Abstract
In response to health concerns and widespread human exposures, two widely used commercial formulations of polybrominated diphenyl ethers (PBDEs) were banned in the United States in 2005. Initial biomonitoring data have provided early indications of reduced human exposures since these bans took effect. Our objective was to evaluate temporal trends in PBDE serum levels among a population of older California women during a four-year period, beginning approximately five years after these formulations were banned. Automated solid phase extraction and gas chromatography/high resolution mass spectrometry were used to measure PBDE levels in blood collected during 2011–2015 among 1253 women (ages 40–94) participating in the California Teachers Study. Only congeners with detection frequencies (DF) ≥ 75% were included in the present analysis: BDE-47 (DF = 88%); BDE-100 (DF = 78%); and BDE-153 (DF = 80%). Results from age- and race/ethnicity-adjusted linear regression analyses indicated modest, but statistically significant, average annual percent increases in the serum concentrations of all three PBDEs over the four-year study period. While not without limitations, these results, in the context of other biomonitoring data, suggest that earlier reported declines in PBDE levels may have plateaued and may now be starting to increase. Further biomonitoring to ascertain current trends and determinants of population exposures is warranted.
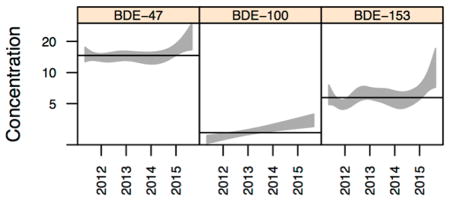
Graphical Abstract
INTRODUCTION
Polybrominated diphenyl ethers (PBDEs) are a class of over 200 congeners introduced in the late 1970s as flame retardant additives to a wide variety of consumer products and building materials.1 Their use skyrocketed over the ensuing three decades and reports began to emerge during the 1990s of rapidly rising body burden levels of PBDEs in human tissues, worldwide and within the United States.2–9 These observations, coupled with laboratory evidence of potentially associated adverse health effects, including neurodevelopmental toxicity, immunotoxicity, endocrine disruption, and carcinogenicity, resulted in the restriction and ban in the production and use of PBDEs, both internationally and in the United States.7,10–12
PBDEs historically have been used in three primary commercial formulations, containing mixtures of compounds; these are named by the average number of bromine atoms of the chemical compounds they contain. The penta- formulation (primarily composed of BDE-99, BDE-47, BDE-100, BDE-153, and BDE-154)13 was predominately used to treat polyurethane foam cushioning in furniture and carpet padding. The Octaformulation (primarily composed of BDE-183, BDE-207, BDE- 203, BDE-209, and BDE-197)13 was used to treat hard plastic casings in computers and other electronic appliances and equipment. The Deca- formulation, almost entirely composed of BDE-209,13 was also used in hard plastic casings, as well as in textiles, adhesives, and wire insulation.6,10,12 In the United States, regulatory and voluntary measures resulted in the discontinuation of the production, use, and sale of the penta-and octa- formulation by the end of 2004 and the Decaformulation by the end of 2013.10
In Europe, where PBDEs were phased-out a few years earlier than in the United States, there is evidence, although somewhat mixed, that PBDE levels in environmental matrices, wildlife and human tissue have been declining.14 In the United States comparatively few data are available to evaluate the success of the PBDE phase-outs. Two small biomonitoring studies in California have indicated secular decreases in PBDE levels in breast milk15 and blood.16 A larger study of newborn blood spots in New York has also suggested secular decreases in body burden levels since the 2005 phase-out of the penta- and octaformulations.17 In contrast, although not specifically designed to evaluate temporal trends, a pair of small breast milk biomonitoring studies in Texas indicated no declines in PBDE levels from 2003 through 2007.18
The objective of the current analysis was to add to the limited data available to help inform the degree to which the phase-out of PBDEs in this country has reduced human exposures. Capitalizing on the availability of serum specimens collected from over 1,000 middle-aged and older California women participating in an ongoing cohort study, we conducted a temporal analysis of PBDE serum levels in blood specimens collected from 2011 through 2015.
MATERIALS AND METHODS
Study Population
The study population consisted of 1253 participants drawn from the California Teachers Study (CTS), a prospective cohort study that recruited 133 479 female professional public school employees in 1995–1996 primarily to study breast cancer. A full description of the cohort is available elsewhere.19 Women included in the current analysis were serving as controls in an ongoing breast cancer case-control study nested within the CTS or were recruited separately in a convenience sample of breast cancer-free CTS participants which targeted nonwhites to enhance racial/ethnic diversity. Controls in the case-control study were drawn from a probability sample of at-risk cohort members frequency matched to breast cancer cases by age, race/ethnicity and broad geographic region (corresponding to the three CTS field collection study sites). The convenience sample of breastcancer free participants was drawn from a probability sample of CTS members under the age of 80 years who self-reported as either White, Black, Hispanic, or Asian/Pacific Islander and were geographically distributed so as to provide approximately equal representation of urban and rural residence. Furthermore, all participants included in the current analysis lived in California and completed a supplementary interviewer-administered questionnaire when their blood was drawn between 2011 and 2015. Participants’ ages were ascertained by the questionnaire and dates of blood collection were recorded by the interviewer at the time blood was collected. Study participants reported their race/ethnicity at recruitment into the study on the baseline CTS questionnaire. The use of human subjects was reviewed and approved by the California Health and Human Services Agency, Committee for the Protection of Human Subjects and all participating centers’ Institutional Review Boards.
Serum Collection
Blood was collected from participants between May 9, 2011 and August 24, 2015 by licensed phlebotomists, usually in the participants’ homes. Blood was collected into a 10 mL BD tube (catalog#367985, Becton Dickinson, Franklin Lakes, NJ) with clot activator, double gel for transport, and silicone coated interior using standard phlebotomy techniques. Prior to field processing, specimens were kept on cool packs for at least 30 min. Within several hours of collection, phlebotomists spun down the clotted blood samples in the field using portable centrifuges to separate the serum portion. Processed samples were then frozen and stored at −20°C for 4–6 weeks until transported either via local courier (on cool-packs) or overnight (on dry ice via FedEx) to the laboratory for chemical analysis. Samples remained frozen during this transportation process. Upon receipt at the laboratory, specimens were stored at −20°C until analysis.
PBDE Analysis
Serum samples were analyzed for 19 PBDE congeners by the Environmental Chemistry Laboratory at the California Department of Toxic Substances Control (Berkeley, CA). Samples were thawed and aliquoted for PBDE and lipid measurements. Automated solid phase extraction (SPE; Biotage, Uppsala, Sweden) and gas chromatography/high resolution mass spectrometry (GC-HRMS, DFS, Thermo- Fisher, Bremen, Germany) were used for the analysis of PBDEs.20 Briefly, thawed serum samples (2 mL) were fortified with a panel of 13C12 labeled surrogate standards and mixed well. Equal volumes (4 mL) of formic acid and water were added into each sample before loading on the SPE modules. Oasis HLB cartridges (3 cc, 500 mg, Waters Co., Milford, MA, USA) and acidified silica (500°C prebaked, manually packed, 3 cc) were used for the sample extraction and cleanup, respectively. The collected final eluates in hexane: dichlomethane (1:1) were concentrated in TurboVap (Biotage, Charlotte, NC), and spiked with recovery standards. Standard reference material (SRM 1958, National Institute of Standards and Technology, Gaithersburg, MD) and bovine serum prespiked with known amounts of target analytes were used as QA/QC samples. The laboratory is proficient in the analysis of PBDEs as demonstrated by its regular participation in the performance evaluation system managed by the Arctic Monitoring & Assessment Program (AMAP).
A small volume of sera from each sample was sent to Boston Children’s Hospital for measurement of total cholesterol and triglycerides by enzymatic methods.21,22 Cholesterol and triglycerides were used to calculate the lipid content based on Phillips’ formula.23 PBDE levels were then normalized for lipids to produce values with units of ng/g lipid. To be consistent with national biomonitoring data,24 we replaced concentrations below the laboratory limit of detections (LODs) with LOD/√2 before lipid adjustment.
In order to minimize potential biases associated with imputing high frequency of nondetectable levels, only the three congeners with detection frequencies (DF) of 75% or more were included in the current analysis. These included 2,2′,4,4′-Tetrabromodiphenyl ether (BDE-47), with an average LOD of 0.032 ng/mL and DF = 88%; 2,2′,4,4′,6-Pentabromodiphenyl ether (BDE-100), with an average LOD of 0.007 ng/mL and DF = 78%; and 2,2′,4,4′,5,5′-hexabromodiphenyl ether (BDE-153), with an average LOD of 0.015 ng/mL and DF = 80%.
Data Analyses
Summary statistics (including means, medians, minimum, and maximum values) were generated for the lipid-normalized concentration of each congener. Initial evaluations of temporal trends were evaluated by plotting the concentration of each PBDE congener versus the date of sample collection. Linear models were used to regress the log10- transformed serum concentration of each PBDE congener on serum collection date. (Serum concentrations were log10- transformed to symmetrize the skewed distributions before using them in regressions). To determine appropriate covariates, we conducted backward stepwise regressions, considering age at blood draw (continuous years, considered both as a linear and a quadratic term), and race/ethnicity (categorical: White, Black, Hispanic, Asian/Pacific Islander, Other/unknown). Regression models were built separately for each PBDE congener and only the factors that were significant at p < 0.20 or that changed the β-coefficient of the serum collection date more than 10% were kept in the final multivariable-adjusted regression models. While there was some indication of variability in serum lipid levels over the course of the study, with the exception of BDE-153, the lipidnormalized values of PBDEs were not significantly related to total serum lipids. Furthermore, incorporation of a term for total serum lipids did not change the β-coefficient for serum collection date for any of the congeners by more than 10% and therefore was not included as covariates in our regression models. For both BDE-47 and BDE-100, the final regression models included only race/ethnicity; for BDE-153 the final regression model included only age (as a linear term). To validate these results, we also performed backward stepwise regressions using Akaike’s Information Criterion (AIC) as the figure of merit for each model. Both approaches resulted in the same set of final models. We present results from both the crude and multivariable-adjusted regressions. Time trend coefficients (β) obtained from regressing log10 concentration on date of sample, expressed as year and fraction of a year, were converted to annual percent increase (API) by the standard method of API = 100 × (10β − 1). Initial exploratory analyses were conducted and graphical representations of the data were generated using R, version 3.3.2.25 Subsequent regression analyses were conducted using PROC GLM in SAS (SAS Institute, Inc., Version 11.0, Cary, NC).26
RESULTS
The majority of study participants were non-Hispanic white (75%); participants ranged in age from 40 to 94 years, with most (70%) in the age range of 60–79 years. The age and racial/ethnic composition of study participants varied somewhat throughout the time course of the study (Table 1) with a lower proportion of non-Hispanic whites providing samples during the first two years than during later years of sample collection, reflecting the effort during these early years to target sampling of nonwhites. In addition, a disproportionate number of samples were collected from study participants in the oldest age category (80+years) during the earlier years of the study.
Table 1.
Age and Race Characteristics of Study Participants (n = 1253) for Entire Study Period and by Year of Blood Collection (2011–2015)
| characteristic | all years | year of blood collection | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| 2011 | 2012 | 2013 | 2014 | 2015 | ||||||||
|
|
|
|
|
|
|
|||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| all | 1253 | 100 | 409 | 100 | 325 | 100 | 250 | 100 | 176 | 100 | 93 | 100 |
| age (years) | ||||||||||||
| 40–49 | 77 | 6 | 26 | 6 | 31 | 9 | 11 | 4 | 7 | 4 | 2 | 2 |
| 50–59 | 179 | 14 | 58 | 14 | 51 | 16 | 39 | 16 | 19 | 11 | 12 | 13 |
| 60–69 | 499 | 40 | 143 | 35 | 132 | 41 | 105 | 42 | 74 | 42 | 45 | 49 |
| 70–79 | 376 | 30 | 114 | 28 | 84 | 26 | 79 | 32 | 70 | 40 | 29 | 31 |
| 80–94 | 122 | 10 | 68 | 17 | 27 | 8 | 16 | 6 | 6 | 3 | 5 | 5 |
| race/ethnicity | ||||||||||||
| white | 938 | 75 | 311 | 76 | 176 | 54 | 205 | 82 | 162 | 92 | 84 | 90 |
| black | 90 | 7 | 31 | 8 | 40 | 12 | 18 | 7 | 0 | 0 | 1 | 1 |
| hispanic | 103 | 8 | 35 | 9 | 51 | 16 | 11 | 4 | 4 | 2 | 2 | 2 |
| asian/paci3c islander | 96 | 8 | 26 | 6 | 57 | 18 | 9 | 4 | 3 | 2 | 1 | 1 |
| other/unknown | 26 | 2 | 6 | 1 | 1 | <1 | 7 | 3 | 7 | 4 | 5 | 6 |
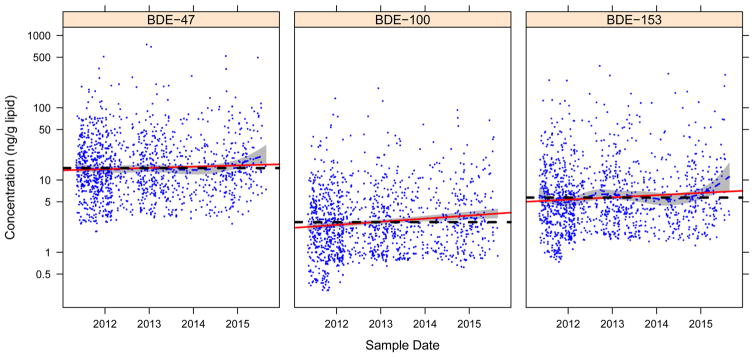
Distributions of the PBDE congeners are summarized in Table 2. Similar to what has been reported in other biomonitoring studies in California27 and the United States,4,6,8,10,14,28 BDE-47 was the most commonly detected congener with the highest levels (median = 13.34 ng/g lipid), followed by BDE-153 (median = 4.89 ng/g lipid) and BDE-100 (median = 2.31 ng/g lipid). Concentrations for each of the PBDE congeners are plotted against date of serum collection in Figure 1. All three congeners displayed considerable variability in concentrations. While no dramatic temporal trends are obvious, linear and spline fits to the data suggest a modest increase in concentrations of all three congeners over the time span of serum collection.
Table 2.
PBDE Serum Concentrations Among 1253 Study Participants
| compound | Detection frequencya | serum concentration (ng/g lipid) | |||
|---|---|---|---|---|---|
|
| |||||
| mean | median | minimumb | maximum | ||
| BDE-47 | 88% | 25.56 | 13.34 | 1.94 | 749.67 |
| BDE-100 | 78% | 5.08 | 2.31 | 0.30 | 186.31 |
| BDE-153 | 80% | 12.03 | 4.89 | 0.74 | 379.31 |
Detection frequency = percent of samples with serum concentration ≥ limit of detection(LOD).
If below the LOD, value was imputed = LOD/√ 2.
Figure 1.
PBDE serum concentrations (ng/g lipid) by date of blood collection. Dashed blue line and gray shading in plots for BDE-47 and BDE-153 denote spline fit and 95% confidence interval (spline for BDE-100 was identical to the linear fit); red line denotes linear fit; Dashed black line denotes overall mean (i.e., a null association). Percent of variance accounted for by marginal spline fits ranged from 1% for BDE-47 to 2% for BDE- 153.
Table 3 presents the results from our linear regression models, expressed as the estimated average annual percent increase (API) in PBDE concentrations. While adjusted APIs for all three congeners were statistically significant (BDE-47 API = 5.6, p = 0.0170; BDE-100 API = 12.0, p < 0.0001; BDE- 153 API = 7.1, p = 0.0047), confidence intervals were wide. Overall, the final adjusted models explained little of the overall variation in PBDE levels: 1.1% for BDE-47; 2.1% for BDE-100; and 1.5% for BDE-153.
Table 3.
Annual Percent Increase (API) in PBDE Serum Concentrations: Results from Linear Models
| compound | Unadjusted | adjusteda | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| APIb | 95% CIc | p-value | APIb | 95% CIc | p-value | |
| BDE-47 | 4.0 | −0.42, 8.6 | 0.0770 | 5.6 | 0.99, 10 | 0.0170 |
| BDE-100 | 10.0 | 5.5, 16 | <0.0001 | 12.0 | 6.8, 17 | <0.0001 |
| BDE-153 | 7.4 | 2.4, 13 | 0.0036 | 7.1 | 2.1, 12 | 0.0047 |
BDE-47 and BDE-100 adjusted for race/ethnicity; BDE-153 adjusted for age.
Annual percent increase (API) converted from time trend coefficients (β) obtained from regressing log10 PBDE concentration ((ng/g lipid) on date of sample collection, expressed as year and fraction of a year, where API = 100*(10β − 1).
CI = Confidence interval.
To evaluate whether congener profiles changed over time, we constructed a variable to represent the ratio of BDE-153 to BDE-47 concentrations; neither graphical representations nor regressions suggested any change in this variable over the time course of the study (data not shown). While initial exploratory analyses suggested some effect modification by age and race might be present (data not shown), numbers within many age/race strata were too sparse during the latter half of the study (as shown in Table 1) to be able to meaningfully model and formally evaluate statistical interaction between these two factors.
DISCUSSION
Our results indicate that in this population of older adult California women, average concentrations in PBDE serum levels are increasing over the course of a four-year time period beginning approximately five years after the two major commercial formulations containing these congeners were phased out of use. Although these trends were statistically significant, confidence intervals were wide and the percent of variation accounted for by the final models was quite small.
Several limitations of these analyses are worth noting. It would have been ideal to have had paired repeat samples in individuals. Although we adjusted for temporal differences in the distribution of age and race/ethnicity (which did not substantially affect our estimates of temporal trends), we were not able to adjust for other potential confounders. Of particular concern could be the inability to account for pregnancy and lactation history which is likely to influence PBDE levels. However, with a minimum age of 40, our study population was largely beyond the childbearing years. Furthermore, a preliminary assessment of these factors based on ascertainment at entry into the cohort (in 1995) indicated these factors were not related to PBDE levels nor did incorporation of variables for these factors in our regression analyses influence the temporal trends observed in our study (data not shown). While it is difficult to imagine other factors that could be related to both sampling date and PBDE serum levels that would be sufficient to change our overall conclusions, we cannot dismiss the possibility that our results could be biased by confounding of unmeasured factors. Our regression models only captured a small proportion of the overall variability in PBDE levels and the confidence intervals for our beta coefficients were wide. Consequently, we suggest that the estimates of average annual percent increases as reported in Table 3 be interpreted with caution. Additionally, because no samples were collected prior to the 2005 PBDE phase-out, our analyses could not directly evaluate whether serum levels were lower than those prior to the phase-out.
A strength of our study is that all of the study participants were selected from the same well-defined and well-specified underlying population of CTS participants. It is difficult, however, to ascertain the degree to which the exposure experiences of our study population, which is comprised entirely of professional and college-educated women, are representative of those of the general population. The most recent published National Health and Nutrition Examination Survey (NHANEs) data on PBDE serum concentrations among the U.S. population were collected several years prior to the sample collection period of our study.29 A comparison of the PBDE serum levels in our study to more contemporaneous biomonitoring data in California is not particularly informative (see Supporting Information (SI), Table S1). As shown in SI Table S1, PBDE serum levels found in our study are similar to, albeit slightly lower, than those reported in blood collected between 2009 and 2012 from first-time mothers residing in Northern California.15 Our levels, however, were substantially lower than those reported in blood collected in 2013 from men and women residing in California’s Central Valley,27 in blood collected between 2010 and 2011 from mostly male firefighters in Southern California,20 and in blood collected between 2011 and 2012 from pregnant patients recruited at a public hospital in San Francisco, California.16 In contrast, our levels were marginally higher than those reported in blood collected between 2010 and 2011 from another group of pregnant women in San Francisco California.30 The distinctly different sociodemographic profiles of these other study populations, the likely occupational sources of PBDE exposures among the firefighters, and the imperfect temporal overlap in sample collection dates, makes it difficult to interpret our findings in the context of the PBDE serum levels reported in these other populations. Given these limitations, and in the absence of more recent population-based California biomonitoring data, it is difficult to ascertain the degree to which the temporal trends we observe in our study may extend to the general California population.
Our results differ from those of three other studies that evaluated temporal trends in body burden levels in the United States after the 2005 ban of the penta- and octa- formulations, all of which reported secular decreases in PBDE levels.15–17 In a study of dried bloodspots collected in New York from approximately 1200 newborns from 1997 to 2011, BDE-47 and BDE-100 levels remained stable through 2002 after which steep declines were reported.17 Another study recruited women during their second trimester of pregnancy from a public hospital in San Francisco CA and compared PBDE serum levels from 25 women recruited in 2008–2009 to those of 36 women recruited in 2011–2012.16 Median serum levels of all five congeners examined, including BDE-47, BDE-100, and BDE- 153, were significantly lower in the blood samples collected in 2011–2012 than in those collected in 2008–2009 (Table 4). Similarly, a significant decrease in PBDE concentrations was reported in a study of breast milk collected from Northern California women that compared samples collected in 2009– 2012 (n = 66) to those collected in 2003–2005 (n = 82) (Table 4).15
Table 4.
Comparison of PBDE Body Burden Levels in Our Study Population to Those Reported in Two Earlier Studies Conducted in Other California Study Populations
| geometric mean of concentration (ng/g lipid) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Guo et al.a | Zota et al.b | this study | ||||
|
|
|
|
||||
| breast milk collected 2003–2005 | breast milk collected 2009–2012 | blood collected 2009–2012 | blood collected 2008–2009 | blood collected 2011–2012 | blood collected 2011–2015 | |
| BDE-47 | 35.1 | 17.4 | 17.4 | 43.1 | 25.75 | 14.60 |
| BDE-100 | 6.16 | 2.87 | 2.89 | 9.0 | 4.66 | 2.62 |
| BDE-153 | 6.71 | 5.22 | 6.08 | 15.5 | 10.87 | 5.72 |
Measured in breast milk (n = 82 for 2003–2005 time period; n = 66 for 2009–2012 time period) and blood (n = 64 collected in 2009–2012) from first time mothers residing in Northern California.15
Measured in blood collected from pregnant patients at a public hospital in San Francisco CA (n = 25 for 2008–2009 time period; n = 36 for 2011–2012 time period).16
There are a number of potential explanations for the inconsistency of our findings with these other studies. Perhaps most notable is the marked difference in age groups that were evaluated. Our study population was comprised entirely of middle-aged and older women while these other studies were conducted in much younger populations (newborns, and women of childbearing age including pregnant and lactating women). Although little is known about how the pharmacokinetics of PBDEs may be influenced by age (or pregnancy or lactation), it is possible that the difference between our findings and these others reflect age-related differences in the uptake, metabolism or elimination of PBDEs such that the lack of declines in body burden levels observed in our study could be due to relatively slower metabolism or elimination of PBDEs among older adults. This represents an understudied, but potentially important, area of inquiry.
Alternatively, it is possible that our discrepant results may reflect differences in the relative importance of different exposure pathways for younger and older populations. Ingestion and inhalation of indoor dust have been identified as the predominant sources of PBDE exposure, with diet playing a lesser but important role.1,10,14,31 PBDE serum levels have been shown to correlate with levels in household dust, especially for BDE-47 and BDE-100, the primary constituents of the penta- formulation.1,6,10,14,32,33 The well-documented elevation in PBDE serum levels among children compared to adults has been largely attributed to greater exposure through hand-to-mouth ingestion of PBDE-contaminated indoor dust.1,10,34,35 Recent, although limited, data suggest that PBDE levels in U.S. household dust may be declining.14,36,37 If in fact, dust PBDE levels are truly decreasing, then the lack of secular declines in PBDE serum levels among our study participants suggests that indoor dust may not be the most important contributor to exposures in this group of older adult women. Overall, few studies to date have examined PBDE serum levels among older women as did ours, where 40% were 70 years or older. An analysis of PBDE serum levels among NHANES participants recruited in 2003–2004 (which included people up to the age of 74), however, did note a 2-fold increase in the proportion of participants over the age of 60 with BDE- 47 concentrations above the 95th-percentile.28 Our results, in the context of these findings from NHANES, suggest that identifying determinants of PBDE levels among older individuals may be of particular importance.
Finally, it is plausible that our results contradict these earlier findings because the temporal trends in PBDE body burden levels have in fact recently shifted. While a comparison of our PBDE levels to those measured in blood collected during earlier time periods from other California populations indicates considerably lower levels during the more recent time period of our study (Table 4), our analysis of temporal trends across the 4-year time interval within our study suggests that this initial decline may have recently plateaued and reversed direction. To our knowledge, no other temporal analyses have been published that span the more recent years encompassed by our study. More studies in other contemporary populations are necessary to ascertain whether exposures to PBDEs are continuing to decline.
Beyond the borders of the United States, most international biomonitoring data generally support declining PBDE body burden levels following international restrictions, although declines have not been universally observed for all congeners and across all regions of the world.10,14 In particular, declining trends seem to be more apparent for the lower-brominated congeners with some reports of stable or even increasing trends for the higher brominated BDE-153 and BDE-209 congeners.38–40 In our study, however, we did not see distinctly different temporal patterns for BDE-153 compared to those of the lower brominated compounds nor did we see any temporal shifts in congener profiles.
After the banning of the polychlorinated biphenyls (PCBs), a similarly persistent class of organic pollutants, body burden levels initially displayed a sharp decline, followed by a gradual leveling off and plateau.41 The fact that PCBs continue to be detected in a substantial proportion of the United States population, nearly 40 years after they were banned,24 is evidence of continuing PCB exposures, likely due to contamination and biomagnification in the food chain.41 It has been hypothesized that following the PBDE phase-out, temporal trends in PBDE body burdens would mirror these trends observed for the PCBs, with an initial sharp decline, followed by a plateau of stable but continuing exposures.41 Our results, in the context of other biomonitoring data, support this hypothesis. Although the timing of blood collection in our study did not allow us to directly assess the immediate effects of the phase out on PBDE serum levels, the levels found in our study population are generally lower than those reported in two other biomonitoring studies conducted in California during earlier time periods, closer in proximity to the 2005 penta- and octa- phase-outs (Table 4), However, the fact that we do not observe declines but rather see evidence for secular increases in PBDE levels during the more recent 4-year time interval of our study period, suggests that any initial secular declines following the PBDE phase-out may no longer be occurring.
Overall, numerous reports of declining PBDE levels in environmental and biologic matrices provide evidence for the initial effectiveness of current PBDE regulations in reducing exposures.10,14 Declines in body burden levels likely reflect reductions in exposure via indoor dust due to replacement of old PBDE-treated consumer products with new PBDE-free products. It is important to consider, however, that many consumer products treated with PBDEs (such as sofas, carpet padding, and fabrics) typically are replaced infrequently and are likely to continue to serve as substantial reservoirs for indoor exposures for many years. Furthermore, as these PBDE-laden products reach the end of their lifespan, are discarded into the waste stream, leach into the outdoor environment, and contaminate and biomagnify in the food chain, dietary ingestion is likely to emerge as the predominant exposure pathway. Our results suggest this shift may have already begun.
In summary, biomonitoring data provide a useful tool for evaluating the effectiveness of regulatory measures to reduce population exposures to toxic chemicals. Initial biomonitoring data, both in the U.S. and elsewhere, suggest that the current PBDE restrictions have reduced exposures.10,14 While the overall PBDE serum levels measured in our study were lower than some earlier studies of California populations, we observed a modest but statistically significant secular increase in PBDE serum levels in our study population over the four years of the study period, indicating a possible shifting in exposure pathways. More research is needed to elucidate the predominant pathways of PBDE exposure in today’s population. If our findings of sustained and possibly increasing PBDE body burden levels are replicated in other contemporary study populations, it would underscore the urgency to take additional regulatory actions to manage the disposal of PBDE-laden products to reduce environmental contamination and minimize dietary exposures.
Supplementary Material
Acknowledgments
This research was supported by funds provided by The Regents of the University of California, California Breast Cancer Research Program, Grant Number 16ZB-8501 and National Cancer Institute (NCI) of the National Institutes of Health (NIH), Grant R01 CA77398. The opinions, findings, and conclusions herein are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, the California Department of Toxic Substances Control, the California Department of Public Health, the Regents of the University of California, or any of its programs. We express our appreciation to all the participants in the California Teachers Study and to the researchers, analysts and staff who have contributed to the success of this research project. We also thank Minhthu Le for administrative support, Christine Duffy for overseeing field data and biospecimen collection, the phlebotomists, and the California Teachers Study Steering Committee members who continue to work on other aspects of the cohort: Jessica Clague-deHart, Christina A. Clarke, Dennis Deapen, James V. Lacey Jr, Huiyan Ma, Susan L. Neuhausen, Hannah Park, Richard Pinder, Sophia S. Wang, and Argyrios Ziogas.
Footnotes
ORCID
Susan Hurley: 0000-0002-5582-1741
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.7b00565.
Table S1. Comparison of PBDE serum levels in our study population to those reported in other California populations during similar time periods (PDF)
References
- 1.Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Polybrominated Diphenyl Ether (draft for public comment) 2015 https://www.atsdr.cdc.gov/toxprofiles/tp207.pdf. [PubMed]
- 2.Costa LG, Giordano G, Tagliaferri S, Caglieri A, Mutti A. Polybrominated diphenyl ether (PBDE) flame retardants: environmental contamination, human body burden and potential adverse health effects. Acta Biomed. 2008;79:172–183. [PubMed] [Google Scholar]
- 3.Darnerud PO, Eriksen GS, Johannesson T, Larsen PB, Viluksela M. Polybrominated diphenyl ethers: occurrence, dietary exposure, and toxicology. Environ Health Perspect. 2001;109(Suppl1):49–68. doi: 10.1289/ehp.01109s149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hites RA. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ Sci Technol. 2004;38:945–956. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- 5.Meironyte D, Noren K, Bergman A. Analysis of polybrominated diphenyl ethers in Swedish human milk. A time-related trend study, 1972–1997. J Toxicol Environ Health, Part A. 1999;58:329– 341. doi: 10.1080/009841099157197. [DOI] [PubMed] [Google Scholar]
- 6.Schecter A, Papke O, Tung KC, Joseph J, Harris TR, Dahlgren J. Polybrominated diphenyl ether flame retardants in the U.S. population: current levels, temporal trends, and comparison with dioxins, dibenzofurans, and polychlorinated biphenyls. J Occup Environ Med. 2005;47:199–211. doi: 10.1097/01.jom.0000158704.27536.d2. [DOI] [PubMed] [Google Scholar]
- 7.Siddiqi MA, Laessig RH, Reed KD. Polybrominated diphenyl ethers (PBDEs): new pollutants-old diseases. Clin Med Res. 2003;1:281–290. doi: 10.3121/cmr.1.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sjodin A, Jones RS, Focant JF, Lapeza C, Wang RY, McGahee EE, 3rd, Zhang Y, Turner WE, Slazyk B, Needham LL, Patterson DG., Jr Retrospective time-trend study of polybrominated diphenyl ether and polybrominated and polychlorinated biphenyl levels in human serum from the United States. Environ Health Perspect. 2004;112:654–658. doi: 10.1289/ehp.112-1241957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sjodin A, Patterson DG, Jr, Bergman A. A review on human exposure to brominated flame retardants–particularly polybrominated diphenyl ethers. Environ Int. 2003;29:829–839. doi: 10.1016/S0160-4120(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 10.Linares V, Belles M, Domingo JL. Human exposure to PBDE and critical evaluation of health hazards. Arch Toxicol. 2015;89:335–356. doi: 10.1007/s00204-015-1457-1. [DOI] [PubMed] [Google Scholar]
- 11.Talsness CE. Overview of toxicological aspects of polybrominated diphenyl ethers: a flame-retardant additive in several consumer products. Environ Res. 2008;108:158–167. doi: 10.1016/j.envres.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Birnbaum LS, Cohen Hubal EA. Polybrominated diphenyl ethers: a case study for using biomonitoring data to address risk assessment questions. Environ Health Perspect. 2006;114:1770–1775. doi: 10.1289/ehp.9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaGuardia MJ, Hale RC, Harvey E. Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta-, octa-, and deca-PBDE technical flameretardant mixtures. Environ Sci Technol. 2006;40:6247–6254. doi: 10.1021/es060630m. [DOI] [PubMed] [Google Scholar]
- 14.Law RJ, Covaci A, Harrad S, Herzke D, Abdallah MA, Fernie K, Toms LM, Takigami H. Levels and trends of PBDEs and HBCDs in the global environment: status at the end of 2012. Environ Int. 2014;65:147–158. doi: 10.1016/j.envint.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Guo W, Holden A, Smith SC, Gephart R, Petreas M, Park JS. PBDE levels in breast milk are decreasing in California. Chemosphere. 2016;150:505–513. doi: 10.1016/j.chemosphere.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 16.Zota AR, Linderholm L, Park JS, Petreas M, Guo T, Privalsky ML, Zoeller RT, Woodruff TJ. Temporal comparison of PBDEs, OH-PBDEs, PCBs, and OH-PCBs in the serum of second trimester pregnant women recruited from San Francisco General Hospital, California. Environ Sci Technol. 2013;47:11776–11784. doi: 10.1021/es402204y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma WL, Yun S, Bell EM, Druschel CM, Caggana M, Aldous KM, Buck Louis GM, Kannan K. Temporal trends of polybrominated diphenyl ethers (PBDEs) in the blood of newborns from New York State during 1997 through 2011: analysis of dried blood spots from the newborn screening program. Environ Sci Technol. 2013;47:8015–8021. doi: 10.1021/es401857v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schecter A, Colacino J, Sjodin A, Needham L, Birnbaum L. Partitioning of polybrominated diphenyl ethers (PBDEs) in serum and milk from the same mothers. Chemosphere. 2010;78:1279–1284. doi: 10.1016/j.chemosphere.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Bernstein L, Allen M, Anton-Culver H, Deapen D, Horn-Ross PL, Peel D, Pinder R, Reynolds P, Sullivan-Halley J, West D, Wright W, Ziogas A, Ross RK. High breast cancer incidence rates among California teachers: results from the California Teachers Study (United States) Cancer Causes Control. 2002;13:625–635. doi: 10.1023/a:1019552126105. [DOI] [PubMed] [Google Scholar]
- 20.Park JS, Voss RW, McNeel S, Wu N, Guo T, Wang Y, Israel L, Das R, Petreas M. High exposure of California firefighters to polybrominated diphenyl ethers. Environ Sci Technol. 2015;49:2948–2958. doi: 10.1021/es5055918. [DOI] [PubMed] [Google Scholar]
- 21.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem Lab Med. 1974;20:470–475. [PubMed] [Google Scholar]
- 22.Stinshoff K, Weisshaar D, Staehler F, Hesse D, Gruber W, Steier E. Relation between concentrations of free glycerol and triglycerides in human sera. Clin Chem. 1977;23:1029–1032. [PubMed] [Google Scholar]
- 23.Phillips D, Burse PJ, Bernert V, Henderson J, Jr, Needham LL. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol. 1989;18:495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Fourth National Report on Human Exposures to Environmental Chemicals. Department of Health and Human Services; Atlanta, GA: 2009. http://www.cdc.gov/exposurereport/pdf/FourthReport.pdf. [Google Scholar]
- 25.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2016. [Google Scholar]
- 26.SAS Institute Inc. SAS 11.0, 9. SAS Institute Inc; Cary, NC: 2007. [Google Scholar]
- 27.Biomonitoring California. A Joint Program of California. Department of Public Health; Department of Toxic Substances Control; and the Office of Environmental Health Hazard Assessment Biomonitoring California Projects; [accessed January 20, 2017]. http://biomonitoring.ca.gov/projects. [Google Scholar]
- 28.Sjodin A, Wong LY, Jones RS, Park A, Zhang Y, Hodge C, Dipietro E, McClure C, Turner W, Needham LL, Patterson DG., Jr Serum concentrations of polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyl (PBB) in the United States population: 2003–2004. Environ Sci Technol. 2008;42:1377–1384. doi: 10.1021/es702451p. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables, (February, 2015) Atlanta, GA: 2015. https://www.cdc.gov/exposurereport/ [Google Scholar]
- 30.Morello-Frosch R, Cushing LJ, Jesdale BM, Schwartz JM, Guo W, Guo T, Wang M, Harwani S, Petropoulou SE, Duong W, Park JS, Petreas M, Gajek R, Alvaran J, She J, Dobraca D, Das R, Woodruff TJ. Environmental Chemicals in an Urban Population of Pregnant Women and Their Newborns from San Francisco. Environ Sci Technol. 2016;50:12464–12472. doi: 10.1021/acs.est.6b03492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lorber M. Exposure of Americans to polybrominated diphenyl ethers. J Exposure Sci Environ Epidemiol. 2008;18:2–19. doi: 10.1038/sj.jes.7500572. [DOI] [PubMed] [Google Scholar]
- 32.Whitehead TP, Crispo Smith S, Park JS, Petreas MX, Rappaport SM, Metayer C. Concentrations of persistent organic pollutants in California women’s serum and residential dust. Environ Res. 2015;136:57–66. doi: 10.1016/j.envres.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zota AR, Rudel RA, Morello-Frosch RA, Brody JG. Elevated house dust and serum concentrations of PBDEs in California: unintended consequences of furniture flammability standards? Environ Sci Technol. 2008;42:8158–8164. doi: 10.1021/es801792z. [DOI] [PubMed] [Google Scholar]
- 34.Trudel D, Scheringer M, von Goetz N, Hungerbuhler K. Total consumer exposure to polybrominated diphenyl ethers in North America and Europe. Environ Sci Technol. 2011;45:2391–2397. doi: 10.1021/es1035046. [DOI] [PubMed] [Google Scholar]
- 35.Bradman A, Castorina R, Sjodin A, Fenster L, Jones RS, Harley KG, Chevrier J, Holland NT, Eskenazi B. Factors associated with serum polybrominated diphenyl ether (PBDE) levels among school-age children in the CHAMACOS cohort. Environ Sci Technol. 2012;46:7373–7381. doi: 10.1021/es3003487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dodson RE, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, Brody JG, Rudel RA. After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California. Environ Sci Technol. 2012;46:13056–13066. doi: 10.1021/es303879n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitehead TP, Brown FR, Metayer C, Park JS, Does M, Petreas MX, Buffler PA, Rappaport SM. Polybrominated diphenyl ethers in residential dust: sources of variability. Environ Int. 2013;57–58:11–24. doi: 10.1016/j.envint.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Darnerud PO, Lignell S, Aune M, Isaksson M, Cantillana T, Redeby J, Glynn A. Time trends of polybrominated diphenylether (PBDE) congeners in serum of Swedish mothers and comparisons to breast milk data. Environ Res. 2015;138:352–360. doi: 10.1016/j.envres.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 39.Fangstrom B, Athanassiadis I, Odsjo T, Noren K, Bergman A. Temporal trends of polybrominated diphenyl ethers and hexabromocyclododecane in milk from Stockholm mothers, 1980– 2004. Mol Nutr Food Res. 2008;52:187–193. doi: 10.1002/mnfr.200700182. [DOI] [PubMed] [Google Scholar]
- 40.He S, Li M, Jin J, Wang Y, Bu Y, Xu M, Yang X, Liu A. Concentrations and trends of halogenated flame retardants in the pooled serum of residents of Laizhou Bay, China. Environ Toxicol Chem. 2013;32:1242–1247. doi: 10.1002/etc.2172. [DOI] [PubMed] [Google Scholar]
- 41.Harrad S, Diamond M. New Directions: Exposure to polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs): current and future scenarios. Atmos Environ. 2006;40:1187–1188. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.