Abstract
Background
To study the effect of estrogen-related receptor α (ERRα) and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) on mesenchymal stem cells (MSCs) apoptosis, and further investigated its detailed molecular mechanisms in the absence of serum, hypoxia, and high glucose conditions.
Material/Methods
In our study, we first evaluated the expression rates of CD14, CD34, CD45, CD44, CD29, and Sca-1 surface markers on MSCs by flow cytometry. Then, the ability of osteogenic and fatty differentiation of MSCs was determined by osteogenic differentiation and adipogenesis reagent kit. Next, Annexin V-APC/7-AAD apoptosis kit was used for detecting the apoptosis rate of MSCs. RT-PCR and Western blotting were used for detection of mRNA expression and proteins expression, respectively.
Results
Our data showed that the MSCs used in our study were capable of self-renewal and differentiating into many cell lineages, such as osteogenic differentiation and adipogenesis. Our results further showed that over-expression of PGC-1α could protect MSCs from apoptosis induced by rotenone. We also found that PGC-1α over-expression could enhance the expression of anti-apoptotic gene Bcl-2, and inhibit the expression of pro-apoptotic gene Bax in MSCs. In addition, our data demonstrated that PGC-1α could induce upregulation of Bcl-2 and further promote the survival of MSCs by interacting with ERRα.
Conclusions
In the absence of serum, hypoxia and high glucose conditions, PGC-1α can regulate the expression of Bcl-2 and promote the survival of MSCs via PGC-1α/ERRα interaction.
MeSH Keywords: Estrogen Receptor alpha; Genes, bcl-2; Mesenchymal Stromal Cells; PPAR gamma
Background
More than 400 million people suffer from diabetes all over the world in 2015, and 15% of which have diabetic foot ulcer (DFU) [1]. With the progression of diabetes, severe ischemia in the lower limb prolongs healing of foot ulcers and gangrene, and may result in amputation and even endanger the lives of patients [2]. The pathophysiology of diabetic peripheral vascular disease is diffuse vascular stenosis and occlusion, resulting in poor angiogenesis and difficulties in rebuilding collateral circulation. Therefore, single drug therapy and revascularization treatments for DFUs can be a long process with high cost and poor efficacy [3,4]. Accordingly, many experts around the world are committed to explore novel strategies for the treatment of diabetic peripheral vascular disease.
In recent years, stem cell transplantation has become a promising strategy for the treatment of diabetes. Mesenchymal stem cells (MSCs) isolated from autologous bone marrow can secrete a variety of pro-angiogenic factors, and directly participate in angiogenesis after transplant to ischemic sites [5,6]. However, the low survival rate of MSCs after transplantation (mainly apoptosis) has become a crucial factor restricting its curative efficacy. How to reduce the apoptosis of MSCs after transplantation is the primary challenge to defeating diabetic lower limb ischemia [7,8]. At present, the mechanism of transplanted stem cell apoptosis is induced by hypoxia as well as the lack of nutrition and nutritional factors caused by insufficient blood supply, inflammatory factors from ischemia or necrosis of cells, and the destruction of cell-to-cell interactions [9]. In diabetic patients with lower limb ischemia coupled with endogenous factors such as high glucose and advanced glycation end products, cell apoptosis will be induce through a series of signal transductions and most of the signals will target mitochondria [10].
The B-cell lymphoma/leukemia-2 (Bcl-2) protein family plays a central role in the regulation of the release of cyt C from mitochondria. The release of cyt C from mitochondria is a key step in the process of mitochondrial apoptosis initiation, which is accomplished by regulation of two channels [11]. First, aggregation of the outer mitochondrial membrane protein forms a high conductance nonselective channel, called membrane permeability transition pore complex. The complex includes Bcl-2 proteins such as Bak, Bax, as well as other components. When the expression of anti-apoptotic protein Bcl-2 increases, the mitochondrial membrane channel composed of Bcl-2 proteins will be closed. In addition, interactions between Bcl-2 protein and the membrane permeability transition pore complex can also close the channel [12,13]. Therefore, the opening and closing of the two channels are all regulated by the Bcl-2 protein family. However, in recent years, researchers have mainly focused on the regulation mechanism of the Bcl-2 protein family after expression; thus, the upstream regulatory mechanism of the Bcl-2 protein family still remains unclear. Moreover, there are no relevant reports regarding the regulation mechanism of the upstream pathways in the process of Bcl-2 protein family expression and the role they play in antagonizing mitochondrial apoptosis [14].
Our previous studies showed that peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) can activate the anti-apoptotic pathways of Bcl-2 in MSCs, and induce the tolerance of MSCs apoptosis. But the detailed mechanism required to regulate the upregulation of the Bcl-2 protein is unclear. Based on protein interaction database retrieval, combined with relevant literature and experimental research, we hypothesized that the upregulation of Bcl-2 protein expression was regulated by PGC-1α through the activation of estrogen-related receptor α (ERRα), eventually leading to antagonistic mitochondrial apoptosis.
ERRα is one of the earliest discovered orphan nuclear receptors. It has not, to date, been recognized as a specific agonist of artificial synthesis or a natural ligand. ERRα plays an important role in the activation of energy metabolism. In addition, ERRα plays a pivotal role in the cellular stress response after the cells suffered from cold, infection, ischemia, or hypoxia [15]. Accordingly, we considered whether ERRα might have a protective effect on stem cell apoptosis after transplantation. Therefore, in the current study, we explored the relationship between PGC-1 and ERRα in the regulation of MSCs apoptosis in the absence of serum, hypoxia, and high glucose conditions, and strived to lay the foundation for defeating diabetes.
Material and Methods
Materials
C57BL/6 mouse MSCs culture medium, C57BL/6 mouse MSCs osteogenic differentiation medium, and mouse MSCs adipogenic differentiation medium were purchased from the Guangzhou Cyagen Biosciences company; Alizarin red calcium staining kit, lipid (oil red O) staining kit was purchased from the Shanghai Genmed Pharmaceutical Technology company. XCT790 was purchased from the Sigma Company. Fetal bovine serum (FBS), DMEM/F12 medium, and trypsin were from the Hyclone Company. Trypsin (excluding EDTA) was purchased from the Gibco Company. Rabbit monoclonal antibody Bcl-2 and Bax were purchased from Abcam Company. CD14-FITC, CD34-FITC, CD45-FITC, CD44-FITC, CD29-FITC, Sca-1-FITC, and isotype control fluorescent antibody were purchased from the BD Company. Mouse monoclonal antibody PGC-1α was purchased from Cell Signaling Technology. Annexin V-APC/7-AAD apoptosis kit was bought from the Sigma Company.
Cell culture
2×105 C57BL/6 mouse MSCs were incubated in DMEM/F12 medium, containing 10% FBS, 1×10 IU/L penicillin, and 100 mg/L streptomycin. When C57BL/6 mouse MSCs grew to 80–90% degrees of convergence, the cells were used for passage or subsequent experiments.
Detection of MSCs surface antigen by flow cytometry
C57BL/6 mouse MSCs were digested with trypsin without EDTA, centrifuged for five minutes at 1,000 rpm at 4°C. The cells were collected, washed two times with PBS (including 1% BSA), and 106 cells/mL suspensions were prepared. The cell suspension had added to it 5 μL of CD14, CD34, CD45, CD44, CD29, or Sca-1 antibody, and incubated in the dark at room temperature for 30 minutes. After incubation, the cells were centrifuged at 1,000 rpm for five minutes, the supernatant was discarded, and the cells were resuspended in 500 μL PBS. Flow cytometry was used to detect the expression rates of surface markers on the MSCs.
Detection of MSCs osteogenic differentiation or adipogenesis ability
The ability to induce MSCs to differentiation was determined by osteogenic and fatty differentiation. In the process of adipogenesis, 4×104 cells/cm2 were seeded into culture plates, and replaced with fresh DMEM/F12 complete medium every two days until the cell density reached 100% fusion degree with alternative use of maintenance medium and induction medium. After three to five cycles, we kept the MSCs in the maintenance medium for seven days, changing the medium every three days. The cells were cultured for two to three weeks. The degree of adipogenesis was evaluated by oil red O staining.
In osteogenic differentiation experiments, 3×103 cells/cm2 were seeded into culture plates, and cultured in DMEM/F12 medium. After 24 hours, the medium was removed, and 2 mL of osteogenic differentiation medium was added. The medium was replaced every two to three days, and maintained for two to three weeks. Alizarin red calcium nodules were used to evaluate the degree of osteogenic differentiation.
Lentivirus-mediated PGC-1α transfer to MSCs
The MSCs were seeded into 6-well plates at a density of 2×105 cells per well. One day after adherence, the MSCs were cultured in DMEM/F12 medium supplemented with 2% FBS and 8 μg/mL polybrene. These cells were infected with control or Lv-PGC-1α-2A-GFP (abbreviated as Lv-PGC-1α) at 10 MOI and 100 MOI, respectively. Virus-containing culture medium was changed with fresh DMEM/F12 medium containing 10% FBS after transfection (12 hours later). The transfection efficiency was detected by flow cytometry. The ideal MOI of 100 was chosen for the following experiments.
CCK-8 assay was used to detect the MSCs survival
MSCs were seeded in 96-well plates at the concentration of 1×104 per well, and treated with 5 μM rotenone for different time points. CCK-8 assay was used to determine the MSCs survival ability. At each specific time point, 10 μL/well CCK-8 solution was added and incubated at 37°C, 5% CO2 humidified incubator for two to four hours. The absorbance value at 450 nm was read using a microplate reader. Cell viability was calculated. The experiment was repeated three times.
Apoptosis rate was detected by Annexin V-APC/7-AAD double staining method
Under normal culture conditions, MSCs were seeded into culture plates and when they had grown to 80% degree of convergence, the medium was replaced with fresh 10% FBS DMEM/F12 medium in control group, and DMEM/F12 medium containing the appropriate concentration of drug in the experimental group. The cells were cultured under normal oxygen partial pressure, and the apoptosis rate was detected by flow cytometry.
In the experiment of apoptotic induction, the MSCs were cultured in medium containing a certain concentration of drugs and the glucose concentration was adjusted to 25 mmol/L, and the cells were transferred to a hypoxia incubator (3% O2). After 24-hour culture, the cells were digested by trypsin without EDTA, and then centrifuged at 2,000 rpm for five minutes in 4°C, the supernatant was discarded, and the cells were washed two times with cold PBS. Then the cells were re-suspended with 500 μL binding buffer, with 5 μL APC labeled Annexin V and 5 μl 7-AAD added, and after mixing, the cells were incubated at room temperature for 10 minutes; the apoptosis rate was detected by flow cytometry.
Quantitative real-time PCR assay
Total RNAs were extracted from MSCs by RNAiso plus agent (Invitrogen) according to manufacturer’s instructions. NanoDrop-2000 was used to determine the quality of total RNAs. A reverse transcription kit (TaKaRa) was used to synthesize the first strand of cDNA. The mRNA expression of PGC-1α, BCL-2, and Bax were detected by RT-PCR. β-actin served as an internal control.
Western blotting
When the adherent cells grew to 80% of the fusion degree, they were transferred to DMEM/F12 medium containing 3% O2, without FBS, and with 25 mmol/L glucose. After 24-hour culture, the culture medium was discarded, cell lysis solution was added, and the resulting liquid placed into a centrifuge tube, transferred to ice for 30 minutes, then centrifuged at 12,000 rpm for 10 minutes in 4°C. Subsequently, the supernatant was collected; and the protein concentration was determined by BCA kit. Then 10 μL samples of protein were taken for 10–12% denaturing SDS-PAGE electrophoresis, transferred to PVDF membrane for two hours at room temperature, followed by addition of the first antibody and then incubated overnight at 4°C. Then the PVDF membrane was washed with TBST five times, each time for 10 minutes. The second antibody was then added and incubated for one to two hours at room temperature, washed with TBST five times, each time for 10 minutes. The final incubation was with ELC reagent for one minute, after which the PVDF membrane was evaluated.
Statistical analysis
SPSS 16.0 statistic software was used for data analysis. All results are presented with average ± standard deviation. Analysis of variance (ANOVA) was used to compare differences between groups. A p value <0.05 was considered statistically significant.
Results
Mesenchymal stem cells (MSCs) were capable of self-renewal and differentiating into many cell lineages
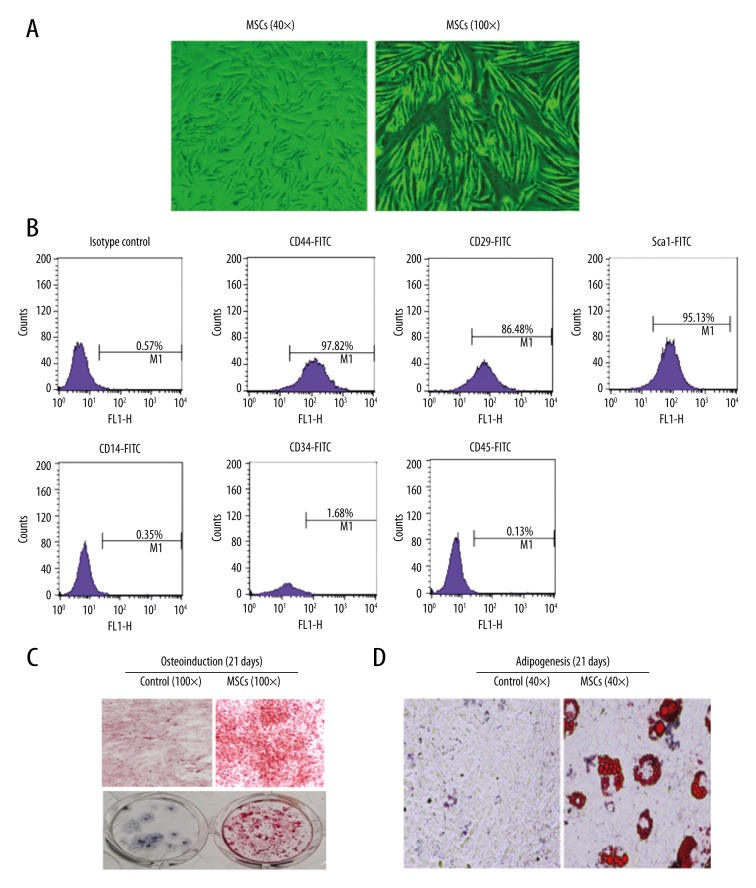
In our current study, we first verified the characteristics of MSCs isolated from C57BL/6 mice, including cell morphological characteristics, cell surface phenotype, and multiple differentiation abilities. Our data indicated that MSCs grew rapidly through the convergence degree. The cell growth ratio, cell morphology, and refractive index did not significantly change during six generations (Figure 1A). We further evaluated the expression rates of CD14, CD34, CD45, CD44, CD29, and Sca-1 surface markers on MSCs by flow cytometry. The results showed that the positive ratio of CD44, CD29, and Sca-1 on MSCs surface were 97.82%, 86.48%, and 95.13%, respectively. However, the positive ratios of CD14, CD34, and CD45 on MSCs surface were 0.35%, 1.68%, and 0.13%, respectively (Figure 1B). Altogether, our results demonstrated that the cells isolated from C57BL/6 mice were in accordance with the expression of MSCs surface markers, and had high purity. Next, we detected the multiple differentiation abilities of MSCs by osteogenic differentiation and adipogenesis assays. In the osteogenic differentiation experiment, MSCs were cultured for 21 days to form cell layer growth, and then red pigment staining was used. The results showed a large number of mineralized nodule particles with different sizes, which suggested that there was a mineralized matrix precipitation (Figure 1C). In the experiment of adipogenesis, oil red O staining showed that there were red and round fat droplets in the cytoplasm. This confirmed that the MSCs isolated from C57BL/6 mice had the ability to differentiate into fat (Figure 1D).
Figure 1.
MSCs were capable of self-renewal and differentiating into many cell lineages. (A) The sixth generation of MSCs cultured for 24 hours after subculture. (B) Expression ratio of CD44, CD14, CD34, CD29, CD45, and Sca-1 surface markers on MSCs detected by flow cytometry. (C) Identification of MSCs through osteoplastic differentiation. (D) Identification of MSCs through adipogenic differentiation.
The expression of PGC-1α was negatively related to MSCs apoptosis induced by rotenone
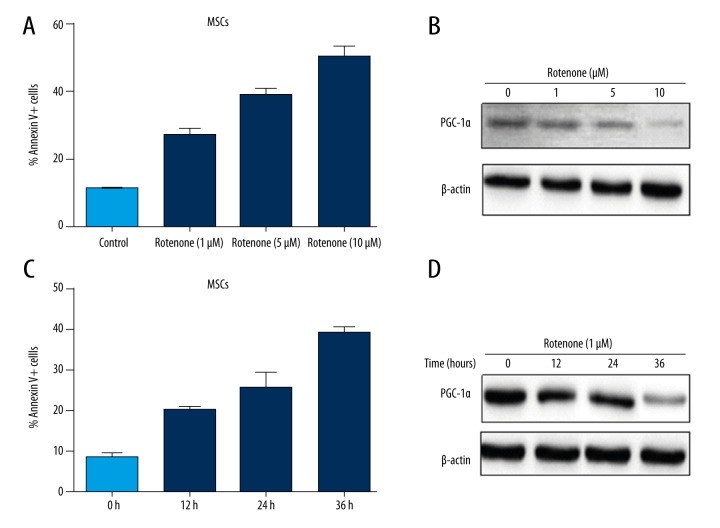
In order to prove whether PGC-1α plays a crucial role in the apoptosis of MSCs, we established a cell apoptosis model for further studies. Cultures of MSCs reached 70%–80% confluency and then were exposed to various concentrations of rotenone for 24 hours; the cell apoptotic rate was analyzed by flow cytometry. The data indicated that the MSCs apoptotic rates at 1 μM rotenone, 5 μM rotenone, and 10 μM rotenone was 27.82%, 42.11%, and 55.27%, respectively, which were an obvious increase compared with the control group and showed a dose-dependent effect (Figure 2A). We further detected PGC-1α expression in MSCs treated with different concentrations of rotenone for 24 hours. Our data showed that PGC-1α expression was significantly downregulated in the 10 μM rotenone treated group compared with the control group (Figure 2B). In order to further verify this phenomenon, we treated MSCs with 1 μM rotenone for 0 hours, 12 hours, 24 hours, and 36 hours, respectively, and evaluated the cell apoptotic rate. Our data also indicated that the apoptotic rate was gradually increased following the prolongation of treatment time (Figure 2C). Then, we further verified the PGC-1α expression, and obtained similar results (Figure 2D). Altogether, our results suggested that PGC-1α expression was negatively related to MSCs apoptosis.
Figure 2.
The expression of PGC-1α was negatively related to MSCs apoptosis induced by rotenone. MSCs were exposed to 0 μM, 1 μM, 5 μM and 10 μM rotenone for 24 hours. (A) The cell apoptosis rates were detected by flow cytometry. (B) PGC-1α protein expression was detected by Western blotting. MSCs were exposed to 1 μM for 0 hours, 12 hours, 24 hours, and 36 hours, respectively. (C) The cell apoptosis rates were detected at different time points. (D) PGC-1α protein expression was detected at different time points.
Over-expression of PGC-1α protected MSCs from apoptosis induced by rotenone
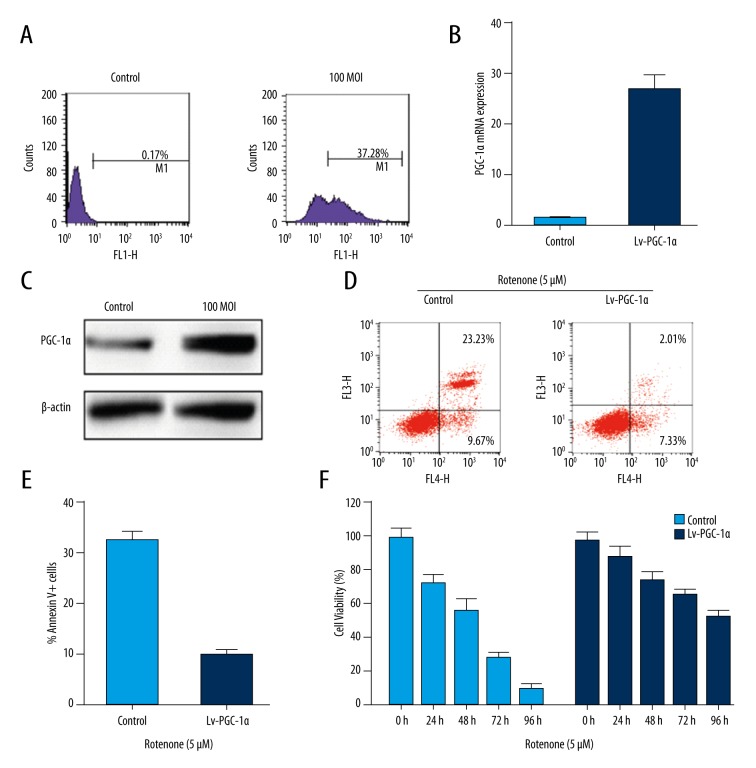
In order to investigate the role of PGC-1α in MSCs, we first evaluated the biological effects of PGC-1α over-expression on MSCs. We constructed a lentivirus vector Lv-PGC-1α to upregulated the expression of PGC-1α. The efficiency of infection was detected by flow cytometry. The data showed that transfection efficiency reached 37.28% at 100 MOI (Figure 3A). We sorted the GFP-positive cells from MSCs by flow cytometry and used the sorted cells for further experiments. The expression of PGC-1α was detected by RT-PCR and Western blot assays. Our results indicated that the expression of PGC-1α mRNA (Figure 3B) and protein (Figure 3C) were markedly upregulated in the Lv-PGC-1α group compared with the control group. We further evaluated whether upregulated PGC-1α played an anti-apoptosis role in MSCs isolated from C57BL/6 mice. We treated MSCs with 5 μM rotenone for 12 hours, then infected with Lv-PGC-1α at 100 MOI for 24 hours, and detected the cell apoptotic rate. Our data showed that the apoptotic rate of MSCs was obviously reduced after infected with Lv-PGC-1α compared with the control group (Figure 3D, 3E). Then we further evaluated the cell viability of MSCs by CCK-8 assay. The results indicated that the cell viability of MSCs was obviously increased in the PGC-1α over-expression group compared with the control group (Figure 3F). In other words, our results implied that upregulating PGC-1α could promote cell survival of MSCs by avoid inducing cell apoptosis.
Figure 3.
Over-expression of PGC-1α can protect MSCs from apoptosis induced by rotenone. (A) Transfection efficiency of MSCs by Lv-PGC-1α was detected by flow cytometry. (B, C) The expression of PGC-1α was detected by RT-PCR (B) and Western blotting (C) assays. (D, E) Cell apoptotic rates of MSCs treated with 5 μM rotenone for 12 hours, then infected Lv-PGC-1α at 100 MOI for 24 hours. (F) The cell viability of MSCs was detected by CCK-8 assay.
PGC-1α over-expression enhanced the expression of anti-apoptotic gene Bcl-2, and inhibited the expression pro-apoptotic gene Bax in MSCs
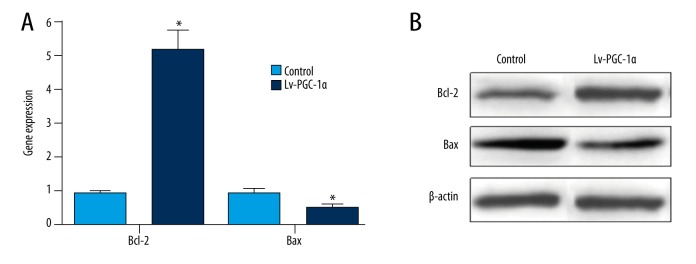
We first investigated the expression of anti-apoptotic genes Bcl-2 and Bcl-XL, and pro-apoptotic gene Bax by RT-PCR in MSCs with over-expression of PGC-1α compared with control cells. Our data indicated that Bcl-2 was significantly upregulated and Bax was significantly downregulated in MSCs with over-expression of PGC-1α (Figure 4A, p<0.05). Furthermore, we also found that the expression of Bcl-XL had no obvious difference in MSCs with over-expression of PGC-1α compared with control cells (data not shown). Moreover, we validated the protein expression of Bcl-2 and Bax by Western blotting. The data also showed that the expression trend of Bcl-2 and Bax proteins was consistent with mRNA (Figure 4B, p<0.05). Altogether, our results implied that MSCs with over-expression of PGC-1α possess stronger anti-apoptotic ability, at least in part because PGC-1α strengthened the expression of the anti-apoptotic gene Bcl-2, and inhibited the expression of the pro-apoptotic gene Bax.
Figure 4.
PGC-1α over-expression enhanced the expression of anti-apoptotic gene Bcl-2, and inhibited the expression pro-apoptotic gene Bax in MSCs. (A) The mRNA expression of Bcl-2 and Bax were detected by RT-PCR. (B) The protein expression of Bcl-2 and Bax was detected by Western blotting.
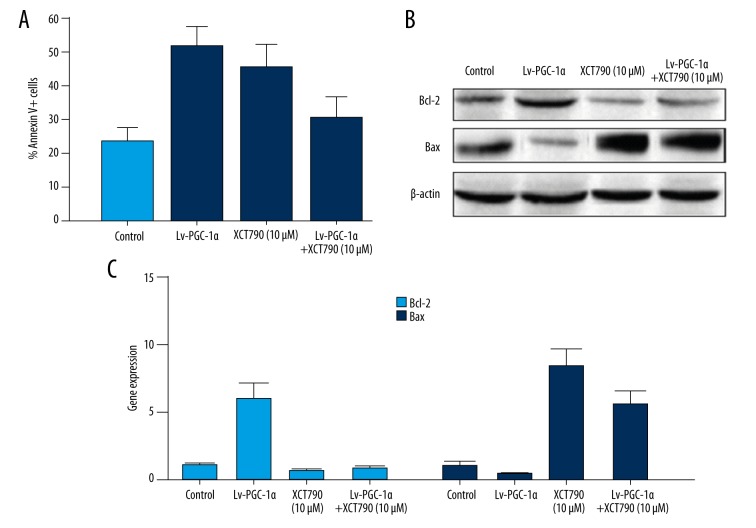
PGC-1α via PGC-1α/ERRα interaction to regulate the expression of Bcl-2 and promoted the survival of MSCs
Our previous studies demonstrated that PGC-1α could enhance the expression of Bcl-2, and obviously improve the survival of MSCs. However, it is not clear how PGC-1α is involved in the regulation of Bcl-2 expression. Estrogen-related receptor α (ERRα) is a nuclear receptor, and recent reports showed it was related to the expression of Bcl-2. Therefore, we hypothesized that PGC-1α via PGC-1α interacts with ERRα to regulate the expression of Bcl-2. We treated MSCs with XCT790 (ERRα inverse agonist) to block the role of ERRα so it could not interact with PGC-1α, and then detected the MSCs apoptotic rate. The data demonstrated that the apoptotic rate of MSCs was obviously reduced after infected with Lv-PGC-1α and significantly enhanced with treatment of 10 μM XCT790 compared with the control group. On the contrary, the apoptotic ratio was evidently increased when treated MSCs combined with upregulated PGC-1α and were exposed to XCT790 compared with over-expression of PGC-1α alone (Figure 5A). We further detected the protein and mRNA expression of Bcl-2 and Bax in different groups. Our results showed that Bcl-2 was obviously decreased and Bax was significantly increased in the combination group compared with the Lv-PGC-1α group (Figure 5B, 5C). Our data indicated that PGC-1α via PGC-1α/ERRα interacted to upregulate the expression of Bcl-2 and promote the survival of MSCs. Once blocked, the interaction between PGC-1α and ERRα, and the protective effect of PGC-1α on MSCs, will be attenuated.
Figure 5.
PGC-1α via PGC-1α/ERRα interacts to regulate the expression of Bcl-2. MSCs were treated by Lv-PGC-1α at 100 MOI, 10 μM XCT790, and combined Lv-PGC-1α and XCT790 for 24 hours, respectively. (A) The cell apoptosis rates were detected by flow cytometry. (B, C) The protein and mRNA expression of Bcl-2 and Bax were detected by Western blotting (B) and RT-PCR (C) in different groups.
Discussion
At present, the treatment strategies for diabetic foot problems include internal medicine, surgical blood flow reconstruction, and interventional therapy. Because diabetic patients are often of older age, and diabetes is often accompanied with cardiovascular disease, diabetic patients often cannot tolerate surgical bypass stimulation [16]. In addition, blood vessel lesions often harm the arteries and some patients may lack distal arterial outflow tract which prevents treatment with vascular bypass surgery and interventional therapy [17,18]. As a new therapy for blood vessel regeneration, stem cell transplantation has brought a new hope for patients with diabetic foot problems, and has become a hot research topic in recent years.
Autologous stem cell transplantation has been recognized in the treatment of ischemic disease. Bone marrow mesenchymal stem cells (MSCs) are a rich source, easy to obtain, and have been safe in vitro amplification techniques, so are widely used in vascular tissue engineering [19]. Gładysz et al. reported that MSCs play a therapeutic role in two ways after transplant to microvascular lesion. First, MSCs will differentiate into vascular cells, such as vascular smooth muscle cells and vascular endothelial cells, and directly generate new blood vessels. Second, MSCs will secrete many growth factors, such as vascular endothelial cell growth factor and basic fibroblast growth factor, and indirectly promote the formation of new blood vessels [20–22].
As a new treatment strategy for diabetic lower limb ischemia, the development of stem cell transplantation is ongoing, and still has many problems. One of the most important problems is a large number of cell apoptosis after stem cell transplantation that restricts its curative effect [23,24]. The survival rate of stem cells is very low after transplantation, which results in greatly weakened angiogenesis, which eventually seriously restricts the clinical application of stem cell transplantation [25]. Therefore, how to reduce the apoptosis of MSCs after transplantation is the primary problem of stem cell transplantation in the treatment of diabetic lower limb ischemia. Hourigan et al. reported that the mechanism of stem cell apoptosis was induced by B-cell lymphoma/leukemia-2 (Bcl-2) protein family and plays a central role in the regulation of mitochondrial cyt C release [26,27]. However, the Bcl-2 family protein role in regulation of apoptosis, especially in the hypoxic environment, still needs to be further studied.
In the current study, a mouse MSCs cell line that could stably up-express PGC-1α was established that could also significantly increase the expression of anti-apoptotic protein Bcl-2 and antagonize the apoptosis of MSCs after transplantation, which further proves that PGC-1α has obvious anti-apoptotic effects on MSCs.
We searched PGC-1α related information through the STRING database and combined the search result with reports related to PGC-1α as an auxiliary activated receptor, and concluded that PGC-1α may directly interacted with the following nuclear receptor or transcription factor in mouse MSCs: ERRα, ERRβ, PPARγ, PPARδ, Nr5al, and Foxo3a, and may eventually regulate Bcl-2 transcription [28,29]. Currently, only ERRα and PPARγ have confirmed expression in mouse MSCs nucleus, however, through our additional literature analysis and experimental verification, we conferred that ERRα probably was closely related to cell apoptosis. In our current study, we hypothesized that PGC-1α via PGC-1α interacts with ERRα to regulate the expression of Bcl-2. We thus treated MSCs with XCT790 (ERRα inverse agonist) to block the role of ERRα and to make it so that it could not interact with PGC-1α, and then we detected the MSCs apoptotic rate. Our data showed that the apoptotic ratio was evidently increased when treated MSCs combined upregulated PGC-1α and were exposed to XCT790, compared with the Lv-PGC-1α group. Our data further indicated that PGC-1α via PGC-1α/ERRα interacted to upregulate the expression of Bcl-2 and promote the survival of MSCs. Once the interaction between PGC-1α and ERRα is blocked, the protective effect of PGC-1α on MSCs will be attenuated.
Conclusions
The biological activity of ERRα is mainly regulated by the interaction between its expression level, the intracellular localization, and the auxiliary activation factors [30]. We have attested that PGC-lα/ERRα axis is a powerful signal pathway to regulate energy balance. In the future, we will further explore the composition and mechanism of the PGC-l/ERR pathway, and look for new ways to improve the ability of MSC transplantation to tolerate apoptosis.
Footnotes
Conflict of interests
All authors declare no conflict of interests in this article.
Source of support: This project is supported by the Chinese National Natural Science Foundation (Grant No. 81270870 and 81200614)
References
- 1.Nickerson DS. Rationale, science, and economics of surgical nerve decompression for diabetic neuropathy foot complications. Clin Podiatr Med Surg. 2016;33(2):267–82. doi: 10.1016/j.cpm.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Peter-Riesch B. The diabetic foot: The never-ending challenge. Endocr Dev. 2016;31:108–34. doi: 10.1159/000439409. [DOI] [PubMed] [Google Scholar]
- 3.Arosi I, Hiner G, Rajbhandari S. Pathogenesis and treatment of callus in the diabetic foot. Curr Diabetes Rev. 2016;12(3):179–83. doi: 10.2174/1573399811666150609160219. [DOI] [PubMed] [Google Scholar]
- 4.Şener LT, Albeniz I. Challenge of mesenchymal stem cells against diabetic foot ulcer. Curr Stem Cell Res Ther. 2015;10(6):530–34. doi: 10.2174/1574888x10666150519092931. [DOI] [PubMed] [Google Scholar]
- 5.Mamidi MK, Das AK, Zakaria Z, Bhonde R, et al. Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthritis Cartilage. 2016;24(8):1307–16. doi: 10.1016/j.joca.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Kim G, Eom YW, Baik SK, et al. Therapeutic effects of mesenchymal stem cells for patients with chronic liver diseases: Systematic review and meta-analysis. J Korean Med Sci. 2015;30(10):1405–15. doi: 10.3346/jkms.2015.30.10.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uccelli A, de Rosbo NK. The immunomodulatory function of mesenchymal stem cells: mode of action and pathways. Ann NY Acad Sci. 2015;1351:114–26. doi: 10.1111/nyas.12815. [DOI] [PubMed] [Google Scholar]
- 8.Ma K, Tan Z, Zhang C, Fu X. Mesenchymal stem cells for sweat gland regeneration after burns: From possibility to reality. Burns. 2016;42(3):492–99. doi: 10.1016/j.burns.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Sonkar R, Powell CA, Choudhury M. Benzyl butyl phthalate induces epigenetic stress to enhance adipogenesis in mesenchymal stem cells. Mol Cell Endocrinol. 2016;431:109–22. doi: 10.1016/j.mce.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 10.Taneja M, Tay KH, Dewan A, et al. Bare nitinol stent enabled recanalization of long-segment, chronic total occlusion of superficial femoral and adjacent proximal popliteal artery in diabetic patients presenting with critical limb ischemia. Cardiovasc Revasc Med. 2010;11(4):232–35. doi: 10.1016/j.carrev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Zhao GX, Pan H, Ouyang DY, He XH, et al. The critical molecular interconnections in regulating apoptosis and autophagy. Ann Med. 2015;47(4):305–15. doi: 10.3109/07853890.2015.1040831. [DOI] [PubMed] [Google Scholar]
- 12.Correia C, Lee SH, Meng XW, et al. Emerging understanding of Bcl-2 biology: Implications for neoplastic progression and treatment. Biochim Biophys Acta. 2015;1853(7):1658–71. doi: 10.1016/j.bbamcr.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.García-Sáez AJ. The secrets of the Bcl-2 family. Cell Death Differ. 2012;19(11):1733–40. doi: 10.1038/cdd.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gross A. BCL-2 family proteins as regulators of mitochondria metabolism. Biochim Biophys Acta. 2016;1857(8):1243–46. doi: 10.1016/j.bbabio.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Banse HE, Frank N, Kwong GP, McFarlane D. Relationship of oxidative stress in skeletal muscle with obesity and obesity-associated hyperinsulinemia in horses. Can J Vet Res. 2015;79(4):329–38. [PMC free article] [PubMed] [Google Scholar]
- 16.Pei E, Li J, Lu C, et al. Effects of lipids and lipoproteins on diabetic foot in people with type 2 diabetes mellitus: A meta-analysis. J Diabetes Complications. 2014;28(4):559–64. doi: 10.1016/j.jdiacomp.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Eggert JV, Worth ER, Van Gils CC. Cost and mortality data of a regional limb salvage and hyperbaric medicine program for Wagner Grade 3 or 4 diabetic foot ulcers. Undersea Hyperb Med. 2016;43(1):1–8. [PubMed] [Google Scholar]
- 18.Wang G, Tian J, Zhu LY, et al. Changes in bacterial profiles and antibiotic sensitivity before and after wound bed preparation for diabetic foot ulcers. Int J Low Extrem Wounds. 2015;14(2):160–67. doi: 10.1177/1534734615574940. [DOI] [PubMed] [Google Scholar]
- 19.Wildes TM, Cashen A. High dose therapy and autologous hematopoietic stem cell transplantation in septuagenarians with non-Hodgkin lymphoma: Feasible, but for which patients? J Geriatr Oncol. 2015;6(5):344–45. doi: 10.1016/j.jgo.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gładysz D, Hozyasz KK. Stem cell regenerative therapy in alveolar cleft reconstruction. Arch Oral Biol. 2015;60(10):1517–32. doi: 10.1016/j.archoralbio.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi C, Nishi K, Minami-Hori M, et al. Multiple poromas following combination chemotherapy and autologous peripheral blood stem cell transplantation. J Dermatol. 2015;42(4):430–32. doi: 10.1111/1346-8138.12794. [DOI] [PubMed] [Google Scholar]
- 22.Lin PC, Chiou TW, Lin ZS, et al. A proposed novel stem cell therapy protocol for liver cirrhosis. Cell Transplant. 2015;24(3):533–40. doi: 10.3727/096368915X687228. [DOI] [PubMed] [Google Scholar]
- 23.Bai L, Xia W, Wong K, et al. Factors predicting haematopoietic recovery in patients undergoing autologous transplantation: 11-year experience from a single centre. Ann Hematol. 2014;93(10):1655–64. doi: 10.1007/s00277-014-2112-2. [DOI] [PubMed] [Google Scholar]
- 24.Munsie M, Hyun I. A question of ethics: Selling autologous stem cell therapies flaunts professional standards. Stem Cell Res. 2014;13(3 Pt B):647–53. doi: 10.1016/j.scr.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Jarocha D, Milczarek O, Kawecki Z, et al. Preliminary study of autologous bone marrow nucleated cells transplantation in children with spinal cord injury. Stem Cells Transl Med. 2014;3(3):395–404. doi: 10.5966/sctm.2013-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hourigan CS, McCarthy P, de Lima M. Reprint of: Back to the future! The evolving role of maintenance therapy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20(2 Suppl):S8–S17. doi: 10.1016/j.bbmt.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Gerli MF, Maffioletti SM, Millet Q, Tedesco FS. Transplantation of induced pluripotent stem cell-derived mesoangioblast-like myogenic progenitors in mouse models of muscle regeneration. J Vis Exp. 2014;83:e50532. doi: 10.3791/50532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang H, Yang R, Liu H, et al. Synergism between PGC-1α and estrogen in the survival of endometrial cancer cells via the mitochondrial pathway. Onco Targets Ther. 2016;9:3963–73. doi: 10.2147/OTT.S103482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostojic SM. Exercise-induced mitochondrial dysfunction: A myth or reality? Clin Sci (Lond) 2016;130(16):1407–16. doi: 10.1042/CS20160200. [DOI] [PubMed] [Google Scholar]
- 30.Salatino S, Kupr B, Baresic M, et al. The genomic context and corecruitment of SP1 affect ERRα coactivation by PGC-1α in muscle cells. Mol Endocrinol. 2016;30(7):809–25. doi: 10.1210/me.2016-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]