Abstract
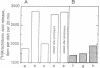
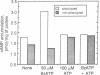
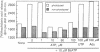
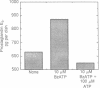
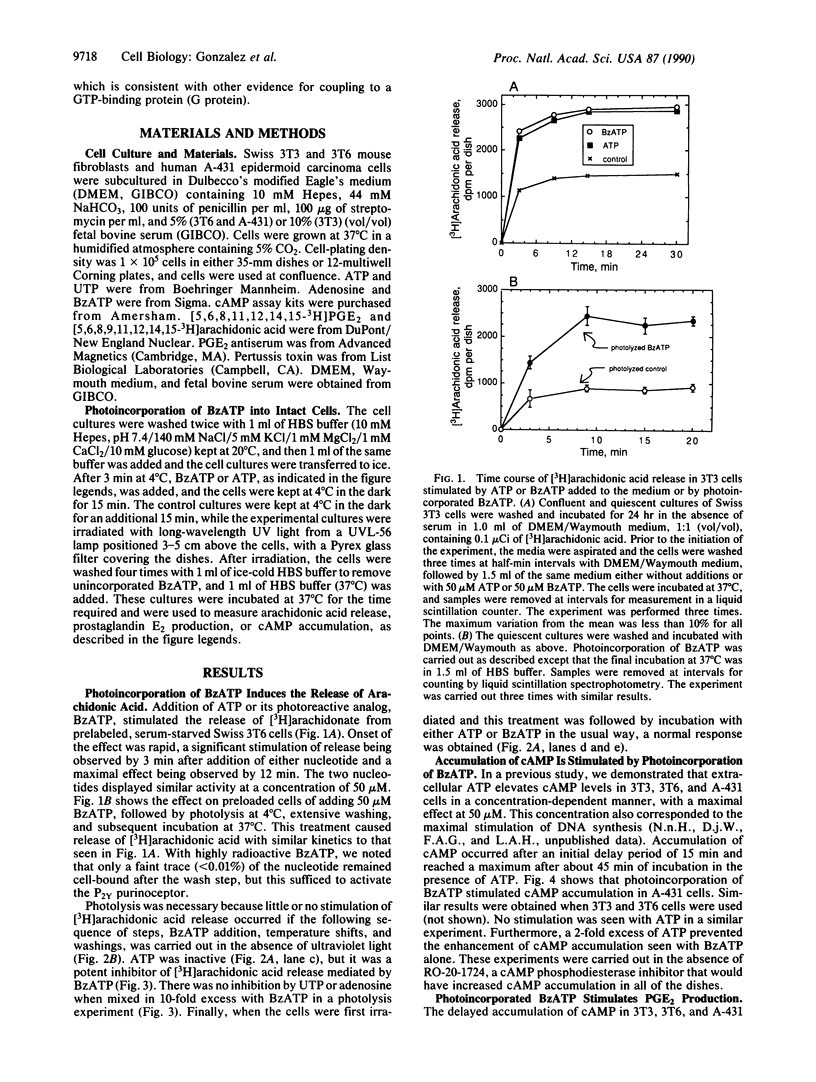
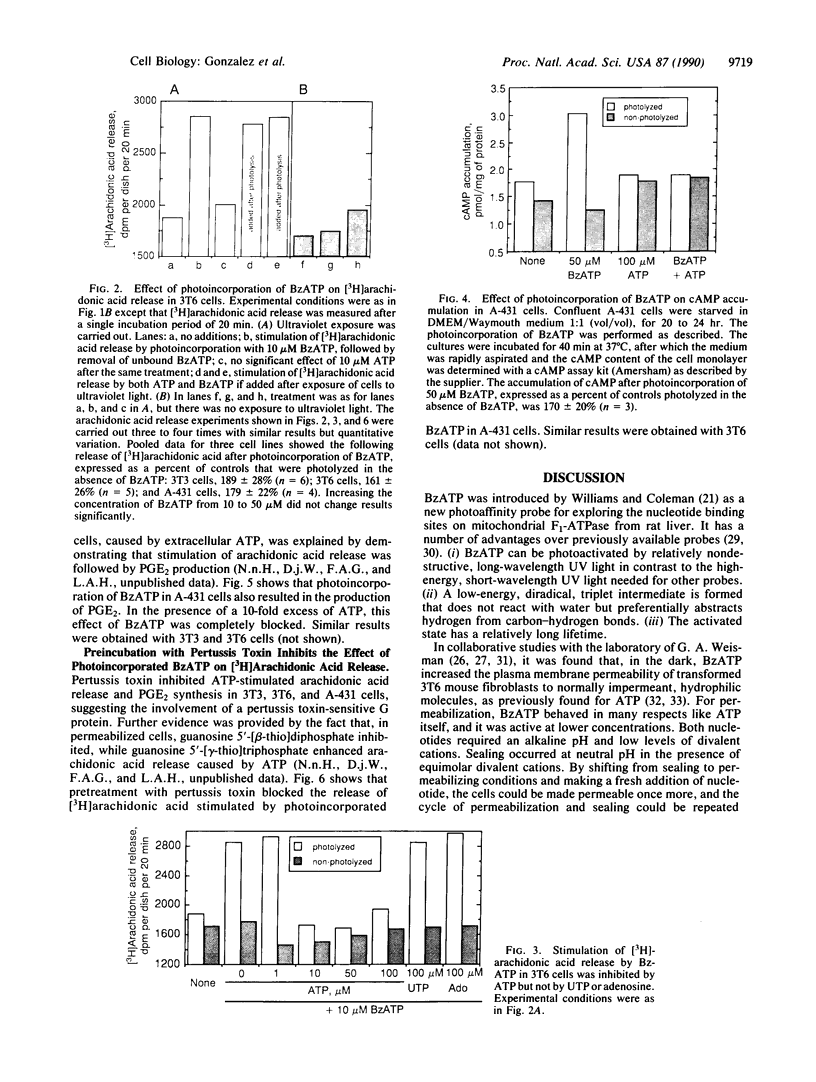
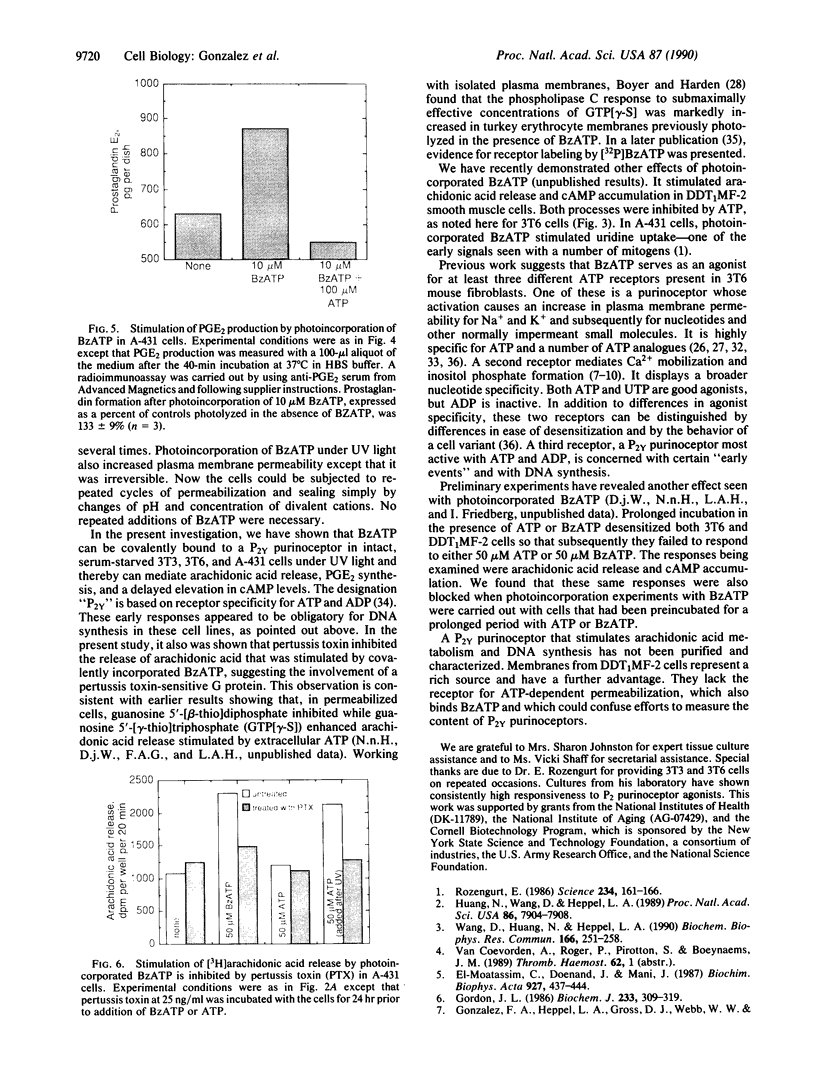
3'-O-(4-Benzoyl)benzoyl-ATP (BzATP), a photoaffinity analog of ATP, was used as a ligand for a P2Y purinoceptor (adenine nucleotide receptor) present in intact Swiss 3T3 and 3T6 cells and A-431 epidermoid carcinoma cells. Photolysis of serum-starved cells in the presence of 10-50 microM BzATP, followed by extensive washing to remove unincorporated BzATP, induced the release of arachidonic acid. A trace (less than 0.01%) of photoincorporated BzATP was as effective as when 50 microM BzATP or ATP was contained in the incubation medium during the assay. Photoincorporated BzATP also stimulated the production of prostaglandin E2 and the accumulation of cyclic AMP. In previous studies, we demonstrated that these three events are obligatory early steps in a pathway leading to DNA synthesis in the above cell lines. The evidence indicated that the purinoceptor activated by extracellular ATP or BzATP was linked to a pertussis toxin-sensitive GTP-binding protein. Consistent with these observations, we now find that pertussis toxin inhibits the effect of photoincorporated BzATP on arachidonic acid release. These results indicate that BzATP is an effective agonist for the P2Y purinoceptor concerned with stimulation of DNA synthesis in 3T3, 3T6, and A-431 cells. Furthermore, after photolysis it becomes irreversibly associated with intact cells and promotes the activation of early events required for DNA synthesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayley H., Knowles J. R. Photoaffinity labeling. Methods Enzymol. 1977;46:69–114. doi: 10.1016/s0076-6879(77)46012-9. [DOI] [PubMed] [Google Scholar]
- Boyer J. L., Cooper C. L., Harden T. K. [32P]3'-O-(4-benzoyl)benzoyl ATP as a photoaffinity label for a phospholipase C-coupled P2Y-purinergic receptor. J Biol Chem. 1990 Aug 15;265(23):13515–13520. [PubMed] [Google Scholar]
- Boyer J. L., Harden T. K. Irreversible activation of phospholipase C-coupled P2Y-purinergic receptors by 3'-O-(4-benzoyl)benzoyl adenosine 5'-triphosphate. Mol Pharmacol. 1989 Dec;36(6):831–835. [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Cable M. B., Briggs F. N. Labeling the adenine nucleotide binding domain of the sarcoplasmic reticulum Ca,Mg-ATPase with photoaffinity analogs of ATP. J Biol Chem. 1984 Mar 25;259(6):3612–3615. [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Exton J. H. Characterization of responses of isolated rat hepatocytes to ATP and ADP. J Biol Chem. 1985 Dec 15;260(29):15789–15794. [PubMed] [Google Scholar]
- Dubyak G. R., De Young M. B. Intracellular Ca2+ mobilization activated by extracellular ATP in Ehrlich ascites tumor cells. J Biol Chem. 1985 Sep 5;260(19):10653–10661. [PubMed] [Google Scholar]
- Erb L., Lustig K. D., Ahmed A. H., Gonzalez F. A., Weisman G. A. Covalent incorporation of 3'-O-(4-benzoyl)benzoyl-ATP into a P2 purinoceptor in transformed mouse fibroblasts. J Biol Chem. 1990 May 5;265(13):7424–7431. [PubMed] [Google Scholar]
- Forsberg E. J., Feuerstein G., Shohami E., Pollard H. B. Adenosine triphosphate stimulates inositol phospholipid metabolism and prostacyclin formation in adrenal medullary endothelial cells by means of P2-purinergic receptors. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5630–5634. doi: 10.1073/pnas.84.16.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F. A., Ahmed A. H., Lustig K. D., Erb L., Weisman G. A. Permeabilization of transformed mouse fibroblasts by 3'-O-(4-benzoyl)benzoyl adenosine 5'-triphosphate and the desensitization of the process. J Cell Physiol. 1989 Apr;139(1):109–115. doi: 10.1002/jcp.1041390116. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. A., Alfonzo R. G., Toro J. R., Heppel L. A. Receptor specific for certain nucleotides stimulates inositol phosphate metabolism and Ca2+ fluxes in A431 cells. J Cell Physiol. 1989 Dec;141(3):606–617. doi: 10.1002/jcp.1041410320. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. A., Bonapace E., Belzer I., Friedberg I., Heppel L. A. Two distinct receptors for ATP can be distinguished in Swiss 3T6 mouse fibroblasts by their desensitization. Biochem Biophys Res Commun. 1989 Oct 31;164(2):706–713. doi: 10.1016/0006-291x(89)91517-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. A., Gross D. J., Heppel L. A., Webb W. W. Studies on the increase in cytosolic free calcium induced by epidermal growth factor, serum, and nucleotides in individual A431 cells. J Cell Physiol. 1988 May;135(2):269–276. doi: 10.1002/jcp.1041350214. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. A., Heppel L. A., Gross D. J., Webb W. W., Parries G. The rapid desensitization of receptors for platelet derived growth factor, bradykinin and ATP: studies on individual cells using quantitative digital video fluorescence microscopy. Biochem Biophys Res Commun. 1988 Mar 30;151(3):1205–1212. doi: 10.1016/s0006-291x(88)80494-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. A., Rozengurt E., Heppel L. A. Extracellular ATP induces the release of calcium from intracellular stores without the activation of protein kinase C in Swiss 3T6 mouse fibroblasts. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4530–4534. doi: 10.1073/pnas.86.12.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S., Di Virgilio F., Steinberg T. H., Silverstein S. C. Extracellular nucleotides mediate Ca2+ fluxes in J774 macrophages by two distinct mechanisms. J Biol Chem. 1988 Jul 25;263(21):10337–10343. [PubMed] [Google Scholar]
- Hallam T. J., Pearson J. D. Exogenous ATP raises cytoplasmic free calcium in fura-2 loaded piglet aortic endothelial cells. FEBS Lett. 1986 Oct 20;207(1):95–99. doi: 10.1016/0014-5793(86)80019-9. [DOI] [PubMed] [Google Scholar]
- Heppel L. A., Weisman G. A., Friedberg I. Permeabilization of transformed cells in culture by external ATP. J Membr Biol. 1985;86(3):189–196. doi: 10.1007/BF01870597. [DOI] [PubMed] [Google Scholar]
- Horstman D. A., Tennes K. A., Putney J. W., Jr ATP-induced calcium mobilization and inositol 1,4,5-triphosphate formation in H-35 hepatoma cells. FEBS Lett. 1986 Aug 18;204(2):189–192. doi: 10.1016/0014-5793(86)80809-2. [DOI] [PubMed] [Google Scholar]
- Huang N., Wang D. J., Heppel L. A. Extracellular ATP is a mitogen for 3T3, 3T6, and A431 cells and acts synergistically with other growth factors. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7904–7908. doi: 10.1073/pnas.86.20.7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambouris N. G., Hammes G. G. Investigation of nucleotide binding sites on chloroplast coupling factor 1 with 3'O-(4-benzoyl)benzoyl adenosine 5'-triphosphate. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1950–1953. doi: 10.1073/pnas.82.7.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Increased free calcium in endothelial cells under stimulation with adenine nucleotides. J Cell Physiol. 1986 Mar;126(3):414–420. doi: 10.1002/jcp.1041260312. [DOI] [PubMed] [Google Scholar]
- Mahmood R., Yount R. G. Photochemical probes of the active site of myosin. Irradiation of trapped 3'-O-(4-benzoyl)benzoyladenosine 5'-triphosphate labels the 50-kilodalton heavy chain tryptic peptide. J Biol Chem. 1984 Nov 10;259(21):12956–12959. [PubMed] [Google Scholar]
- Manolson M. F., Rea P. A., Poole R. J. Identification of 3-O-(4-benzoyl)benzoyladenosine 5'-triphosphate- and N,N'-dicyclohexylcarbodiimide-binding subunits of a higher plant H+-translocating tonoplast ATPase. J Biol Chem. 1985 Oct 5;260(22):12273–12279. [PubMed] [Google Scholar]
- Pirotton S., Raspe E., Demolle D., Erneux C., Boeynaems J. M. Involvement of inositol 1,4,5-trisphosphate and calcium in the action of adenine nucleotides on aortic endothelial cells. J Biol Chem. 1987 Dec 25;262(36):17461–17466. [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Heppel L. A., Friedberg I. Effect of exogenous ATP on the permeability properties of transformed cultures of mouse cell lines. J Biol Chem. 1977 Jul 10;252(13):4584–4590. [PubMed] [Google Scholar]
- Sistare F. D., Picking R. A., Haynes R. C., Jr Sensitivity of the response of cytosolic calcium in Quin-2-loaded rat hepatocytes to glucagon, adenine nucleosides, and adenine nucleotides. J Biol Chem. 1985 Oct 15;260(23):12744–12747. [PubMed] [Google Scholar]
- Sung S. S., Young J. D., Origlio A. M., Heiple J. M., Kaback H. R., Silverstein S. C. Extracellular ATP perturbs transmembrane ion fluxes, elevates cytosolic [Ca2+], and inhibits phagocytosis in mouse macrophages. J Biol Chem. 1985 Nov 5;260(25):13442–13449. [PubMed] [Google Scholar]
- Wang D. J., Huang N. N., Heppel L. A. Extracellular ATP shows synergistic enhancement of DNA synthesis when combined with agents that are active in wound healing or as neurotransmitters. Biochem Biophys Res Commun. 1990 Jan 15;166(1):251–258. doi: 10.1016/0006-291x(90)91938-o. [DOI] [PubMed] [Google Scholar]
- Williams N., Ackerman S. H., Coleman P. S. Benzophenone-ATP: a photoaffinity label for the active site of ATPases. Methods Enzymol. 1986;126:667–682. doi: 10.1016/s0076-6879(86)26070-x. [DOI] [PubMed] [Google Scholar]
- Williams N., Coleman P. S. Exploring the adenine nucleotide binding sites on mitochondrial F1-ATPase with a new photoaffinity probe, 3'-O-(4-benzoyl)benzoyl adenosine 5'-triphosphate. J Biol Chem. 1982 Mar 25;257(6):2834–2841. [PubMed] [Google Scholar]
- el-Moatassim C., Dornand J., Mani J. C. Extracellular ATP increases cytosolic free calcium in thymocytes and initiates the blastogenesis of the phorbol 12-myristate 13-acetate-treated medullary population. Biochim Biophys Acta. 1987 Mar 11;927(3):437–444. doi: 10.1016/0167-4889(87)90110-8. [DOI] [PubMed] [Google Scholar]