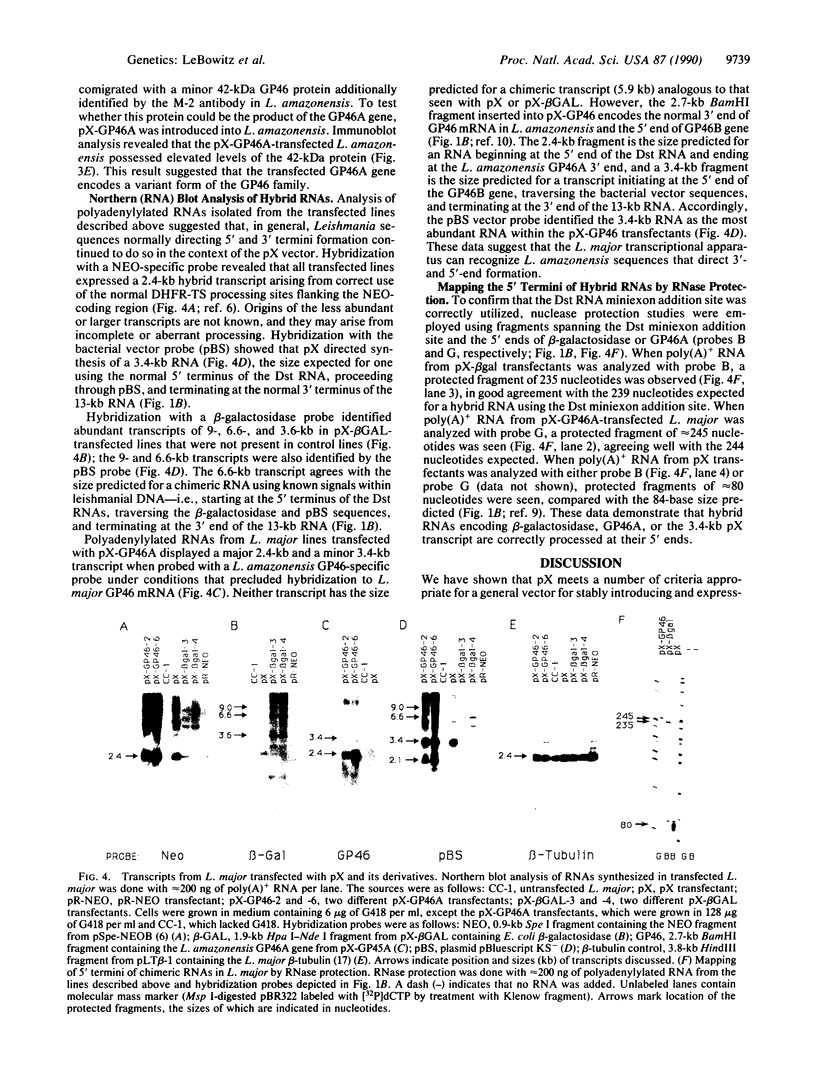
Abstract
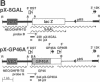
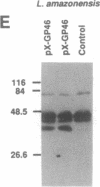
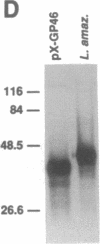
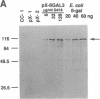
Trypanosomatid protozoan parasites cause several important tropical diseases and have been a fertile ground for the discovery of molecular paradigms such as trans-splicing and RNA editing. Transfection-based methods for the study of these organisms have recently been developed, and we have now designed an expression vector, pX, which contains only 2.3 kilobases of Leishmania DNA and can be stably transfected with high efficiency. Genes encoding Escherichia coli beta-galactosidase or a Leishmania amazonensis protective membrane glycoprotein (GP46A/M-2) were inserted into the pX expression site and transfected into Leishmania major, where they directed the synthesis of high levels of mRNAs formed by 5' and 3' processing events occurring predominantly at the sites used by the normal transcripts. Colony assays and immunoblot analysis showed that both proteins were produced; enzymatically active beta-galactosidase comprised approximately 1% of total protein. Sizes of the GP46A protein synthesized in transfected L. major or L. amazonensis were similar and differed from the predominant L. amazonensis GP46, suggesting that the GP46A gene may encode a variant GP46 family member. Because these vectors function efficiently in pathogenic species of Leishmania, pX will facilitate the genetic analyses of parasite proteins crucial for infectivity as well as the identification of cis-acting elements mediating transcription and replication.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellofatto V., Cross G. A. Expression of a bacterial gene in a trypanosomatid protozoan. Science. 1989 Jun 9;244(4909):1167–1169. doi: 10.1126/science.2499047. [DOI] [PubMed] [Google Scholar]
- Borst P. Discontinuous transcription and antigenic variation in trypanosomes. Annu Rev Biochem. 1986;55:701–732. doi: 10.1146/annurev.bi.55.070186.003413. [DOI] [PubMed] [Google Scholar]
- Champsi J., McMahon-Pratt D. Membrane glycoprotein M-2 protects against Leishmania amazonensis infection. Infect Immun. 1988 Dec;56(12):3272–3279. doi: 10.1128/iai.56.12.3272-3279.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton C. E., Fueri J. P., Itzhaki J. E., Bellofatto V., Sherman D. R., Wisdom G. S., Vijayasarathy S., Mowatt M. R. Transcription of the procyclic acidic repetitive protein genes of Trypanosoma brucei. Mol Cell Biol. 1990 Jun;10(6):3036–3047. doi: 10.1128/mcb.10.6.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton C. E. The molecular biology of the Kinetoplastidae. Genet Eng. 1988;(7):1–56. [PubMed] [Google Scholar]
- Ellenberger T. E., Beverley S. M. Biochemistry and regulation of folate and methotrexate transport in Leishmania major. J Biol Chem. 1987 Jul 25;262(21):10053–10058. [PubMed] [Google Scholar]
- Huang P. L., Roberts B. E., Pratt D. M., David J. R., Miller J. S. Structure and arrangement of the beta-tubulin genes of Leishmania tropica. Mol Cell Biol. 1984 Jul;4(7):1372–1383. doi: 10.1128/mcb.4.7.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl L. P., McMahon-Pratt D. Structural and antigenic characterization of a species- and promastigote-specific Leishmania mexicana amazonensis membrane protein. J Immunol. 1987 Mar 1;138(5):1587–1595. [PubMed] [Google Scholar]
- Kapler G. M., Beverley S. M. Transcriptional mapping of the amplified region encoding the dihydrofolate reductase-thymidylate synthase of Leishmania major reveals a high density of transcripts, including overlapping and antisense RNAs. Mol Cell Biol. 1989 Sep;9(9):3959–3972. doi: 10.1128/mcb.9.9.3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapler G. M., Coburn C. M., Beverley S. M. Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol Cell Biol. 1990 Mar;10(3):1084–1094. doi: 10.1128/mcb.10.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laban A., Tobin J. F., Curotto de Lafaille M. A., Wirth D. F. Stable expression of the bacterial neor gene in Leishmania enriettii. Nature. 1990 Feb 8;343(6258):572–574. doi: 10.1038/343572a0. [DOI] [PubMed] [Google Scholar]
- Laban A., Wirth D. F. Transfection of Leishmania enriettii and expression of chloramphenicol acetyltransferase gene. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9119–9123. doi: 10.1073/pnas.86.23.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman K. L., Langer P. J., McMahon-Pratt D. Molecular cloning and characterization of the immunologically protective surface glycoprotein GP46/M-2 of Leishmania amazonensis. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8393–8397. doi: 10.1073/pnas.87.21.8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt D. M., David J. R. Monoclonal antibodies recognizing determinants specific for the promastigote state of Leishmania mexicana. Mol Biochem Parasitol. 1982 Nov;6(5):317–327. doi: 10.1016/0166-6851(82)90064-0. [DOI] [PubMed] [Google Scholar]
- Rudenko G., Le Blancq S., Smith J., Lee M. G., Rattray A., Van der Ploeg L. H. Procyclic acidic repetitive protein (PARP) genes located in an unusually small alpha-amanitin-resistant transcription unit: PARP promoter activity assayed by transient DNA transfection of Trypanosoma brucei. Mol Cell Biol. 1990 Jul;10(7):3492–3504. doi: 10.1128/mcb.10.7.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]