Abstract
Mutations in the cystathionine beta-synthase (CBS) gene are the cause of classical homocystinuria, the most common inborn error in sulfur metabolism. The c.797 G>A (p.R266K) mutation in CBS was originally described in several Norwegian pyridoxine responsive CBS deficient patients, and heterologous gene expression studies have shown that the protein has near wild-type levels of enzyme activity. Here, we characterize a transgenic mouse lacking endogenous Cbs and expressing p.R266K human CBS protein from a zinc inducible metallothionein promoter (Tg-R266K Cbs−/−). Unlike mice expressing other mutant CBS alleles, the Tg-R266K transgene is unable to efficiently rescue neonatal lethality of Cbs−/− on a C57BL/6J background. On a C3H/HeJ background, zinc-induced Tg-R266K Cbs−/− mice express CBS mRNA, but have very low levels of CBS protein and enzyme activity, resulting in extreme elevations in serum total homocysteine (tHcy). Treatment with pyridoxine did not have any appreciable effect on tHcy, indicating this allele is not pyridoxine responsive in mice. However, treatment with the proteasome inhibitor bortezomib resulted in an 97% reduction in tHcy and a 2381% increase in liver CBS activity. These studies show that the p.R266K mutation causes increased proteasomal degradation in vivo, and that treatments that stabilize the protein can be used to reverse its effect.
Keywords: CBS, bortezomib, pyridoxine, methionine, homocysteine
Introduction
Cystathionine β-synthase (CBS) deficiency (MIM# 236200) is the most common inborn error of sulfur metabolism and is the cause of classical homocystinuria [Mudd, 2011]. CBS catalyzes the condensation of homocysteine with serine to form cystathionine, which is the first step in the de novo production of cysteine. CBS deficient patients are characterized by extreme elevations in plasma total homocysteine (tHcy). In healthy adults, tHcy concentration in plasma ranges from 5 to 15 µM, but untreated patients with CBS deficiency often have tHcy in excess of 200 µM. CBS-deficient patients suffer at an early age from various pathologies including thrombosis, osteoporosis, mental retardation, and dislocated lenses. The major cause of mortality and morbidity in these patients is thrombosis. Current treatment strategies involve dietary restriction and vitamin therapy, but these are only partially effective and do not work in all patients. Recent preclinical studies suggest that CBS enzyme replacement may be a possible in the future treatment [Bublil, et al., 2016].
CBS encodes a 63 kDa protein that is 551 amino acids in length and is normally functional as a homotetramer [Kraus, et al., 1978]. Human CBS protein contains three functional domains: An N-terminal domain that binds heme (aa 1-75), a core domain (aa 76-398) that contains the catalytic site which binds pyridoxal 5’-phosphate (PLP) and shares similarity with other PLP-utilizing enzymes, and a C-terminal regulatory domain that is responsible for allosteric regulation by S-adenosylmethionine (aa 399-551) [Jhee and Kruger, 2005]. In patients with CBS deficiency, at least 924 mutant alleles have been characterized with 85% of these being missense mutations (see http://cbs.lf1.cuni.cz/index.php). Many missense mutant CBS enzymes have been expressed in cell-based heterologous expression systems such as E. coli and S. cerevisiae [Kozich and Kraus, 1992; Kraus, et al., 1999; Kruger, 1994]. Although most expressed mutant proteins have greatly reduced enzyme activities, several do not. This suggests that either the mutations are not disease causing, or that the heterologous expression system is failing to capture the true effect of the mutation [Kim, et al., 1997; Kopecka, et al., 2011; Maclean, et al., 2002].
A mouse model for CBS deficiency was originally created by Watanabe and colleagues, but >90% of the homozygous animals died by 4 weeks of age due to liver damage [Watanabe, et al., 1995]. To overcome the issue of neonatal lethality, our lab created a transgenic mouse in which the human CBS encoding cDNA was placed downstream of the mouse metallothionein (MT-I) promoter (Tg-hCBS) [Wang, et al., 2004]. These mice were then crossed to Cbs+/− mice to generate heterozygous transgene positive animals (Tg-hCBS Cbs+/−), which were then intercrossed on zinc water to generate Tg-hCBS Cbs−/− mice. These animals are born in the expected Mendelian ratio and have near 100% survival through adulthood. Surprisingly, neonatal lethality was also rescued by transgenes expressing two different patient derived missense alleles, p.I278T and p.S466L, despite both enzymes having <5% of the enzyme activity of the wild-type enzyme [Gupta, et al., 2013; Wang, et al., 2005]. Interestingly, the p.S466L mutation shows high levels of enzyme activity in E. coli and S. cerevisiae, but is subject to degradation when expressed in mouse liver [Gupta, et al., 2008; Janosik, et al., 2001].
p.R266K is a mutation that was initially identified in multiple patients from Norway. Untreated homozygous patients had very high tHcy levels, but all were highly responsive to treatment with pyridoxine [Kim, et al., 1997]. The mutation is located near the heme-binding site and biophysical studies indicate that the mutation slightly alters heme binding to the enzyme [Melenovska, et al., 2015; Smith, et al., 2012]. However, in heterologous expression systems, the enzyme is extremely active and has very modest differences compared to the wild-type enzyme [Majtan and Kraus, 2012]. Thus, it is not clear why this mutation causes severe homocystinuria.
Here, we have created a transgenic mouse (Tg-R266K Cbs−/−) that is lacking endogenous mouse Cbs and expresses p.R266K encoding human CBS (hCBS) using a zinc-inducible promoter. Like untreated human homozygous patients, our mice have extremely elevated serum tHcy and lack significant levels of CBS activity in the liver. Treatment of our mice with a proteasome inhibitor, but not pyridoxine, restores liver CBS activity and lowers serum tHcy to near normal levels. Our studies indicate that in vivo p.R266K hCBS is unstable and degraded by the proteasome.
Materials and Methods
DNA and Constructs
All nucleotide numbering is based on the GenBank RefSeq NM_000071.1. The DNA mutation numbering system is based on the cDNA with +1 corresponding to the A or the ATG translation initiation codon in the reference sequence. Codon 1 is the initiator in the protein sequence. Site-directed mutagenesis was used to introduce a c.797 G>A (p.R266K) change into pLW2, which contains a hemagglutinin epitope tagged version of the human CBS cDNA [Wang, et al., 2004] using the Quick Change XL site-directed mutagenesis kit from Stratagene (La Jolla, CA). The entire open reading frame of the resulting clone, pLW2:R266K, was sequenced to verify no additional changes occurred due to the mutagenesis process. Plasmid pLW2:R266K was subsequently digested with MfeI and cloned into the EcoR1 site of MT-LCR expression vector 2999 [Palmiter, et al., 1993]. The resulting plasmid was designated pLW3:R266K.
Mouse Generation
Transgenic mice containing pLW3:R266K were created as previously described [Wang, et al., 2004]. In brief, DNA was digested with SalI to release bacterial sequences, and the purified fragment was microinjected into the pronuclei of day 0.5 C57BL/6JxC3H/HeJ F2 embryos. Approximately 70 injected embryos were then transferred to pseudopregnant mice, which resulted in the birth of 21 pups. Tails from the resulting pups were then screened for the presence of the transgene by PCR as described [Wang, et al., 2004], and only one positive animal (female) was obtained. This DNA positive female was then backcrossed to a C57BL/6J male and six of eleven offspring were transgene positive, indicating germline inheritance.
To create Tg-R266K Cbs−/− mice, transgene positive offspring were bred to C57BL/6J Cbs− heterozygous animals [Watanabe, et al., 1995] originally obtained from Jackson Laboratories (Bar Harbor, ME) to obtain Tg+ Cbs−/+ animals. These animals were then backcrossed again to C57BL/6J Cbs− heterozygous animals to obtain Tg+ Cbs−/− animals. Mice were on Zn water for these crosses (25 mM ZnSO4). Once it became clear that the vast majority of Tg-R266K Cbs−/− mice were not surviving into adulthood, Tg-R266K Cbs+/− mice were backcrossed with C3H/HeJ mice for at least three generations before again intercrossing.
Genotyping of offspring was generally done between 10 and 14 days of age as previously described [Wang, et al., 2004]. Animals were fed standard rodent chow (Teklad 2018SX) containing 0.6% methionine by weight.
Pyridoxine and Bortezomib Studies in Mice
A cohort of Tg-R266K Cbs−/− mice were studied under control (regular water), zinc water (25mM; 14 days), and zinc water supplemented with pyridoxine (0.4g/L; Sigma Aldrich P5669; 14 days) conditions. For most experiments only female mice were used as previous studies have shown that female mice more consistently induce Mt-I driven transgenes [Wang, et al., 2004; Wang et al., 2005]. Mice ranged in age from 88–250 days of age. Blood was collected by retro-orbital bleed to measure serum tHcy and methionine.
Bortezomib (Velcade, Millennium Pharmaceuticals, Cambridge, MA, USA) was obtained from the pharmacy at Fox Chase Cancer Center. Adult female Tg-R266K Cbs−/− mice (n=8) were subcutaneously implanted with Alzet microosmotic pumps (Model 2001); pumping rate 1 microliter/hr) containing bortezomib diluted in 0.9% NaCl to deliver a final dose of 0.49 mg/kg/day. Mice were on zinc water 11 days before the pump implant and through the duration of drug studies, and were also eye bled before the implant to extract serum. Mice were sacrificed after two days on pump to harvest liver and serum.
Metabolite Measurements
Serum tHcy and methionine were measured using a Biochrom 30 amino acid analyzer as previously described [Wang, et al., 2004]. The sum total of all forms of homocysteine including protein bound, free reduced and free oxidized, is referred to as tHcy.
Western Blotting and CBS Enzyme Activity
Liver homogenates were prepared as previously described [Shan, et al., 2001; Wang, et al., 2005]. For Western blotting, 30 µg of total protein extract was electrophoresed on a 7% NuPAGE Tris-Acetate gel (Novex, Life Technologies) under denaturing conditions followed by transfer on to a PVDF membrane (Bio Rad) CBS was detected using a polyclonal rabbit anti-human primary antibody (1:10,000) and secondary anti-rabbit antibody (1:30,000) conjugated with horse radish peroxidase (GE Healthcare). Signal was visualized by SuperSignal West Pico Chemiluminescent kit (Thermo Scientific), and signal was measured by using Alpha Innotech image analyzer. Relative CBS protein levels (Figure 1d) were calculated by taking the absolute pixel count for CBS, dividing by the actin pixel count for the same sample, and multiplying by 10.
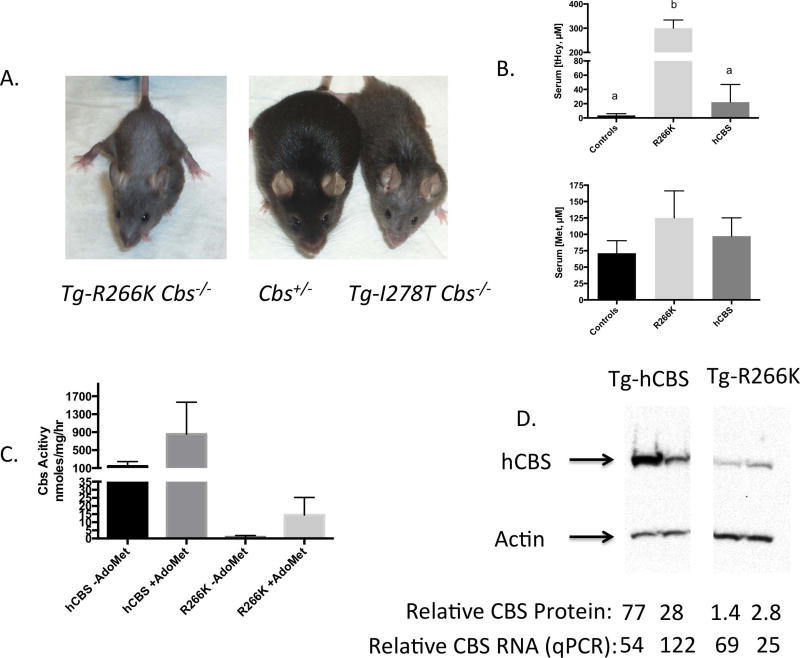
Figure 1. Characterization of Tg-R266K Cbs−/− (C57BL/6J) mice.
A: Pictures of 150 day old Tg-R266K Cbs−/−, Tg− Cbs+/−, and Tg-I278T Cbs−/− mice. B: Serum tHcy and methionine in controls (Cbs+/+), Tg-R266K Cbs−/−, and Tg-hCBS Cbs−/− mice. All mice were on zinc water. Error bars show standard deviation (n=4 or 5). If letters above column are different, means are statistically significant as determined by post-hoc Tukey’s test. C: Liver CBS activity in the presence and absence of AdoMet (n=4). D: Western blot of CBS protein. All four lanes were on the same blot. At bottom is shown relative quantitation of protein (normalized to actin) and relative transcript levels as determined by qRT-PCR (normalized to actin).
CBS activity was analyzed in the presence and absence of AdoMet (250 µM) as previously described [Wang, et al., 2004]. Reactions contained 30µg of dialyzed liver extracts, 5 mM L-serine, 10 mM DL-homocysteine and 50µM pyridoxal phosphate. One unit of activity is defined as nmoles of cystathionine formed per milligram of protein per hour.
Quantitative Real Time PCR
RNA was extracted from livers using TRIzol (Life Technologies) followed by cleanup using Qiagen RNeasy Mini kit. Gene expression analysis was done using Taq Man probes for human CBS (Hs00163925_m1, Applied Biosystems) and mouse β-actin (Mm01205647_g1). Quantification of signal was achieved using an Applied Biosystems 7900 HT detection system. Each sample was assayed in duplicate for both probes, and averages were used for statistical analysis. Relative signal strength was calculated using the ΔΔCt method [Livak and Schmittgen, 2001].
Histology
Haematoxylin and eosin (H and E) staining was performed on livers fixed in 10% formalin as previously described [Wang, et al., 2005].
Statistics
All values cited in text and figures are arithmetic mean ± standard deviation. T-tests are all two-sided with a p<0.05 considered significant. One-way ANOVA (analysis of variance) followed by a post-hoc Tukey’s test was used in experiments that involved multiple comparisons.
Results
Tg-R266K Does Not Rescue Neonatal Lethality and Mice Have Hyperhomocysteinemia
To understand the effect of the p.R266K mutation in vivo, we created a DNA construct that contains a hemagglutinin (HA)-tagged p.R266K CBS encoding cDNA downstream of the mouse MT-I promoter, in a manner analogous to that used previously to express other human CBS alleles [Wang, et al., 2004]. A single founder was obtained which was then backcrossed to C57BL/6J animals to confirm Mendelian transmission and transgene expression in response to zinc (Supp. Fig. S1). Tg-R266K mice were then backcrossed to Cbs+/− (C57BL/6J) to generate Tg-R266K Cbs+/− pups, which were intercrossed to generate Tg-R266K Cbs−/− mice. Out of 74 pups, 15 TgR266K Cbs−/− animals were detected by genotyping at 10–14 days of age, which is quite close to the expected Mendelian value (13.875). However, by the time of weaning (around 30 days) 12 of these animals had died, suggesting that the transgene had not rescued the high degree of neonatal lethality associated with homozygosity for Cbs− in C57BL/6J mice [Watanabe, et al., 1995]. In mice that were followed closely, death generally occurred between days 18–21, with no noticeable change in behavior occurring until about 24 hours before death. These findings indicate that, unlike the p.I278T and p.S466L alleles, p.R266K is unable to rescue the neonatal lethality of Cbs−/− mice on the C57BL/6J background. Livers from either moribund or recently deceased Tg-R266K Cbs−/− mice, showed evidence of lipid accumulation and abnormal liver pathology characterized by enlarged hepatic nuclei (Suppl. Fig. S2).
The few Tg-R266K Cbs−/− mice that survived into adulthood tended to be smaller than their Cbs+/− or Cbs+/+ littermates. In addition, as they aged, they showed the loss of hair of both the face and upper torso, similar to that observed in Tg-I278T Cbs−/− mice (Fig. 1A). These adult survivors had significantly elevated serum tHcy and methionine and barely detectable levels of liver CBS activity. However, this residual activity was stimulated by the allosteric effector AdoMet (Figs. 1B, C). Examination of the levels of CBS protein and mRNA in the liver indicates that Tg-R266K Cbs−/− mice had at least a 10-fold lower level of steady state CBS protein, but only a small decrease in CBS transcript level compared to Tg-hCBS Cbs−/− mice (Fig. 1D). These findings show that there is less p.R266K protein relative to transcript compared to hCBS protein, suggesting that either protein stability or translational efficiency is impaired.
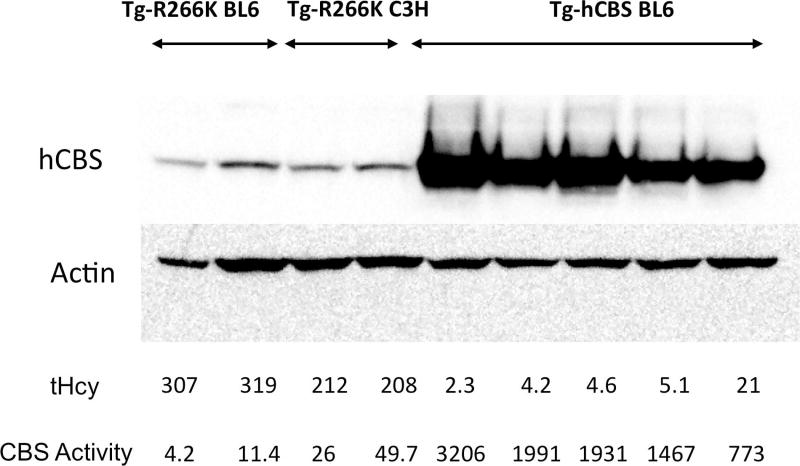
To circumvent the high degree of neonatal lethality, we crossed our mice with C3H/HeJ mice, as Cbs−/− neonatal lethality rates are reduced on this background [Akahoshi, et al., 2008]. After three backcrosses, 31 out of 35 (89%) Tg-R266K Cbs−/− mice lived greater than six weeks of age. Zinc-treated adult Tg-R266K Cbs−/− mice on the C3H/HeJ background tended to have slightly lower serum tHcy and slightly more liver CBS activity than surviving Tg-R266K Cbs−/− mice on the C57BL6/J background (Fig. 2). However, the amount of CBS activity in the C3H/HeJ livers was only about 2% of that found in hCBS expressing livers. Although activity was slightly higher in the C3H/HeJ samples compared to the C57BL/6J samples, the amount of CBS protein appeared to be comparable.
Figure 2.
Comparison of liver CBS expression, activity, and serum tHcy of Tg-R266K Cbs−/− mice on C57BL/6J and C3H/HeJ backgrounds. Total Hcy in micromolar is shown below western. Bottom line shows units of CBS activity (nmoles cystathionine formed/mg total soluble liver protein/hr).
Pyridoxine Supplementation Does Not Restore Function to p.R266K hCBS
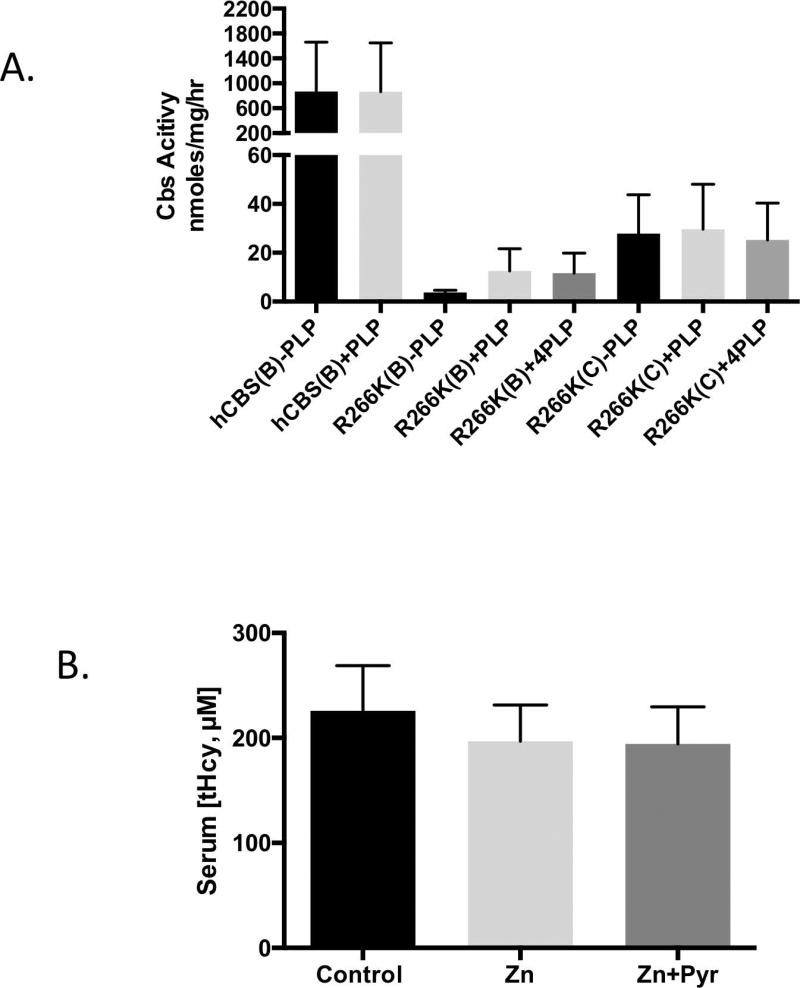
Since p.R266K was associated with strong pyridoxine responsive behavior in human patients, we decided to investigate the effect of PLP on CBS activity in dialyzed liver extracts from mice expressing either hCBS or p.R266K in both C57BL/6J and C3H/HeJ backgrounds (Fig. 3A). Addition of either 50 or 200 µM PLP to the in vitro enzyme reaction slightly increased the activity in the Tg-R266K Cbs−/− C57BL/6J-derived extracts, but the increases were not statistically significant. Tg-R266K Cbs−/− C3H/HeJ-derived extracts showed no increase at all. However, even in the presence of high PLP concentrations, CBS enzyme activity in p.R266K enzyme extracts was minimal, with none of the samples containing more than 5% of the activity observed in WT hCBS extracts.
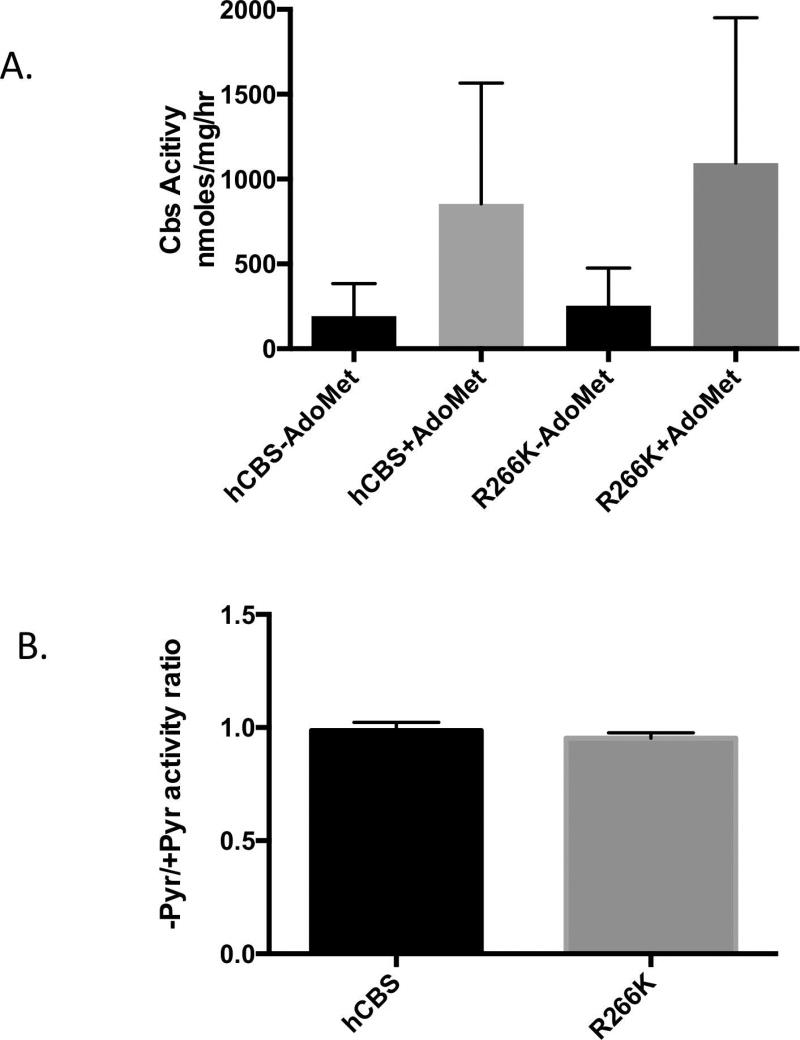
Figure 3. Response to pyridoxine.
A: Dialyzed liver extracts from Zn-treated mice carrying the indicated transgene in the indicated strain (B=C57BL/6J; C=C3H/HeJ). Each reaction was done either in the absence (-PLP), or presence of 50 µM (+PLP) or 200 µM (+4PLP) PLP. Error bars show SD (n=3 or 4). B: Comparison of tHcy in Tg-R266K Cbs−/− mice (C3H/HeJ,) treated with either regular water, zinc-water (25 mM), or zinc-water plus pyridoxine (400 mg/L). Error bars show SD (n=8 per group)
We also examined the effect of pyridoxine supplementation in vivo. A cohort of C3H/HeJ mice were examined under three different conditions: (1) control, (2) zinc water supplemented, and (3) zinc water containing 0.04% pyridoxine. It should be noted that the amount of pyridoxine added was four times greater than used in our previous study on Tg-I278T Cbs−/−, which caused a 7-fold increase in serum PLP levels [Chen, et al., 2006]. Despite this fact, we observed no statistically significant differences in by any of the three treatments (Fig. 3B). Thus, neither the in vitro or in vivo studies demonstrated that mouse-liver produced p.R266K was responsive to pyridoxine.
Bortezomib Restoration of p.R266K Function
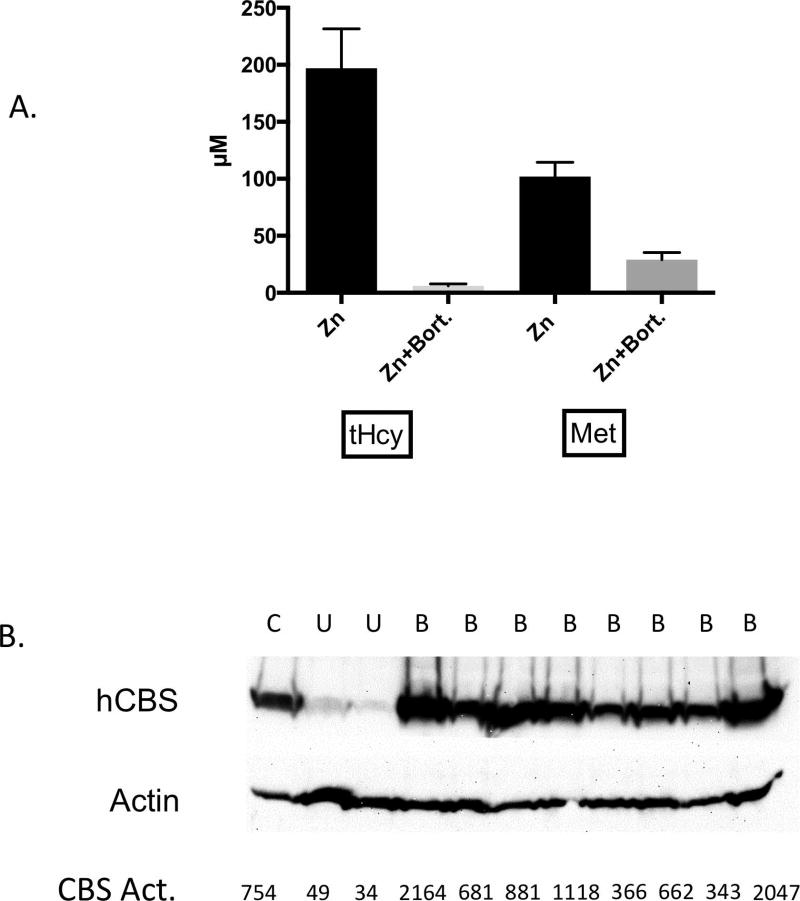
Because p.R266K appeared to have reduced stability compared to hCBS, we examined if treatment with the proteasome inhibitor bortezomib could rescue CBS function. Zinc-treated Tg-R266K Cbs−/− mice (C3H/HeJ) were surgically implanted with an osmotic pump that delivered bortezomib at a dose of 0.49 mg/kg/day. After two days, the mice were euthanized, and blood and liver were collected for analysis. Bortezomib treated animals exhibited a dramatic lowering in both serum tHcy and a more modest lowering in Met in response to the treatment (Fig. 4A). Serum tHcy levels went from 197 µM before pump implantation to 6.1 µM, which is only slightly higher than normal mouse tHcy levels. CBS protein in liver extracts from treated animals was much higher than in untreated animals and was similar to the amount observed in a zinc-induced Tg-hCBS Cbs−/− (C57BL/6J) mouse (Fig. 4B). Activity studies showed that liver extracts containing bortezomib rescued p.R266K had similar levels of activity as hCBS protein derived from the livers of non-bortezomib treated Tg-hCBS Cbs−/− mice (Fig. 5A). In addition, the bortezomib rescued p.R266K protein responded similarly to allosteric stimulation by AdoMet.
Figure 4. Bortezomib effect on Tg-R266K Cbs−/− (C3H/HeJ) mice.
A: Serum tHcy and Methionine in mice before and after bortezomib treatment (0.49/mg/kg/day, n=8). Error bars show SD. B: CBS Western blot of liver extracts from treated (B) and untreated mice (U). Control (C) is liver extract from Tg-hCBS Cbs−/− mouse. CBS activity is expressed in nmoles cystathionine formed/mg total soluble liver protein/hr.
Figure 5. CBS activity and pyridoxine response in bortezomib-treated liver extracts.
A: CBS enzyme activity in extracts from bortezomib-treated mice of the indicated genotype in the presence and absence of AdoMet. Error bar shows SD (n=4 for each group). B: Ratio of CBS enzyme activity of indicated samples in the presence and absence of added PLP in reaction mixture.
We also examined the sensitivity of the bortezomib rescued p.R266K to PLP in vitro. Our reasoning was that the protein instability of p.R266K might be something specific to mice and, if the protein was stabilized, we might now be able to see a differential response to PLP. Therefore, we measured the ratio of enzyme activity in the presence and absence of PLP in the reaction mix. There was no difference in the sensitivity to PLP depletion in the reaction mixture (Fig. 5B), suggesting that the bortezomib stabilized p.R266K was also not rescuable by PLP.
Discussion
In this paper, we have analyzed the p.R266K mutation in the mouse model of CBS deficiency. This mutation is interesting because it has been found homozygous in multiple CBS deficient patients, but in vitro studies of the mutant enzyme indicate that it is nearly as active as wild-type CBS [Majtan and Kraus, 2012]. In addition, patients homozygous of this allele strongly respond to pyridoxine, with their post-treatment tHcy levels reaching normal levels [Kim, et al., 1997]. To study this mutation, we have made a transgenic mouse that expresses human p.R266K hCBS under control of the zinc inducible MT-I promoter. These mice were then back crossed with Cbs+/− (C57BL/6J) mice to generate mice that only express the p.R266K hCBS allele. The characterization of these mice revealed four important observations. First, unlike mice expressing either wild-type CBS or other patient derived missense-containing CBS proteins, the p.R266K CBS can not rescue the neonatal lethal phenotype associated with Cbs−/− in a C57BL6/J background. Second, mice expressing p.R266K have severe hyperhomocysteinemia due to the fact that the enzyme is unstable in the liver. Third, despite being rescuable by pyridoxine in humans, there is no evidence indicating that the mouse liver produced protein is pyridoxine responsive. Lastly, treatment with the proteasome inhibitor bortezomib can rescue the hyperhomocysteinemia in Cbs−/− mice expressing p.R266K. Below, we will discuss each of these points in more detail.
Cbs−/− animals on a mostly C57BL/6J background were present in litters at 10–14 days, but 80% of the animals died by 30 days of age. Our lab previously showed that not only could hCBS rescue this neonatal lethality, but so could two patient derived missense alleles (p.I278T and p.S466L) [Gupta, et al., 2008; Wang, et al., 2005]. Both of these alleles showed minimal levels of enzyme activity in liver extracts, similar to the activity found in p.R266K livers. With regards to steady-state protein levels, p.I278T animals tended to have more protein than p.R266K animals, but in some p.S466L animals, we observed levels similar to the p.R266K mice [Gupta, et al., 2013; Gupta, et al., 2008]. A possible explanation for the difference in neonatal lethality is that neonatal death is not directly related to CBS’s known enzymatic function, but may actually reflect an additional “moonlighting” function. Support for this idea comes from the observation that tHcy levels in surviving Tg− Cbs−/− adult mice are no different than those of Tg-I278T Cbs−/− mice [Wang, et al., 2005]. In addition, we did not observe that tHcy levels of 18 day old Tg-R266K Cbs−/− mice were any higher than in 18 day old Tg-I278T Cbs−/− mice (unpublished data), suggesting that elevated tHcy is not directly related to the neonatal lethality. These findings are consistent with the idea that p.R266K affects an undefined moonlighting function, but p.I278T and p.S466L do not. Interestingly, our finding that out crossing onto the C3H/HeJ background can largely rescue the phenotype, reducing lethality from 80 to 10%, indicates that whatever the cause of the lethality is, it is largely strain specific.
Adult Tg-R266K Cbs−/− mice (on zinc) in both C57BL6/J and C3H/HeJ backgrounds have extremely elevated tHcy levels, which is similar to human p.R266K homozygotes [Kim, et al., 1997]. On the surface, this finding seems at odds with a variety of studies on recombinant p.R266K, which shows the enzyme to be highly active [Chen, et al., 2006; Majtan and Kraus, 2012]. In fact, when expressed in mammalian CHO-K1 cells, the protein was quite stable and formed tetramers at 77% of the level found in cells expressing wild-type human CBS, and had 50% of the enzyme activity [Melenovska, et al., 2015]. However, our examination of liver CBS protein indicates that Tg-R266K Cbs−/− mice have much lower steady state levels than Tg-hCBS Cbs−/− mice. This difference in CBS protein level does not seem to be caused by differences in RNA expression as the levels of mRNA between Tg-hCBS Cbs−/− and Tg-R266K Cbs−/− mice are roughly comparable (see Fig. 1D). Thus, the difference in protein levels either reflects differences in protein synthesis (translational efficiency) or protein degradation. The finding that treatment with proteasome inhibitors resulted in a large increase in protein levels in Tg-R266K Cbs−/− mice supports the view that increased degradation is the most likely explanation. These findings illustrate an important limitation of bacterial and yeast-based assays in assessing the functional relevance of specific mutations. More specifically, our studies show that the proteostasis environment [Balch, et al., 2008] can play a key role in determining the stability effects of a specific missense mutation, i.e., the intracellular environment of E. coli and S. cerevisiae allows p.R266K to fold into an active functional form, while the environment in mouse liver does not.
In human patients, the p.R266K alteration is associated with an extremely robust response to pyridoxine. All five patients containing this allele (four homozgyotes and one presumed compound heterozygote) had post treatment tHcy in the normal range [Kim, et al., 1997]. However, mouse liver produced p.R266K exhibited no significant response to either pyridoxine supplementation in vivo or PLP addition in vitro. These findings were identical to our studies of the p.I278T allele, which are also associated with pyridoxine response in human patients. One possible explanation is that there are differences in the proteostasis environment between mouse and human liver, such that p.R266K has sufficient time to achieve a stable folded form in human, but does not in mouse. Therefore, we further speculated that if given time to fold properly, this form might then show increased sensitivity to PLP. However when we tried to mimic this, using proteasome inhibitors to stabilize p.R266K and then comparing in vitro response to PLP addition, we found that bortezomib stabilized p.R266K was no more sensitive to pyridoxine depletion than WT hCBS. The inability to mimic pyridoxine response in mice suggests there may be underlying differences between mouse and human liver physiology with regards to pyridoxine.
Perhaps the most interesting finding was that treatment with bortezomib could restore both serum tHcy and liver CBS activity to wild-type levels in Tg-R266K Cbs−/− mice. This result shows that other than it being unstable, p.R266K hCBS is essentially fully active, and in agreement with other heterologous expression studies. We have previously tested bortezomib with two other patient derived alleles (p.S466L and p.I278T) and found that they also were rescuable. All of these studies involved relatively short (two to five day) treatment durations using implantable osmotic pumps to give continuous dosing to the mice. However, continuous dosing is too toxic to consider for the long term studies. Further studies will need to be done to determine if non-continuous dosing over a longer period could give similar results. If so, then use of bortezomib on CBS deficient patients might be worth pursuing.
Supplementary Material
Acknowledgments
Grant Sponsors: This work was supported by grant NIH CA06927, DK101404, GM098772 and an appropriation from the Commonwealth of Pennsylvania.
We wish to thank the Sequencing, Genomic, Transgenic Mouse and Laboratory Animal Facilities of Fox Chase Cancer Center for their assistance.
Footnotes
Disclosure Statement: The authors declare no conflict of interest.
References
- Akahoshi N, Kobayashi C, Ishizaki Y, Izumi T, Himi T, Suematsu M, Ishii I. Genetic background conversion ameliorates semi-lethality and permits behavioral analyses in cystathionine beta-synthase-deficient mice, an animal model for hyperhomocysteinemia. Hum Mol Genet. 2008;17:1994–2005. doi: 10.1093/hmg/ddn097. [DOI] [PubMed] [Google Scholar]
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- Bublil EM, Majtan T, Park I, Carrillo RS, Hulkova H, Krijt J, Kozich V, Kraus JP. Enzyme replacement with PEGylated cystathionine beta-synthase ameliorates homocystinuria in murine model. J Clin Invest. 2016;126:2372–2384. doi: 10.1172/JCI85396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wang L, Fazlieva R, Kruger WD. Contrasting behaviors of mutant cystathionine beta-synthase enzymes associated with pyridoxine response. Hum Mutat. 2006;27:474–482. doi: 10.1002/humu.20320. [DOI] [PubMed] [Google Scholar]
- Gupta S, Wang L, Anderl J, Slifker MJ, Kirk C, Kruger WD. Correction of Cystathionine beta-Synthase Deficiency in Mice by Treatment with Proteasome Inhibitors. Hum Mutat. 2013 doi: 10.1002/humu.22335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Wang L, Hua X, Krijt J, Kozich V, Kruger WD. Cystathionine β-synthase p.S466L mutation causes hyperhomocysteinemia in mice. Hum Mutat. 2008;29:1048–1054. doi: 10.1002/humu.20773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janosik M, Kery V, Gaustadnes M, Maclean KN, Kraus JP. Regulation of human cystathionine beta-synthase by S-adenosyl-L-methionine: evidence for two catalytically active conformations involving an autoinhibitory domain in the C-terminal region. Biochemistry (Mosc) 2001;40:10625–10633. doi: 10.1021/bi010711p. [DOI] [PubMed] [Google Scholar]
- Jhee KH, Kruger WD. The role of cystathionine beta-synthase in homocysteine metabolism. Antioxid Redox Signal. 2005;7:813–822. doi: 10.1089/ars.2005.7.813. [DOI] [PubMed] [Google Scholar]
- Kim CE, Gallagher PM, Guttormsen AB, Refsum H, Ueland PM, Ose L, Folling I, Whitehead AS, Tsai MY, Kruger WD. Functional modeling of vitamin responsiveness in yeast: a common pyridoxine-responsive cystathionine beta-synthase mutation in homocystinuria. Hum Mol Genet. 1997;6:2213–2221. doi: 10.1093/hmg/6.13.2213. [DOI] [PubMed] [Google Scholar]
- Kopecka J, Krijt J, Rakova K, Kozich V. Restoring assembly and activity of cystathionine beta-synthase mutants by ligands and chemical chaperones. J Inherit Metab Dis. 2011;34:39–48. doi: 10.1007/s10545-010-9087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozich V, Kraus JP. Screening for mutations by expressing patient cDNA segments in Ecoli: homocystinuria due to cystathionine beta-synthase deficiency. Hum Mutat. 1992;1:113–123. doi: 10.1002/humu.1380010206. [DOI] [PubMed] [Google Scholar]
- Kraus J, Packman S, Fowler B, Rosenberg LE. Purification and properties of cystathionine beta-synthase from human liver. Evidence for identical subunits. J Biol Chem. 1978;253:6523–6528. [PubMed] [Google Scholar]
- Kraus JP, Janosik M, Kozich V, Mandell R, Shih V, Sperandeo MP, Sebastio G, de Franchis R, Andria G, Kluijtmans LA, Blom H, Boers GH, et al. Cystathionine beta-synthase mutations in homocystinuria. Hum Mutat. 1999;13:362–375. doi: 10.1002/(SICI)1098-1004(1999)13:5<362::AID-HUMU4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Kruger WD. A yeast system for expression of human cystathionine beta-synthase: structual and functional conservation of the human and yeast genes. Proc Natl Acad Sci USA. 1994;91:6614–6618. doi: 10.1073/pnas.91.14.6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maclean KN, Gaustadnes M, Oliveriusova J, Janosik M, Kraus E, Kozich V, Kery V, Skovby F, Rudiger N, Ingerslev J, Stabler SP, Allen RH, et al. High homocysteine and thrombosis without connective tissue disorders are associated with a novel class of cystathionine beta-synthase (CBS) mutations. Hum Mutat. 2002;19:641–655. doi: 10.1002/humu.10089. [DOI] [PubMed] [Google Scholar]
- Majtan T, Kraus JP. Folding and activity of mutant cystathionine beta-synthase depends on the position and nature of the purification tag: characterization of the R266K CBS mutant. Protein Expr Purif. 2012;82:317–324. doi: 10.1016/j.pep.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melenovska P, Kopecka J, Krijt J, Hnizda A, Rakova K, Janosik M, Wilcken B, Kozich V. Chaperone therapy for homocystinuria: the rescue of CBS mutations by heme arginate. J Inherit Metab Dis. 2015;38:287–294. doi: 10.1007/s10545-014-9781-9. [DOI] [PubMed] [Google Scholar]
- Mudd SH. Hypermethioninemias of genetic and non-genetic origin: A review. American journal of medical genetics Part C, Seminars in medical genetics. 2011;157:3–32. doi: 10.1002/ajmg.c.30293. [DOI] [PubMed] [Google Scholar]
- Palmiter RD, Sandgren EP, Koeller DM, Brinster RL. Distal regulatory elements from the mouse metallothionein locus stimulate gene expression in transgenic mice. Mol Cell Biol. 1993;13:5266–5275. doi: 10.1128/mcb.13.9.5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan X, Dunbrack RL, Jr, Christopher SA, Kruger WD. Mutations in the regulatory domain of cystathionine beta-synthase can functionally suppress patient-derived mutations in cis. Hum Mol Genet. 2001;10:635–643. doi: 10.1093/hmg/10.6.635. [DOI] [PubMed] [Google Scholar]
- Smith AT, Su Y, Stevens DJ, Majtan T, Kraus JP, Burstyn JN. Effect of the disease-causing R266K mutation on the heme and PLP environments of human cystathionine beta-synthase. Biochemistry. 2012;51:6360–6370. doi: 10.1021/bi300421z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chen X, Tang B, Hua X, Klein-Szanto A, Kruger WD. Expression of mutant human cystathionine beta-synthase rescues neonatal lethality but not homocystinuria in a mouse model. Hum Mol Genet. 2005;14:2201–2208. doi: 10.1093/hmg/ddi224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Jhee KH, Hua X, DiBello PM, Jacobsen DW, Kruger WD. Modulation of cystathionine beta-synthase level regulates total serum homocysteine in mice. Circ Res. 2004;94:1318–1324. doi: 10.1161/01.RES.0000129182.46440.4a. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Osada J, Aratani Y, Kluckman K, Reddick R, Malinow MR, Maeda N. Mice deficient in cystathionine beta-synthase: animal models for mild and severe homocyst(e)inemia. Proc Natl Acad Sci U S A. 1995;92:1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.