Abstract
Studies have reported that electroacupuncture (EA) may reduce learning and memory impairment following cerebral ischemic injury. However, the precise mechanism of action remains unclear. In the present study, the attenuation of focal cerebral ischemia/reperfusion injury by EA in rats was investigated. EA at the Baihui (DU 20) and Shenting (DU 24) acupoints was demonstrated to significantly improve performance in the Morris water maze task, with shortened latency time and increased frequency of passing the platform. Molecular analysis revealed that EA activated the expression of α7 nicotinic acetylcholine receptors (α7nAChR) in the hippocampus. In addition, EA led to a decreased expression of the microglia/macrophage marker Iba1 and the astrocyte marker glial fibrillary acidic protein in the hippocampus. EA treatment also led to decreased production of the inflammatory cytokines tumor necrosis factor-α and interleukin-1β. Treatment with methyllycaconitine, an α7nAChR antagonist, attenuated the improvement of learning and memory following EA treatment and the inhibitory effects of EA on glial cell activation and inflammatory cytokine production. In conclusion, the findings of the present study demonstrate that EA is able to improve learning and memory function following cerebral ischemic injury via activation of α7nAChR, which significantly decreases the neuroinflammatory response.
Keywords: electroacupuncture, cerebral ischemia/reperfusion, α7 nicotinic acetylcholine receptor, cholinergic anti-inflammatory pathway
Introduction
Stroke is one of the most common causes of mortality and a leading cause of disability worldwide (1). Survivors are typically afflicted by certain levels of functional impairment, including motor, sensory and cognitive dysfunction (2–4). Evidence suggests that up to 64% of patients exhibit a certain degree of cognitive impairment, with 20–30% of patients demonstrating dementia at 3 months following stroke (5–7). Learning and memory deficits are among the most common cognitive impairments and severely affect patients' daily activities and quality of life, which leads to them becoming a significant burden on their families and society (8,9).
Acupuncture is one of the most commonly used and important therapies in traditional Chinese medicine and has been applied clinically for over a millennium (10). Electroacupuncture (EA), a combination of electrical stimulation with acupuncture, has demonstrated efficacy in alleviating cognitive impairments and improving learning and memory in patients and animal models post-stroke (11–14). However, the exact mechanism of its benefits on impaired cognition remains unclear.
Neuroinflammation is a prime pathological factor in stroke, involving a number of complex cellular processes and activities, including astrocyte and microglial proliferation and the production of inflammatory cytokines, including tumor necrosis factor (TNF)-α and interleukin (IL)-1β (15,16). Previous animal studies suggested a mechanistic link between neuroinflammation and cognitive function following traumatic brain injury (TBI) and also in Alzheimer's disease (AD) models (17–19). In addition, hippocampal neuroinflammation is responsible for mediating cognitive dysfunction in the aging brain, in post-cerebral ischemia, and also in AD (20–22).
Cholinergic anti-inflammatory pathways are critical regulators of inflammation. These pathways primarily involve the interaction of vagus nerve cholinergic signaling with α7 nicotinic acetylcholine receptors (α7nAChRs) on immune cells, which leads to inhibition of pro-inflammatory cytokine production, thereby preventing excessive inflammatory responses (23). The α7nAChR serves as an important signaling receptor in cholinergic anti-inflammatory pathways and is closely associated with learning and memory (24–26). Counteracting neuroinflammatory processes via cholinergic anti-inflammatory signaling prevents progressive tissue damage in the brain following stroke (27). EA is known to act in a similar manner by inhibiting neuroinflammation and preventing development of cognitive dysfunction in patients post-stroke (14,28). In addition, a previous study demonstrated that treatment with EA prior to ischemia/reperfusion (I/R) injury is neuroprotective because EA prevents the downregulation of α7nAChR in neurons within the ischemic penumbra (29). The present study aimed to elucidate whether EA ameliorates learning and memory through α7nAChR-mediated inhibition of neuroinflammation in a rat model of focal cerebral I/R injury.
Materials and methods
Animals
A total of 65 male Sprague-Dawley (SD) rats (250–280 g, ages 10–12 weeks) were provided by Shanghai SLAC Laboratory Animal Co., Ltd. [Laboratory Animal Use Certificate no. SCXK (SH) 2012–0002] and housed under controlled conditions with a 12-h light/dark cycle, 22±2°C temperature and 55±15% humidity for at least 1 week prior to surgery and treatment. All animals were allowed ad libitum access to standard rodent food and water. All animal treatments and experiments were conducted in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of Fujian University of Traditional Chinese Medicine (FUTCM; Fujian, China).
Cerebral I/R injury model
Transient focal cerebral ischemia was established via middle cerebral artery occlusion (MCAO) as previously described (30) in 55 rats. Briefly, rats were anesthetized with an intraperitoneal injection of 10% chloral hydrate (3 ml/kg body weight; catalogue no. 30037516; Changzhou Haituo Experimental Instrument., Co., Ltd., Changzhou, China) diluted with 0.9% saline in a sterilized surgical site. Following a midline incision in the neck, the left common carotid artery (CCA), left external carotid artery (ECA) and the internal carotid artery (ICA) were carefully exposed and dissected. The left middle cerebral artery (MCA) was occluded by introducing an embolus through the ICA. To occlude the origin of the left MCA, a nylon filament (0.36±0.02 tip diameter; Guangzhou Jialing Co. Ltd., Guangzhou, China) was inserted (~20 mm) from the ECA and ICA into the MCA. Reperfusion was accomplished by withdrawing the filament after 2 h of occlusion-mediated ischemia. The incision was sutured and sterilized. During and following surgery, the internal temperature of the animals was maintained at 37°C using a heating pad. For rats in the control group (n=10), the arteries were similarly exposed, but not immobilized in a blinded manner as previously described (30). The neurological deficits of the rats were scored as follows: A score of 0 represented no neurological deficit, 1 indicated mild deficits (failure to fully extend the right forepaw), 2 (circling to the right) and 3 (falling to the right) indicated moderate deficits, and a score of 4 represented severe deficits (complete loss of walking ability). Rats that received MCAO and a score of 0 or 4 were excluded from the experiment.
Groups
When the MCAO model was established, the rats were randomly assigned into four groups according to neurological deficit scores: i) MCAO group (n=13); ii) MCAO + EA group (EA group, n=13); iii) MCAO + EA + normal saline (EA + NS group, n=12) and iv) MCAO + EA + methyllycaconitine group (EA + MLA group, n=12). Control rats received surgery without artery ligation (n=10). Therefore, there were five groups in total in the present study.
EA treatment
Rats were administered EA for 30 min daily for 7 days, starting 2 days after I/R surgery. The acupuncture needles (0.3 mm diameter) were inserted at a depth of 2–3 mm into the Baihui (DU 20) and Shenting (DU 24) acupoints, which are commonly used to treat post-stroke cognitive impairment in China (31). Electrical stimulation was then generated using the EA apparatus (Model G6805; Shanghai Medical Instrument Factory, Shanghai, China) with disperse-dense waves of a frequency of 2–10 Hz and an intensity of 2–4 mA. The rats in the control and MCAO groups remained in their cages without special intervention.
Drug administration
Methyllycaconitine (MLA; 5 mg/kg; Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) was diluted in 0.9% saline and administered intraperitoneally 30 min prior to each EA treatment (32). In the EA + NS group, 0.9% saline was injected, with a volume equivalent to that of the methyllycaconitine solution.
Morris water maze
Rats were subjected to the Morris water maze 3 days following surgery to assess spatial learning and memory as previously described (33). The water maze apparatus (Chinese Academy of Sciences, Beijing, China) consisted of a circular, black-painted pool (diameter, 120 cm; depth, 50 cm) filled with water tinted with black ink (depth, 30 cm; temperature, 26±2°C). The tank was divided into four equal quadrants and a video camera attached to a computer was placed above the center of the tank to record the rats. A fixed, 6-cm platform was submerged 2 cm below the surface of the water. A number of visual cues were placed in each quadrant. During the first set of trials, each rat was placed in the water at four equidistant locations to the platform. When the rats arrived at the platform and remained on it for 3 sec they were considered to have found the platform. When the rats were unable to find the platform within 90 sec, they were placed on the platform for 10 sec and the time score was 90 sec. The latency time to find the submerged platform and the total swimming distance were recorded for 4 days.
Rats were subjected to the Morris water maze test with the platform removed 7 days following surgery. Rats were placed in the quadrant located diagonally from the target quadrant and allowed to swim for a maximum of 90 sec. The frequency of swimming across the former location of the platform in the target quadrant was recorded.
Immunohistochemistry
Rats were anesthetized with 10% chloral hydrate by intraperitoneal injection and perfused transcardially with 0.9% NaCl followed by 4% paraformaldehyde through the left ventricle. The brain was removed and fixed in 4% paraformaldehyde at 4°C for 24 h. Samples underwent dehydration with an ethanol gradient, 70, 80 and 90% for 1 h each and 100% ethanol for 20 min, 1 h and a final 20 min. Following washing with xylene, 20 min and 1 h followed by 20 min, the specimens were embedded in paraffin and cut into 5-µm sections (RM2235 slice machine; Leica Microsystems GmbH, Wetzlar, Germany). Following deparaffinization, antigen retrieval was accomplished by immersing and boiling the sections in a Tris-EDTA Buffer (10 mM Tris, 1 mM EDTA, 0.05% Tween-20, pH 9.0; for α7nAChR) or citrate buffer solution [10 mM Tris, 20% citrate, pH 6.0; for glial fibrillary acidic protein (GFAP) and microglial marker Iba1] in a microwave oven.
α7nAChR, GFAP and Iba1 levels were analyzed using immunohistochemistry assay kits [diaminobenzidine (DAB) kit-0017; Maixin-Bio, Fujian, China] according to the manufacturer's protocol. Primary antibodies binding to α7nAChR (cat. no. ab24644; 1:100; Abcam, Cambridge, UK), GFAP (cat. no. 3670; 1:500; Cell Signaling Technologies, Inc., Danvers, MA, USA), and Iba1 (cat. no. NB100-1028; 1:250; Novus Biologicals, LLC, Littleton, CO, USA) were incubated with the sections at 4°C overnight and then the sections were incubated with secondary antibodies, provided in the DAB kit-0017, incubated at room temperature for 10 min. The positive cells were stained brown with DAB. Images were captured using a fluorescence microscope (DFC310 FX; Leica Microsystems GmbH) at ×400 magnification. Positive cells were counted in four randomly selected microscopic fields using the Motic Med 6.0 CMIAS pathology image analysis system (Beihang Motic Inc., Beijing, China).
Western blot analysis
Hippocampal tissues were homogenized in non-denaturing lysis buffer (Beyotime Institute of Biotechnology Co., Ltd., Beijing, China; no. P0013B). Tissues were then ground on ice and incubated for 30 min prior to 10 min centrifugation at 1,465 × g at 4°C to separate the supernatant. Total protein in each sample was measured using the bicinchoninic acid (BCA) assay. A total of 50 µg protein was separated on a 10% SDS-PAGE gel and transferred to a polyvinylidene fluoride (PVDF) membrane. The membranes were blocked for 2 h with blocking buffer (P0023B; Beyotime Institute of Biotechnology Co., Ltd.) and then incubated with primary antibodies targeting α7nAChR (cat. no. ab24644; 1:10,000; Abcam), TNF-α (cat. no. 3707; 1:500; Cell Signaling Technologies, Inc.), IL-1β (1:200; sc-12742; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) or GAPDH (cat. no. ab8245; 1:8,000; Abcam) at 4°C overnight. Following washing with TBS containing 0.05% Tween-20, the membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibody (cat. no. 7076 for GAPDH; and cat. no. 7074 for α7nAChR, TNF-α and IL-1β; 1:5,000; Cell Signaling Technologies, Inc.) for 1 h at room temperature. Blots were developed using enhanced chemiluminescence (Beyotime Institute of Biotechnology, Co., Ltd.) and images were obtained and analyzed using a Bio-Image Analysis System, version 1.42q (Bio-Rad Laboratories, Hercules, CA, USA). The optical densities of the target protein were normalized against the GAPDH band and the analysis was replicated 3 times.
Statistical analysis
The experimental results for each group are expressed as the mean ± standard error of the mean. Statistical analysis was performed with one-way analysis of variance using the SPSS package for Windows (Version 18.0; SPSS, Inc., Chicago, IL, USA), homogeneity of variance with a LSD test, and heterogeneity of variance with Games-Howell (A) test. P<0.05 was considered to represent a statistically significant difference.
Results
EA reduces learning and memory impairment following focal cerebral ischemic injury
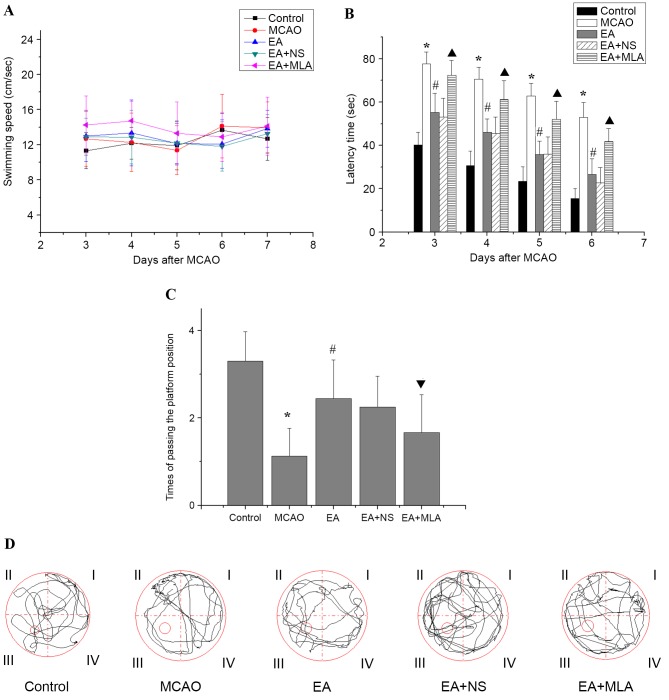
Following modeling, the rats in the control group were of good health with no fatalities. In the model group, 3 rats died due to epilepsy. A further 3 mortalities were observed in the EA group, 2 in the EA + NS group and 2 in the EA + MLA group. Concerning the Morris water maze performance, as presented in Fig. 1, no significant differences were detected in swimming speed on days 3, 4, 5, 6 and 7 following MCAO among the five groups (Fig. 1A; P>0.05). This result indicates that the MCAO model did not affect rat motor function in the Morris water maze. Rats in the MCAO group demonstrated a longer latency time (Fig. 1B) to reach the hidden platform and passed the platform position fewer times (Fig. 1C and D) in the water maze tests than did those in the control group. In EA-treated rats, latency time was significantly reduced and frequency in passing the platform was increased compared with that in the MCAO group (Fig. 1B and C; P<0.05). However, rats treated with EA + MLA demonstrated prolonged latency with a decreased number of times crossing the platform compared with the EA + NS group.
Figure 1.
Effects of EA on the learning and memory of transient focal cerebral ischemic injured rats in the water maze. (A) No significant differences in swimming speed were observed among the control (n=10), MCAO (n=8), EA (n=9), EA + NS (n=9) and EA+ MLA (n=9) groups up to day 7. (B) The latency time to reach the hidden platform, (C) the number of times the rats passed the platform position and (D) typical traces during the test. *P<0.01 vs. control, #P<0.01 vs. MCAO, ▲P<0.01 vs. EA + NS and ▼P<0.05 vs. EA + NS. Data are expressed as mean ± standard error of the mean. EA, electroacupuncture; MCAO, middle cerebral artery occlusion; NS, normal saline; MLA, methyllycaconitine.
EA activates α7nAChR expression in the hippocampus
As indicated in Fig. 2, immunohistochemical analysis revealed a significant reduction in the number of α7nAChR-positive cells in the CA1 region of the hippocampus in the MCAO group compared with the control group (P<0.05). However, EA reversed the α7nAChR reduction caused by I/R injury. Furthermore, MLA decreased α7nAChR expression levels compared with those in the EA + NS group.
Figure 2.
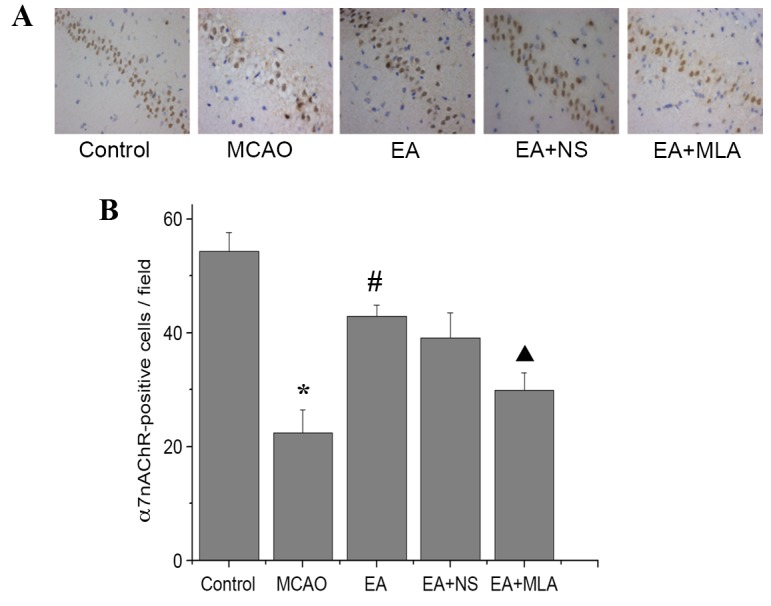
EA increases the expression of α7nAChR in the hippocampus. Distribution of α7nAChR-positive cells in the CA1 region of the hippocampus in the five groups stained with DAB (A) observed under a microscope (magnification, ×400) and (B) quantified using an image analysis system. *P<0.01 vs. control, #P<0.01 vs. MCAO and ▲P<0.01 vs. EA + NS. Data are expressed as mean ± standard error of the mean (n=5). EA, electroacupuncture; α7nAChR, α7 nicotinic acetylcholine receptor; MCAO, middle cerebral artery occlusion; NS, normal saline; MLA, methyllycaconitine; DAB, 3,3′-Diaminobenzidine.
EA suppresses neuroinflammation via α7-dependent cholinergic pathways in the hippocampus
Cerebral ischemic injury triggers a serious inflammatory response in the brain. As presented in Fig. 3, elevated expression of the astrocyte marker GFAP and microglia/macrophage marker Iba1 was detected in the hippocampus in the MCAO group compared with the control group. Western blot analysis of α7nAChR was consistent with the immunohistochemical analysis, and confirmed that the EA-induced increase in α7nAChR was attenuated by MLA (Fig. 4A and B). Furthermore, elevated expression of the key inflammatory factors TNF-α and IL-1β (Fig. 4A and C) was detected in the hippocampus in the MCAO group compared with the control group. In the EA treatment group, Iba1, GFAP and inflammatory factors were significantly reduced compared with those in the MCAO group (Figs. 3C and 4C; P<0.05). However, those inhibitions were markedly eradicated in the EA + MLA group compared with the EA + NS group.
Figure 3.
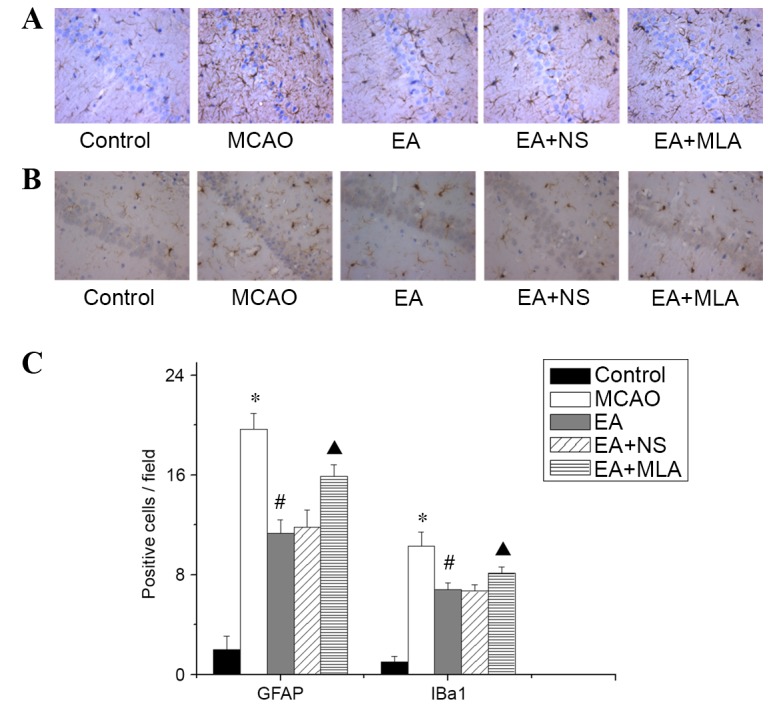
EA reduces the activity of microglia and astrocytes in the hippocampus. (A) GFAP-positive astrocytes and (B) Iba1-positive microglia in the CA1 region of the hippocampus in the five groups, stained with DAB. (C) Quantified data for the five groups. *P<0.01 vs. control, #P<0.01 vs. MCAO and ▲P<0.01 vs. EA + NS. Data are expressed as mean ± standard error of the mean (n=5). Magnification of ×400. EA, electroacupuncture; GFAP, glial fibrillary acidic protein; Iba1, microglial marker; MCAO, middle cerebral artery occlusion; NS, normal saline; MLA, methyllycaconitine; DAB, 3,3′-Diaminobenzidine.
Figure 4.
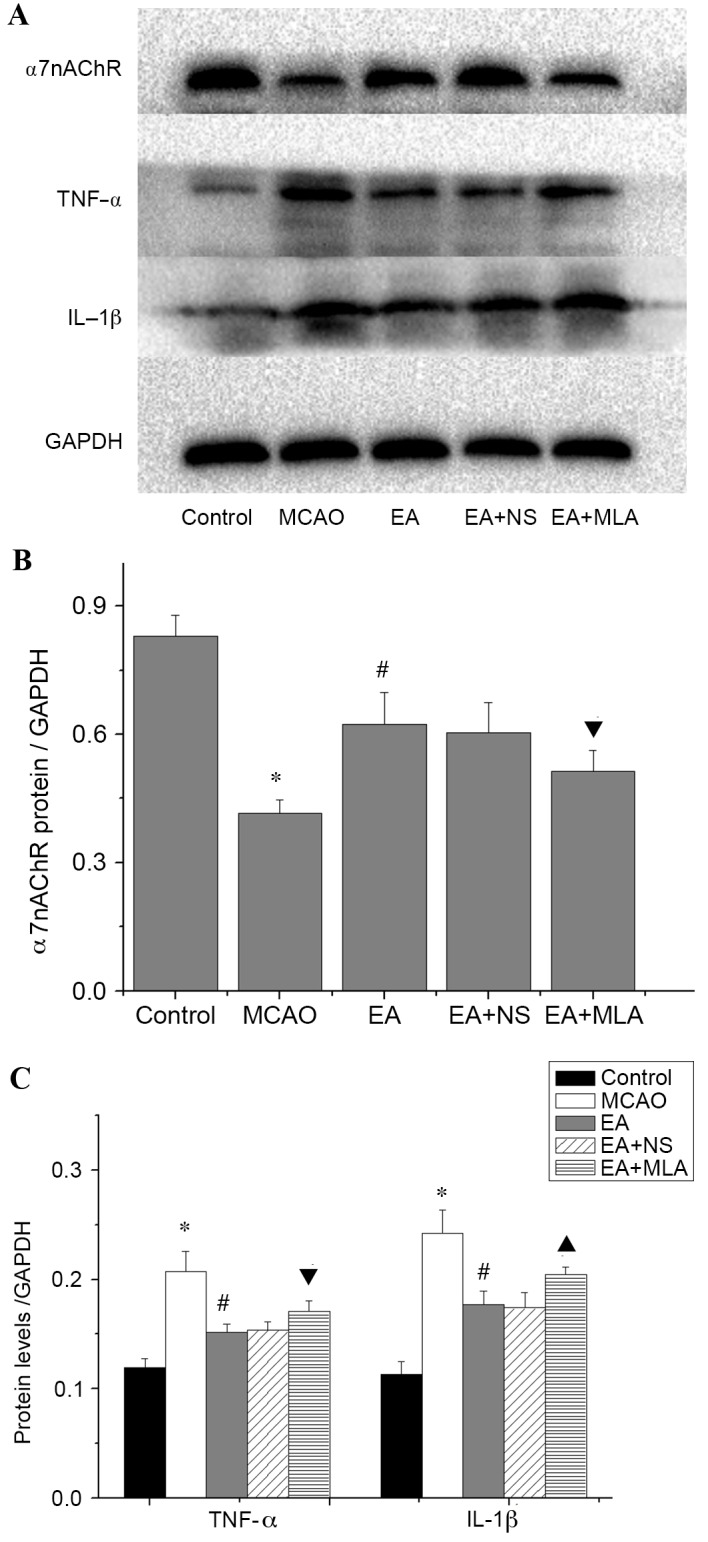
Western blot analysis of inflammatory protein expression in the hippocampus. (A) Western blot analysis demonstrating the concentrations of α7nAChR, TNF-α, IL-1B and GAPDH. (B) Quantification of the western blots for the levels of α7nAChR and (C) proinflammatory cytokines TNF-α and IL-1β in the five groups. *P<0.01 vs. control, #P<0.01 vs. MCAO, ▲P<0.01 vs. EA + NS and ▼P<0.05 vs. EA + NS. Data are expressed as mean ± standard error of the mean (n=5). α7nAChR, α7 nicotinic acetylcholine receptors; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MCAO, middle cerebral artery occlusion; EA, electroacupuncture; NS, normal saline; MLA, methyllycaconitine.
Discussion
In the present study, EA was demonstrated to reduce learning and memory deficits via activation of α7nAChR-dependent anti-inflammatory pathways. In the Morris water maze assessment, the rats demonstrated a prolonged latency to find the hidden platform and decreased times of passing the platform position following MCAO treatment. In rats treated with EA, latency was shortened and the platform crossing time was increased. These results suggest that EA at the DU 20 and DU 24 acupoints improved learning and memory ability in cerebral ischemia-injured rats, which is consistent with previous studies (33,34).
A large hippocampal neuroinflammatory response was observed following MCAO, and EA reduced this inflammatory response as demonstrated by the reduction of Iba1 and GFAP expression and TNF-α and IL-1β production in the injured hippocampus. The histological evidence further suggests that α7nAChR mediates the reduction of neuroinflammation. It is known that α7nAChR is essential for the cholinergic anti-inflammatory response because loss of the α7 nicotinic receptor subunit fails to inhibit cytokine synthesis (35). Studies indicate that two critical signaling pathways, nuclear factor-κB (NF-κB) and janus kinase/signal transducer and activator of transcription (Jak/STAT), are required for the α7nAChR-dependent anti-inflammatory response. Activation of the α7nAChR prevents Iκ-B breakdown and promotes p65 nuclear translocation to suppress the transcription of inflammatory cytokines (36,37). α7nAChR may recruit the tyrosine kinase Jak2, and activate the transcription factor STAT3 to inhibit pro-inflammatory gene transcription (38). Previous research reports that EA may suppress NF-κB activation to induce an anti-inflammation response (39) and enhance STAT3 activation (40).
Global cerebral ischemia diminishes the capacity of the cholinergic anti-inflammatory pathway to control inflammation, but the application of an α7nAChR agonist protects against ischemia-induced cell death and inflammation in the hippocampus. This significantly decreases the mRNA expression of proinflammatory cytokines and reduces microglial activation, but not astrocyte activation (41,42). Conversely, treatment with an α7nAChR antagonist following global cerebral ischemia worsened neuronal death and led to increased microglial activation (41,42). In a mouse model of Parkinson's disease, an α7nAChR agonist decreased the activation of astrocytes and microglia in the substantia nigra, and these protective effects were abolished by administration of an α7nAChR-selective antagonist in vitro and in vivo (43). The current study indicates that the neuroprotective effects of EA are due to its actions as a α7nAChR agonist. Future studies may involve investigating an α7nAChR agonist in comparison with EA to validate this hypothesis.
Another potential mechanism for the action of EA-induced improvements in memory function involves effects on synaptic plasticity. Neuroinflammation has been confirmed to have a negative effect on learning and memory processes by blocking long-term potentiation (LTP) in the hippocampus in vitro and in vivo (44–46). Synaptic plasticity, neurotransmitter release and fast synaptic transmission are also modulated by the activation of neuronal α7nAChR (47,48). In addition, EA pretreatment has been demonstrated to protect the brain against transient cerebral ischemic injury via increased α7nAChR expression on neurons (29). The current study suggests that EA-mediated regulation of neuroinflammation may enhance LTP, and combined with EA regulation of α7nAChR expression on neurons, improve spatial learning and memory as a result.
In the present study, EA-induced expression of α7nAChR following temporary MCAO may serve the same role as an α7nAChR agonist in focal cerebral ischemia. Similarly, co-treatment with MLA, an α7nAChR antagonist, significantly affected the inhibitory effects of EA on glial activation and expression of inflammatory factors. In addition, MLA inhibited the improvement of spatial learning and memory following EA treatment. From these data, it may be inferred that EA activates an α7nAChR-dependent anti-inflammatory pathway to control neuroinflammation, which, in turn, leads to improvement in learning and memory outcomes after I/R brain injury.
In conclusion, the current study demonstrates that EA may reduce learning and memory impairment following cerebral I/R injury. The protective effects of EA appear to be mediated via the α7nAChR-mediated anti-inflammatory pathway. EA improves cognitive function by an α7nAChR-mediated mechanism that decreases neuroinflammation, as demonstrated by reduced glial activation and inflammatory cytokine production. However, A potential limitation of the present study is the lack of a positive control using an a7nAChR agonist group, which should be considered in future studies to compare the effects of EA and a7nAChR agonists.
Acknowledgements
This study was supported by the Fujian Rehabilitation Technology of Collaborative Innovation Center Support Project (grant no. X2012004), the Natural Science Foundation of China (grant no. 81403462), the Fujian Natural Science Foundation of China (grant no. 2015J01335) and the Fujian Key Laboratory of Rehabilitation Technology.
References
- 1.Bae CY, Sun HS. Current understanding of TRPM7 pharmacology and drug development for stroke. Acta Pharmacol Sin. 2013;34:10–16. doi: 10.1038/aps.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niemi ML, Laaksonen R, Kotila M, Waltimo O. Quality of life 4 years after stroke. Stroke. 1988;19:1101–1107. doi: 10.1161/01.STR.19.9.1101. [DOI] [PubMed] [Google Scholar]
- 3.Carey LM. Somatosensory loss after stroke. Crit Rev Phys Rehabil Med. 1995;7:51–91. doi: 10.1615/CritRevPhysRehabilMed.v7.i1.40. [DOI] [Google Scholar]
- 4.Jin YP, Di Legge S, Ostbye T, Feightner JW, Hachinski V. The reciprocal risks of stroke and cognitive impairment in an elderly population. Alzheimers Dement. 2006;2:171–178. doi: 10.1016/j.jalz.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Pendlebury ST, Rothwell PM. Risk of recurrent stroke, other vascular events and dementia after transient ischaemic attack and stroke. Cerebrovasc Dis. 2009;27(Suppl 3):S1–S11. doi: 10.1159/000209260. [DOI] [PubMed] [Google Scholar]
- 6.Pohjasvaara T, Erkinjuntti T, Vataja R, Kaste M. Dementia three months after stroke. Baseline frequency and effect of different definitions of dementia in the Helsinki Stroke Aging Memory Study (SAM) cohort. Stroke. 1997;28:785–792. doi: 10.1161/01.STR.28.4.785. [DOI] [PubMed] [Google Scholar]
- 7.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: A systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 8.Chan KY, Wang W, Wu JJ, Liu L, Theodoratou E, Car J, Middleton L, Russ TC, Deary IJ, Campbell H, et al. Epidemiology of Alzheimer's disease and other forms of dementia in China, 1990–2010: A systematic review and analysis. Lancet. 2013;381:2016–2023. doi: 10.1016/S0140-6736(13)60221-4. [DOI] [PubMed] [Google Scholar]
- 9.Aggarwal NT, Tripathi M, Dodge HH, Alladi S, Anstey KJ. Trends in Alzheimer's disease and dementia in the asian-pacific region. Int J Alzheimers Dis. 2012;2012:171327. doi: 10.1155/2012/171327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu JN. A short history of acupuncture. J Altern Complement Med. 1996;2:19–21. doi: 10.1089/acm.1996.2.19. [DOI] [PubMed] [Google Scholar]
- 11.Liu F, Li ZM, Jiang YJ, Chen LD. A meta-analysis of acupuncture use in the treatment of cognitive impairment after stroke. J Altern Complement Med. 2014;20:535–544. doi: 10.1089/acm.2013.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Zhou J, Li J, Yang SB, Mo LQ, Hu JH, Yuan WL. Electroacupuncture pretreatment prevents cognitive impairment induced by limb ischemia-reperfusion via inhibition of microglial activation and attenuation of oxidative stress in rats. Brain Res. 2012;1432:36–45. doi: 10.1016/j.brainres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Zhang C, Wen Y, Fan XN, Tian G, Zhou XY, Deng SZ, Meng ZH. Therapeutic effects of different durations of acupuncture on rats with middle cerebral artery occlusion. Neural Regen Res. 2015;10:159–164. doi: 10.4103/1673-5374.150727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan XW, Chen F, Chen Y, Chen GH, Liu HH, Guan SK, Deng Y, Liu Y, Zhang SJ, Peng WJ, et al. Electroacupuncture prevents cognitive impairments by regulating the early changes after brain irradiation in rats. PLoS One. 2015;10:e0122087. doi: 10.1371/journal.pone.0122087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoll G, Jander S, Schroeter M. Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol. 1998;56:149–171. doi: 10.1016/S0301-0082(98)00034-3. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Webster SJ, Van Eldik LJ, Watterson DM, Bachstetter AD. Closed head injury in an age-related Alzheimer mouse model leads to an altered neuroinflammatory response and persistent cognitive impairment. J Neurosci. 2015;35:6554–6569. doi: 10.1523/JNEUROSCI.0291-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson VE, Stewart W, Smith DH. Traumatic brain injury and amyloid-β pathology: A link to Alzheimer's disease? Nat Rev Neurosci. 2010;11:361–370. doi: 10.1038/nrn2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, Gentleman S, Heckemann RA, Gunanayagam K, Gelosa G, Sharp DJ. Inflammation after trauma: Microglial activation and traumatic brain injury. Ann Neurol. 2011;70:374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- 20.Buchanan JB, Sparkman NL, Chen J, Johnson RW. Cognitive and neuroinflammatory consequences of mild repeated stress are exacerbated in aged mice. Psychoneuroendocrinology. 2008;33:755–765. doi: 10.1016/j.psyneuen.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chin Y, Kishi M, Sekino M, Nakajo F, Abe Y, Terazono Y, Hiroyuki O, Kato F, Koizumi S, Gachet C, Hisatsune T. Involvement of glial P2Y1 receptors in cognitive deficit after focal cerebral stroke in a rodent model. J Neuroinflammation. 2013;10:95. doi: 10.1186/1742-2094-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tweedie D, Ferguson RA, Fishman K, Frankola KA, Van Praag H, Holloway HW, Luo W, Li Y, Caracciolo L, Russo I, et al. Tumor necrosis factor-α synthesis inhibitor 3,6′-dithiothalidomide attenuates markers of inflammation, Alzheimer pathology and behavioral deficits in animal models of neuroinflammation and Alzheimer's disease. J Neuroinflammation. 2012;9:106. doi: 10.1186/1742-2094-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 24.Boccia MM, Blake MG, Krawczyk MC, Baratti CM. Hippocampal α7 nicotinic receptors modulate memory reconsolidation of an inhibitory avoidance task in mice. Neuroscience. 2010;171:531–543. doi: 10.1016/j.neuroscience.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 25.Acheson DT, Twamley EW, Young JW. Reward learning as a potential target for pharmacological augmentation of cognitive remediation for schizophrenia: A roadmap for preclinical development. Front Neurosci. 2013;7:103. doi: 10.3389/fnins.2013.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kadir A, Almkvist O, Wall A, Langstrom B, Nordberg A. PET imaging of cortical 11C-nicotine binding correlates with the cognitive function of attention in Alzheimer's disease. Psychopharmacology (Berl) 2006;188:509–520. doi: 10.1007/s00213-006-0447-7. [DOI] [PubMed] [Google Scholar]
- 27.Lee ST, Chu K, Jung KH, Kang KM, Kim JH, Bahn JJ, Jeon D, Kim M, Lee SK, Roh JK. Cholinergic anti-inflammatory pathway in intracerebral hemorrhage. Brain Res. 2010;1309:164–171. doi: 10.1016/j.brainres.2009.10.076. [DOI] [PubMed] [Google Scholar]
- 28.Liu XY, Zhou HF, Pan YL, Liang XB, Niu DB, Xue B, Li FQ, He QH, Wang XH, Wang XM. Electro-acupuncture stimulation protects dopaminergic neurons from inflammation-mediated damage in medial forebrain bundle-transected rats. Exp Neurol. 2004;189:189–196. doi: 10.1016/j.expneurol.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 29.Wang Q, Wang F, Li X, Yang Q, Li X, Xu N, Huang Y, Zhang Q, Gou X, Chen S, Xiong L. Electroacupuncture pretreatment attenuates cerebral ischemic injury through α7 nicotinic acetylcholine receptor-mediated inhibition of high-mobility group box 1 release in rats. J Neuroinflammation. 2012;9:24. doi: 10.1186/1742-2094-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.STR.20.1.84. [DOI] [PubMed] [Google Scholar]
- 31.Chen LP, Wang FW, Zuo F, Jia JJ, Jiao WG. Clinical research on comprehensive treatment of senile vascular dementia. J Tradit Chin Med. 2011;31:178–181. doi: 10.1016/S0254-6272(11)60036-8. [DOI] [PubMed] [Google Scholar]
- 32.Tyagi E, Agrawal R, Nath C, Shukla R. Cholinergic protection via alpha7 nicotinic acetylcholine receptors and PI3K-Akt pathway in LPS-induced neuroinflammation. Neurochem Int. 2010;56:135–142. doi: 10.1016/j.neuint.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Feng X, Yang S, Liu J, Huang J, Peng J, Lin J, Tao J, Chen L. Electroacupuncture ameliorates cognitive impairment through inhibition of NF-κB-mediated neuronal cell apoptosis in cerebral ischemia-reperfusion injured rats. Mol Med Rep. 2013;7:1516–1522. doi: 10.3892/mmr.2013.1392. [DOI] [PubMed] [Google Scholar]
- 34.Han X, Zhao X, Lu M, Liu F, Guo F, Zhang J, Huang X. Electroacupuncture ameliorates learning and memory via activation of the CREB signaling pathway in the hippocampus to attenuate apoptosis after cerebral hypoperfusion. Evid Based Complement Alternat Med. 2013;2013:156489. doi: 10.1155/2013/156489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 36.Yoshikawa H, Kurokawa M, Ozaki N, Nara K, Atou K, Takada E, Kamochi H, Suzuki N. Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-kappaB phosphorylation and nuclear factor-kappaB transcriptional activity through nicotinic acetylcholine receptor alpha7. Clin Exp Immunol. 2006;146:116–123. doi: 10.1111/j.1365-2249.2006.03169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, Huston J, Tracey KJ, Al-Abed Y, Metz CN. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005;201:1113–1123. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, Boeckxstaens GE. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–851. doi: 10.1038/ni0905-954b. [DOI] [PubMed] [Google Scholar]
- 39.Liu F, Fang J, Shao X, Liang Y, Wu Y, Jin Y. Electroacupuncture exerts an anti-inflammatory effect in a rat tissue chamber model of inflammation via suppression of NF-κB activation. Acupunct Med. 2014;32:340–345. doi: 10.1136/acupmed-2013-010460. [DOI] [PubMed] [Google Scholar]
- 40.Zhou H, Zhang Z, Wei H, Wang F, Guo F, Gao Z, Marsicano G, Wang Q, Xiong L. Activation of STAT3 is involved in neuroprotection by electroacupuncture pretreatment via cannabinoid CB1 receptors in rats. Brain Res. 2013;1529:154–164. doi: 10.1016/j.brainres.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Norman GJ, Morris JS, Karelina K, Weil ZM, Zhang N, Al-Abed Y, Brothers HM, Wenk GL, Pavlov VA, Tracey KJ, Devries AC. Cardiopulmonary arrest and resuscitation disrupts cholinergic anti-inflammatory processes: A role for cholinergic α7 nicotinic receptors. J Neurosci. 2011;31:3446–3452. doi: 10.1523/JNEUROSCI.4558-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guan YZ, Jin XD, Guan LX, Yan HC, Wang P, Gong Z, Li SJ, Cao X, Xing YL, Gao TM. Nicotine inhibits microglial proliferation and is neuroprotective in global ischemia rats. Mol Neurobiol. 2015;51:1480–1488. doi: 10.1007/s12035-014-8825-3. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, Hu J, Wu J, Zhu C, Hui Y, Han Y, Huang Z, Ellsworth K, Fan W. α7 nicotinic acetylcholine receptor-mediated neuroprotection against dopaminergic neuron loss in an MPTP mouse model via inhibition of astrocyte activation. J Neuroinflammation. 2012;9:98. doi: 10.1186/1742-2094-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bellinger FP, Madamba S, Siggins GR. Interleukin 1 beta inhibits synaptic strength and long-term potentiation in the rat CA1 hippocampus. Brain Res. 1993;628:227–234. doi: 10.1016/0006-8993(93)90959-Q. [DOI] [PubMed] [Google Scholar]
- 45.Kelly A, Vereker E, Nolan Y, Brady M, Barry C, Loscher CE, Mills KH, Lynch MA. Activation of p38 plays a pivotal role in the inhibitory effect of lipopolysaccharide and interleukin-1 beta on long term potentiation in rat dentate gyrus. J Biol Chem. 2003;278:19453–19462. doi: 10.1074/jbc.M301938200. [DOI] [PubMed] [Google Scholar]
- 46.Tong L, Prieto GA, Kramér EA, Smith ED, Cribbs DH, Lynch G, Cotman CW. Brain-derived neurotrophic factor-dependent synaptic plasticity is suppressed by interleukin-1β via p38 mitogen-activated protein kinase. J Neurosci. 2012;32:17714–17724. doi: 10.1523/JNEUROSCI.1253-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ondrejcak T, Wang Q, Kew JN, Virley DJ, Upton N, Anwyl R, Rowan MJ. Activation of α7 nicotinic acetylcholine receptors persistently enhances hippocampal synaptic transmission and prevents Ass-mediated inhibition of LTP in the rat hippocampus. Eur J Pharmacol. 2012;677:63–70. doi: 10.1016/j.ejphar.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 48.Huang M, Felix AR, Kwon S, Lowe D, Wallace T, Santarelli L, Meltzer HY. The alpha-7 nicotinic receptor partial agonist/5-HT3 antagonist RG3487 enhances cortical and hippocampal dopamine and acetylcholine release. Psychopharmacology (Berl) 2014;231:2199–2210. doi: 10.1007/s00213-013-3373-5. [DOI] [PubMed] [Google Scholar]