Abstract
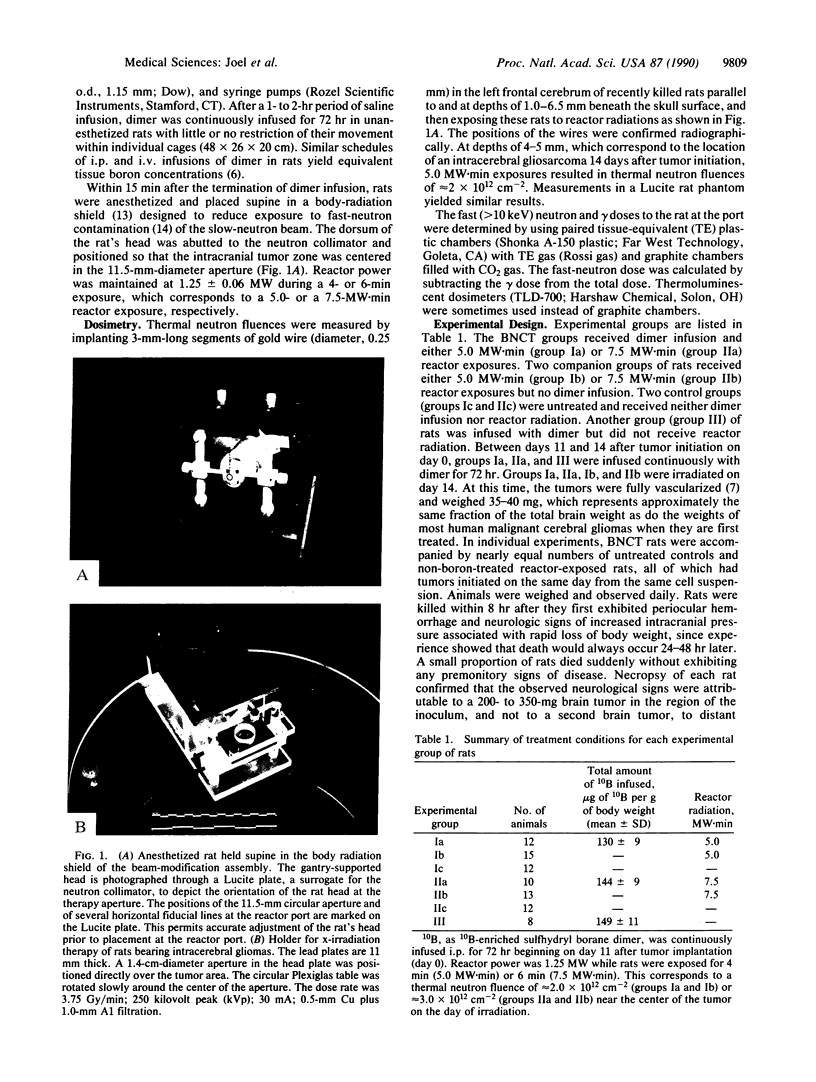
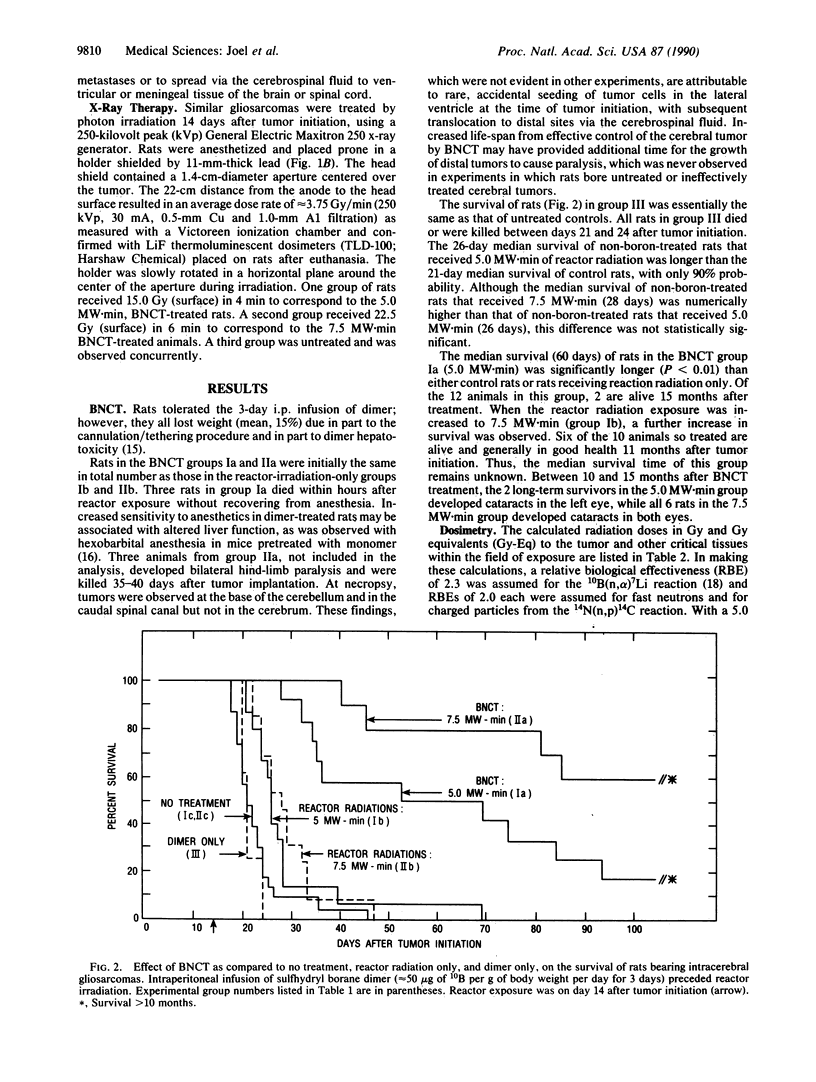
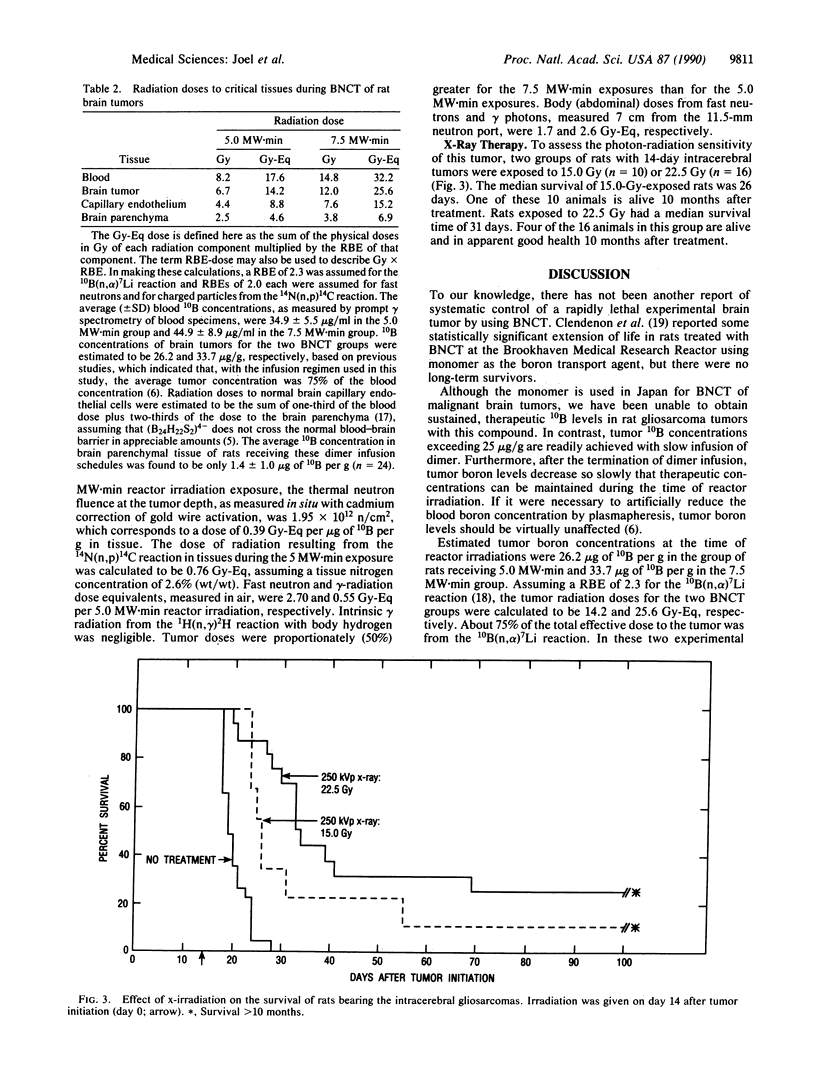
The efficacy of boron neutron capture therapy (BNCT) for the treatment of intracerebrally implanted rat gliosarcomas was tested. Preferential accumulation of 10B in tumors was achieved by continuous infusion of the sulfhydryl borane dimer, Na4(10)B24H22S2, at a rate of 45-50 micrograms of 10B per g of body weight per day from day 11 to day 14 after tumor initiation (day 0). This infusion schedule resulted in average blood 10B concentrations of 35 micrograms/ml in a group of 12 gliosarcoma-bearing rats and 45 micrograms/ml in a group of 10 similar gliosarcoma-bearing rats treated by BNCT. Estimated tumor 10B levels in these two groups were 26 and 34 micrograms/g, respectively. On day 14, boron-treated and non-boron-treated rats were exposed to 5.0 or 7.5 MW.min of radiation from the Brookhaven Medical Research Reactor that yielded thermal neutron fluences of approximately 2.0 x 10(12) or approximately 3.0 x 10(12) n/cm2, respectively, in the tumors. Untreated rats had a median postinitiation survival time of 21 days. Reactor radiation alone increased median postinitiation survival time to 26 (5.0 MW.min) or 28 (7.5 MW.min) days. The 12 rats that received 5 MW.min of BNCT had a median postinitiation survival time of 60 days. Two of these animals survived greater than 15 months. In the 7.5 MW.min group, the median survival time is not calculable since 6 of the 10 animals remain alive greater than 10 months after BNCT. The estimated radiation doses to tumors in the two BNCT groups were 14.2 and 25.6 Gy equivalents, respectively. Similar gliosarcoma-bearing rats treated with 15.0 or 22.5 Gy of 250-kilovolt peak x-rays had median survival times of only 26 or 31 days, respectively, after tumor initiation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker M., Deen D. F., Baker D. G. BCNU and X-ray therapy of intracerebral 9L rat tumors. Int J Radiat Oncol Biol Phys. 1979 Sep;5(9):1581–1583. doi: 10.1016/0360-3016(79)90776-4. [DOI] [PubMed] [Google Scholar]
- Barker M., Hoshino T., Wheeler K. T., Wilson C. B. Chemotherapeutic implications of early tumor cell growth in an animal brain-tumor model. J Natl Cancer Inst. 1975 Apr;54(4):851–853. [PubMed] [Google Scholar]
- Calvo W., Hopewell J. W., Reinhold H. S., Yeung T. K. Time- and dose-related changes in the white matter of the rat brain after single doses of X rays. Br J Radiol. 1988 Nov;61(731):1043–1052. doi: 10.1259/0007-1285-61-731-1043. [DOI] [PubMed] [Google Scholar]
- Clendenon N. R., Barth R. F., Gordon W. A., Goodman J. H., Alam F., Staubus A. E., Boesel C. P., Yates A. J., Moeschberger M. L., Fairchild R. G. Boron neutron capture therapy of a rat glioma. Neurosurgery. 1990 Jan;26(1):47–55. doi: 10.1097/00006123-199001000-00007. [DOI] [PubMed] [Google Scholar]
- Fairchild R. G., Gabel D., Laster B. H., Greenberg D., Kiszenick W., Micca P. L. Microanalytical techniques for boron analysis using the 10B(n,alpha)7Li reaction. Med Phys. 1986 Jan-Feb;13(1):50–56. doi: 10.1118/1.595962. [DOI] [PubMed] [Google Scholar]
- Fairchild R. G., Robertson J. S., Levine D. A., Goodman L. J. A tissue-equivalent ionization chamber for inphantom dosimetry: comparison of measured values with calculated values. Health Phys. 1966 Jun;12(6):787–792. doi: 10.1097/00004032-196606000-00007. [DOI] [PubMed] [Google Scholar]
- Fontenelle L., Garner G., Graham B., Goettsch R., Soltani B. In-vivo and in-vitro studies of the effects of mercaptoundecahydrododecaborate (BSH) on hepatic cytochrome P-450 mono-oxygenase enzyme activity and on hexobarbital (HXB) induced sleep in mice. Strahlenther Onkol. 1989 Feb-Mar;165(2-3):172–173. [PubMed] [Google Scholar]
- Gabel D., Fairchild R. G., Larsson B., Börner H. G. The relative biological effectiveness in V79 Chinese hamster cells of the neutron capture reactions in boron and nitrogen. Radiat Res. 1984 May;98(2):307–316. [PubMed] [Google Scholar]
- Joel D., Slatkin D., Fairchild R., Micca P., Nawrocky M. Pharmacokinetics and tissue distribution of the sulfhydryl boranes (monomer and dimer) in glioma-bearing rats. Strahlenther Onkol. 1989 Feb-Mar;165(2-3):167–170. [PubMed] [Google Scholar]
- Marshall P. G., Miller M. E., Grand S., Micca P. L., Slatkin D. N. Toxicities of Na2B12H11SH and Na4B24H22S2 in mice. Basic Life Sci. 1989;50:333–351. doi: 10.1007/978-1-4684-5622-6_37. [DOI] [PubMed] [Google Scholar]
- Reinhold H. S., Calvo W., Hopewell J. W., van der Berg A. P. Development of blood vessel-related radiation damage in the fimbria of the central nervous system. Int J Radiat Oncol Biol Phys. 1990 Jan;18(1):37–42. doi: 10.1016/0360-3016(90)90264-k. [DOI] [PubMed] [Google Scholar]
- SWEET W. H. The uses of nuclear disintegration in the diagnosis and treatment of brain tumor. N Engl J Med. 1951 Dec 6;245(23):875–878. doi: 10.1056/NEJM195112062452301. [DOI] [PubMed] [Google Scholar]
- Schmidek H. H., Nielsen S. L., Schiller A. L., Messer J. Morphological studies of rat brain tumors induced by N-nitrosomethylurea. J Neurosurg. 1971 Mar;34(3):335–340. doi: 10.3171/jns.1971.34.3.0335. [DOI] [PubMed] [Google Scholar]
- Slatkin D. N., Kalef-Ezra J. A., Saraf S. K., Joel D. D. A beam-modification assembly for experimental neutron capture therapy of brain tumors. Basic Life Sci. 1990;54:317–320. doi: 10.1007/978-1-4684-5802-2_24. [DOI] [PubMed] [Google Scholar]
- Slatkin D. N., Stoner R. D., Rosander K. M., Kalef-Ezra J. A., Laissue J. A. Central nervous system radiation syndrome in mice from preferential 10B(n, alpha)7Li irradiation of brain vasculature. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4020–4024. doi: 10.1073/pnas.85.11.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatkin D., Micca P., Forman A., Gabel D., Wielopolski L., Fairchild R. Boron uptake in melanoma, cerebrum and blood from Na2B12H11SH and Na4B24H22S2 administered to mice. Biochem Pharmacol. 1986 May 15;35(10):1771–1776. doi: 10.1016/0006-2952(86)90342-4. [DOI] [PubMed] [Google Scholar]
- Soloway A. H., Hatanaka H., Davis M. A. Penetration of brain and brain tumor. VII. Tumor-binding sulfhydryl boron compounds. J Med Chem. 1967 Jul;10(4):714–717. doi: 10.1021/jm00316a042. [DOI] [PubMed] [Google Scholar]




