Abstract
Background
Adverse drug events (ADEs) associated with over-the-counter (OTC) medications cause 178,000 hospitalizations each year. Older adults, aged 65 and older, are particularly vulnerable to ADEs. Of the 2.2 million older adults considered at risk for a major ADE, more than 50% are at risk due to concurrent use of an OTC and prescription medication.
Objectives
To refine the intervention and implementation strategy through diagnostic and formative evaluation; to evaluate the effectiveness of the intervention for preventing misuse of high-risk OTC medications by older adults; and to evaluate the implementation of the intervention in community pharmacies.
Methods
A system redesign intervention to decrease high-risk OTC medication misuse will be tested to reduce misuse by improving communication between older adults and community pharmacists via the following features: a redesign of the physical environment to sensitize older adults to high-risk OTC medications, and the implementation of a clinical decision tool to support the pharmacist when critically evaluating the older adult’s health status. The study will be conducted in three phases: a participatory design phase, a beta phase, and a test phase. The test phase will be conducted in three mass-merchandise stores. A total of 144 older adults will be recruited. A pre (control)/post (intervention) test will determine the effectiveness of the intervention. The primary outcome will be a comparison of proportion of older adults who misuse OTC medication from baseline to post-intervention. The process of implementation in the community pharmacy setting will be evaluated using the taxonomy proposed Proctor et al. The participatory design phase has been approved by the institution’s IRB (2016-0743).
Projected Impact
We anticipate that this project, which focuses on achieving systems-based improvement in an underemphasized area of the medication use process, will reduce ADEs associated with inappropriate OTC medication use in older adults.
Keywords: Medication safety, Community pharmacy, Dissemination and implementation, Older Adults, Hybrid design
Background
Older adults are at high risk of significant harm associated with over the counter (OTC) medication use. Of the estimated 2.2 million older adults who are at risk of a major adverse drug event (ADE), more than 50% of these interactions involve an OTC medication.1 Four of the 10 most frequently used drugs are available OTC. They are: ibuprofen, aspirin, acetaminophen, and diphenhydramine. These four drugs also are available in multiple-ingredient preparations, accounting for 45% of acetaminophen and 26% of aspirin use, thus increasing the potential for dangerous overdosing.2 Older adult use of non-steroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen and aspirin, results in 80,000 preventable ADEs each year. NSAID use accounts for a larger burden of ADEs (15.4%) than anticoagulants (10.2%), one of the Department of Health and Human Services’ top priority drugs in its National Action Plan for ADE prevention.3,4 Unintentional overdoses of acetaminophen result in 14,000 emergency department visits, and up to 50% of all acute liver failures per year.5,6 Diphenhydramine has anticholinergic properties which causes dizziness and loss of balance in 25% of older adults, which increases their risk of falling.7 Of older adults taking diphenhydramine for sleep, 40% were also taking one or more anticholinergic medications concurrently, compounding the risk of ADEs.8 These four drugs represent a significant ADE burden, and are the focus of this study.
Most older adults are not familiar with the appropriate dosing of OTC medications or how OTC medications interact with their other medications. Compounding the lack of patient knowledge about OTC medications, providers do not know which OTC medications their patients are consuming.9 Such lack of awareness and documentation about OTC medication use may lead to duplication of therapies and dangerous overdosing. In fact, the Centers for Medicare and Medicaid Services single out diphenhydramine and NSAIDs for review specifically because of their OTC availability and potential for therapeutic duplication.10,11 Despite the fact that OTC medication misuse is prevalent and costly, this potentially enormous patient safety gap remains relatively invisible. No effective interventions exist to help older adults prevent OTC medication misuse.
The availability of pharmacists at the point of sale of OTC medication has great potential for decreasing OTC medication misuse by older adults. A study found that 57% of elderly patients taking chronic prescription and OTC medications were not taking their OTC safely and required a pharmacist intervention with their OTC medication.12 Moveover, 80% of Americans report they would purchase (or avoid) a particular OTC medicine based on their pharmacist’s recommendation.13 Unfortunately, the current suboptimal design of community pharmacies and lack of standardized processes contribute to misuse of high-risk OTC medications. OTC medications are displayed to improve profitability,14 not prevent medication misuse. Older adults, who tend to have more visual and cognitive impairment, can become overwhelmed when faced with poorly designed aisles of confusing OTC choices.15
In response to The Affordable Care Act legislation that expands health care coverage, many chain community pharmacy organizations are retooling themselves as a vital part of the health care system, by discontinuing the sale of tobacco, adding primary care health clinics, and redesigning the pharmacy to allow pharmacists to more easily interact with patients.16,17 Although “health and wellness” is a focus of this nationwide movement, there is no specific emphasis on OTC medication safety. The present study provides a timely opportunity to expand the industry’s new initiatives to include system redesign to improve OTC medication use by older adults.
Few interventions have attempted to decrease misuse of high-risk OTC medications in older adults, and none have addressed system barriers.18 Compared to efforts to improve prescription medication safety,19,20 efforts to decrease OTC medication misuse in community-dwelling older adults have been practically ignored. To address this alarming and critical gap in medication safety, a system redesign intervention has been developed to mitigate system barriers. This intervention, which is grounded in human factors engineering principles and methods, includes a redesign of the community pharmacy’s OTC aisles, and the implementation of a pharmacist clinical decision tool. The intervention will decrease misuse by heightening older adults’ awareness of OTC risk, allowing pharmacy staff to see and initiate conversations with older adults in the aisles, and incorporating an efficient standardized process for pharmacists to gather necessary information to make recommendations.
The long term goal is to prevent OTC medication misuse and subsequent harm by targeting system barriers. A three-phase, pre (control)/post (intervention) study design to determine the intervention’s effectiveness in decreasing high-risk OTC medication misuse by older adults will be conducted. The hypothesis is that older adults who are more aware of risks and can more easily determine if those risks pertain to their own health situation by speaking with a pharmacist will safely select and not misuse high-risk OTC medications. Specifically, the aims are:
Aim 1. To refine the system redesign intervention and implementation strategy through diagnostic and formative evaluation. The two phase approach will comprise of a participatory design phase, and a beta test and refinement phase to produce a refined intervention and feasible implementation strategy.
Aim 2. To evaluate the effectiveness of a refined system redesign intervention for preventing misuse of high risk OTC medications by older adults. The hypothesis is that communication with a pharmacist, facilitated by the pharmacy redesign and clinical decision tool, will prevent older adult misuse of OTC medications. This hypothesis will be tested by evaluating the potential misuse of OTC medications selected by older adults and comparing results prior to and after the implementation of the intervention.
Aim 3. To evaluate the implementation of a refined system redesign intervention in community pharmacies. By employing summative evaluation and assessing evidence-based implementation outcomes, key intermediate outcomes will be identified and measured,21 including the intervention’s impact on pharmacists’ work and older adults’ decision making processes. These measures will inform the understanding of the potential feasibility and sustainability of widespread dissemination and implementation of the system intervention.
Conceptual Model
The conceptual framework for this intervention is adapted from the Systems Engineering Initiative for Patient Safety (SEIPS) 2.0 work system model to improve patient outcomes, a human factors engineering model developed by Holden et al.22 The SEIPS Model has been applied to frame the design and analysis of many patient safety studies, including research conducted in community pharmacies.23,24 The SEIPS 2.0 model is appropriate for this study because it emphasizes how work system barriers can impact the “work” conducted by pharmacists and older adults. Work is defined as goal-oriented, effortful activities.25 For pharmacists, work would include clarifying an older adult’s medication list or determining if the older adult should self-treat their stated problem. Older adult work would include determining how often to take an OTC medication or if an OTC medication interacted with prescription medication. This model is also appropriate because it highlights the importance of collaborative work, in which pharmacists and older adults are actively engaged agents, working together to achieve their goals. These work processes in turn affect older adult, pharmacist, and organizational outcomes.
Study Design Overview
In order to assess the effectiveness of the intervention while also understanding the context for implementation, an effectiveness-implementation hybrid design proposed by Curran et al will be utilized.26 A hybrid design, which blends design components of effectiveness and implementation research, improves the speed of knowledge creation and increases the usefulness and policy relevance of the research being conducted.27 In addition to a diagnostic and formative evaluation to refine the intervention (Aim 1), Curran’s hybrid Type 2 design will be used in which Aim 2 is to determine the effectiveness of the intervention. An independent aim (Aim 3) is to pilot test an implementation strategy and to understand the context for widespread implementation through an examination of barriers and facilitators.
The study design includes three phases (Figure 1). Aim 1 (To refine the intervention and implementation strategy through diagnostic and formative evaluation) will comprise the first two phases. The two phases are a participatory design phase and a beta test and refinement phase. In the first phase, a participatory design approach will be used to fine-tune the intervention. This phase will utilize a stakeholder group comprised of pharmacists, technicians, and older adults. The second phase will be a beta test of the intervention in one pilot store. During the beta phase, the intervention will be pilot tested with a total of 24 older adult participants. OTC medication selection and reported use information will be collected at baseline and post-intervention. Members of the participatory design group will convene to discuss results from the beta test. Actionable barriers identified during the beta test will be discussed and formative evaluation will be used to refine and improve the implementation process and optimize the intervention.28
Figure 1.
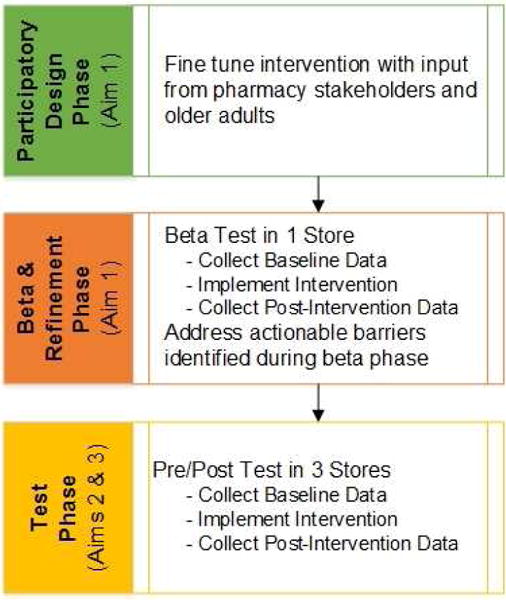
Research Design Overview
In the third phase, a pre (control)/post (intervention) test will be conducted to determine the effectiveness of a refined system redesign intervention to decrease OTC medication misuse in older adults (Aim 2) and understand the process of implementation in the community pharmacy setting (Aim 3). The third phase will include the collection of baseline data followed by the implementation of the refined intervention and collection of post-intervention data in three different stores. A total of 144 older adult participants will be recruited.
The System Redesign Intervention
A system redesign intervention, grounded in the SEIPS 2.0 model that addresses both pharmacist and older adult barriers to decreasing OTC medication misuse has been conceptualized. The intervention will maintain the autonomy and self-care engagement, while improving the selection behaviors of older adults. The intervention will improve communication by facilitating a collaborative process and will include two components:
- Physical layout redesign: The physical layout of the OTC aisles will be redesigned to facilitate pharmacist-older adult communication. The redesign will have two parts:
- The high-risk OTC medications (e.g., pain, sleep, cough/cold) currently displayed in one aisle that is not within the line of sight of and relatively far away from the prescription department, will be moved to the aisle closest to the prescription department and within line of sight of the pharmacists and technicians.
- Signage will be installed and aisle redesign will take place to denote the OTC medications that are typically not safe for older adults. Human factors principles associated with vision, contrast, symbols, and color to denote warning will be incorporated to architect safe decision making.29 Given that much of OTC medication selection occurs based on prior experiences, the content of the signs will trigger older adults to question their selection of an OTC with regards to a safe and effective choice. This content helps older adults recognize they may lack the knowledge necessary to make an appropriate decision.
Clinical Decision Tool: Because pharmacists have almost no information when making OTC recommendations, they must ask a number of time-consuming questions to the older adult to determine what might be appropriate. The OTC clinical decision tool was developed from pharmacist focus groups.30 The purpose for the clinical decision tool is to help the pharmacist efficiently gather relevant information by using an algorithm for the information that the pharmacist seeks to establish about an older adult, and the sequence for gathering specific information. Consistent with previously reported successful interventions,31 the tool provides a list of criteria arranged in a systematic manner, allowing the pharmacist to ensure that all criteria are considered. The tool serves as a cognitive aid which provides a framework for grouping specific information. This can help improve memory recall and provide a standardized framework for evaluation.
Study Sites
This study will take place in four mass merchandise stores in Wisconsin that are part of the same pharmacy organization (one store in the beta phase, and three stores in the test phase). These stores were selected because they represent a variety of communities that vary by the size of the population, proportion of older adults, and socioeconomic status. Pharmacists working at these stores have been identified as potential champions of this intervention, and could serve as mentor coaches when the project is scaled up to more stores.
Phase 1: Participatory Design Approach to Fine-tune Intervention and Implementation Strategy
Participatory design is an approach to design that actively involves all stakeholders at several stages of the innovation process to help ensure the result meets their needs and is usable.32 The participatory design approach will be taken using a stakeholder group consisting of up to five older adults, four pharmacists, and one technician. Pharmacy staff and older adults will be recruited from stores in the same pharmacy organization.
Six meetings with all stakeholders will be scheduled to fine-tune the intervention components and implementation strategy. Initially, the meetings with pharmacy stakeholders and older adults will be separate, as their concerns may be different. The focus will be on articulating and verifying the needs of older adults. The initial meetings will be used to build trust and buy-in, and to discuss their ideas about how data collection and the intervention can be incorporated into their current work processes. At least one of the later meetings will take place at the store that will serve as the beta site. This will allow pharmacy stakeholders and older adults to visualize how the OTC aisle will be moved and redesigned, and how the pharmacist and/or technician may easily and efficiently approach and interact with older adults when selecting and recommending OTC medications. Stakeholders will help inform the signage that will be used to denote potentially unsafe OTC medications.
It is anticipated that the majority of older adult stakeholders will be white. In order to capture the perspectives of a more diverse group, the Community Advisors on Research Design and Strategy (CARDS)33, a core service of the Wisconsin Network for Research Support in the UW School of Nursing will be consulted. CARDS are focus groups of community members from diverse racial, ethnic, socioeconomic, and educational backgrounds. They are trained to give constructive feedback to researchers on how to make materials accessible for diverse groups of people.
Phase 2: The Beta and Refinement Phase
In order to optimize the intervention and the likelihood of affecting change, a beta test of the intervention will be conducted. The results of the beta test will inform the development of a refined intervention. Before, during, and after the beta test implementation, effectiveness and implementation outcomes will be analyzed to resolve actionable barriers, enhance identified levers of change, and refine components of the intervention.
The research team will use an iterative approach to discuss the results and to assess the data gathered during the beta test. Stakeholders from the participatory design group and CARDS will be consulted to address unanticipated barriers. Actionable barriers identified in the beta test will be discussed and formative evaluation data will be used to refine and improve the implementation process and optimize the intervention.28 Insights gained in the recruitment strategy, data collection tools, how the clinical decision tool is used, pharmacist training, and signage and aisle redesign will be incorporated into the refined intervention and implementation strategy that will be used in the test phase.
Pharmacist, Technician, Store Manager Recruitment
Two pharmacists, two technicians, and one store manager will be recruited. The two pharmacists and one technician will fulfill two roles in the study: research personnel and study participants. Research personnel will participate in eight hours of human subjects and clinical decision tool training.
Older Adult Participant Recruitment
The study population will be older adults aged 65+ who have received at least one prescription at one of the study stores in the last 12 months, and indicate that they intend to purchase an OTC medication from one of the following categories: pain, sleep, cough/cold. The participants must be able to provide written informed consent and speak English. Prior to baseline and post-intervention, up to two mailings of a random sample of 100 patients will be sent to patients aged 65+ who have received a prescription at the beta site store in the last 12 months. The mailing will invite them to participate, and will include an information sheet and study flier. The random sample invited to participate in the baseline data collection at each store will be a different sampling frame than the post-intervention sample.
The letter will direct older adults to call the research coordinator on the telephone if they are interested in participating. The letter will also identify a study liaison at the beta store. This individual will be one of the technicians who works at the beta store. This study liaison will answer questions, and assist the older adult in contacting the research coordinator if he/she chooses to participate. If the older adult agrees to participate, the research coordinator will set up an appointment to meet the participant at the store, and mail the informed consent form and medical/demographic form for the participant to complete prior to the appointment. Participants will receive $20 cash for participating.
During the beta phase, a total of 24 participants from one store will be recruited: 12 participants during baseline data collection and 12 participants during the post-intervention data collection.
Data Collection
During the beta phase, information that both sheds light on how to optimize the intervention (effectiveness outcomes), and information that will facilitate a smooth implementation (implementation outcomes) will be collected. The taxonomy of implementation outcomes proposed by Proctor et al will be applied.21 A summary of all outcome variables and data sources are provided in Table 1.
Table 1.
Effectiveness and Implementation Outcomes
| Effectiveness Outcome | Definition | Data Source |
|---|---|---|
| OTC Medication Misuse | One or more of the following:
|
|
| Older Adult Decision Making Process | Knowledge and strategies required to select a safe OTC medication |
|
| Recommendation characteristics | Type and confidence of recommendation |
|
| Implementation Outcome | Definition | Data Source |
| Acceptability | Satisfaction with aspects of innovation |
|
| Adoption | Intention to try the innovation |
|
| Appropriateness | Perceived fit, compatibility, practicability |
|
| Feasibility | Actual fit; the extent to which an innovation can be successfully used |
|
| Fidelity | Innovation delivered as intended; quality of innovation delivery |
|
| Cost | Innovation delivery costs, overhead costs |
|
Older Adult Medical/Demographics form
The form will include the self-report of disease information using the OAR questionnaire,34 prescription and OTC medications, and demographic information.35 Participants recruited at both baseline and post-intervention will complete the form.
Older Adult Interviews
Each consented older adult will participate in a face to face “walking” interview in which a trained researcher will ask the older adult to articulate the medication they are searching for and the decisions involved in making the selection. These probes will help us understand the older adult’s so-called “work,” (goal-oriented effortful activities) as they select an OTC medication. Following the walking interview, a short semi-structured interview will be conducted to gather information on how the older adult intends to use the medication (e.g. dosing and duration).35 Additionally, for those older adults participating in the study post-intervention, questions will be posed specifically related to the feasibility, usefulness, and acceptance of the intervention.
Older Adult Satisfaction Survey
A community pharmacy satisfaction scale developed by Kassam et al was adapted.36 This survey will be administered at both baseline and post-intervention during the beta phase. The survey measures three important components that are hypothesized will show improvement post-intervention: care plan development, information and education, and collaborative care. This survey will be completed by older adults at the end of the interview.
Pharmacist/Technician Observations
The purpose of the observations is to gain insight on fit and compatibility of the intervention within the pharmacist’s and technician’s work system. A total of four 3-hour observations (two during baseline and post-intervention) will be conducted at the beta site. The focus of the observations will be how the intervention impacts pharmacists’ and technicians’ work with a focus on how they incorporate this added responsibility into their workflow. Observational data will be recorded by hand and then be transcribed into typed notes.23,37,38
Pharmacist/Technician Tool Acceptance Survey
A technology acceptance survey that has been previously used by Holden et al. was adapted to explore perceived ease of use, perceived usefulness to the pharmacist and older adult, behavioral intention to use, and satisfaction with the clinical decision tool.39 The two pharmacists and two technicians from the beta site store will be administered the survey four weeks after the intervention is implemented.
Pharmacist/Technician/Store Manager/Administrator Interviews
Interviews will be conducted with two pharmacists, two technicians, one store manager, and one administrator to elicit descriptions of each respondent’s role and how the intervention was implemented in their store. Guided by the components of the SEIPS 2.0 model, facilitators and barriers related to each implementation outcome will be probed.23,37,40–42
Pharmacist OTC Encounter Form
The purpose of the Pharmacist OTC Encounter Form are: 1) to document the interactions that pharmacists have with consumers (study participants and non-participants) regarding OTC medications, 2) provide a reliability check for the data collected during the older adult participant interviews, and 3) pilot-test a quick and simple data collection tool that may be used during a future scaled up intervention.
The encounter form will be used to collect recommendation characteristic outcomes that are in addition to the primary outcome of OTC medication misuse (e.g., referral to a physician, behavioral or medication recommendations). Further, the encounter form will be used to quantify the time required to interact with consumers before and after the intervention is implemented.
Following each interaction, the pharmacist will document the interaction into a web-based application loaded on a tablet computer. The questions on the form include who initiated the conversation, whether the pharmacist left the prescription department to help the consumer, the problem or issue, recommendations that the pharmacist made, level of confidence in OTC guidance, and perceived time required to help the consumer. There will also be an optional open text box for pharmacists to type free text. In order to minimize the data collection burden, the beta phase pharmacists will collect encounter data for one week at three time points: 1) two weeks prior to intervention implementation, 2) two weeks after implementation, and 3) four weeks after implementation.
Data Analysis
The focus of the data analysis during the beta phase will be to learn how to improve the intervention and implementation strategy for the test phase.
Primary Outcome - OTC Medication Misuse
The OTC medication misuse outcome will be dichotomized into “safe use” or “misuse”. The same criteria used in a pilot study will be used to determine if an OTC medication that was selected is considered appropriate use or misuse. The four types of potential misuse include: 1) drug-drug interaction,43 2) drug-disease interaction44 3) drug-age interaction44 and 4) reported usage that exceeded product labeling guidelines. A simple chi-square analysis will be done to conduct a univariate comparison of proportions of older adults who misuse OTC medications from baseline to post-intervention. The total and type of misuse will be quantified and compared to determine if the intervention reduces the frequency or instances of concurrent misuse.
OTC Decision Process Mapping
Using data from the older adult interviews, a cognitive task analysis45–47 will be conducted to compare the decision making processes of the older adults at baseline and post-intervention. The hypothesis is that the intervention will impact the decisions of older adult and will predict medication choice appropriateness. This method of analysis will serve as a mediator of the effect of the intervention and will allow the research team to identify where the intervention improves decision making (e.g. reduces the number of choices) and where it does not (e.g. information is still hard to process).
Pharmacist OTC Encounter Form
At 2 and 4 weeks post-intervention, the number of pharmacist recommendations made, the types of recommendations, drug recommendations, non-drug recommendations including behavioral modifications or referral to physician, and confidence in making recommendation will be compared.
Quantitative Survey Data Analysis
For the pharmacist/technician tool acceptance survey, scores for each domain will be calculated by taking the mean of all item responses from that scale, and descriptive statistics will be calculated. For the older adult satisfaction survey, descriptive statistics and t-tests will be conducted to compare mean domain scores between the baseline and post-intervention time points.
Qualitative Data Analysis
Qualitative data from observations and interviews will be subjected to rigorous qualitative data analysis using techniques employed in previous pharmacy medication safety qualitative research.23,30,37,40–42,48,49 A deductive content analysis, discovering patterns, themes and categories in the data, will be used and guided by the theory-based approach.50 A conceptual coding structure will be created and as each interview or observation transcript is added to the analysis, passages will be classified according to existing codes, and codes will be added as needed.
Phase 3: The Test Phase
The test phase will be conducted to evaluate the effectiveness and implementation of the refined system redesign intervention in community pharmacies. A pre (control)/post (implementation) test will be conducted in three different stores.
Aim 2. Evaluate the effect of a system redesign intervention on preventing misuse of high-risk OTC medications by older adults
The primary study outcome of this study is the change in the proportion of older adults who misuse OTC medications. Secondary outcomes include an evaluation of older adult decision making processes and the pharmacist recommendation characteristics.
Sample Size Calculations
The sample size power calculation was focused on the primary outcome of Aim 2, to “evaluate the effect of a system redesign intervention on improved OTC medication selection by older adults,” collected during the test phase only. One small study found that 43% of older adults used an OTC medication safely. This is higher than the pilot work which found that only 5% of the study population took an OTC medication safely.35 Therefore, it is believed that older adult safe OTC medication use to be in the 5–43% range. In comparison, another study found that 82% of patients report that they would purchase (or avoid) a particular OTC medication based on their pharmacist’s recommendation.
The proportion of older adults who safely use OTC medications was conservatively estimated to increase from 40% to 65%. Based on a two-level (subjects level-1 nested under pharmacist level-2) logistic regression model with 80% power at a 0.05 significance level to detect pre-post group differences, the study would meet power requirements (1−β =.80, α=0.05) with a sample size of 8 older adults (per pharmacist assessed twice, for nine pharmacists) for each baseline and post-intervention data collection period. The total number of older adults required for the test phase of study will be 144 (48 older adults recruited in each of three test site stores).
Pharmacist, Technician, and Older Adult Recruitment, and Data Collection
The same recruitment strategy will be used as in the beta test. A total of three pharmacists, two technicians, and one store manager in each of the three test phase stores will be recruited. A total of 144 participants in the test phase will be recruited through up to three waves of mailings for the baseline and post-intervention data collection, at each store.
The same effectiveness outcomes of OTC medication misuse, older adult decision making processes, and recommendation characteristics will be collected, as in the beta phase. A total of nine pharmacists and six technicians will complete the interview and Tool Acceptance Survey.
Data Analysis
Primary Outcome - OTC Medication Misuse
The OTC medication misuse outcome will be dichotomized into “safe use” or “misuse”. The four types of potential misuse include: 1) drug-drug interaction,43 2) drug-disease interaction44 3) drug-age interaction44 and 4) reported usage that exceeded product labeling guidelines.
In order to assess if the OTC medication is considered “safe use” or “misuse”, a review committee made up of two geriatric pharmacotherapists and one OTC medication expert will be used to independently adjudicate the primary outcome. The review committee will be provided with the name of the OTC medication that participants select, a description of how the participant intends to use the OTC medication from the older adult interviews, and the self-reported disease, prescription, and OTC medication list provided on the medical/demographic form. The review committee will be blinded to the participant’s status as a baseline or post-intervention participant to help guard against information bias.
The impact of the intervention will be assessed using a multi-level logit model with a two-level structure. Comparisons between the baseline and post-intervention cohorts will be made, nested within pharmacist. Total and type of misuse will be quantified and compared, to determine if the intervention reduces the frequency or instances of concurrent misuse.
Older Adult Decision Making Process and Pharmacist Recommendation Characteristics
These outcomes will be analyzed the same way as in the beta phase.
Aim 3. Evaluate the implementation of a system redesign intervention in community pharmacies
The purpose of the test phase implementation assessment is to determine the degree of success or failure through an analysis of the summative data results collected at the end of the project. The summative data collected will be guided by the taxonomy of implementation outcomes proposed by Proctor et al, with both quantitative and qualitative data sources.21
Sample Size Calculation
Unlike a hypothesis-testing quantitative study, there is no method for calculating power or sufficient sample sizes for qualitative data such as interviews or observations. Based on the experience of having conducted extensive pharmacy observation and interview studies, the allotted time of one hour each for 9 pharmacist interviews, 6 technician interviews, 3 store manager interviews, and one administrator interview and the number of observations (four 3-hour observations per pharmacy for a total of 12 observations) is believed to be sufficient.
Data Collection and Analysis
The same implementation outcomes will be collected and analyzed, as in the beta test. These included outcomes of acceptability, adoption, appropriateness, feasibility, fidelity, and cost.
Dissemination and Implementation Plan
It is believed if this intervention works, as it should, the dissemination and implementation plan, will lead to widespread adoption, which is the long term goal. Scaling up the intervention throughout 80+ stores in Wisconsin will be the first step, and the plan is designed to facilitate such scaling within the company organization. Accordingly, the primary targets will be company pharmacists and older adults.
There are three key features needed to disseminate the results to pharmacists.
A champion at the organizational level: A champion at the organizational level has been shown in numerous health care innovation studies to be an essential element.51
Mentored coaching by experienced pharmacists: In addition to an organizational champion, widespread implementation will require credible coaches to promote the success and sustainability of the intervention. Coaches will be recruited from the study pharmacists who have experience with the intervention.52
An implementation toolkit: An intervention toolkit will be developed specifically for scalability for stores, and made available through websites affiliated with the Agency for Healthcare Research and Quality and other entities championing patient safety.
Further, the successful implementation of this intervention in the partnering store in Wisconsin may generate significant positive publicity regarding the importance of OTC medication safety in older adults, and the vital role of community pharmacists in decreasing misuse. Similar to the widespread publicity and response by the medical community surrounding CVS’s removal of tobacco products,16,53,54 this study could lead to a shift in the marketplace to add an OTC medication safety emphasis into health and wellness initiatives and policies.
In order to set the stage for further spread to other organizations, the research team will begin disseminating preliminary data and formative implementation insights in year 2 and 3 of the study in scientific journal and at national meetings. Results will also be shared with older adults in the community through organizations that serve older adults.
Limitations
A randomized controlled trial and cohort designs were not considered in this study due to the methodological limitation of being unable to randomize and to observe and track changes within a given participant. Limitations of using a pre/post study design include the potential for confounders, and the risk that participants in the baseline and post-intervention data collections are not equal.
In prior studies using the same recruitment plan,35 there was a rapid recruitment of 20 older adults in 4 weeks. Of older adults who were interested in participating, 100% completed data collection. Additional recruitment steps were added for this proposal including the addition of a store research liaison who will be a familiar face to potential participants and will field questions about the study prior to consent. Further, since this recruitment strategy is familiar to the research team, no delays when seeking human subject approval are anticipated. As a result, it is believed that recruitment for this study will be very feasible.
There is a recognition that OTC medications can be purchased at retail establishments that do not have pharmacies (e.g., convenience stores, gas stations). Therefore, the intervention is not generalizable to those settings. However, if successful, there is great potential for the intervention to be implemented in community pharmacies in national chain, independent, mass merchandise, and grocery stores that are ubiquitous in both urban and rural communities. Further, the recruitment strategy will result in asking older adults to opt-in to participating in the study. The research team recognizes that self-section of this group that may not be generalizable to the entire population.
Integration of the clinical decision tool in practice will take time, and enforcement of the clinical decision tool may be difficult to achieve. While the purpose of the tool is to decrease OTC medication misuse, it is believed that the introduction of the tool is an opportunity to improve efficiency, helping the pharmacist to quickly gather relevant information to make a safe recommendation. The pharmacy stakeholders in the participatory design process will inform how it is integrated.
There is potential that pharmacists and technicians will interact with study participants differently than nonparticipants, if they note that participants are with researchers. The data collected on the OTC encounter form will be used as a reliability check to assess this concern.
Self-report and recall bias are limitations with using the older adult medical/demographic form; however, the design will most likely result in underestimating potential misuse. Additionally, the surveys were selected in order to limit their burden.
Projected Impact
We anticipate that this project, which focuses on achieving systems-based improvement in an underemphasized area of the medication use process, will generate critical data that will shed light on a potentially enormous but invisible patient safety concern.
Our preliminary work allows us to test an intervention that is guided by both theory and pilot data. We will leverage the knowledge, skills, and accessibility of pharmacists, who are a ubiquitous, yet underutilized community resource, and who are valued as one of the most trusted health care professionals.55
Completion of this study will provide evidence that OTC medication safety in older adults can be improved, and will provide a road map for pharmacy organizations to do so. Ultimately, we believe that the successful completion of this study will reduce ADEs associated with inappropriate OTC medication use in older adults.
Adverse drug events (ADEs) associated with over-the-counter (OTC) medications cause 178,000 hospitalizations each year. In this study, a system redesign intervention to decrease high-risk OTC medication misuse is proposed. This paper describes a protocol for a hybrid design study evaluating both the effectiveness and implementation of a system redesign intervention in community pharmacies. A pre (control)/post (intervention) test will determine the effectiveness of the intervention. The primary outcome will be OTC medication misuse. The process of implementation in the community pharmacy setting will be described using the implementation taxonomy proposed by Proctor et al.
Acknowledgments
The authors would like to thank members of the research team who provided valuable comment on the research protocol: Steven Albert, Roger Brown, Pascale Carayon, Nora Jacobson, Corey Lester, Jane Mahoney, Cynthia Phelan, and Ken Walker.
Funding
This work was supported by the Agency for Healthcare Research and Quality (AHRQ), grant 1R18HS024490-01, and by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. RJH is supported by grant K01AG044439 from the National Institute on Aging (NIA) of the US National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michelle A. Chui, Systems Approach to Medication Safety Research Laboratory, Social & Administrative Sciences Division, University of Wisconsin – Madison, School of Pharmacy, 777 Highland Avenue, Madison, WI 53705.
Jamie A. Stone, Systems Approach to Medication Safety Research Laboratory, Social & Administrative Sciences Division, University of Wisconsin – Madison, School of Pharmacy, 777 Highland Avenue, Madison, WI 53705.
Richard Holden, School of Informatics and Computing, Indiana University-Purdue University Indianapolis, 535 W. Michigan Street, IT 475, Indianapolis, IN 46202.
Bibliography & References Cited
- 1.Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA. 2008;300:2867–2878. doi: 10.1001/jama.2008.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287:337–344. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 3.Gurwitz JH, Field TS, Harrold LR, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003;289:1107–1116. doi: 10.1001/jama.289.9.1107. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Department of Health and Human Services: Office of Disease Prevention and Health Promotion. National action plan for adverse drug event prevention. Washington, DC: 2014. [Google Scholar]
- 5.Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 6.Nourjah P, Ahmad SR, Karwoski C, Willy M. Estimates of acetaminophen (Paracetomal)-associated overdoses in the United States. Pharmacoepidemiol Drug Saf. 2006;15:398–405. doi: 10.1002/pds.1191. [DOI] [PubMed] [Google Scholar]
- 7.Serper M, McCarthy DM, Patzer RE, et al. What patients think doctors know: beliefs about provider knowledge as barriers to safe medication use. Patient Educ Couns. 2013;93:306–311. doi: 10.1016/j.pec.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kantar Health. National health and wellness survey: US, 2012. Princeton, NJ: 2013. [Google Scholar]
- 9.Sleath B, Rubin RH, Campbell W, Gwyther L, Clark T. Physician-patient communication about over-the-counter medications. Soc Sci Med. 2001;53:357–369. doi: 10.1016/s0277-9536(00)00341-5. [DOI] [PubMed] [Google Scholar]
- 10.Andersen RM, Yu H, Wyn R, Davidson PL, Brown ER, Teleki S. Access to medical care for low-income persons: how do communities make a difference? Med Care Res Rev. 2002;59:384–411. doi: 10.1177/107755802237808. [DOI] [PubMed] [Google Scholar]
- 11.Gandhi TK, Weingart SN, Borus J, et al. Adverse drug events in ambulatory care. N Engl J Med. 2003;348:1556–1564. doi: 10.1056/NEJMsa020703. [DOI] [PubMed] [Google Scholar]
- 12.Guirguis K. The use of nonprescription medicines among elderly patients with chronic illness and their need for pharmacist interventions. Consult Pharm. 2010;25:433–439. doi: 10.4140/TCP.n.2010.433. [DOI] [PubMed] [Google Scholar]
- 13.NCPIE. Uses and attitudes about taking over-the-counter medicines: Findings of a 2003 national opinion survey. 2003 http://www.bemedwise.org/survey/summary_survey_findings.pdf.
- 14.Alexander A. Hamacher Resource Group enhances category management program to assist independents. 2014 http://www.drugstorenews.com/article/hamacher-resource-group-enhances-category-management-program-assist-independents. Accessed 2014.
- 15.Johnson MM, Drungle SC. Purchasing over-the-counter medications: the influence of age and familiarity. Exp Aging Res. 2000;26:245–261. doi: 10.1080/036107300404886. [DOI] [PubMed] [Google Scholar]
- 16.Brennan TA, Schroeder SA. Ending sales of tobacco products in pharmacies. JAMA. 2014;311:1105–1106. doi: 10.1001/jama.2014.686. [DOI] [PubMed] [Google Scholar]
- 17.Twigg MJ, Bhattacharya D, Desborough JA, Wright D. A drop-in clinic for patients with poorly-controlled diabetes: a community pharmacy feasibility study. Int J Clin Pharm. 2015;37:395–402. doi: 10.1007/s11096-015-0076-5. [DOI] [PubMed] [Google Scholar]
- 18.The Gerontological Society of America. GSA and CHPA National summit: OTC medication behaviors of older adults. Washington DC: 2013. Over-the-counter medication behaviors of older adults: research is needed to better understand and promote safe and effective use. [Google Scholar]
- 19.Knudsen P, Herborg H, Mortensen AR, Knudsen M, Hellebek A. Preventing medication errors in community pharmacy: root-cause analysis of transcription errors. Qual Saf Health Care. 2007;16:285–290. doi: 10.1136/qshc.2006.022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nester TM, Hale LS. Effectiveness of a pharmacist-acquired medication history in promoting patient safety. Am J Health Syst Pharm. 2002;59:2221–2225. doi: 10.1093/ajhp/59.22.2221. [DOI] [PubMed] [Google Scholar]
- 21.Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65–76. doi: 10.1007/s10488-010-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holden RJ, Carayon P, Gurses AP, et al. SEIPS 2.0: a human factors framework for studying and improving the work of healthcare professionals and patients. Ergonomics. 2013;56:1669–1686. doi: 10.1080/00140139.2013.838643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Odukoya OK, Stone JA, Chui MA. Barriers and facilitators to recovering from e-prescribing errors in community pharmacies. J Am Pharm Assoc (2003) 2015;55:52–58. doi: 10.1331/JAPhA.2015.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chui MA, Mott DA, Maxwell L. A qualitative assessment of a community pharmacy cognitive pharmaceutical services program, using a work system approach. Res Social Adm Pharm. 2012;8:206–216. doi: 10.1016/j.sapharm.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holden RJ, Schubert CC, Mickelson RS. The patient work system: an analysis of self-care performance barriers among elderly heart failure patients and their informal caregivers. Appl Ergon. 2015;47:133–150. doi: 10.1016/j.apergo.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50:217–226. doi: 10.1097/MLR.0b013e3182408812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glasgow RE, Lichtenstein E, Marcus AC. Why don’t we see more translation of health promotion research to practice? Rethinking the efficacy-to-effectiveness transition. Am J Public Health. 2003;93:1261–1267. doi: 10.2105/ajph.93.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stetler CB, Legro MW, Wallace CM, et al. The role of formative evaluation in implementation research and the QUERI experience. J Gen Intern Med. 2006;21(Suppl 2):S1–8. doi: 10.1111/j.1525-1497.2006.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wickens CD, Gordon SE, Liu Y. An introduction to human factors engineering. Upper Saddle River, N.J.: Pearson Prentice Hall; 2004. [Google Scholar]
- 30.Chui MA, Stone JA, Martin BA, Croes KD, Thorpe JM. Safeguarding older adults from inappropriate over-the-counter medications: the role of community pharmacists. Gerontologist. 2014;54:989–1000. doi: 10.1093/geront/gnt130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hales BM, Pronovost PJ. The checklist–a tool for error management and performance improvement. J Crit Care. 2006;21:231–235. doi: 10.1016/j.jcrc.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Karsh BT. Beyond usability: designing effective technology implementation systems to promote patient safety. Qual Saf Health Care. 2004;13:388–394. doi: 10.1136/qshc.2004.010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Community Advisors on Research Design and Strategy. http://winrs.son.wisc.edu/services/cards/. Accessed 2016.
- 34.Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol. 1981;36:428–434. doi: 10.1093/geronj/36.4.428. [DOI] [PubMed] [Google Scholar]
- 35.Stone JA, Lester CA, Aboneh EA, Phelan CH, Welch LL, Chui MA. A preliminary examination of over the counter medication misuse rates in older adults. Res Social Adm Pharm. 2016 doi: 10.1016/j.sapharm.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kassam R, Collins JB, Berkowitz J. Patient satisfaction with pharmaceutical care delivery in community pharmacies. Patient Prefer Adherence. 2012;6:337–348. doi: 10.2147/PPA.S29606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Odukoya OK, Stone JA, Chui MA. How do community pharmacies recover from e-prescription errors? Res Social Adm Pharm. 2014;10:837–852. doi: 10.1016/j.sapharm.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wetterneck TB, Lapin JA, Krueger DJ, Holman GT, Beasley JW, Karsh BT. Development of a primary care physician task list to evaluate clinic visit workflow. BMJ Qual Saf. 2012;21:47–53. doi: 10.1136/bmjqs-2011-000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holden RJ, Brown RL, Scanlon MC, Karsh BT. Pharmacy workers’ perceptions and acceptance of bar-coded medication technology in a pediatric hospital. Res Social Adm Pharm. 2012;8:509–522. doi: 10.1016/j.sapharm.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chui MA, Stone JA, Thorpe JM, Martin BA. Exploring work system barriers to effective over-the-counter counseling. Human Factors and Ergonomics Annual Meeting; 2013; San Diego. [Google Scholar]
- 41.Maxwell L, Odukoya OK, Stone JA, Chui MA. Using a conflict conceptual framework to describe challenges to coordinated patient care from the physicians’ and pharmacists’ perspective. Res Social Adm Pharm. 2014;10:824–836. doi: 10.1016/j.sapharm.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chui MA, Stone JA. Exploring information chaos in community pharmacy handoffs. Res Social Adm Pharm. 2014;10:195–203. doi: 10.1016/j.sapharm.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lexi-Comp I. (Lexi-Drugs(R)) Lexi-Comp, Inc; [Google Scholar]
- 44.American Geriatrics Society. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616–631. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crandall B, Klein GA, Hoffman RR, editors. Working minds a practitioner’s guide to cognitive task analysis. Cambridge, Mass: MIT Press; 2006. [Google Scholar]
- 46.Hoffman RR, Militello LG. Perspectives on cognitive task analysis: historical origins and modern communities of practice. New York: Psychology Press; 2009. [Google Scholar]
- 47.Shepherd A. Hierarchical task analysis. London; New York: Taylor & Francis; 2001. [Google Scholar]
- 48.Odukoya OK, Stone JA, Chui MA. E-prescribing errors in community pharmacies: exploring consequences and contributing factors. Int J Med Inform. 2014;83:427–437. doi: 10.1016/j.ijmedinf.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chui MA, Stone JA. The prescription handoff in community pharmacy: a study of its form and function. J Am Pharm Assoc (2003) 2012;52:e161–167. doi: 10.1331/JAPhA.2012.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patton MQ. Qualitative research and evaluation methods. Thousand Oaks, Calif: Sage Publications; 2002. [Google Scholar]
- 51.Holland R, Meyers D, Hildebrand C, Bridges AJ, Roach MA, Vogelman B. Creating champions for health care quality and safety. Am J Med Qual. 2010;2:102–108. doi: 10.1177/1062860609352108. [DOI] [PubMed] [Google Scholar]
- 52.Li J, Hinami K, Hansen LO, Maynard G, Budnitz T, Williams MV. The physician mentored implementation model: a promising quality improvement framework for health care change. Acad Med. 2015;90:303–310. doi: 10.1097/ACM.0000000000000547. [DOI] [PubMed] [Google Scholar]
- 53.Young CA. Walgreens expands flagships, other Well Experience stores. 2013 http://www.pharmacist.com/walgreens-expands-flagships-other-well-experience-stores. Accessed 2015.
- 54.Johnsen M. Rite Aid continues evolving Wellness store format 2014. http://www.retailingtoday.com/article/rite-aid-continues-evolving-wellness-store-format. Accessed 2015.
- 55.Smith M, Bates DW, Bodenheimer T, Cleary PD. Why pharmacists belong in the medical home. Health Aff (Millwood) 2010;29:906–913. doi: 10.1377/hlthaff.2010.0209. [DOI] [PubMed] [Google Scholar]


