Abstract
Background
It has been reported that trifluoperazine (TFP) inhibits proliferation of cancer cells, however, the effects of TFP in renal proliferation diseases are still unclear. This study examined the effects of TFP on proliferation of human renal mesangial cells and analyzed the underlying mechanisms.
Material/Methods
Cell proliferation in vivo was determined by HE staining, immunohistochemistry of proliferating cell nuclear antigen (PCNA), and Western blot analysis (Ki-67 and PCNA). Effects of different TFP concentrations and treatment duration on cell proliferation and cell cycle were analyzed using the MTT assay and flow cytometry. Expression of G0/G1 phase cell cycle-related proteins and TFP-induced MAPK and PI3K/AKT signaling pathways was estimated with Western blot analysis.
Results
Our findings suggest that TFP inhibits cell proliferation in a dose- and time-dependent manner and decreased PCNA and Ki-67 levels in lupus MRL/lpr mice. TFP arrested the cell cycle in the G0/G1 phase, down-regulating cyclin D1, CDK2, and CDK4, and up-regulating p21 expression in a dose-dependent manner. In addition, TFP inhibited p-AKT and p-JNK, possibly by suppressing the activation of PI3K/AKT and JNK/MAPK signaling pathways. TFP treatment remarkably reduced the levels of serum creatinine (Cr) in lupus mice.
Conclusions
TFP exhibits inhibitory activity against mesangial cells in vivo and in vitro, which is associated with G1 cell cycle arrest by inactivation of PI3K/AKT and JNK/MAPK signaling pathways. These results suggest the potential of TFP in treatment of mesangial proliferative diseases.
MeSH Keywords: Cell Cycle, Mesangial Cells, Trifluoperazine
Background
Mesangial cells (MC) are specialized cells that form the glomerular mesangium and, combined with the mesangial matrix, shape the vascular pole of the glomerulus. Mesangial cells play an essential role in many aspects, for example, formation of capillary loops during development, contraction to regulate capillary flow, and removal of macromolecules [1–3], but the severe proliferation of MC causes functional damage of the kidneys.
Mesangial proliferative glomerulonephritis (MsPGN) is characterized by MC proliferation and various degrees of extracellular matrix (ECM) thickening [4,5]. Immunologically, MsPGN can be an IgA- or non-IgA nephropathy. Inhibition of MC proliferation was shown to slow the progression of MsPGN [6,7].
MRL/lpr lupus mice are a well-known pathological model of mesangial cell proliferative nephropathy [8–10]. Due to mutations of the lpr gene, MRL/lpr mice do not produce functional Fas receptor, which allows autoreactive T cells to escape negative selection in the thymus and be released into the peripheral organs, causing spontaneous lupus nephritis (LN) in animals at the age of approximately 24 weeks. Renal pathology of LN involves MC proliferation and thickening of the mesangial matrix [11]. As high concentrations of fetal bovine serum (FBS) stimulate abnormal proliferation of mesangial cells in vitro, mesangial cells stimulated by 20% FBS were used in this study [12].
The role of cell cycle regulation in MsPGN pathogenesis is widely recognized in cell and in vitro studies. In cultured human MC, expression of cyclin-dependent kinases 2 and 4 (CDK2 and CDK4) and cyclins A, D1, and E was up-regulated by stimulation with different mitogens (6–8), whereas opposing p27Kip1 and p21Waf1 were down-regulated [13–15]. Ke et al. [16] reported that morin inhibits MC proliferation and ECM accumulation in vivo in rats, by promoting the activation of p27Kip1 and p21Waf1 and thus has potential use in the treatment of MC proliferative nephropathy. Tian et al. [17] reported that early application of low-dose rapamycin, an mTOR inhibitor, slows the progression of MsPGN and protects renal function by arresting the cell cycle in rats. These data suggest that modulating cell cycle regulation in glomerular MC may slow the progression of MsPGN.
Trifluoperazine (TFP) is a calmodulin inhibitor and a classic anxiolytic and antipsychotic drug. Calmodulin is involved in cellular proliferation, inflammation, neurodegeneration, and other pathological processes. TFP was shown to suppress proliferation of breast cancer, fibrosarcoma HT1080, leukemia, and human A549 lung adenocarcinoma cells, by regulating different signaling pathways [18–22]. However, the role of TFP in MC is unclear.
The present study was planned to investigate the effects of TFP on cell proliferation and cell cycle progression in human mesangial cell line in vivo and in vitro.
Material and Methods
Animals
Young (6–7-week-old) female C57BL/6 mice weighing 20±5 g were purchased from the Experimental Animal Center of Shanxi Medical University (Taiyuan, China). Female MRL/lpr LN mice were purchased from the Model Animal Institute of Nanjing University (induced from Jackson Laboratories, USA). The mice were housed under controlled environmental conditions (temperature 22°C, 12-h light-dark cycle), given free access to water, and fed a standard laboratory diet. The animals were active and had glossy hair before the start of the experiments. The study was conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee of Shanxi Medical University. Twenty-four female mice were randomly divided into 3 groups: control group, LN group, and LN+TFP group. Mice in the LN+TFP group were intraperitoneally injected with TFP (20 mg/kg·d, 12 weeks, Sigma, St. Louis, MO, USA), whereas the LN group received saline for 12 weeks. The animals were sacrificed 12 h after the last injection. Serum was collected by centrifuging at 5000 rpm for 15 min at 4°C, and stored at –20°C for determining CRE levels. Renal tissues were obtained and kept in 10% neutral-buffered formalin and embedded in paraffin for histopathological and immunohistochemistry (IHC) analysis. Additional renal samples were immediately frozen in liquid nitrogen and stored at −80°C until analysis.
PCNA immunohistochemistry
Renal tissues were fixed in formalin and embedded with paraffin. Tissue sections were deparaffinized in xylene. Unspecific staining was blocked with 1.5% standard goat serum for 20 min before incubation with 1: 100 diluted primary PCNA antibody overnight at 4°C. Sections incubated with PBS were used as negative control. The samples were incubated with a biotinylated secondary antibody for 1 h at room temperature. Glomeruli of mice (10 per mouse) were blindly assessed at each time point using high-power light microscopy (×400). PCNA relative density was calculated as the ratio of PCNA-positive cells to total glomerular cells.
Cell culture and treatments
The human mesangial cell line T-SV40 was donated by Dr Li Xuewang at Union Medical College Hospital (Beijing, China) and grown in RPMI-1640 medium (HyClone, Logan, UT, USA) supplemented with 10% FBS (HyClone) at 37°C with 5% CO2. Approximately 70%-confluent cells were incubated in serum-free medium for 24 h and treated with 10% and 20% FBS as control and model groups, respectively. Different concentrations of TFP were added to the corresponding groups.
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay
Cell proliferation was analyzed using the MTT assay. The cells were grown in 96-well plates at the density of 106 cells/well and treated with 10% or 20% FBS and different concentrations of TFP (0, 5, 10, 20, and 30 μmol/L) for 24 h. Following treatment, the cells were incubated with 15 μL MTT (0.5%, Sigma) for 3 h at 37°C (n=6 per group). The medium was removed and 150 μL of dimethyl sulfoxide (Sigma) was added to each sample. A microplate reader (Bio-Rad Model 550, Hercules, CA, USA) was used to determine absorbance at 560 nm.
Flow cytometry analysis
Effects of TFP on the cell cycle were analyzed using flow cytometry. The cells (6×105 cells/well) were seeded in 6-well plates and cultured without FBS for 24 h to achieve cell synchronization. Next, the cells were treated with 10% or 20% FBS and TFP (0, 5, 10, and 20 μmol/L) for 24 h. After washing, the cells were fixed in 75% at 4°C overnight. The fixed cells were collected and stained with propidium iodide (50 μg/mL; Sigma) containing RNase (50 μg/mL). An FC500 flow cytometer (Coulter, Beckman, Palo Alto, CA, USA) was used to determine the proportion of cells in different phases of the cell cycle (G1, S, and G2/M).
Western blot analysis
The cells were lysed in radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime Biotech, Jiangsu, China), and centrifuged at 12000× g for 45 min at 4°C. After harvesting the supernatant, protein concentration was determined using the BCA assay kit KeyGEN (Beyotime Biotech). Samples containing 60 μg of protein were resolved on 12% SDS-PAGE electrophoresis and transferred to nitrocellulose membranes (Whatman International Ltd., Germany). After blocking for 1 h, the membranes were incubated with the following primary antibodies overnight at 4°C: Ki-67 (H-300), PCNA (FL-261), cyclin E (M20), cyclin D1 (H-295), p27 (C-19), p21 (C-19), CDK2 (D-12), CDK4 (C-22), CDK6 (C-21), p-ERK 1/2 (E-4), ERK 1/2 (H-72), p-JNK (G-7), JNK (D-2), p-AKT/Thr308 (p-AKT), AKT, and β-actin (C4): dilution 1: 200 (Santa Cruz Biotechnology) antibody. After being washed 3 times with TBST buffer, the membranes were incubated with corresponding horseradish peroxidase-conjugated secondary antibodies for 1.5 h at room temperature and analyzed using the Quantity One analysis system (Bio-Rad, Hercules, CA, USA).
Measurement of renal function
Creatinine (Cr), a key renal function marker, was determined with commercially available kits according to the manufacturer’s instructions (Shanghai Elisa Biotech Co., Ltd.). The observation absorbance of Cr was read at 450 nm.
Statistical analyses
Statistical analyses were performed using SPSS 19.0 (IBM, Armonk, NY, USA). The values are expressed as the means ±SD. The differences between the 2 groups were determined by the two-tailed Student’s t-test. A p value <0.05 was considered statistically significant.
Results
TFP inhibits cellular proliferation in glomeruli of LN mice
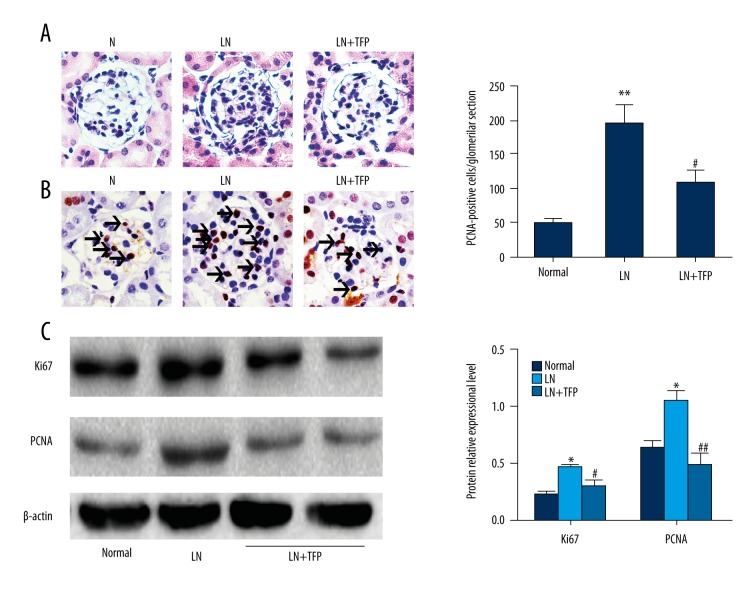
Hematoxylin and eosin (HE) staining indicated diffuse proliferation of mesangial cells and matrix in the glomeruli of LN mice, compared to control, which decreased after 3-month treatment with TFP (Figure 1A). Proliferating cell nuclear antigen (PCNA) immunohistochemistry confirmed these findings (Figure 1B). In addition, Western blotting showed that TFP significantly decreased the expression of Ki-67 and PCNA in LN mice (Figure 1C).
Figure 1.
TFP inhibits cellular proliferation in glomeruli of LN mice. TFP inhibited cellular proliferation in LN mice. (A) HE staining (400× magnification. Scale bar, 50 μm); (B) PCNA immunohistochemistry (400× magnification. Scale bar, 50 μm); (C) Expression of PCNA and Ki-67 was analyzed with Western blotting. (n=6; * p<0.05 vs. N, ** p<0.01 vs. N, # p<0.05 vs. LN, ## p<0.01 vs. LN; N – control; LN – lupus nephritis groups; LN+TFP – TFP-treated lupus nephritis groups.)
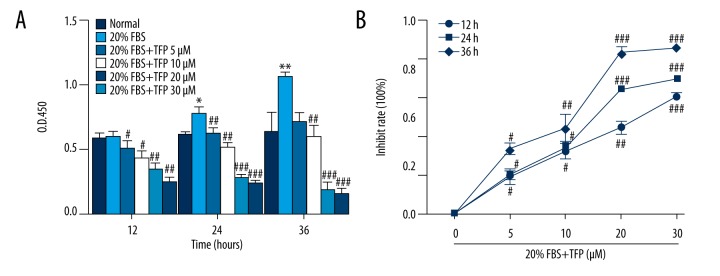
TFP reduces MC proliferation in vitro
The MTT assay showed time- and dose-dependent inhibition of MC proliferation by TFP (Figure 2A). Meanwhile, the inhibitive rate of MC was correspondingly added the increase of the concentration of TFP (Figure 2B).
Figure 2.
TFP reduces MC proliferation in vitro. TFP reduced MC proliferation. Quiescent MCs were treated with 10 and 20% FBS in the absence or presence of TFP (5, 10, 15, 20, and 30 μmol/L). Cell viability was assessed using the MTT assay after 12, 24, and 36 h. Data are presented as means ±SD values of 3 independent experiments. (* p<0.05 vs. control, ** p<0.01 vs. control, # p<0.05 vs. 20% FBS group, ## p<0.01 vs. 20% FBS group, ### p<0.001 vs. 20% FBS group).
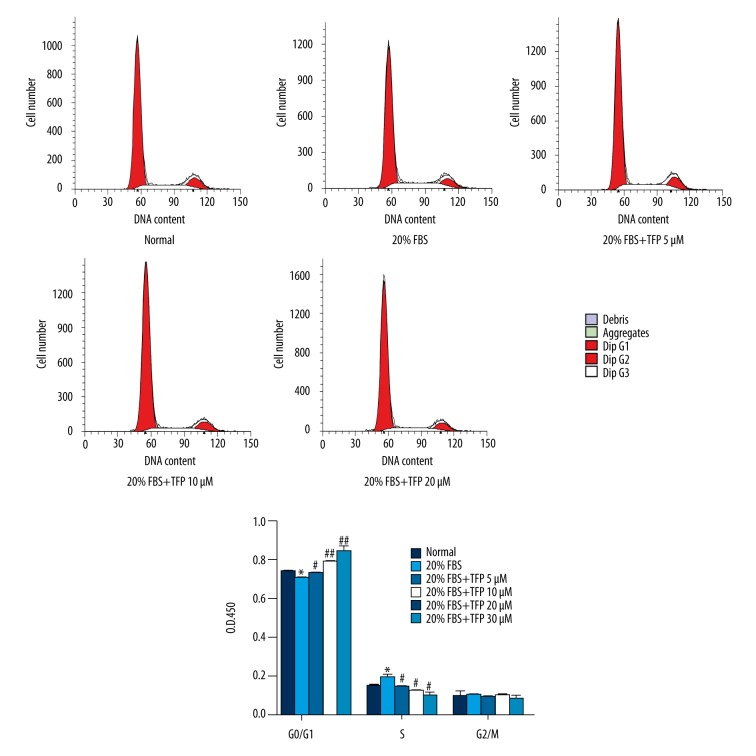
TFP arrests the MC cycle in the G1 phase
After 24-h treatment with TFP in 20% FBS, the number of MCs in G0/G1 and S phases was significantly increased and decreased, respectively, compared to the 20% FBS group. The effect was dependent on the dose of TFP (Figure 3).
Figure 3.
TFP arrests the MC cycle in the G1 phase. TFP-induced G0/G1 phase cell cycle arrest of mesangial cells. Data are presented as means ±SD values of 3 independent experiments. (* p<0.05 vs. control, # p<0.05 vs. 20% FBS group, ## p<0.01 vs. 20% FBS group).
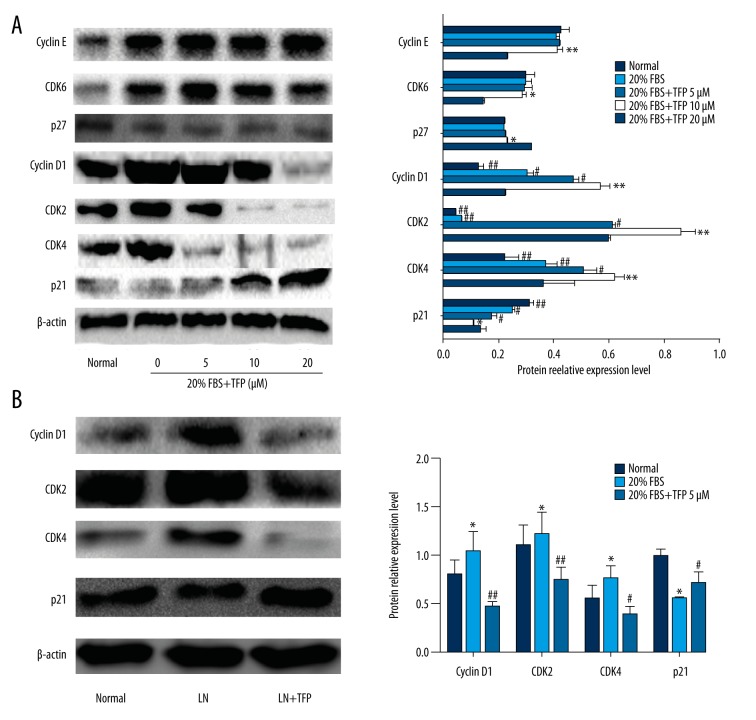
Effects of TFP on expression of cell cycle-related proteins
Western blot analysis was used to confirm the effects of TFP on expression of cell cycle-related proteins. Compared to control (10% FBS), high concentration (20%) of FBS markedly increased the expression of cyclin D1, CDK2, CDK4, and CDK6 and decreased the expression of p21 and p27. TFP (5, 10, and 20 μM) pretreatment decreased the levels of cyclin D1, CDK2, and CDK4 and increased p21 expression, in a dose-dependent manner (Figure 4A). CDK6, cyclin E, and p27 expression was not affected by TFP. These results were further validated by Western blot analysis in LN mice (Figure 4B).
Figure 4.
Effects of TFP on expression of cell cycle-related proteins. Effects of TFP on expression of G0/G1 phase cell cycle-related proteins in mesangial cells. (A) Expression of cell cycle-related proteins in MCs was determined by Western blot analysis. Data are expressed as means ± SD for 3 experiments per group. (* p<0.05 vs. control, ** p<0.05 vs. control, # p<0.05 vs. 20% FBS group, ## p<0.01 vs. 20% FBS group, ### p<0.001 vs. 20% FBS group); (B) Western blot analysis of cell cycle-related proteins in LN mice. (n=6/group; * p<0.05 vs. N, ** p<0.01 vs. N, # p<0.05 vs. LN, ## p<0.01 vs. LN; N – control; LN – lupus nephritis groups; LN+TFP – TFP-treated lupus nephritis groups).
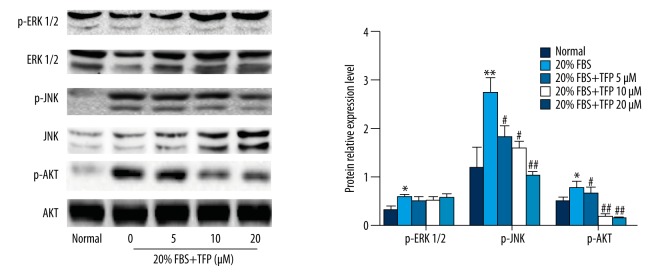
TFP suppressed p-AKT and p-JNK without affecting p-ERK1/2
Western blot analysis showed that 20% FBS activated extracellular signal-regulated kinase (ERK)-MAPK, c-Jun N-terminal kinase (JNK)-MAPK, and PI3K/AKT signaling pathways, compared to control (10% FBS). TFP pretreatment significantly and dose-dependently decreased p-JNK and p-AKT levels in 20% FBS-cultured MCs, with no observable change in p-ERK levels, compared to the 20% FBS group (Figure 5). These results suggest that TFP inhibits JNK-MAPK and PI3K/AKT signaling pathways without affecting ERK-MAPK.
Figure 5.
2.5 TFP suppressed p-AKT and p-JNK without affecting p-ERK1/2. TFP-mediated inhibition of AKT and JNK phosphorylation. Protein levels were determined by Western blot. Data are expressed as means ±SD for 3 experiments per group. (* p<0.05 vs. control, ** p<0.01 vs. control, # p<0.05 vs. 20% FBS group, ## p<0.01 vs. 20% FBS group, ### p<0.001 vs. 20% FBS group).
The effect of TFP in renal function in LN mice
The effect of TFP on renal function in LN mice was investigated. The level of serum Cr was markedly increased in the LN group compared to the control groups. However, serum Cr was significantly decreased in TFP-treated LN mice (Figure 6). The data demonstrate that TFP improved renal function in LN mice.
Figure 6.
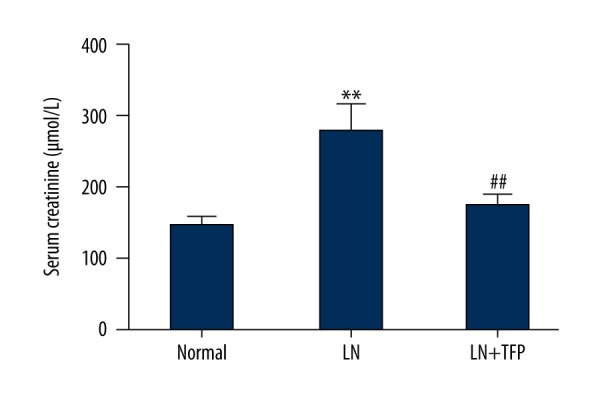
The effect of TFP in renal function in LN mice. TFP reduced level of serum Cr in serum of LN mice. (n=6; * p<0.05 vs. N, ** p<0.01 vs. N, # p<0.05 vs. LN, ## p<0.01 vs. LN; N – control; LN – lupus nephritis groups; LN+TFP – TFP-treated lupus nephritis groups).
Discussion
MC proliferation is a key feature of glomerular diseases, including IgA nephropathy, lupus nephritis, and diabetic nephropathy. In experimental animal models of nephritis, MCs are used as target cells of immune injury because stimulation-induced MC proliferation accelerates kidney damage and promotes glomerulosclerosis because of the release of inflammatory factors and ECM components. Therefore, inhibition of MC proliferation has become an important treatment approach for proliferative glomerular diseases. As TFP was recently shown to inhibit proliferation of cancer cells, this study focused on the effects of TFP on MC proliferation, using MC cells in 20% FBS and LN mice.
In LN mice, HE staining indicated that 12-week daily TFP treatment attenuated MC proliferation. Western blot analysis confirmed the inhibition of cell proliferation markers PCNA and Ki-67, compared to untreated LN mice. These results indicate that TFP suppresses MC proliferation in vivo.
In vitro, TFP inhibited MC proliferation, as shown by the MTT assay. TFP effects on the cell cycle were analyzed using flow cytometry. In TFP-treated cells, prolonged G0/G1 phase and shortened S phase were observed, indicating that TFP extended the G1 phase and inhibited cell proliferation. These results are consistent with previous studies on TFP in other tissues and proliferative diseases [23,24].
The 4 stages of the cell cycle – G0/G1, S, G2, and M – are regulated by growth factors, oncogenic stimulation, or regulatory units such as cyclin/CDK complexes [25,26]. As flow cytometry indicated that TFP mainly affected MCs in the G0/G1 phase, we focused on G0/G1 regulators. Cyclins D and E, together with CDK2, CDK4, or CDK6, promote progression of cells from the G1 to the G2 phase, whereas p21 and p27 have an opposite effect. Our results showed that upon stimulation with 20% FBS, expression of cyclins D and E, and CDKs 2, 4, or 6 in MCs was increased, whereas expression of p27 and p21 was significantly inhibited, in agreement with previous work [12]. TFP treatment decreased cyclin D1, CDK2, and CDK4 expression, whereas p21 expression was increased in a dose-dependent manner. No changes in the protein levels of cyclin E, CDK6, and p27 were observed. In vivo, Western blotting indicated that cyclin D1, CDK2, and CDK4 expression levels were up-regulated in LN mice, whereas p21 expression was decreased, compared to control. For cyclin D1, CDK2, and p21, the trend was reversed after 12 weeks of TFP treatment. These results indicate that, in proliferating MCs, TFP down-regulates the expression of cyclin D1, CDK2, and CDK4 and up-regulates p21 expression, in a dose-dependent manner.
MAPK and PI3K/AKT signaling pathways participate in regulation of cell apoptosis and proliferation. The MAPK family of serine/threonine kinases consists of 3 subtypes: ERK, JNK, and p38MAPK. ERK is activated by growth factors and is associated with differentiation and cell death. In contrast, JNK responds to environmental stress, pro-cytokines, and mitogens [27,28]. ERK and JNK are activated during abnormal proliferation of mesangial cells, in vivo and in vitro, affecting cell cycle progression [17,29]. AKT is a promoter of cell proliferation and survival and is overexpressed in tumors, suggesting that activation of AKT may promote abnormal proliferation of cells [30,31]. Previous studies suggest that inactivation of AKT decreases the expression of cyclin D1, CDK2, and CDK4, inhibiting G1/S cell cycle progression [26,32]. In this study, ERK, JNK, and AKT were activated by 20% FBS. TFP treatment inactivated AKT and JNK in MCs, consistent with TFP-mediated suppression of cyclin D1 and CDK2 expression and up-regulation of p21 expression. ERK levels were not affected by TFP treatment. Therefore, we conclude that TFP arrests cell cycle progression in the G0/G1 phase by down-regulating phosphorylated AKT and JNK, and subsequently increasing p21 levels and decreasing cyclin D1, CDK2, and CDK4 expression.
Serum Cr (a blood measurement) is an important indicator of renal health because it is an easily measured byproduct of muscle metabolism that is excreted unchanged by the kidneys. A rise of creatinine in blood always predicts marked damage to functioning nephrons [33]. In our study, the level of serum Cr was associated with markedly increased injury in the LN mice group; however, there was an obviously decrease after TFP treatment TFP (20 mg/kg·d, 12 weeks). These results indicate that TFP slows LN progression in the MRL/lpr mice.
Conclusions
In summary, we demonstrated that TFP exhibits anti-proliferative effects on mesangial cells in vivo and in vitro. TFP inhibited JNK and AKT-mediated signaling pathways, suppressing the expression of cyclin D1, CDK2, and CDK4 complexes, and increasing p21 expression, leading to G0/G1 cell cycle arrest (Figure 7). These findings indicate that TFP is a promising anti-proliferative agent for the treatment of mesangial proliferative glomerulonephritis and related diseases.
Figure 7.
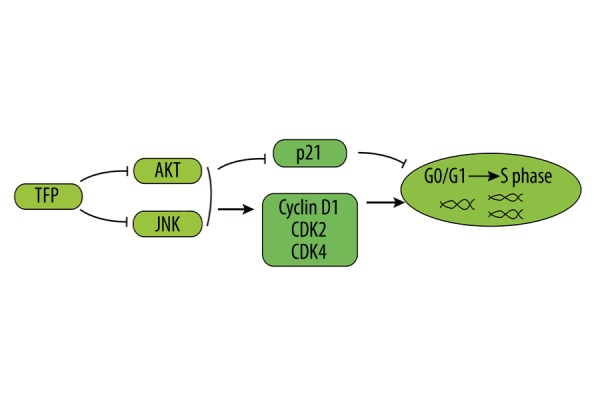
Proposed mechanisms of TFP effects on mesangial cell proliferation.
Acknowledgments
The authors would like to thank Dr Xuewang Li for providing the human mesangial cells.
Abbreviations
- MsPGN
mesangial proliferative glomerulonephritis
- TFP
trifluoperazine
- PCNA
proliferating cell nuclear antigen
- Cr
creatinine
Footnotes
Conflicts of Interest
The authors declare no conflict of interest.
Source of support: This study was supported by the National Natural Science Foundation of China (no. 81570626 and 81450033)
References
- 1.Takano K, Kawasaki Y, Imaizumi T, et al. Development of glomerular endothelial cells, podocytes and mesangial cells in the human fetus and infant. Tohoku J Exp Med. 2007;212(1):81–90. doi: 10.1620/tjem.212.81. [DOI] [PubMed] [Google Scholar]
- 2.Schlondorff D, Banas B. The mesangial cell revisited: No cell is an island. J Am Soc Nephrol. 2009;20:1179–87. doi: 10.1681/ASN.2008050549. [DOI] [PubMed] [Google Scholar]
- 3.Scindia YM, Deshmukh US, Bagavant H. Mesangial pathology in glomerular disease: Targets for therapeutic intervention. Adv Drug Deliv Rev. 2010;62:1337–43. doi: 10.1016/j.addr.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cove-Smith A, Hendry BM. The regulation of mesangial cell proliferation. Nephron Exp Nephrol. 2008;108:e74–79. doi: 10.1159/000127359. [DOI] [PubMed] [Google Scholar]
- 5.Kute VB, Vanikar AV, Shah PR, et al. Mesangial proliferative glomerulonephritis with acute tubule interstitial nephritis leading to acute kidney injury in influenza a (h1n1) infection. Indian J Nephrol. 2014;24:114–16. doi: 10.4103/0971-4065.127902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou X, Workeneh B, Hu Z, Li R. Effect of immunosuppression on the human mesangial cell cycle. Mol Med Rep. 2015;11:910–16. doi: 10.3892/mmr.2014.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kriz W, Hahnel B, Hosser H, et al. Pathways to recovery and loss of nephrons in anti-thy-1 nephritis. J Am Soc Nephrol. 2003;14:1904–26. doi: 10.1097/01.asn.0000070073.79690.57. [DOI] [PubMed] [Google Scholar]
- 8.Lamoureux JL, Watson LC, Cherrier M, et al. Reduced receptor editing in lupus-prone mrl/lpr mice. J Exp Med. 2007;204:2853–64. doi: 10.1084/jem.20071268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reilly CM, Mishra N, Miller JM, et al. Modulation of renal disease in mrl/lpr mice by suberoylanilide hydroxamic acid. J Immunol. 2004;173:4171–78. doi: 10.4049/jimmunol.173.6.4171. [DOI] [PubMed] [Google Scholar]
- 10.Peairs A, Dai R, Gan L, et al. Epigallocatechin-3-gallate (egcg) attenuates inflammation in mrl/lpr mouse mesangial cells. Cell Mol Immunol. 2010;7:123–32. doi: 10.1038/cmi.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumoto K, Yoshikai Y, Asano T, et al. Defect in negative selection in lpr donor-derived t cells differentiating in non-lpr host thymus. J Exp Med. 1991;173:127–36. doi: 10.1084/jem.173.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen D, Li Y, Mei Y, et al. Mir-34a regulates mesangial cell proliferation via the pdgfr-beta/ras-mapk signaling pathway. Cell Mol Life Sci. 2014;71:4027–42. doi: 10.1007/s00018-014-1599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shankland SJ, Pippin J, Flanagan M, et al. Mesangial cell proliferation mediated by pdgf and bfgf is determined by levels of the cyclin kinase inhibitor p27kip1. Kidney Int. 1997;51:1088–99. doi: 10.1038/ki.1997.151. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Y, Cheng DW, Levi E, Singh LP. Igf-1 increases laminin, cyclin d1, and p21cip1 expression in glomerular mesangial cells: An investigation of the intracellular signaling pathway and cell-cycle progression. J Cell Biochem. 2006;98:208–20. doi: 10.1002/jcb.20771. [DOI] [PubMed] [Google Scholar]
- 15.Qiu LQ, Sinniah R, Hsu SI. Role of differential and cell type-specific expression of cell cycle regulatory proteins in mediating progressive glomerular injury in human iga nephropathy. Lab Invest. 2004;84:1112–25. doi: 10.1038/labinvest.3700144. [DOI] [PubMed] [Google Scholar]
- 16.Ke YQ, Liu C, Hao JB, et al. Morin inhibits cell proliferation and fibronectin accumulation in rat glomerular mesangial cells cultured under high glucose condition. Biomed Pharmacother. 2016;84:622–27. doi: 10.1016/j.biopha.2016.09.088. [DOI] [PubMed] [Google Scholar]
- 17.Tian J, Wang Y, Liu X, et al. Rapamycin ameliorates iga nephropathy via cell cycle-dependent mechanisms. Exp Biol Med. 2015;240:936–45. doi: 10.1177/1535370214555666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gulino A, Barrera G, Vacca A, et al. Calmodulin antagonism and growth-inhibiting activity of triphenylethylene antiestrogens in mcf-7 human breast cancer cells. Cancer Res. 1986;46:6274–78. [PubMed] [Google Scholar]
- 19.Shin SY, Kim SY, Kim JH, et al. Induction of early growth response-1 gene expression by calmodulin antagonist trifluoperazine through the activation of elk-1 in human fibrosarcoma ht1080 cells. J Biol Chem. 2001;276:7797–805. doi: 10.1074/jbc.M009465200. [DOI] [PubMed] [Google Scholar]
- 20.Hait WN, Grais L, Benz C, Cadman EC. Inhibition of growth of leukemic cells by inhibitors of calmodulin: Phenothiazines and melittin. Cancer Chemother Pharmacol. 1985;14:202–5. doi: 10.1007/BF00258116. [DOI] [PubMed] [Google Scholar]
- 21.Chen QY, Wu LJ, Wu YQ, et al. Molecular mechanism of trifluoperazine induces apoptosis in human a549 lung adenocarcinoma cell lines. Mol Med Rep. 2009;2:811–17. doi: 10.3892/mmr_00000177. [DOI] [PubMed] [Google Scholar]
- 22.Polischouk AG, Holgersson A, Zong D, et al. The antipsychotic drug trifluoperazine inhibits DNA repair and sensitizes non small cell lung carcinoma cells to DNA double-strand break induced cell death. Mol Cancer Ther. 2007;6:2303–9. doi: 10.1158/1535-7163.MCT-06-0402. [DOI] [PubMed] [Google Scholar]
- 23.Singh A, Sharma S, Khuller GK. Camp regulates vegetative growth and cell cycle in candida albicans. Mol Cell Biochem. 2007;304:331–41. doi: 10.1007/s11010-007-9516-4. [DOI] [PubMed] [Google Scholar]
- 24.Singh J, Chatterjee S. Cell cycle dependent variation of calmodulin in tetrahymena. Cytobios. 1988;55:95–103. [PubMed] [Google Scholar]
- 25.Hilakivi-Clarke L, Wang C, Kalil M, et al. Nutritional modulation of the cell cycle and breast cancer. Endocr Relat Cancer. 2004;11:603–22. doi: 10.1677/erc.1.00665. [DOI] [PubMed] [Google Scholar]
- 26.Li T, Zhao X, Mo Z, et al. Formononetin promotes cell cycle arrest via downregulation of akt/cyclin d1/cdk4 in human prostate cancer cells. Cell Physiol Biochem. 2014;34:1351–58. doi: 10.1159/000366342. [DOI] [PubMed] [Google Scholar]
- 27.Park HJ, Lee HJ, Choi MS, et al. Jnk pathway is involved in the inhibition of inflammatory target gene expression and nf-kappab activation by melittin. J Inflamm (Lond) 2008;5:7. doi: 10.1186/1476-9255-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakata K, Suzuki Y, Inoue T, et al. Deficiency of shp1 leads to sustained and increased erk activation in mast cells, thereby inhibiting il-3-dependent proliferation and cell death. Mol Immunol. 2011;48:472–80. doi: 10.1016/j.molimm.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Goruppi S, Bonventre JV, Kyriakis JM. Signaling pathways and late-onset gene induction associated with renal mesangial cell hypertrophy. EMBO J. 2002;21:5427–36. doi: 10.1093/emboj/cdf535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez-Barbero A, Dorado F, Velasco S, et al. Tgf-beta1 induces cox-2 expression and pge2 synthesis through mapk and pi3k pathways in human mesangial cells. Kidney Int. 2006;70:901–9. doi: 10.1038/sj.ki.5001626. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell D, Rodgers K, Hanly J, et al. Lipoxins inhibit akt/pkb activation and cell cycle progression in human mesangial cells. Am J Pathol. 2004;164:937–46. doi: 10.1016/S0002-9440(10)63181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo D, Ye J, Dai J, et al. Notch-1 regulates akt signaling pathway and the expression of cell cycle regulatory proteins cyclin d1, cdk2 and p21 in t-all cell lines. Leuk Res. 2009;33:678–85. doi: 10.1016/j.leukres.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 33.Saratlija Novakovic Z, Glavina Durdov M, et al. The interstitial expression of alpha-smooth muscle actin in glomerulonephritis is associated with renal function. Med Sci Monit. 2012;18(4):CR235–40. doi: 10.12659/MSM.882623. [DOI] [PMC free article] [PubMed] [Google Scholar]