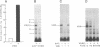Abstract
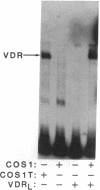
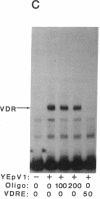
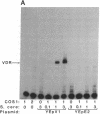
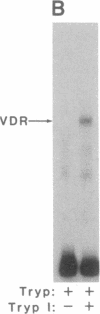
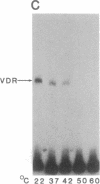
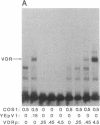
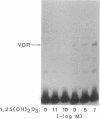
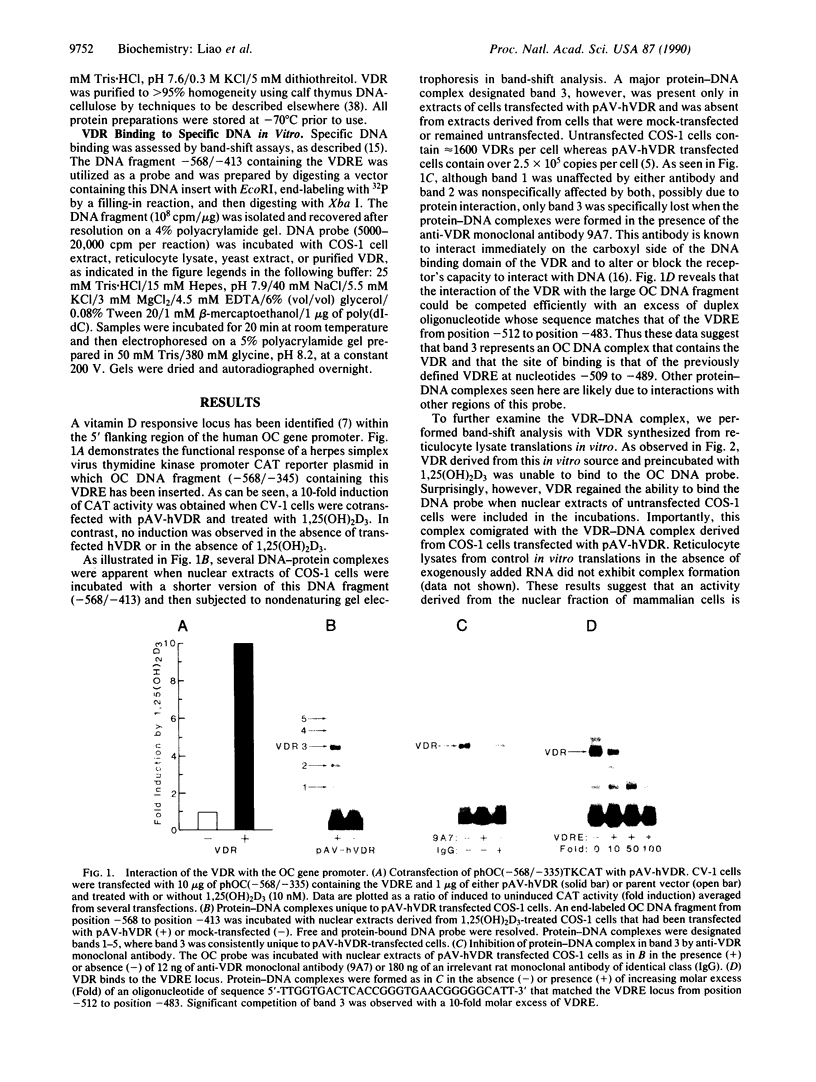
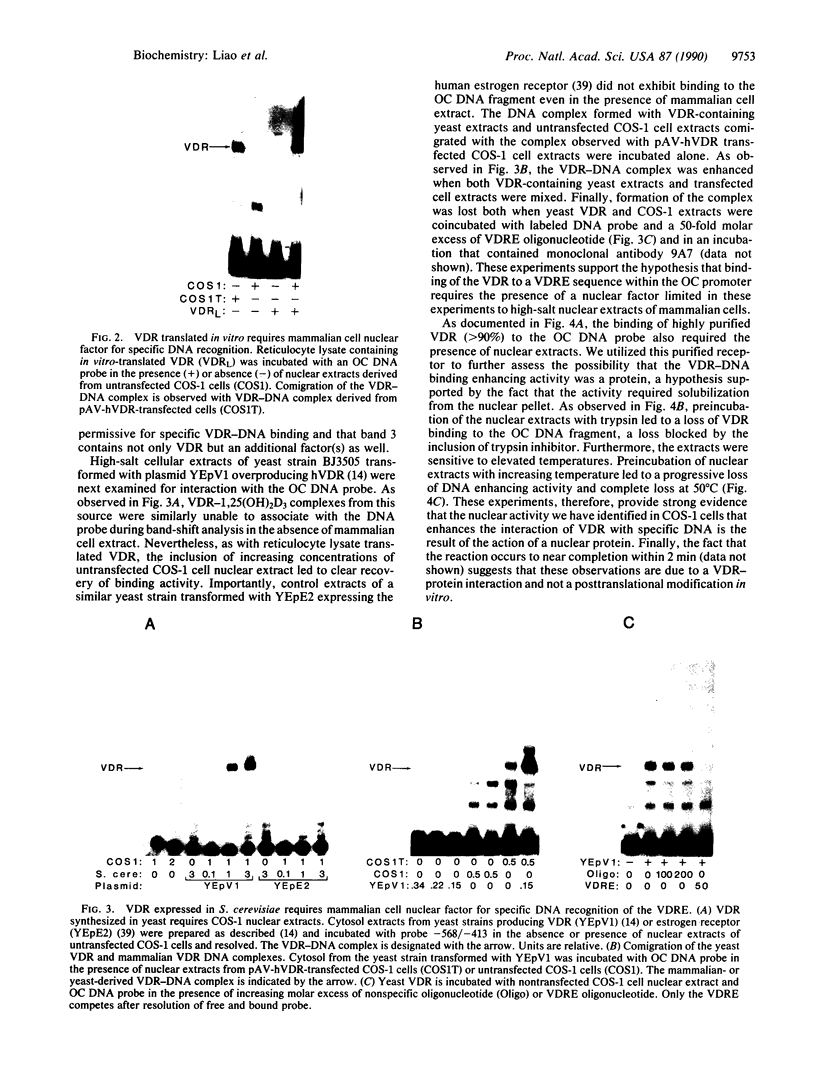
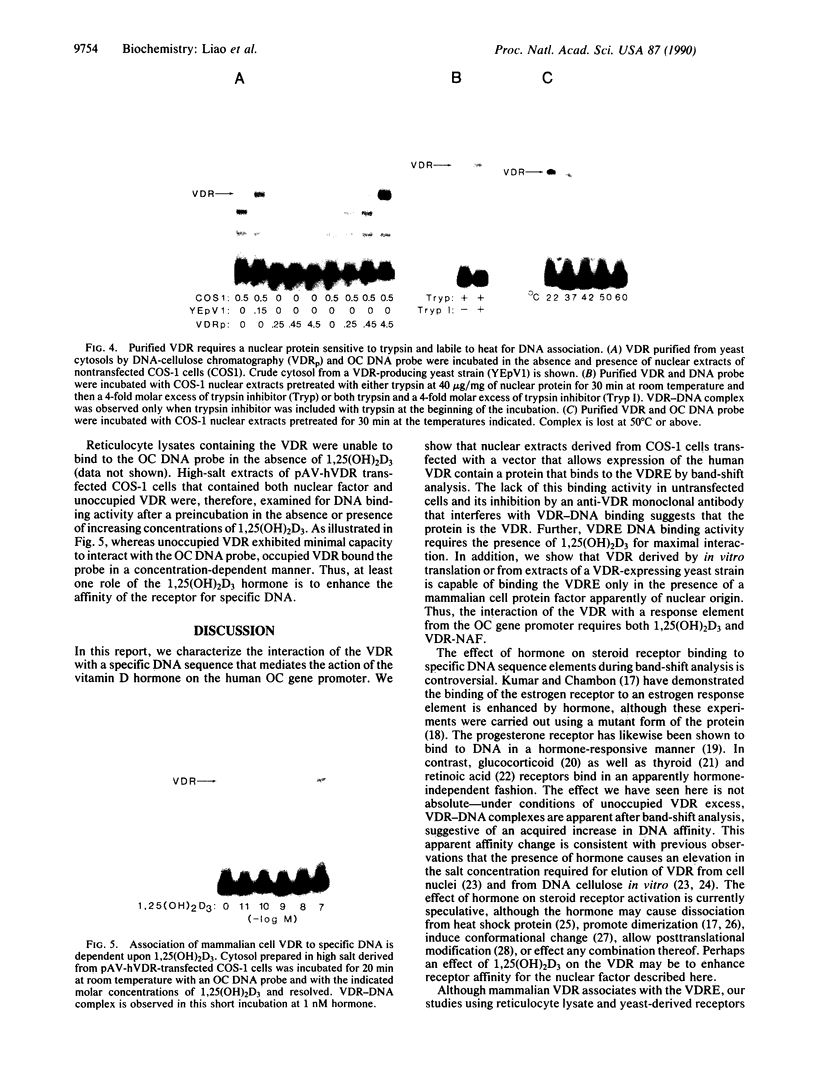
The regulation of osteocalcin gene expression by 1,25-dihydroxyvitamin D3 is mediated by the vitamin D receptor and a cis-acting DNA response element that has been identified within the 5' region of the osteocalcin promoter. In this report, we show that vitamin D receptors derived from nuclear extracts of mammalian cells bind directly to this cis-acting element in vitro and do so in a manner requiring hormone. Vitamin D receptors derived from reticulocyte lysate translations in vitro or from extracts of a Saccharomyces cerevisiae strain that expresses the recombinant protein also bind the osteocalcin responsive element, but only when nuclear extracts of mammalian cells are provided. The vitamin-D-receptor-DNA-binding accessory factor is isolated by salt extraction, labile to temperature, and sensitive to tryptic digestion. These studies suggest that the high-affinity interaction of the vitamin D receptor with the osteocalcin vitamin D response element in vitro requires both 1,25-dihydroxyvitamin D3 and an accessory protein derived from the mammalian cell nucleus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Luk D., Gentz R., Rauscher F. J., 3rd, Curran T. Expression and purification of the leucine zipper and DNA-binding domains of Fos and Jun: both Fos and Jun contact DNA directly. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1032–1036. doi: 10.1073/pnas.87.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi M. K., Tsai S. Y., Tsai M. J., O'Malley B. W. Identification of a functional intermediate in receptor activation in progesterone-dependent cell-free transcription. Nature. 1990 Jun 7;345(6275):547–550. doi: 10.1038/345547a0. [DOI] [PubMed] [Google Scholar]
- Baker A. R., McDonnell D. P., Hughes M., Crisp T. M., Mangelsdorf D. J., Haussler M. R., Pike J. W., Shine J., O'Malley B. W. Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proc Natl Acad Sci U S A. 1988 May;85(10):3294–3298. doi: 10.1073/pnas.85.10.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Burnside J., Darling D. S., Chin W. W. A nuclear factor that enhances binding of thyroid hormone receptors to thyroid hormone response elements. J Biol Chem. 1990 Feb 15;265(5):2500–2504. [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Damm K., Thompson C. C., Evans R. M. Protein encoded by v-erbA functions as a thyroid-hormone receptor antagonist. Nature. 1989 Jun 22;339(6226):593–597. doi: 10.1038/339593a0. [DOI] [PubMed] [Google Scholar]
- Deans R. J., Denis K. A., Taylor A., Wall R. Expression of an immunoglobulin heavy chain gene transfected into lymphocytes. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1292–1296. doi: 10.1073/pnas.81.5.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demay M. B., Gerardi J. M., DeLuca H. F., Kronenberg H. M. DNA sequences in the rat osteocalcin gene that bind the 1,25-dihydroxyvitamin D3 receptor and confer responsiveness to 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1990 Jan;87(1):369–373. doi: 10.1073/pnas.87.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawell S. E., Lees J. A., White R., Parker M. G. Characterization and colocalization of steroid binding and dimerization activities in the mouse estrogen receptor. Cell. 1990 Mar 23;60(6):953–962. doi: 10.1016/0092-8674(90)90343-d. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Lipkin S. M., Devary O. V., Rosenfeld M. G. Positive and negative regulation of gene transcription by a retinoic acid-thyroid hormone receptor heterodimer. Cell. 1989 Nov 17;59(4):697–708. doi: 10.1016/0092-8674(89)90016-0. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W., Walters M. R., Bishop J. E., Norman A. W. Unoccupied and in vitro and in vivo occupied 1,25-dihydroxyvitamin D3 intestinal receptors. Multiple biochemical forms and evidence for transformation. J Biol Chem. 1983 Jul 25;258(14):8642–8648. [PubMed] [Google Scholar]
- Johnson P. F., McKnight S. L. Eukaryotic transcriptional regulatory proteins. Annu Rev Biochem. 1989;58:799–839. doi: 10.1146/annurev.bi.58.070189.004055. [DOI] [PubMed] [Google Scholar]
- Kawai S., Nishizawa M. New procedure for DNA transfection with polycation and dimethyl sulfoxide. Mol Cell Biol. 1984 Jun;4(6):1172–1174. doi: 10.1128/mcb.4.6.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerner S. A., Scott R. A., Pike J. W. Sequence elements in the human osteocalcin gene confer basal activation and inducible response to hormonal vitamin D3. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4455–4459. doi: 10.1073/pnas.86.12.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988 Oct 7;55(1):145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Kumar V., Green S., Stack G., Berry M., Jin J. R., Chambon P. Functional domains of the human estrogen receptor. Cell. 1987 Dec 24;51(6):941–951. doi: 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- Markose E. R., Stein J. L., Stein G. S., Lian J. B. Vitamin D-mediated modifications in protein-DNA interactions at two promoter elements of the osteocalcin gene. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1701–1705. doi: 10.1073/pnas.87.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell D. P., Pike J. W., Drutz D. J., Butt T. R., O'Malley B. W. Reconstitution of the vitamin D-responsive osteocalcin transcription unit in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Aug;9(8):3517–3523. doi: 10.1128/mcb.9.8.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell D. P., Scott R. A., Kerner S. A., O'Malley B. W., Pike J. W. Functional domains of the human vitamin D3 receptor regulate osteocalcin gene expression. Mol Endocrinol. 1989 Apr;3(4):635–644. doi: 10.1210/mend-3-4-635. [DOI] [PubMed] [Google Scholar]
- Murray M. B., Towle H. C. Identification of nuclear factors that enhance binding of the thyroid hormone receptor to a thyroid hormone response element. Mol Endocrinol. 1989 Sep;3(9):1434–1442. doi: 10.1210/mend-3-9-1434. [DOI] [PubMed] [Google Scholar]
- O'Malley B. The steroid receptor superfamily: more excitement predicted for the future. Mol Endocrinol. 1990 Mar;4(3):363–369. doi: 10.1210/mend-4-3-363. [DOI] [PubMed] [Google Scholar]
- Pike J. W., Haussler M. R. Association of 1,25-dihydroxyvitamin D3 with cultured 3T6 mouse fibroblasts. Cellular uptake and receptor-mediated migration to the nucleus. J Biol Chem. 1983 Jul 25;258(14):8554–8560. [PubMed] [Google Scholar]
- Pike J. W., Haussler M. R. Purification of chicken intestinal receptor for 1,25-dihydroxyvitamin D. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5485–5489. doi: 10.1073/pnas.76.11.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike J. W. Monoclonal antibodies to chick intestinal receptors for 1,25-dihydroxyvitamin D3. Interaction and effects of binding on receptor function. J Biol Chem. 1984 Jan 25;259(2):1167–1173. [PubMed] [Google Scholar]
- Pike J. W., Sleator N. M. Hormone-dependent phosphorylation of the 1,25-dihydroxyvitamin D3 receptor in mouse fibroblasts. Biochem Biophys Res Commun. 1985 Aug 30;131(1):378–385. doi: 10.1016/0006-291x(85)91813-3. [DOI] [PubMed] [Google Scholar]
- Pratt W. B. Transformation of glucocorticoid and progesterone receptors to the DNA-binding state. J Cell Biochem. 1987 Sep;35(1):51–68. doi: 10.1002/jcb.240350105. [DOI] [PubMed] [Google Scholar]
- Shapiro D. J., Sharp P. A., Wahli W. W., Keller M. J. A high-efficiency HeLa cell nuclear transcription extract. DNA. 1988 Jan-Feb;7(1):47–55. doi: 10.1089/dna.1988.7.47. [DOI] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Skafar D. F., Notides A. C. Modulation of the estrogen receptor's affinity for DNA by estradiol. J Biol Chem. 1985 Oct 5;260(22):12208–12213. [PubMed] [Google Scholar]
- Tora L., Mullick A., Metzger D., Ponglikitmongkol M., Park I., Chambon P. The cloned human oestrogen receptor contains a mutation which alters its hormone binding properties. EMBO J. 1989 Jul;8(7):1981–1986. doi: 10.1002/j.1460-2075.1989.tb03604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. Y., Sagami I., Wang H., Tsai M. J., O'Malley B. W. Interactions between a DNA-binding transcription factor (COUP) and a non-DNA binding factor (S300-II). Cell. 1987 Aug 28;50(5):701–709. doi: 10.1016/0092-8674(87)90328-x. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Tsai S. Y., Cook R. G., Beattie W. G., Tsai M. J., O'Malley B. W. COUP transcription factor is a member of the steroid receptor superfamily. Nature. 1989 Jul 13;340(6229):163–166. doi: 10.1038/340163a0. [DOI] [PubMed] [Google Scholar]
- Willmann T., Beato M. Steroid-free glucocorticoid receptor binds specifically to mouse mammary tumour virus DNA. Nature. 1986 Dec 18;324(6098):688–691. doi: 10.1038/324688a0. [DOI] [PubMed] [Google Scholar]
- de Thé H., Vivanco-Ruiz M. M., Tiollais P., Stunnenberg H., Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990 Jan 11;343(6254):177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]