risk among participants with less social deprivation. There was a graded increase in the incidence of ASCVD with more social deprivation, and adding information on social deprivation resulted in a modest improvement in risk classification of the ASCVD Pooled Cohort risk equations.
The ASCVD Pooled Cohort risk equations were developed using data from the Framingham Original and Offspring studies, ARIC (Atherosclerosis Risk in Communities) study, CHS (Cardiovascular Health Study), and CARDIA (Coronary Artery Risk Development in Young Adults) study.1 These US cohorts, which included participants from the general population, were conducted in the era before pharmacologic therapies including statins and aspirin were commonly used for ASCVD prevention.1, 29 After their publication in 2013, the ASCVD Pooled Cohort risk equations were assessed using data from more contemporary US cohorts. The Pooled Cohort risk equations overestimated ASCVD risk in the WHS, PHS, and WHI, which mainly included adults without social deprivation.3, 4 In addition, the Pooled Cohort risk equations overestimated ASCVD risk in MESA (Multi‐Ethnic Study of Atherosclerosis) study.30 In contrast, the ASCVD Pooled Cohort risk equations had good calibration among individuals for whom predicted risk may lead to consideration of statin initiation using data from the REGARDS study, a cohort with a high degree of generalizability to black and white US adults.8 Several factors have been suggested that could explain the mismatch between the ASCVD risk predicted by the Pooled Cohort risk equations with that observed in some contemporary US cohorts, including an increased use of pharmacologic risk reduction therapies (eg, statins, aspirin), a decline in ASCVD incidence in the general population, and a multiethnic composition.29, 31, 32 The current analysis suggests that selecting populations without social deprivation may have also contributed to this mismatch.
Social deprivation has been defined using many different indicators including income, education, social connections, and neighborhood of residency.9, 10 Prior studies have shown that social deprivation is associated with ASCVD incidence independently of cardiovascular risk factors commonly used for risk prediction.33, 34, 35 In the ARIC study, after adjustment for the Framingham CHD Risk Score, participants without a history of CHD or diabetes diabetes mellitus who had <12 years of education or an annual income <150% of the federal poverty level had a hazard ratio of 1.58 (95% CI, 1.31–1.90) for CHD compared with their counterparts with ≥12 years of education and an annual income ≥150% of the federal poverty level.33 In the current analysis, social deprivation was also associated with incident ASCVD after adjusting for variables included in the Pooled Cohort risk equations. Pharmacologic therapies including statins and aspirin are effective to reduce ASCVD risk.36, 37 However, individuals with social deprivation may be less likely to initiate and be adherent to these therapies.10, 38 In a previous analysis of the REGARDS study, the multivariable‐adjusted prevalence ratio for statin use at baseline associated with an annual income <$20 000 versus ≥$20 000 was 0.94 (95% CI, 0.88–1.00) among high‐risk participants.39 Therefore, a lower use of pharmacologic therapies may contribute to the higher ASCVD risk associated with social deprivation. Other mechanisms that may contribute to an increased risk for ASCVD among individuals with social deprivation include lifestyle factors (eg, unhealthy diet and low physical activity), less social support, an unfavorable residential environment, and high levels of stress.10, 11
Adding a new factor to risk prediction models may be perceived as unimportant as this commonly leads to a very small improvement in discrimination.40 However, adding factors to risk prediction models could also contribute to improvements in the calibration in specific subpopulations. Results from the REGARDS study suggest that, although the Pooled Cohort risk equations seem to have a good calibration among US adults for whom ASCVD‐predicted risk should lead to a discussion of statin initiation, these equations may overestimate risk among those with less social deprivation. Results in the REGARDS study are consistent with prior studies conducted in Europe showing that prediction models, which do not incorporate data on social deprivation status, could underestimate cardiovascular risk among adults with deprivation and/or overestimate risk among those without deprivation.12, 13 This could occur when social deprivation remains associated with higher ASCVD risk after adjusting for variables included in the prediction model. Therefore, at each level of predicted risk, observed risk would be higher among individuals with more social deprivation, leading to a differential calibration by social deprivation levels. A mismatch between observed and predicted ASCVD risk by social deprivation levels could have substantial consequences when used to guide statin therapy initiation. Underestimation of ASCVD risk among adults with social deprivation may lead to underuse of statins in this population, contributing to disparities in ASCVD. Overestimation of ASCVD risk among those without deprivation may lead to unnecessary treatment, more statin‐related adverse events and higher costs. Therefore, future studies aimed at developing or improving ASCVD risk prediction models in the United States should consider including social deprivation data to achieve good calibration across populations with and without deprivation.
Prior studies have found area‐level indicators of social deprivation to be associated with a higher ASCVD risk.41, 42, 43 Using area‐level information for ASCVD risk prediction in clinical practice is compelling because individuals may be reluctant to report social deprivation data.11 However, people living in the same area may show substantial heterogeneity in their individual levels of deprivation, which may result in area‐level indicators providing low discrimination. An area‐level indicator of social deprivation, the Townsend score, was associated with cardiovascular risk after adjusting for risk factors in the United Kingdom and is incorporated into the QRISK.43, 44 Also, the Scottish risk prediction model ASSIGN incorporates an area‐level indicator of social deprivation, the Scottish Index of Multiple Deprivation.41 In the current study, we used the ADI, a composite score derived from US census data, as an area‐level indicator of social deprivation. Among US adults, the ADI has been associated with all‐cause mortality and 30‐day rehospitalization.18, 45 Results from the current analysis suggest that the ADI may not be independently associated with ASCVD risk and should not be used for ASCVD risk prediction among US adults.
The 2013 ACC/AHA cholesterol management guidelines recommend that ASCVD‐predicted risk should be used to start a clinician‐patient discussion on statin therapy initiation.2 In addition to predicted ASCVD risk, clinicians and patients should discuss potential risk‐reduction benefits and adverse effects associated with statin therapy, heart‐healthy lifestyles, management of other risk factors not included in the Pooled Cohort risk equations, and patient preferences. Results from the current analysis suggest that clinicians should also consider social deprivation when discussing statin initiation. Few data are currently available on adherence to the 2013 ACC/AHA cholesterol management guideline in clinical practice, including the use of the Pooled Cohort risk equations to inform a shared decision to initiate statins. Adherence to the ACC/AHA cholesterol management guideline may be lower among patients with social deprivation as it has been suggested that those who have lower income or education are less likely to be involved in treatment decisions.46 Future studies should investigate adherence to the 2013 ACC/AHA cholesterol management guideline and whether this differs by patients' social deprivation status.
Study Strengths and Limitations
Strengths of the current analysis include the large number of participants with information on 3 indicators of social deprivation and factors to calculate ASCVD risk using the Pooled Cohort risk equations. Data collection in the REGARDS study followed standardized protocols and CHD and stroke events were adjudicated by trained personnel following published recommendations. Despite these strengths, the results of our study should be interpreted in the context of known and potential limitations. We used digoxin use as a proxy for heart failure as this was not assessed at baseline in the REGARDS study. Therefore, our analysis may have included some participants with heart failure who were not taking digoxin. Follow‐up of REGARDS study participants is currently ongoing and data were only available to calculate observed ASCVD risk at 5 years. The REGARDS study does not have active surveillance to detect ASCVD events that may have not been reported by participants or proxies, which could have led to an underestimation of the ASCVD risk in this cohort. The ADI uses 2000 US census data and may not represent the environment of REGARDS study participants at the time of their enrollment in 2003–2007.
Conclusions
Results from the current study suggest that ASCVD risk predicted by the Pooled Cohort risk equations may mismatch observed risk by social deprivation levels. Specifically, the Pooled Cohort risk equations may overestimate ASCVD risk among individuals with less social deprivation but may have good calibration or underestimate ASCVD risk among those with more social deprivation. Future ASCVD risk prediction equations could add information on social deprivation status to reduce the mismatch across groups with different levels of deprivation. Meanwhile, clinicians and patients should take social deprivation into consideration, in addition to ASCVD‐predicted risk and other risk factors, when discussing statin initiation.
Sources of Funding
This research project is supported by a cooperative agreement U01‐NS041588 from the National Institute of Neurological Disorders and Stroke, the National Institutes of Health, and the Department of Health and Human Service. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis, or interpretation of the data. Additional support was provided by grants R01‐HL080477 and K24‐HL111154 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Disclosures
Carson, Safford and Muntner receive grant support from Amgen Inc. Colantonio, Richman, Lloyd‐Jones, Howard, Deng, Howard, and Goff have no disclosures to report.
Supporting information
Table S1. Estimation of Race‐ and Sex‐Specific Risk for Atherosclerosis Cardiovascular Disease Using the Pooled Cohort Risk Equations
Table S2. Calibration of the Atherosclerotic Cardiovascular Disease Pooled Cohort Risk Equations by the Cumulative Number of Indicators Showing Deprivation
Table S3. Calibration of the Atherosclerotic Cardiovascular Disease Pooled Cohort Risk Equations by Annual Household Income
Table S4. Calibration of the Atherosclerotic Cardiovascular Disease Pooled Cohort Risk Equations by Education
Table S5. Calibration of the Atherosclerotic Cardiovascular Disease Pooled Cohort Risk Equations by Relationship Status
Table S6. Observed and Predicted Incidence Rates of Atherosclerotic Cardiovascular Disease and Calibration and Discrimination of the Pooled Cohort Risk Equations by Social Deprivation Status (Using Multiple Imputation With 12 Imputed Data Sets, n=10 768)
Table S7. Observed and Predicted Incidence Rates of Atherosclerotic Cardiovascular Disease and Calibration and Discrimination of the Pooled Cohort Risk Equations by Area Deprivation Index
Table S8. Calibration of the Atherosclerotic Cardiovascular Disease Pooled Cohort Risk Equations by Area Deprivation Index
Table S9. Hazard Ratios for Atherosclerosis Cardiovascular Disease Associated With Categories of Area Deprivation Index
Figure S1. Flow‐chart of REGARDS study participants included in the analysis.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions and further information about the study can be found at http://www.regardsstudy.org.
(J Am Heart Assoc. 2017;6:e005676. DOI: 10.1161/JAHA.117.005676.)
This work was presented as a poster at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health Scientific Sessions, March 1–4, 2016, in Phoenix, AZ.
References
- 1. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice G . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 2. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. [DOI] [PubMed] [Google Scholar]
- 3. Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013;382:1762–1765. [DOI] [PubMed] [Google Scholar]
- 4. Cook NR, Ridker PM. Further insight into the cardiovascular risk calculator: the roles of statins, revascularizations, and underascertainment in the Women's Health Study. JAMA Intern Med. 2014;174:1964–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Final report on the aspirin component of the ongoing Physicians' Health Study. Steering Committee of the Physicians' Health Study Research Group. N Engl J Med. 1989;321:129–135. [DOI] [PubMed] [Google Scholar]
- 6. Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low‐dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. [DOI] [PubMed] [Google Scholar]
- 7. Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women's Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107–S121. [DOI] [PubMed] [Google Scholar]
- 8. Muntner P, Colantonio LD, Cushman M, Goff DC Jr, Howard G, Howard VJ, Kissela B, Levitan EB, Lloyd‐Jones DM, Safford MM. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA. 2014;311:1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lang T, Lepage B, Schieber A‐C, Lamy S, Kelly‐Irving M. Social determinants of cardiovascular diseases. Public Health Rev. 2012;33:601–622. [Google Scholar]
- 10. Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz‐Flores S, Davey‐Smith G, Dennison‐Himmelfarb CR, Lauer MS, Lockwood DW, Rosal M, Yancy CW. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132:873–898. [DOI] [PubMed] [Google Scholar]
- 11. Fiscella K, Tancredi D. Socioeconomic status and coronary heart disease risk prediction. JAMA. 2008;300:2666–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brindle PM, McConnachie A, Upton MN, Hart CL. Davey Smith G, Watt GC. The accuracy of the Framingham risk‐score in different socioeconomic groups: a prospective study. Br J Gen Pract. 2005;55:838–845. [PMC free article] [PubMed] [Google Scholar]
- 13. Ramsay SE, Morris RW, Whincup PH, Papacosta AO, Thomas MC, Wannamethee SG. Prediction of coronary heart disease risk by Framingham and SCORE risk assessments varies by socioeconomic position: results from a study in British men. Eur J Cardiovasc Prev Rehabil. 2011;18:186–193. [DOI] [PubMed] [Google Scholar]
- 14. Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. [DOI] [PubMed] [Google Scholar]
- 15. Safford MM, Brown TM, Muntner PM, Durant RW, Glasser S, Halanych JH, Shikany JM, Prineas RJ, Samdarshi T, Bittner VA, Lewis CE, Gamboa C, Cushman M, Howard V, Howard G. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308:1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 17. Pullicino PM, McClure LA, Wadley VG, Ahmed A, Howard VJ, Howard G, Safford MM. Blood pressure and stroke in heart failure in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Stroke. 2009;40:3706–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh GK. Area deprivation and widening inequalities in US mortality, 1969–1998. Am J Public Health. 2003;93:1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, Cushman M, Moy CS, Soliman EZ, Kissela BM, Howard G. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011;69:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole‐Wilson PA, Gurfinkel EP, Lopez‐Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernandez‐Aviles F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio‐Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck‐Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez‐Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al‐Attar N. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. [DOI] [PubMed] [Google Scholar]
- 21. Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall‐Pedoe H. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549. [DOI] [PubMed] [Google Scholar]
- 22. Prineas RJ, Crow RS, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings: Standards and Procedures for Measurement and Classification. Boston, MA: Wright‐OSG; 1982. [Google Scholar]
- 23. Prineas RJ, Crow RS, Zhang ZM. Minnesota Code Manual of Electrocardiographic Findings. London, England: Springer‐Verlag; 2010. [Google Scholar]
- 24. D'Agostino R, Nam B‐H. Evaluation of the performance of survival analysis models: discrimination and calibration measures. Handb Stat. 2004;23:1–25. [Google Scholar]
- 25. Colette D. Modeling Survival Data in Medical Research. London, England: Chapman & Hall; 1994. [Google Scholar]
- 26. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 27. Pencina MJ, D'Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 29. Stone NJ, Lloyd‐Jones DM. The Pooled Cohort equations for predicting risk of myocardial infarction and stroke: validated in representative natural history populations. Mayo Clin Proc. 2016;91:692–694. [DOI] [PubMed] [Google Scholar]
- 30. DeFilippis AP, Young R, Carrubba CJ, McEvoy JW, Budoff MJ, Blumenthal RS, Kronmal RA, McClelland RL, Nasir K, Blaha MJ. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162:266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cook NR, Ridker PM. Calibration of the Pooled Cohort equations for atherosclerotic cardiovascular disease: an update. Ann Intern Med. 2016;165:786–794. [DOI] [PubMed] [Google Scholar]
- 32. Blaha MJ. The critical importance of risk score calibration: time for transformative approach to risk score validation? J Am Coll Cardiol. 2016;67:2131–2134. [DOI] [PubMed] [Google Scholar]
- 33. Fiscella K, Tancredi D, Franks P. Adding socioeconomic status to Framingham scoring to reduce disparities in coronary risk assessment. Am Heart J. 2009;157:988–994. [DOI] [PubMed] [Google Scholar]
- 34. Ferrario MM, Veronesi G, Chambless LE, Tunstall‐Pedoe H, Kuulasmaa K, Salomaa V, Borglykke A, Hart N, Soderberg S, Cesana G. The contribution of educational class in improving accuracy of cardiovascular risk prediction across European regions: the MORGAM Project Cohort Component. Heart. 2014;100:1179–1187. [DOI] [PubMed] [Google Scholar]
- 35. Rawshani A, Svensson AM, Rosengren A, Eliasson B, Gudbjornsdottir S. Impact of socioeconomic status on cardiovascular disease and mortality in 24,947 individuals with type 1 diabetes. Diabetes Care. 2015;38:1518–1527. [DOI] [PubMed] [Google Scholar]
- 36. Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R. Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. [DOI] [PubMed] [Google Scholar]
- 37. Guirguis‐Blake JM, Evans CV, Senger CA, Rowland MG, O'Connor EA, Whitlock EP. Aspirin for the Primary Prevention of Cardiovascular Events: A Systematic Evidence Review for the U.S. Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2015. [PubMed] [Google Scholar]
- 38. Brown AF, Gross AG, Gutierrez PR, Jiang L, Shapiro MF, Mangione CM. Income‐related differences in the use of evidence‐based therapies in older persons with diabetes mellitus in for‐profit managed care. J Am Geriatr Soc. 2003;51:665–670. [DOI] [PubMed] [Google Scholar]
- 39. Gamboa CM, Safford MM, Levitan EB, Mann DM, Yun H, Glasser SP, Woolley JM, Rosenson R, Farkouh M, Muntner P. Statin underuse and low prevalence of LDL‐C control among U.S. adults at high risk of coronary heart disease. Am J Med Sci. 2014;348:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172; discussion 207‐112. [DOI] [PubMed] [Google Scholar]
- 41. Woodward M, Brindle P, Tunstall‐Pedoe H; SIGN group on risk estimation . Adding social deprivation and family history to cardiovascular risk assessment: the ASSIGN score from the Scottish Heart Health Extended Cohort (SHHEC). Heart. 2007;93:172–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, Sorlie P, Szklo M, Tyroler HA, Watson RL. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345:99–106. [DOI] [PubMed] [Google Scholar]
- 43. Hippisley‐Cox J, Coupland C, Vinogradova Y, Robson J, May M, Brindle P. Derivation and validation of QRISK, a new cardiovascular disease risk score for the United Kingdom: prospective open cohort study. BMJ. 2007;335:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hippisley‐Cox J, Coupland C, Vinogradova Y, Robson J, Brindle P. Performance of the QRISK cardiovascular risk prediction algorithm in an independent UK sample of patients from general practice: a validation study. Heart. 2008;94:34–39. [DOI] [PubMed] [Google Scholar]
- 45. Kind AJ, Jencks S, Brock J, Yu M, Bartels C, Ehlenbach W, Greenberg C, Smith M. Neighborhood socioeconomic disadvantage and 30‐day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014;161:765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Willems S, De Maesschalck S, Deveugele M, Derese A, De Maeseneer J. Socio‐economic status of the patient and doctor‐patient communication: does it make a difference? Patient Educ Couns. 2005;56:139–146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Estimation of Race‐ and Sex‐Specific Risk for Atherosclerosis Cardiovascular Disease Using the Pooled Cohort Risk Equations
Table S2. Calibration of the Atherosclerotic Cardiovascular Disease Pooled Cohort Risk Equations by the Cumulative Number of Indicators Showing Deprivation
Table S3. Calibration of the Atherosclerotic Cardiovascular Disease Pooled Cohort Risk Equations by Annual Household Income
Table S4. Calibration of the Atherosclerotic Cardiovascular Disease Pooled Cohort Risk Equations by Education
Table S5. Calibration of the Atherosclerotic Cardiovascular Disease Pooled Cohort Risk Equations by Relationship Status
Table S6. Observed and Predicted Incidence Rates of Atherosclerotic Cardiovascular Disease and Calibration and Discrimination of the Pooled Cohort Risk Equations by Social Deprivation Status (Using Multiple Imputation With 12 Imputed Data Sets, n=10 768)
Table S7. Observed and Predicted Incidence Rates of Atherosclerotic Cardiovascular Disease and Calibration and Discrimination of the Pooled Cohort Risk Equations by Area Deprivation Index
Table S8. Calibration of the Atherosclerotic Cardiovascular Disease Pooled Cohort Risk Equations by Area Deprivation Index
Table S9. Hazard Ratios for Atherosclerosis Cardiovascular Disease Associated With Categories of Area Deprivation Index
Figure S1. Flow‐chart of REGARDS study participants included in the analysis.


