Abstract
Extensively drug-resistant tuberculosis is a burgeoning global health crisis mainly affecting economically active young adults, and has high mortality irrespective of HIV status. In some countries such as South Africa, drug-resistant tuberculosis represents less than 3% of all cases but consumes more than a third of the total national budget for tuberculosis, which is unsustainable and threatens to destabilise national tuberculosis programmes. However, concern about drug-resistant tuberculosis has been eclipsed by that of totally and extremely drug-resistant tuberculosis—ie, resistance to all or nearly all conventional first-line and second-line antituberculosis drugs. In this Review, we discuss the epidemiology, pathogenesis, diagnosis, management, implications for health-care workers, and ethical and medicolegal aspects of extensively drug-resistant tuberculosis and other resistant strains. Finally, we discuss the emerging problem of functionally untreatable tuberculosis, and the issues and challenges that it poses to public health and clinical practice. The emergence and growth of highly resistant strains of tuberculosis make the development of new drugs and rapid diagnostics for tuberculosis—and increased funding to strengthen global control efforts, research, and advocacy—even more pressing.
Introduction
Although the incidence of tuberculosis is decreasing in several parts of the world (eg, the Americas, Europe, western Pacific, and southeast Asia), in other regions (eg, Africa) tuberculosis is out of control, and is responsible for about 1·5 million deaths annually.1 The incidence of multidrug-resistant tuberculosis (ie, resistance to at least isoniazid and rifampicin) has increased during the past decade in many parts of the world.2 More recently, this concern has been overtaken by the threat of extensively drug-resistant tuberculosis (resistance to isoniazid, rifampicin, one fluoroquinolone, and one second-line injectable drug; figure 1).3–5 Appropriate recognition, prevention, and treatment of this new threat is crucial for several reasons. Drug-resistant tuberculosis and its progressive forms have poorer outcomes than does drug-susceptible tuberculosis; drug-resistant disease frequently affects economically active young adults, and although multidrug-resistant and extensively drug-resistant tuberculosis represent a very small fraction of the total tuberculosis burden in resource-poor settings, these strains consume a disproportionate percentage of resources in tuberculosis programmes. For example, in South Africa multidrug-resistant and extensively drug-resistant strains of tuberculosis represent less than 3% of the total tuberculosis caseload, but consume about 35% of the total budget of the national tuberculosis programme; a substantial proportion of this spending is attributable to extensively drug-resistant tuber culosis.6 In this Review, we discuss several important aspects of extensively drug-resistant tuberculosis and resistance beyond extensively drug-resistant tuberculosis.
Figure 1. Global distribution of extensively drug-resistant tuberculosis by genotype and country.
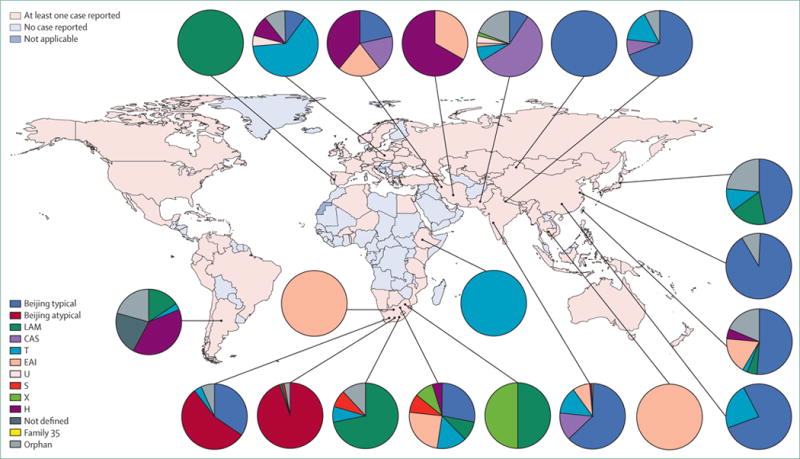
The proportion of isolates with a defined genotype are shown for South Africa, Ethiopia, Argentina, Portugal, Poland, Iran, Pakistan, India, Nepal, Cambodia, China, Taiwan, and Japan. We classified Beijing genotype strains from South Africa as typical or atypical to show regional differences in the population structure of extensively drug-resistant tuberculosis. Data sources provided in the appendix. LAM=Latino-American-Mediterranean family. CAS=Central-Asian family. T=T family. EAI=East-African-Indian family. U=U family. S=S family. X=X family. H=Haarlem family.
Definitions and terminology
Drug-resistant tuberculosis encompasses multidrug-resistant, extensively drug-resistant, extremely drug-resistant,7 and totally drug-resistant strains (panel 1).3–5 Extremely drug-resistant tuberculosis was first described in two Italian women who died after 422 and 625 days spent in hospital, and 94 and 60 months of treatment, respectively.7 Totally drug-resistant tuberculosis has been described in Iran, India, and South Africa.3–5 Resistance beyond extensively drug-resistant tuberculosis has not been defined by WHO, and the comparative severity of each form is unclear.
Panel 1. Established and emerging definitions of drug-resistant tuberculosis.
Multidrug-resistant tuberculosis
Resistance to at least isoniazid and rifampicin, the two most effective first-line antituberculosis drugs; treatment is more costly and toxic, and has worse cure rates (40–80%) than for drug-susceptible strains (cure rates of >90%) in effective programmes8–15
Extensively drug-resistant tuberculosis
Multidrug-resistant tuberculosis plus resistance to any fluoroquinolone and any second-line injectable drug (ie, kanamycin, amikacin, or capreomycin); cure and survival rates are worse for extensively drug-resistant tuberculosis than for multidrug-resistant tuberculosis16–20
Resistance beyond extensively drug-resistant tuberculosis
Preliminary data suggest that cure and survival rates could be worse for resistance to all second-line injectable drugs, all WHO group 4 drugs, ethambutol, and pyrazinamide in addition to extensively drug-resistant tuberculosis,21 but needs confirmation in prospective studies; two definitions (extremely and totally drug-resistant tuberculosis), not endorsed by WHO, have been proposed to describe the most severe forms of drug resistance
Extremely drug-resistant tuberculosis
Reported in two patients with strains resistant to all known first-line and second-line drugs, including all group 3 drugs (fluoroquinolones), and to additional group 5 drugs of uncertain efficacy (rifabutin, clofazimine, dapsone, clarithromycin, and thioacetazone; linezolid was not available at the time of testing);9 both patients died after protracted illness
Totally drug-resistant tuberculosis
Reported in Iran, where 15 isolates were resistant to all first-line or second-line drugs tested,5 India, where 4 cases were resistant to all first-line and second-line drugs tested (cycloserine was not tested),4 and South Africa, where resistance was defined by high-confidence genotypic mutations (with the exception of para-aminosalicylic acid);3 treatment outcomes were poor with a high death rate in all studies
Multidrug-resistant and extensively drug-resistant tuberculosis are defined by international consensus.22–25 Indeed, in a European tuberculosis cohort study25 of multidrug-resistant and extensively drug-resistant strains, the accepted definition of extensively drug-resistant tuberculosis had both clinical value (ie, outcomes for extensively drug-resistant tuberculosis were worse than were those for multidrug-resistant tuberculosis) and programmatic value (ie, emphasising that suboptimum adherence drives acquired resistance to second-line drugs). By contrast, the international community still needs to agree a definition for resistance beyond extensively drug-resistant tuberculosis, including which drugs should compose the panel defining this strain, whether new drugs (eg, delamanid, bedaquiline [Janssen Pharmaceuticals, NJ, USA], and PA-824 [TB Alliance, NY, USA]) should be included (panel 2), and how often should this panel be reassessed to allow inclusions of new compounds. Whether resistance beyond extensively drug-resistant tuberculosis is associated with a worse prognosis than is extensively drug-resistant tuberculosis is not clear.
Panel 2. First-line and second-line drugs for tuberculosis as classified by WHO26.
Group 1—first-line oral drugs
Isoniazid
Pyrazinamide
Ethambutol
Rifampicin
Rifabutin
Group 2—second-line injectable drugs
Kanamycin
Amikacin
Capreomycin
Streptomycin
Group 3—fluoroquinolones
Levofloxacin
Moxifloxacin
Ofloxacin
Gatifloxacin
Group 4—oral bacteriostatic second-line drugs
Para-aminosalicylic acid
Cycloserine
Terizidone
Ethionamide
Prothionamide
Group 5—drugs with unclear efficacy or role in treatment of drug-resistant tuberculosis
Clofazimine
Linezolid
Amoxicillin and clavulanate
Thioacetazone
Clarithromycin
Imipenem and cilastatin
Meropenem clavulanate
High-dose isoniazid
Bedaquiline
Delaminid
Based on guidelines by WHO and the Stop TB initiative.26
Recently published data support the WHO recommendation not to use the term totally drug-resistant tuberculosis, but rather to define new categories of resistance beyond extensively drug-resistant tuberculosis.21 In a study21 of 405 patients with extensively drug-resistant tuberculosis divided into 4 groups on the basis of susceptibility data, the rate of cure was significantly lower in patients with extensively drug-resistant tuberculosis and resistance to all second-line drugs than for patients with extensively drug-resistant tuberculosis alone (19% vs 43%), whereas the risk of treatment failure and death was higher for patients with additional resistance (≥48% vs 35%). These data suggest that patients with additional resistance beyond extensively drug-resistant tuberculosis might have worse outcomes than do those with multidrug-resistant or extensively drug-resistant tuberculosis alone. Findings from another study of extensively drug-resistant tuberculosis in South Africa16 showed that the number of drugs that a Mycobacterium tuberculosis isolate was resistant to was an independent predictor of mortality. Thus, internationally agreed definitions based on outcomes are needed to inform public health efforts to monitor and prevent further emergence of drug resistance.
Molecular epidemiology of extensively and totally drug-resistant tuberculosis
The development of methods to genotypically classify strains of M tuberculosis has provided insight into the movement of strains through space and time.27 On the basis of spacer oligonucleotide typing,28 IS6110 DNA fingerprinting,29 or typing with mycobacterial inter spersed repetitive unit-variable number tandem repeats,30 strains can be identified as genotypically identical to (clustered) or genotypically distinct from other strains. Accordingly, clustering suggests recent spread or transmission of tuberculosis, regardless of the presence or absence of primary drug resistance. By contrast, for drug-sensitive tuberculosis, identification of distinct genotypes suggests endogenous reactivation of latent infection (ie, the patient was infected in the past and subsequently developed disease).31 Distinct genotypes among cases of drug-resistant tuberculosis could result from either endogenous reactivation or new acquisition of drug resistance if patients were previously treated with antituberculosis drugs (ie, secondary resistance acquired in the course of treatment in an individual patient).32 Application of these methods has addressed important knowledge gaps,33–38 and was key to the description of the transmission of extensively drug-resistant tuberculosis in Tugella Ferry, KwaZulu-Natal, South Africa.36 In that study,36 despite acquiring resistance to at least four drugs, the strains retained both their fitness to spread and to exogenously re-infect previously treated patients.33
Of the 84 countries that reported extensively drug-resistant tuberculosis in 2011 (figure 1),1 only 14 had reported genotyping of isolates by the end of 2012 (appendix). South Africa remains the only country where comprehensive molecular epidemiological investigations have been done, and these investigations have informed several WHO and local policy decisions about emergence, transmission, and amplification of drug-resistant tuberculosis (appendix).
Studies done in Iran5 and South Africa3 provide the only available data about the molecular epidemiology of totally drug-resistant tuberculosis. Findings from the Iranian study5 showed that all of the strain genotypes of totally drug-resistant tuberculosis were unique; the investigators concluded that these cases emerged through the use of second-line drugs to treat respiratory diseases other than tuberculosis.5 By contrast, findings from the South African study3 showed that the genotypes of the totally drug-resistant strains were mostly clustered (84%) and were clustered with genotypes of extensively drug-resistant strains. The investigators concluded that the acquisition of additional resistance in these cases resulted from inadequate treatment of extensively drug-resistant tuberculosis because of the small number of effective drugs available.3 These findings emphasise the need for improved laboratory capacity in association with universal use of rapid diagnostic testing, improved patient management, and strengthened treatment regimens for multidrug-resistant tuberculosis to halt the emergence and subsequent spread of extensively or totally drug-resistant tuberculosis.
Clinical epidemiology and future trends
Findings from several studies have shown that previous tuberculosis treatment is the strongest risk factor for multidrug-resistant tuberculosis and that, among previously treated patients, previous treatment failure, the number of previous regimens, and the quality of previous tuberculosis care predict drug resistance.39–44 Other individual risk factors vary across settings, but often include young age,41,42 male sex,41 history of incarceration,42 history of previous admission to hospital,39,45 alcohol misuse,42,45 poverty,40,44 HIV infection,41 and smoking.42 Few studies have differentiated between risk factors for primary and acquired (or secondary) resistance. Therefore, whether these determinants are proxies for exposure to patients with drug-resistant tuberculosis, or instead are associated with suboptimum care that selects for drug resistance, is unclear. Molecular epidemiology has been used to identify multidrug resistance as a risk factor for clustering of cases,46,47 and has shown that primary resistance can occur in patients who have been recently treated for tuberculosis (especially in the context of hospital admission), suggesting nosocomial reinfection with a drug-resistant strain.39,48 As expected, extensively drug-resistant tuberculosis is strongly predicted by previous use of second-line drugs in almost all studies that have assessed this strain.39,40,49 In some settings, previous treatment that did not conform to national guidelines,40 the purchase of fluoroquinolones before diagnosis of tuberculosis,50 lack of adherence to a previous regimen, and poverty44 were also independent predictors of extensively drug-resistant tuberculosis.
Mathematical models have been developed to explore the epidemic behaviour of drug-resistant tuberculosis and the potential effectiveness of interventions. Most of these models use deterministic compartmental simulations to capture both the acquisition of resistance by a small proportion of treated patients and the further spread of drug-resistant tuberculosis after emergence.51,52 Epidemic theory posits that the number of people infected by a single infectious host depends not only on the infectiousness of the pathogen but also the duration of infectiousness, which in turn depends on the timing and effectiveness of diagnosis and treatment of the disease. Although multidrug-resistant strains of tuberculosis have often been assumed to have an evolutionary fitness cost that could reduce their transmissibility,53 models have shown that this possible reduction is counterbalanced by the much longer than expected duration of the infectious period in patients with drug resistance who receive first-line drugs to which they respond more slowly than would a person with drug-sensitive disease.54 These models suggest that early diagnosis and effective treatment of drug-resistant tuberculosis, either through improved access to culture and drug-sensitivity testing54,55 or through rapid diagnostics,54,56 could have a profound effect on reduction of the burden of resistant tuberculosis in high-prevalence settings. Other models that link dynamic epidemic simulations with economic analysis show that these interventions can be highly cost effective, because the interventions provide not only potentially lifesaving care but also prevent further spread of drug-resistant disease.57 In view of the substantial burden of drug-resistant tuberculosis in Asia, India and China deserve special mention (appendix).
Pathogenesis and mechanisms of drug-resistant tuberculosis
Selection of drug-resistant mutants—conventional thinking
The conventional understanding of the mechanisms for emergence of multidrug-resistant and extensively drug-resistant tuberculosis (eg, in India, China, and South Africa) is based on research by Luria and Delbrück58 and David.59 The Luria-Delbrück experiment showed that genetic mutations in M tuberculosis that confer resistance arise independently of selection pressure.58 In the 1970s, David59 used Luria and Delbrück’s tests to show very low average rates of mutation (2·6 × 10−⁸ mutations per M tuberculosis bacterium per generation for isoniazid, 2·3 × 10−10 for rifampicin, and 1 × 10−7 for ethambutol).59 Thus, tuberculosis resistance arises spontaneously but at a low and predictable de-novo rate, and not by horizontal gene transfer (figure 2).59 In view of the large bacterial burdens of up to 10⁹ colony-forming units per patient and bacterial replication, pre-existing M tuberculosis subpopulations resistant to one drug might be expected to occur in some patients, although the probability of pre-existing drug resistance to two or three drugs (as calculated by multiplication of mutation rates) is infinitesimally small. In the classic understanding of emergence of drug resistance with monotherapy, initial therapy kills off most of the susceptible bacterial subpopulation, but allows the pre-existing drug-resistant subpopulation to continue to replicate, eventually replacing the drug-susceptible population (figure 2). This scenario could occur with fragmented treatment (panel 3) or lack of adherence to treatment regimens.
Figure 2. The pathogenesis of drug-resistant tuberculosis.
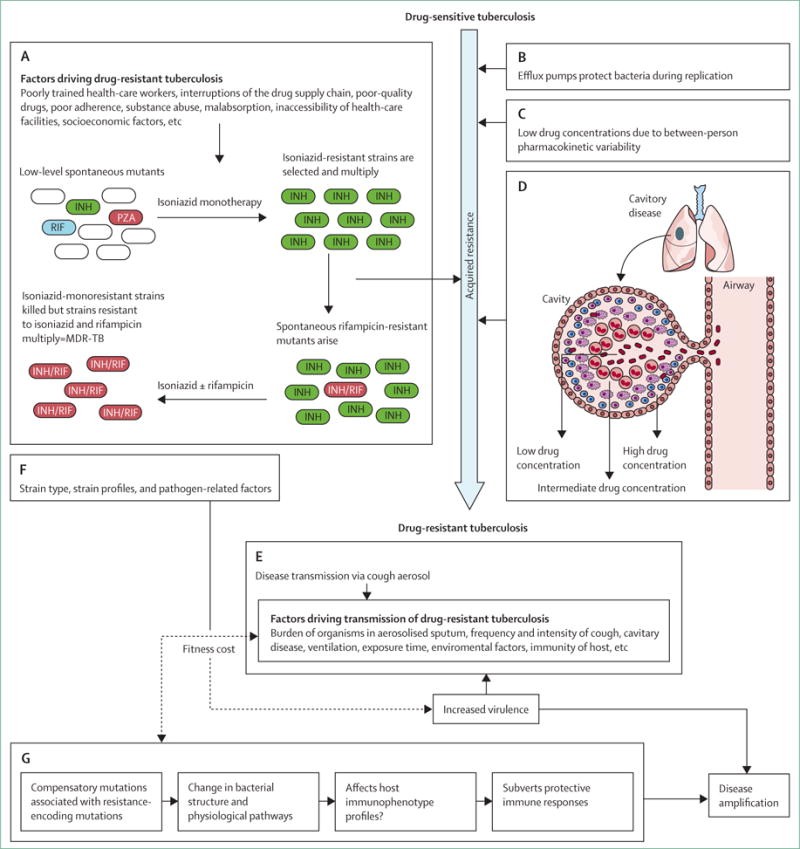
The traditional interpretation of resistance development is that sequential drug resistance develops through fragmented treatment (A), which can be fuelled by several programmatic and socioeconomic factors. However, resistance can develop despite excellent adherence. Several factors, including efflux pumps (B), between-person pharmacokinetic variability (C), and extensive immunopathology in the lung resulting in differential drug penetration into granulomas and cavities (D) might all drive site-specific drug concentrations below minimum inhibitory concentrations, thus probably enabling drug resistance. After acquired drug resistance develops, person-to-person transmission might constitute the major route of spread (E). Strain-specific genotype, newly acquired drug-encoding mutations, and compensatory mutations that can affect fitness cost (and hence transmission) might also interact (F). Compensatory mutations could be associated with changes in structure and physiological pathways, which could affect host immune response and thereby potentially subvert protective responses and drive progressive disease (G). INH=isoniazid. RIF=rifampicin. PZA=pyrazinamide. MDR-TB=multidrug-resistant tuberculosis.
Panel 3. Pathogenesis and mechanisms of the development of drug-resistant mutations.
Poor drug regimens and non-adherence select resistant mutants
Continuation phase in patients with sputum-culture-positive isoniazid-resistant strains (20% of tuberculosis cases in many parts of South Africa) is effectively rifampicin monotherapy, leading to rifampicin resistance
Social factors including interruptions (figure 2) all interact to increase the risk of non-adherence and further acquired drug resistance
Subtherapeutic drug concentrations drive resistance despite excellent adherence
Induction of efflux pumps
Variability in drug metabolic rates, causing subtherapeutic serum concentrations
Variability in drug absorption rates, leading to subtherapeutic peak concentrations
Exposure of mycobacteria to suboptimum drug concentrations in fibro-cavitary lesions
Increased transmission of drug-resistant Mycobacterium tuberculosis
High bacterial burden
M tuberculosis genotype
Metabolic status of organisms in aerosolised sputum
Frequency and intensity of cough and sputum viscosity
Cavitary disease and number of cavities
Degree of ventilation, length of exposure, humidity, and other factors
Effects of genotype and compensatory mutations
Epistatic interactions might modulate fitness (increased, decreased, or unchanged)
Compensatory mutations affect structural and physiological pathways because of changes in the proteome60
Strain and types of mutations associated with different cell-wall characteristics, thickness, and budding characteristics61,62
Changes to structure and bacterial proteome in drug-resistant isolates affect host response
For combination treatments, pharmacokinetic mismatch could occur—ie, a drug in the combination treatment that has a short pharmacokinetic half-life disappears, leaving a companion drug with a longer half-life to act as monotherapy.63 This situation causes sequential acquisition of drug-resistant mutations, with multidrug-resistant and extensively drug-resistant tuberculosis due to an accumulation of mutations acquired one at a time. In this conventional view, the main determinant of drug resistance is gene mutations, either in the genes converting pro-drugs to active drugs, or in the genes of drug targets that are selected because of lack of adherence. However, findings from experimental and clinical studies have challenged this traditional understanding.
Drug concentrations, efflux pumps, and evolution based understanding of acquired drug resistance
Drug resistance can develop even when adherence to treatment is excellent, and multidrug-resistant tuberculosis still occurs even under stringent supervision of patients.35 Several mechanisms might account for this finding, some of which were established with use of the hollow-fibre-system model of tuberculosis, a preclinical tool in which different metabolic populations of M tuberculosis are exposed to concentration–time profiles of antituberculosis drugs. 1–2 years before the description of extensively drug-resistant tuberculosis by Gandhi and colleagues,36 findings from studies using the hollow-fibre-system model of tuberculosis showed that quinolone resistance could easily occur even with drug concentrations that maximised microbial killing.64,65 Acquired drug resistance occurred within 2 weeks of monotherapy, leading to a biphasic kill curve, especially when ciprofloxacin and ofloxacin are used with the less effective second-line drugs.64,65 Findings from subsequent studies using the hollow-fibre-system model with isoniazid, rifampicin, pyrazinamide, and ethambutol showed the same rapid acquisition of resistance, and suggested two further related notions.66–70 First, efficacy and acquired drug resistance were strongly associated with the area-under-the-curve and peak drug concentrations, indexed to minimum inhibitory concentration—ie, there were drug concentrations below which drug resistance was amplified and microbial killing failed. Second, the acquired drug resistance was either accompanied by, or preceded by, very early induction of many low-level-resistance efflux pumps, which also conferred multidrug resistance or tolerance.70,71 These efflux pumps could have protected the bacilli during several rounds of replication, allowing for the eventual generation of chromosomal mutations associated with high-level acquired drug resistance in a process termed the “antibiotic resistance arrow of time” (figure 3).70,72 In this “antibiotic resistance arrow of time” model in the laboratory, subtherapeutic drug concentrations initiated a one-directional sequence of events during long-term therapy that induced efflux pumps as a first step towards multidrug resistance and genetic mutations as a final step.
Figure 3. The arrow of time of antibiotic resistance.
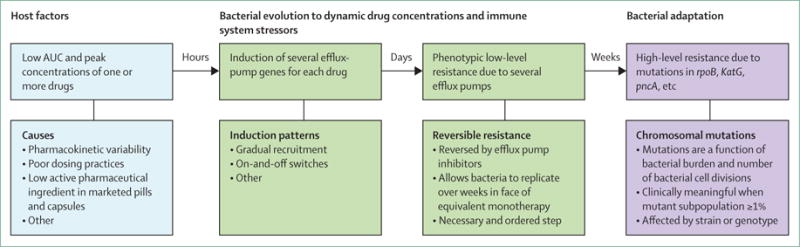
Several factors initiate the process, the most important of which is low drug concentrations due to pharmacokinetic variability. Variability is encountered at each step of drug absorption, distribution, metabolism, and elimination, and could be due to different single-nucleotide polymorphisms in enzymes for drug transport and xenobiotic metabolism, comorbid conditions (eg, AIDS), or increased patient weight. This variability leads in some patients to low drug concentrations of one or more drugs in the regimen (effectively equivalent to monotherapy). Bacteria then adapt to these concentrations, initially through epigenetic mechanisms (eg, induction of several efflux pumps). These efflux pumps are associated with low-level multidrug resistance and enable several rounds of bacterial replication, allowing for development of mutations in canonical genes associated with drug resistance. AUC=area under the curve.
Findings from clinical studies lend support to the role of efflux-pump induction in emergence of multidrug resistance. Several researchers have examined drug-resistant clinical isolates (including multidrug-resistant tuberculosis), and reported the coexistence of genetic mutations and multidrug efflux pumps.73,74 Repetitive sputum samples from the same patient were reported to have the same early induction of efflux pumps, and later establishment of chromosomal mutations often associated with acquired drug resistance.75 However, findings from studies using whole-genome sequencing would not be expected to show this effect; because the induced efflux pumps are already encoded by the M tuberculosis genome, the induction is epigenetic.
Although acquired drug resistance has clearly been shown to occur even under strict supervision of patients, many public health researchers and doctors believe that poor adherence accounts for most cases of acquired drug resistance. Studies using the hollow-fibre-system model have directly examined the role of lack of adherence in emergence of drug resistance during standard short-course chemotherapy.76 Although therapeutic failure occurred only when more than 60% of doses were missed, no multidrug-resistant isolates were reported in patients with any lack of adherence, except transient monoresistance that disappeared with time.76 Findings from a follow-up study77 that used a murine tuberculosis model also showed no emergence of multidrug-resistant tuberculosis with high rates of non-adherence. These findings, together with reports of acquired drug resistance even under strict supervision,35 and the emergence of drug resistance below particular drug threshold concentrations, suggest that pharmacokinetic variability could be a proximate cause for acquired drug resistance.
To investigate this hypothesis, we simulated the pharmacokinetics of patients with tuberculosis from the Western Cape, South Africa, in computer-aided models of clinical trials. We noted that multidrug-resistant tuberculosis would still occur in 0·68% of patients during the first 2 months of treatment, despite 100% adherence,76 because drug metabolism rates for rifampicin or isoniazid were high in some patients due to between-patient pharmacokinetic variability; these patients would effectively receive monotherapy for long periods of time. Such pharmacokinetic variability is driven mainly by genetic polymorphisms in xenobiotic metabolism and drug transporters.78 These simulation findings have since been replicated in three clinical studies.79–81 The first was a meta-analysis79 that showed higher rates of acquired drug resistance among fast isoniazid acetylators than among slow acetylators in multidrug regimens. A second meta-analysis80 of prospective studies showed that, despite higher rates of non-adherence, self administered therapy did not lead to increased rates of acquired drug resistance compared with directly observed short-course treatment. The third study81 was a prospective study of 142 patients with tuberculosis in Western Cape, South Africa, in which the pharmacokinetics of pyrazinamide, rifampicin, and isoniazid were measured and patients were followed up for up to 2 years. Between-patient pharmacokinetic variability was wide; although the highest ratio of the highest dose to lowest dose for all three drugs was 2·7, the ratios of the highest-to-lowest peak concentrations were more than 102 for rifampicin, more than 31 for isoniazid, and more than 63 for pyrazinamide. The peak and area-under-the-curve concentrations of pyrazinamide, rifampicin, and isoniazid predicted more than 91% of therapy failure; 0·7% of all cases of acquired drug resistance were reported by the third month of therapy, and was preceded by low peak and area-under-the-curve drug concentrations in all patients who developed resistance despite 100% adherence;81 non-adherence did not predict acquired drug resistance. Thus, low drug concentrations due to between-patient pharmacokinetic variability is a proximate cause of acquired drug resistance. Pharmacokinetic variability can also be caused by differential penetration of drugs into the lung microcompartments (figure 2). The finding of isolates from different lesions in the same patient that have different resistance profiles (ie, heteroresistance), lends support to the hypothesis of differential drug penetration or exposure.82 Collectively, these data from laboratory experiments, clinical trial simulations, and prospective clinical studies provide support for the notion that biological variability (eg, pharmacokinetic variability) is the main culprit for emergence of multidrug-resistant tuberculosis. Therefore, innovative and new methods are needed to optimally dose patients. These methods could include a pharmacogenetic approach, measurement of drug concentrations in patients, improved targeting of drugs to the disease site when given orally, or targeting of drugs through the inhalational route.83
Genotype, strain type, and compensatory mutations
Biological variability within the M tuberculosis genotype is an important cause of emergence of multidrug resistance in tuberculosis. The typical mutation rates identified by David59 were established with use of the M tuberculosis laboratory strain, H37Rv. Findings from a 2013 study84 showed that among different M tuberculosis genotypes, as defined by large-sequence polymorphism phylogenetic analyses, lineage 2 (to which the Beijing strain belongs) had much higher mutation rates than did lineage 4 (common in Western Europe and the USA, to which H37Rv belongs). In view of this finding, emergence of multidrug-resistant tuberculosis is 22 times more likely for lineage 2 than for lineage 4, and multidrug resistance could even preexist in some patients originally infected with a drug-susceptible strain before therapy initiation.84 Calculations of average mutation rates that suggested that acquired drug resistance would not easily result from therapy with more than one drug are therefore probably not applicable to the most common M tuberculosis genotypes associated with multidrug-resistant tuberculosis. In addition to differences in mutation rates between different genotypes, the mutation rate also varies between strains of the same genotype. However, the reasons for this variability are still unclear. Although some evidence suggests that certain strains (eg, the Beijing strain) are associated with increased likelihood of emergence of multidrug resistance,85 little evidence supports the contention that some strains are more likely to mutate as a result of dysfunctional DNA repair mechanisms.85 Additionally, interaction between hypermutable bacterial lineages and patients who rapidly metabolise first-line drugs might account for the high rates of multidrug-resistant tuberculosis reported in some settings despite patients receiving directly observed short-course treatment.86
Findings from studies of whole-genome sequencing have shown that resistance-encoding mutations are associated with compensatory mutations elsewhere in the tuberculosis genome.87,88 Similar to compensatory mutations in pseudomonas species that can modulate virulence and transmissibility,87,88 compensatory mutations in M tuberculosis could be associated with physiological and structural changes (panel 3, figure 2). Collectively, these findings raise the possibility that drug resistance could affect micro-anatomical structure and antigenic specificity, and hence perhaps the T-cell immune response.
Potential role for immune-modulating therapy
Data about the immunology of drug-resistant tuberculosis are scarce, but there have been some reports of immune-mediated clearance.89–91 Using an in-vitro killing assay, we showed that depletion of peripheral blood CD4+/FOXP3+ regulatory T cells in patients with drug-sensitive92 and drug-resistant (unpublished) tuberculosis augmented mycobacterial containment, suggesting an important subversive biological role for these cells. This finding suggests that immunomodulation by manipulation of regulatory T cells (eg, targeting of antagonists for interleukin 2 receptors, autologous transfusion after expansion, infusion of mesenchymal stromal cells,93 or blocking of other regulatory T-cell functions)94 might affect disease progression. Effective tuberculosis treatment might therefore require not only drug therapy, but also realignment or redirection of the immune system to deal more effectively with mycobacteria. Roughly 20% of tuberculosis cases in the prechemotherapeutic era spontaneously resolved, which supports the notion of immune-mediated clearance. Several immunotherapeutic interventions have been proposed for the treatment of drug-resistant tuberculosis—eg, M vaccae, vitamin D, and intravenous immunoglobulin—and many of these therapies modulate regulatory T cells.95 An alternative approach could be to use immunosuppressive drugs (eg, steroids or TNF antagonists) to enhance the chemotherapeutic effect by driving the replication of long-term persisters, thus exposing these bacteria by disruption of the protective shelter of the granuloma.95 Thus, counterintuitively, immunosuppressive drugs might enhance bacterial clearance.
Transmission dynamics
Several clinical, bacterial, behavioural, and environmental factors probably drive transmission (figure 2),96–98 but most cases of tuberculosis result from transmission by a small proportion of patients (so-called super-spreaders).99,100 The determinants of this super-spreader status are poorly characterised, but culturable bacterial load is important,101 and epidemiological modelling studies suggest that infection-control interventions96 (especially those aimed at health-care workers and the small number of patients who probably bring about most transmission100,102) will probably reduce the probability of epidemics of drug-resistant tuberculosis emerging. The high transmissibility36 and virulence (ie, high mortality and extensive clinical lung immunopathology)16 of highly drug-resistant strains, including extensively drug-resistant tuberculosis, and their extensive spread even in regions with low HIV prevalence suggests that resistance-conferring mutations could be associated with compensatory mutations that result in normal or possibly even enhanced evolutionary fitness levels.87 Epistatic interactions (in which the phenotypic effect of a mutation such as fitness changes dependent on the presence or absence of other mutations in the same genome103) between several resistance-conferring and non-resistance-conferring mutations, the genotype or strain type, and associated compensatory mutations might modulate fitness costs associated with drug resistance.103,104
Diagnostic aspects
The rollout of the Xpert MTB/RIF assay105 (Cepheid, Sunnyvale, CA, USA)—a semi-nested quantitative real-time PCR assay that simultaneously detects tuberculosis and resistance to rifampicin—in many countries with a high burden of tuberculosis is projected to substantially reduce the tuberculosis epidemic (figure 4).57 Xpert MTB/RIF is validated for use with sputum, although evidence is emerging to support its use with extrapulmonary samples.106 Xpert MTB/RIF increases the number of tuberculosis cases detected through increased sensitivity and improves time to treatment initiation.107 Through increased detection of rifampicin resistance compared with GeneXpert and earlier detection than for culture, the assay is expected to enable early initiation of appropriate therapy and thereby reduce transmission of drug-resistant tuberculosis.57 However, substantial operational challenges need to be overcome; in South Africa, even with the availability of a rapid line-probe assay, initiation of second-line treatment still took a median of 55 days.108
Figure 4. Molecular (A, B) and culture-based (C) tests for diagnosis of drug resistance.
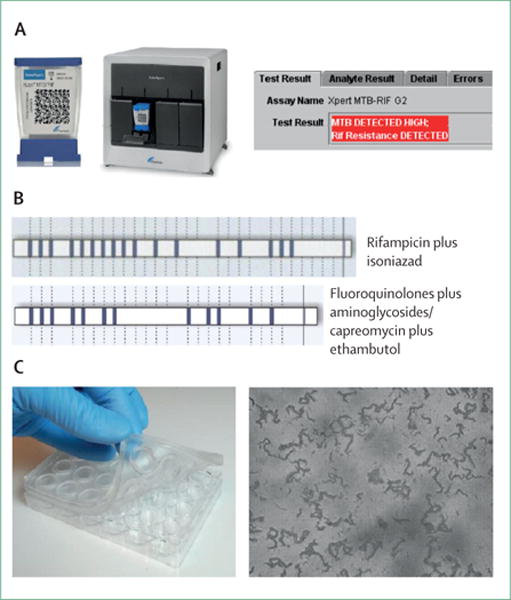
(A) The Xpert MTB/RIF assay, an automated real-time PCR assay that can detect genotypic resistance to rifampicin and Mycobacterium tuberculosis complex DNA. A test cartridge, four-module machine, and an example of a tuberculosis-positive rifampicin-resistant result are shown. (B) Line-probe hybridisation patterns, resulting from the detection of specific DNA fragments containing mutations associated with drug resistance, for the Hain Lifesciences MTBDRplus (top row) and Hain Lifesciences MTBDRsl(bottom row) test. (C) An example of a commercially available kit for microscopic observation drug susceptibility, in which bacteria are cultured in a microtitre-well-plate format, and visually inspected with light microscopy for distinctive cording patterns that are indicative of M tuberculosis growth in the presence of the drug of interest.
The Xpert MTB/RIF assay can be done by minimally trained health-care workers in primary care facilities, which suggests that similar assays could be used to tackle tuberculosis in prisons and clinical hotspots, especially where rates of drug-resistant tuberculosis are high.107 However, the Xpert MTB/RIF assay does not detect isoniazid monoresistance, which has a frequency of about 10–15% in high-burden settings.109,110 Furthermore, in countries such as South Africa where almost 10 000 cases of drug-resistant tuberculosis are treated annually and the proportion of drug-resistance in new tuberculosis cases is about 5–6%,111 the positive predictive value for rifampicin resistance is suboptimum (about 70%)—ie, about 1 in 3 or 4 rifampicin-resistant results will probably be false positive. However, where recommended algorithmically (eg, in South Africa) or where patients are unwell or true multidrug-resistant disease is clinically plausible, patients with a positive Xpert MTB/RIF assay are often started on treatment for multidrug-resistant tuberculosis while confirmatory test results are awaited.
Other diagnostic methods include the line-probe assays (eg, the MTBDRplus assay [Hain Lifesciences, Nehren, Germany]); version 2 of the MTBDRplus assay offers similar performance to Xpert MTB/RIF for tuberculosis detection directly from patient specimens, and has excellent performance for the detection of multidrug-resistant tuberculosis.112,113 Thus, molecular assays such as Xpert MTB/RIF and MTBDRplus can offer a rapid and accurate diagnosis of multidrug-resistant tuberculosis within days of patient contact. More recently, the MTBDRsl assay (Hain Lifesciences, Nehren, Germany) has been introduced, which assesses resistance to second-line injectable drugs (mutations on the rrs gene), fluoroquinolones (mutations on the gyrA gene), and ethambutol (mutations on the embB gene).114 However, this assay is not recommended for use directly on smear-negative specimens because of decreased accuracy,115 meaning that culture isolates often need to be used to rule-in resistance. Sensitivity for resistance to second-line injectable drugs and fluoroquinolones is about 90% and 95%, respectively.115–117 Alternative testing platforms that could be used in resource-poor settings and have excellent accuracy include microscopic observation drug susceptibility, the nitrate reductase assay, and thin-layer agar (appendix).118
Medical and surgical management of extensively drug-resistant tuberculosis and treatment failure
In patients who do not respond to treatment for multidrug-resistant tuberculosis (generally culture positive after at least 6 months of treatment) and who persistently remain susceptible to fluoroquinolones and aminoglycosides, treating physicians should consider non-compliance, malabsorption, drug quality, and other factors.95,119–121 Surgery or a regimen of alternative second-line drugs and injectables should be considered for these patients if appropriate (panel 4).
Panel 4. Management of extensively drug-resistant tuberculosis and more drug-resistant strains.
Principles for medical management of multidrug-resistant tuberculosis
Regimens based, when possible, on proven or probable susceptibility to at least four drugs
Regimen generally based on a later-generation fluoroquinolone (eg, moxifloxacin or levofloxacin), plus an injectable drug, usually an aminoglycoside (ie, either amikacin or kanamycin), any first-line drug to which the isolate is susceptible (appendix), and addition of group 4 drugs (eg, cycloserine, terizidone, or ethionamide), such that at least four drugs to which the isolate is probably susceptible are used
Injectable drugs are used for 6–8 months or longer, and the total duration of treatment is suggested to be 24 months122
Drugs that patients have previously received for 3 months or longer are generally avoided
Psychological factors should be addressed to ensure compliance
Patients should be monitored for adverse drug reactions, which are common103
A single drug should not be added to a failing regimen
Principles for management of extensively drug-resistant tuberculosis
Regimens should be constructed on the basis of prevailing patterns in drug susceptibility testing
One injectable is chosen from group 2, any drug that the isolate is susceptible to from group 1, and any remaining available drugs from group 3 or 4
In view of the high background rates of tuberculosis and multidrug resistance in several countries, regimens are often constructed around a backbone of capreomycin and para-aminosalicylic acid
Patients should be carefully monitored for adverse drug reactions, particularly when receiving capreomycin (eg, renal failure, hypokalaemia, and hypomagnesaemia), which are common103
Patients receiving capreomycin should have urea and electrolytes monitored weekly for the first 8 weeks, and then monthly thereafter; attention should be paid to correction of risk factors for renal failure (ie, dehydration, nausea, vomiting and diarrhoea, and avoidance of other nephrotoxic drugs [co-trimoxazole and nevirapine]), and early identification of underlying renal disease (eg, diabetes and HIV-associated nephropathy)
Surgical management of extensively drug-resistant tuberculosis
Candidates include patients with unilateral disease (or apical bilateral diseases in selected cases) with adequate lung function who have not responded to treatment for multidrug-resistant tuberculosis, any patient with extensively drug-resistant tuberculosis, or any patient who has not responded to treatment for extensively drug-resistant tuberculosis119,123
Facilities for surgical lung resection are scarce in high-burden settings and, even when available, surgery is an option only for very few patients
Patients with contralateral disease are usually not surgical candidates, but establishment of whether contralateral disease is due to old tuberculosis or fibrosis rather than active disease is often difficult; the role of PET and CT scanning for these patients needs further investigation
Construction of a treatment regimen for extensively drug-resistant tuberculosis is more challenging than for multidrug-resistant tuberculosis (panels 2, 4). Because rates of previous multidrug resistance in patients with extensively drug-resistant tuberculosis are high,16 capreomycin resistance often occurs because of cross-resistance with aminoglycosides—in 178 isolates of extensively drug-resistant tuberculosis, the rate of capreomycin resistance was about 80% (K Dheda, unpublished)124—and drugs such as ethionamide and cycloserine are often not an option. Other drug options include high-dose isoniazid (which can be successful for tuberculosis strains with inhA promoter mutations, and has been used to treat extensively drug-resistant tuberculosis in South Africa),125 clofazimine, and other drugs from group 3 or 4 (panel 2). However, some of these drugs are of doubtful value. Moxifloxacin is often added to the regimen despite documented resistance to fluoroquinolones.126 Where appropriate, meropenem can be useful,127 and if mutational screening is used rifabutin can be an option.128 Linezolid, although not available to national tuberculosis programmes worldwide, can substantially improve culture-con version outcomes in patients with extensively drug-resistant tuberculosis, but longer-term outcomes are unknown and cost and toxic effects are major concerns.129 The recent approval by the US Food and Drug Administration of TMC-207 (bedaquiline) is encouraging, but this drug is not yet available in resource-poor settings. In South Africa the registration of the drug was turned down by the Medicines Control Council, in favour of a clinical registry to document its safety and effectiveness in a regimen to treat some forms of drug-resistant tuberculosis. Concerns have been raised about high mortality in the placebo arm of the trial of TMC-207.130 Delamanid is also promising, although it is still undergoing approval.131,132
Collectively, this evidence suggests that patients with extensively drug-resistant tuberculosis and more resistant strains of tuberculosis are functionally untreatable. The poor prognosis (20% culture conversion during the study period and 40% 1-year mortality) of extensively drug-resistant tuberculosis in high-burden settings such as South Africa is therefore not surprising.16–18,133 Resistance beyond extensively drug-resistant tuberculosis is difficult to interpret because of the poor reliability of susceptibility testing for capreomycin, para-aminosalicylic acid, cycloserine, and other drugs, and because there are insuffi cient data about whether resistance beyond extensively drug-resistant tuberculosis affects outcomes.21,134 A further complication is the high rates of adverse events in patients with extensively drug-resistant tuberculosis, with potentially fatal results (panel 4).135
Definition of treatment failure in patients with extensively drug-resistant tuberculosis is contentious but important, because it defines when treatment should potentially be withdrawn because of futility. In South Africa, treatment is regarded as being unlikely to produce a favourable outcome if culture conversion has not occurred within 12 months. The median time to culture conversion in this patient population is about 6 months.16 However, prospective data from larger cohorts are needed to clarify the predictive value of this suggested definition. About 25% of converters can subsequently revert to culture positivity.124
Management of patients with HIV
With the emergence of the epidemic of drug-resistant tuberculosis in southern Africa, and the increasing prevalence of HIV in eastern Europe, large numbers of patients with multidrug-resistant and extensively drug-resistant tuberculosis also have HIV.16,136–139 Mortality rates are substantially increased for these patients.16,134,140–142 Reduction of mortality needs prompt treatment, although few data are available to guide therapeutic approaches to treat both diseases. Concerns exist, as for treatment of drug-susceptible tuberculosis and HIV, that concurrent treatment of drug-resistant tuberculosis and HIV will lead to drug–drug interactions, increased toxic effects, reduced adherence, and increased incidence of immune reconstitution inflammatory syndrome.143
Few studies have prospectively examined each of these issues in the setting of drug-resistant tuberculosis and HIV. No data are available for drug–drug interactions between second-line tuberculosis drugs and anti-retroviral therapy, although interactions are suspected.144 Small retrospective studies about over lapping toxic effects with treatment for both multidrug-resistant tuberculosis and HIV have been reported;135,145–147 however, although adverse events were common in these studies, they were no more frequent for combination with antiretroviral therapy than for treatment for multidrug-resistant tuberculosis alone. Adherence to treatment of multidrug-resistant tuberculosis alone is probably low, in view of the complexity, duration, and toxic effects of the drugs used, but this has not been well studied. The addition of antiretroviral therapy is likely to further worsen adherence. The frequency of immune reconstitution inflammatory syndrome (defined as clinical deterioration despite appropriate treatment, caused by restoration of pathogen-specific immune responses) in the setting of concurrent treatment of drug-resistant tuberculosis and antiretroviral therapy is also unknown. Whether immune reconstitution inflammatory syndrome is more frequent or severe in patients with both HIV and drug-resistant tuberculosis is not clear, because bacterial loads will probably be increased, the potency of second-line tuberculosis drugs is reduced, and time to culture conversion is lengthened.143,148,149
In the absence of high-quality data, guidelines for the management of drug-resistant tuberculosis and HIV are based mostly on expert opinion and data from studies of drug-susceptible tuberculosis and HIV.150 Findings from several well designed clinical trials151–154 and prospective observational studies155–158 have shown that concurrent tuberculosis treatment and anti-retroviral therapy is associated with improved treatment outcomes and low rates of adverse events and immune reconstitution inflammatory syndrome. Patients receiving concurrent treatment for drug-sensitive tuberculosis and HIV in the SAPiT study151 also had lower mortality than did patients for whom antiretroviral therapy was deferred until after tuberculosis treatment was completed.151 On the basis of these data, concurrent treatment is recommended for all patients co-infected with drug-resistant tuberculosis and HIV, irrespective of baseline CD4 cell count.150 Tuberculosis treatment guidelines are the same for patients with drug-resistant strains co-infected with HIV as for patients without HIV.150
Outcomes for multidrug-resistant tuberculosis compared with extensively and totally drug-resistant tuberculosis
Survival and treatment outcomes in tuberculosis are associated with the degree of drug resistance. Generally, patients with drug-susceptible tuberculosis have higher survival and cure rates than do those with multidrug-resistant tuberculosis; similarly, success rates are higher in multidrug-resistant tuberculosis than in extensively drug-resistant tuberculosis.134,159,160 Treatment success rates in drug-susceptible tuberculosis generally vary from 70% to 95%,161 whereas in multidrug-resistant tuberculosis, success rates are usually between 40 and 70%.160,162 Treatment success rates in extensively drug-resistant tuberculosis have been reported to be as high as 60%;142 however, in most settings, the success rates typically range from 20% to 50%.16,18,160 Treatment success is linked not only to drug-resistance category, but also to resistance to additional first-line and second-line drugs. Patients with multidrug-resistant tuberculosis with only resistance to isoniazid and rifampicin have better survival than do those with additional resistance to ethambutol or streptomycin (figure 5).134 Additional resistance to fluoroquinolones or second-line injectable drugs results in worse odds of treatment success in a stepwise fashion than does multidrug resistance alone, multidrug resistance plus fluoroquinolone resistance, multidrug resistance plus resistance to second-line injectable drugs, or extensively drug-resistant tuberculosis.163 Resistance to second-line and third-line drugs beyond the minimum definition of extensively drug-resistant tuberculosis is also associated with further reductions in the likelihood of treatment success. Findings from a meta-analysis21 showed that patients with the minimum definition of extensively drug-resistant tuberculosis had higher cure rates (43%) than did patients with more drug-resistant tuberculosis; cure rates were lower for extensively drug-resistant tuberculosis with resistance to all other injectables (30%), resistance to all injectables and at least one group 4 drug (34%), and resistance to all injectables, one group 4 drug, and either pyrazinamide or ethambutol (19%).
Figure 5. Kaplan-Meier survival plot by drug-resistance pattern for patients with multidrug-resistant and extensively drug-resistant tuberculosis, 2005–07 (log-rank p<0·0001).
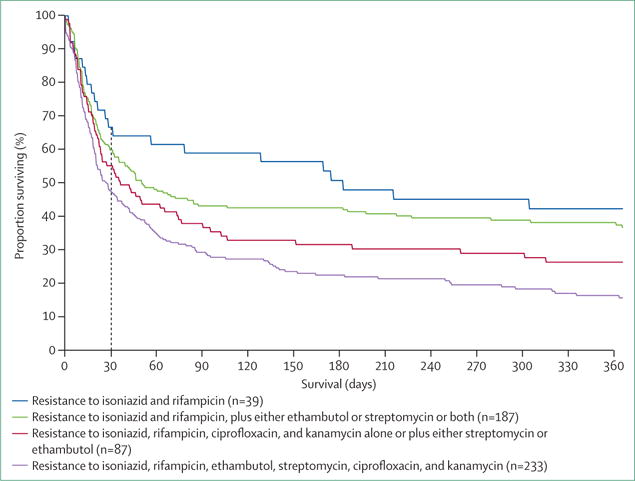
Median survival was 182 days (95% CI 31–395) for patients with tuberculosis resistant to isoniazid and rifampicin; 50 days (95% CI 35–106) for patients with tuberculosis resistant to isoniazid and rifampicin, plus either ethambutol or streptomycin or both; 36 days (95% CI 23–74) for patients with tuberculosis resistant to isoniazid, rifampicin, ciprofloxacin, and kanamycin, alone or plus either streptomycin or ethambutol; and 27 days (95% CI 20–38) for patients with tuberculosis resistant to isoniazid, rifampicin, ethambutol, streptomycin, ciprofloxacin, and kanamycin. Figure reproduced from Gandhi and colleagues134 by permission of the American Thoracic Society.
On the basis of the available evidence, the factors associated with improved odds of treatment success are use of a large number of active drugs,16,142,164 concurrent use of antiretroviral therapy in patients co-infected with HIV,16,17,40,165 use of community based models of treatment,141,166–168 and use of a later-generation fluoroquinolone.16 Further rigorous studies are needed to establish the best regimens and treatment protocols to optimise treatment success, dependent on the degree of drug resistance.
The emergence of untreatable tuberculosis—ethical and medicolegal aspects
No effective drugs are available to treat patients with extensively drug-resistant tuberculosis who do not respond to therapy (ie, no culture conversion after 12–18 months of intensive inpatient treatment) and who are unsuitable for surgical lung resection, rendering this patient population functionally untreatable. Large numbers of these patients have been reported in South Africa, and present a management, medicolegal, and ethical challenge. In the Western Cape Province of South Africa, about 100 of these patients have been discharged back into the community since 2008 because of treatment futility and insufficient bed space.124 The median time to death in the community after discharge was just under 2 years, and almost a third of patients were smear positive at discharge and therefore pose a high risk of transmission.124 At present, no systematic programme in South Africa is available to ensure that these patients have appropriate infection control in their home environments, and there are no reasonable alternatives (eg, long-stay community facilities) where they can live. Both long-term community facilities (where multi disciplinary care can be delivered, and patients stay on a voluntary basis) and palliative care facilities for patients who are terminally ill are needed.169 Often, these patients reside in single-room dwellings and are employed as casual labourers; can their sending home be justified? The need to support their families means that engaging in employment, use of public transport, and visiting public places is unavoidable, which has serious implications for the uncontrolled spread of highly drug-resistant forms of tuberculosis.
There are several other unresolved questions.119,120,123 Should these patients be left on treatment if they are deemed to be non-responsive to treatment for extensively drug-resistant tuberculosis? Should treatment be withdrawn for patients who do not adhere to regimens, to prevent the evolution of higher-grade drug resistance? What should be done with the small number of patients who continue to default their treatment, usually in the setting of substance abuse, and are a danger to fellow patients and health-care workers? Should there be facilities to incarcerate such patients in appropriate cases? What about the rights of communities and individuals to be protected from a virtually untreatable and potentially fatal disorder? In countries such as the USA and the Netherlands, incarceration has been used highly selectively to prevent unchecked spread of drug-resistant tuberculosis, and to mitigate the danger to the community.170 In a recent international survey,170 only 17% of respondents were against incarceration in principle. Mothers with extensively drug-resistant tuberculosis who are pregnant also present difficulties in management, particularly in resource-poor settings where social services are not developed and relatives might not be available to accept care of the child. The beds in many tuberculosis facilities in South Africa are occupied by patients with extensively drug-resistant tuberculosis who have not responded to treatment and have nowhere to go. Although some patients have relatively short lifespans after treatment failure, others live for months to years, can continue to raise families, and engage in employment and use of public transport. The issue is not confined to high-burden settings, but is also becoming problematic in Russia, eastern Europe, and other parts of the world. Urgent and cohesive action is needed from WHO and governments to deal with this growing problem.
Drug-resistant tuberculosis in health-care workers and infection control
Several reports have documented very high rates of tuberculosis in health-care workers in high-burden settings, with an incidence of more than 1000 per 100 000 workers.171 Recently, reports of drug-resistant tuberculosis in health-care workers have started to emerge. In the province of KwaZulu-Natal, South Africa,172 we identified more than 300 health-care workers with multidrug-resistant and extensively drug-resistant tuberculosis; these rates were about six times higher than were those of the general population, suggesting that drug-resistant tuberculosis was probably nosocomially acquired. We have also documented drug-resistant tuberculosis in health-care workers (eg, nurses, medical students, medical officers, and senior doctors) in Western Cape Province, South Africa.173 Fears about contraction of drug-resistant tuberculosis could further exacerbate the shortage of health-care workers in settings where they are needed the most. Thus, health-care workers in appropriate settings should be screened at least annually (with symptom questionnaires and chest radiography), should have access to rapid diagnostics, and appropriate measures for environmental control (ie, adequate ventilation) and personal protection (ie, availability of 1 μm filter masks with fit testing) should be available and implemented.174 Health-care facilities should have a written administrative plan about how patients with tuberculosis should be triaged, and how health-care workers should be screened and managed for drug-resistant tuberculosis.174 However, comprehensive measures to prevent tuberculosis in health-care workers have yet to be widely implemented in many resource-poor settings.174
Prophylaxis for people exposed to patients with drug-resistant tuberculosis
How people who are in close contact with patients with highly drug-resistant forms of tuberculosis should be managed remains controversial. The risk of substantial toxic effects should be balanced against the less than 5–10% likelihood of household contacts developing active disease from airborne transmission.175,176 The list of suitable drugs for chemoprophylaxis is small, but could include combinations of clofazimine and moxifloxacin, clofazimine and para-aminosalicylic acid, and linezolid and clofazimine. All these drugs have potential serious toxic effects, including cardiac arrhythmias (clofazamine and moxifloxacin), skin discoloration (clofazamine), and neurological and bone-marrow toxicity (linezolid). Appropriate counselling with regular follow-up and imaging is often the route followed by most clinicians.
Conclusions
Multidrug-resistant tuberculosis emerged in several high-burden settings in the early 1980s.19 Roughly 20 years later, the first cases of extensively drug-resistant tuberculosis emerged,19 and more than a decade later functionally untreatable cases of tuberculosis have been reported.124 While existing patients are appropriately managed, health-systems infrastructure and capacity need to be drastically strengthened with a multidisciplinary approach to curb the development of further drug-resistant tuberculosis. Several new approaches to study the pathogenesis and mechanisms driving resistance will probably inform new strategies for prevention and management of this disease, including identification of new drug-specific mutations, epistasis and compensatory evolution, and delineation of the fundamental biology of transmission. Rapid diagnostics urgently need to be rolled out, and the research community needs to be supported by international funding agencies to develop comprehensive, rapid, and point-of-care diagnostics for drug-resistant tuberculosis. Because new diagnostic tests have been implemented in resource-poor, high-burden settings where they were previously unavailable, treatment capacity for new cases of drug-resistant tuberculosis needs to be urgently scaled up. New drugs could shorten regimens for multidrug-resistant tuberculosis to less than 1 year without requiring an injectable drug, and several multicentre trials (including studies of optimum combinations of antiretroviral therapy in patients with drug-resistant tuberculosis) are underway. However, specific efforts for tuberculosis control will not succeed if broader issues—eg, reduction of poverty, overcrowding, political stability, and rates of HIV—are not addressed. There is also an urgent and comprehensive need to scale up protective measures and strategies against tuberculosis in health-care workers. The large-scale emergence of functionally untreatable tuberculosis needs governments and international agencies to take serious steps to reform the global economy to reduce poverty, make existing drugs available in resource-poor settings, and accelerate drug development.
Supplementary Material
Key messages.
Extensively drug-resistant and more drug-resistant strains of tuberculosis pose a serious threat to global health, result in high mortality, and are extremely costly to treat
Data from molecular epidemiological studies show gradual acquisition and emergence of extensively drug-resistant tuberculosis followed by transmission and amplification of the epidemic
Modelling studies suggest that early diagnosis and effective treatment of drug-resistant tuberculosis could profoundly reduce the burden of disease
Several new insights (eg, within-person pharmacokinetic variability, efflux pumps, intrapulmonary drug gradients, adaptive mutations that differentially affect fitness, and the notion of disease superspreaders) challenge the present understanding of the pathogenesis of drug-resistant tuberculosis
Several new technologies (eg, the GeneXpert MTB/RIF assay) enable the rapid diagnosis of drug-resistant tuberculosis
Treatment outcomes for patients with extensively drug-resistant tuberculosis (particularly in endemic settings) are dismal because of a lack of effective drugs
Absence of effective drugs and scarce resources mean that patients with incurable tuberculosis are discharged into the community where they are at high risk of transmitting disease
Several new effective antimicrobials (eg, delamanid, bedaquiline, and linezolid) have recently become available for clinical use, but a combination regimen for drug-resistant tuberculosis needs urgent validation in a prospective clinical study in intermediate-burden and high-burden settings
Search strategy and selection criteria.
We searched PubMed for articles published in all languages between January, 1995, and November, 2013, with the terms “tuberculosis”, “drug-resistant”, “MDR-TB”, and “XDR-TB”. We also identified relevant articles through searches of the authors’ personal files, review articles, landmark papers, and other relevant search engines (eg, Google Scholar).
Footnotes
Contributors
KD conceived the idea and mainly wrote the abstract, introduction, conclusions, and sections about pathogenesis, medical and surgical management, outcomes, medicolegal aspects, health-care workers, and prophylaxis. GBM mainly wrote the sections about definitions and terminology, RW wrote the section about molecular epidemiology, MM wrote the section about clinical epidemiology, ZU wrote the section about drug-resistant tuberculosis in India and China, and NRG wrote the section about management of patients infected with HIV. TG cocontributed to the section about pathogenesis, and GT cocontributed to the section about diagnostics and pathogenesis. All authors edited and proofread the Review, and cocontributed to other sections as appropriate.
Declaration of interests
We declare that we have no competing interests.
Contributor Information
Keertan Dheda, Lung Infection and Immunity Unit, Division of Pulmonology and UCT Lung Institute, Department of MedicineInstitute of Infectious Diseases and Molecular Medicine, University of Cape Town, Cape Town, South Africa.
Tawanda Gumbo, Office of Global Health and Department of Medicine, University of Texas Southwestern Medical Center, Dallas, TX, USA.
Neel R Gandhi, Departments of Epidemiology, Global Health, and Infectious Diseases, Rollins School of Public Health, Emory University, Atlanta, GA, USA.
Megan Murray, Department of Global Health and Social Medicine, Harvard Medical School, Boston, MA, USA.
Grant Theron, Lung Infection and Immunity Unit, Division of Pulmonology and UCT Lung Institute, Department of Medicine.
Zarir Udwadia, Hinduja Hospital and Research Center, Mumbai, India.
G B Migliori, WHO Collaborating Centre for TB and Lung Diseases, Fondazione S Maugeri, Care and Research Institute, Tradate, Italy.
Robin Warren, DST/NRF Centre of Excellence for Biomedical Tuberculosis Research, MRC Centre for Molecular and Cellular Biology, Division of Molecular Biology and Human Genetics, Department of Biomedical Sciences, Faculty of Medicine and Health Sciences, Stellenbosch University, Tygerberg, South Africa.
References
- 1.WHO. Global Tuberculosis Report 2012. Geneva: World Health Organization; 2012. [Google Scholar]
- 2.WHO. The new WHO report on anti-tuberculosis drug resistance—multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. Geneva: World Health Organization; 2011. [Google Scholar]
- 3.Klopper M, Warren RM, Hayes C, et al. Emergence and spread of extensively and totally drug-resistant tuberculosis, South Africa. Emerg Infect Dis. 2013;19:449–55. doi: 10.3201//EID1903.120246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Udwadia ZF, Amale RA, Ajbani KK, Rodrigues C. Totally drug-resistant tuberculosis in India. Clin Infect Dis. 2012;54:579–81. doi: 10.1093/cid/cir889. [DOI] [PubMed] [Google Scholar]
- 5.Velayati AA, Masjedi MR, Farnia P, et al. Emergence of new forms of totally drug-resistant tuberculosis bacilli: super extensively drug-resistant tuberculosis or totally drug-resistant strains in Iran. Chest. 2009;136:420–25. doi: 10.1378/chest.08-2427. [DOI] [PubMed] [Google Scholar]
- 6.Pooran A, Pieterson E, Davids M, Theron G, Dheda K. What is the cost of diagnosis and management of drug resistant tuberculosis in South Africa? PLoS One. 2013;8:e54587. doi: 10.1371/journal.pone.0054587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Migliori GB, De Iaco G, Besozzi G, Centis R, Cirillo DM. First tuberculosis cases in Italy resistant to all tested drugs. Euro Surveill. 2007;12:E070517.1. doi: 10.2807/esw.12.20.03194-en. [DOI] [PubMed] [Google Scholar]
- 8.Espinal MA, Kim SJ, Suarez PG, et al. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA. 2000;283:2537–45. doi: 10.1001/jama.283.19.2537. [DOI] [PubMed] [Google Scholar]
- 9.Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLoS One. 2009;4:e6914. doi: 10.1371/journal.pone.0006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim DH, Kim HJ, Park SK, et al. Treatment outcomes and survival based on drug resistance patterns in multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2010;182:113–19. doi: 10.1164/rccm.200911-1656OC. [DOI] [PubMed] [Google Scholar]
- 11.Migliori GB, Espinal M, Danilova ID, Punga VV, Grzemska M, Raviglione MC. Frequency of recurrence among MDR-tB cases ‘successfully’ treated with standardised short-course chemotherapy. Int J Tuberc Lung Dis. 2002;6:858–64. [PubMed] [Google Scholar]
- 12.Orenstein EW, Basu S, Shah NS, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis. 2009;9:153–61. doi: 10.1016/S1473-3099(09)70041-6. [DOI] [PubMed] [Google Scholar]
- 13.Skrahina A, Hurevich H, Zalutskaya A, et al. Alarming levels of drug-resistant tuberculosis in Belarus: results of a survey in Minsk. Eur Respir J. 2012;39:1425–31. doi: 10.1183/09031936.00145411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zignol M, van Gemert W, Falzon D, et al. Surveillance of anti-tuberculosis drug resistance in the world: an updated analysis, 2007–2010. Bull World Health Organ. 2012;90:111–119D. doi: 10.2471/BLT.11.092585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akcakir Y. Correlates of treatment outcomes of multidrug-resistant tuberculosis (MDR-TB): a systematic review and meta-analysis. Montreal, QC: McGill University; 2010. [Google Scholar]
- 16.Dheda K, Shean K, Zumla A, et al. Early treatment outcomes and HIV status of patients with extensively drug-resistant tuberculosis in South Africa: a retrospective cohort study. Lancet. 2010;375:1798–807. doi: 10.1016/S0140-6736(10)60492-8. [DOI] [PubMed] [Google Scholar]
- 17.Kvasnovsky CL, Cegielski JP, Erasmus R, Siwisa NO, Thomas K, der Walt ML. Extensively drug-resistant TB in Eastern Cape, South Africa: high mortality in HIV-negative and HIV-positive patients. J Acquir Immune Defic Syndr. 2011;57:146–52. doi: 10.1097/QAI.0b013e31821190a3. [DOI] [PubMed] [Google Scholar]
- 18.O’Donnell MR, Padayatchi N, Kvasnovsky C, Werner L, Master I, Horsburgh CR., Jr Treatment outcomes for extensively drug-resistant tuberculosis and HIV co-infection. Emerg Infect Dis. 2013;19:416–24. doi: 10.3201/eid1903.120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Symons G, Shean K, Pietersen E, et al. A historical review of XDR tuberculosis in the Western Cape province of South Africa. S Afr Med J. 2011;101:636–38. [PubMed] [Google Scholar]
- 20.Shean KP, Willcox PA, Siwendu SN, et al. Treatment outcome and follow-up of multidrug-resistant tuberculosis patients, West Coast/Winelands, South Africa, 1992–2002. Int J Tuberc Lung Dis. 2008;12:1182–89. [PubMed] [Google Scholar]
- 21.Migliori GB, Sotgiu G, Gandhi NR, et al. Drug resistance beyond XDR-TB: results from a large individual patient data meta-analysis. Eur Respir J. 2013;42:169–79. doi: 10.1183/09031936.00136312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs—worldwide, 2000–2004. MMWR Morb Mortal Wkly Rep. 2006;55:301–05. [PubMed] [Google Scholar]
- 23.Holtz TH. XDR-TB in South Africa: revised definition. PLoS Med. 2007;4:e161. doi: 10.1371/journal.pmed.0040161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holtz TH, Cegielski JP. Origin of the term XDR-TB. Eur Respir J. 2007;30:396. doi: 10.1183/09031936.00042607. [DOI] [PubMed] [Google Scholar]
- 25.Migliori GB, Besozzi G, Girardi E, et al. the SMIRA/TBNET Study Group Clinical and operational value of the extensively drug-resistant tuberculosis definition. Eur Respir J. 2007;30:623–26. doi: 10.1183/09031936.00077307. [DOI] [PubMed] [Google Scholar]
- 26.WHO. Stop TB Initiative Treatment of tuberculosis: guidelines. 4th. Geneva: World Health Organization; 2009. [Google Scholar]
- 27.Mathema B, Kurepina N, Fallows D, Kreiswirth BN. Lessons from molecular epidemiology and comparative genomics. Semin Respir Crit Care Med. 2008;29:467–80. doi: 10.1055/s-0028-1085699. [DOI] [PubMed] [Google Scholar]
- 28.Kamerbeek J, Schouls L, Kolk A, et al. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–14. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Embden JD, Cave MD, Crawford JT, et al. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–09. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allix C, Supply P, Fauville-Dufaux M. Utility of fast mycobacterial interspersed repetitive unit-variable number tandem repeat genotyping in clinical mycobacteriological analysis. Clin Infect Dis. 2004;39:783–89. doi: 10.1086/423383. [DOI] [PubMed] [Google Scholar]
- 31.Small PM, Hopewell PC, Singh SP, et al. The epidemiology of tuberculosis in San Francisco. A population-based study using conventional and molecular methods. N Engl J Med. 1994;330:1703–09. doi: 10.1056/NEJM199406163302402. [DOI] [PubMed] [Google Scholar]
- 32.Van Rie A, Warren R, Richardson M, et al. Classification of drug-resistant tuberculosis in an epidemic area. Lancet. 2000;356:22–25. doi: 10.1016/S0140-6736(00)02429-6. [DOI] [PubMed] [Google Scholar]
- 33.Andrews JR, Gandhi NR, Moodley P, et al. the Tugela Ferry Care and Research Collaboration Exogenous reinfection as a cause of multidrug-resistant and extensively drug-resistant tuberculosis in rural South Africa. J Infect Dis. 2008;198:1582–89. doi: 10.1086/592991. [DOI] [PubMed] [Google Scholar]
- 34.Bifani PJ, Mathema B, Liu Z, et al. Identification of a W variant outbreak of Mycobacterium tuberculosis via population-based molecular epidemiology. JAMA. 1999;282:2321–27. doi: 10.1001/jama.282.24.2321. [DOI] [PubMed] [Google Scholar]
- 35.Calver AD, Falmer AA, Murray M, et al. Emergence of increased resistance and extensively drug-resistant tuberculosis despite treatment adherence, South Africa. Emerg Infect Dis. 2010;16:264–71. doi: 10.3201/eid1602.090968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–80. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 37.Jacobson KR, Theron D, Victor TC, Streicher EM, Warren RM, Murray MB. Treatment outcomes of isoniazid-resistant tuberculosis patients, Western Cape Province, South Africa. Clin Infect Dis. 2011;53:369–72. doi: 10.1093/cid/cir406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Rie A, Victor TC, Richardson M, et al. Reinfection and mixed infection cause changing Mycobacterium tuberculosis drug-resistance patterns. Am J Respir Crit Care Med. 2005;172:636–42. doi: 10.1164/rccm.200503-449OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andrews JR, Shah NS, Weissman D, Moll AP, Friedland G, Gandhi NR. Predictors of multidrug- and extensively drug-resistant tuberculosis in a high HIV prevalence community. PLoS One. 2010;5:e15735. doi: 10.1371/journal.pone.0015735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balaji V, Daley P, Anand AA, et al. Risk factors for MDR and XDR-TB in a tertiary referral hospital in India. PLoS One. 2010;5:e9527. doi: 10.1371/journal.pone.0009527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faustini A, Hall AJ, Perucci CA. Risk factors for multidrug resistant tuberculosis in Europe: a systematic review. Thorax. 2006;61:158–63. doi: 10.1136/thx.2005.045963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skrahina A, Hurevich H, Zalutskaya A, et al. Multidrug-resistant tuberculosis in Belarus: the size of the problem and associated risk factors. Bull World Health Organ. 2013;91:36–45. doi: 10.2471/BLT.12.104588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Y, Xu S, Wang L, et al. National survey of drug-resistant tuberculosis in China. N Engl J Med. 2012;366:2161–70. doi: 10.1056/NEJMoa1108789. [DOI] [PubMed] [Google Scholar]
- 44.Zhao P, Li XJ, Zhang SF, Wang XS, Liu CY. Social behaviour risk factors for drug resistant tuberculosis in mainland China: a meta-analysis. J Int Med Res. 2012;40:436–45. doi: 10.1177/147323001204000205. [DOI] [PubMed] [Google Scholar]
- 45.Ricks PM, Mavhunga F, Modi S, et al. Characteristics of multidrug-resistant tuberculosis in Namibia. BMC Infect Dis. 2012;12:385. doi: 10.1186/1471-2334-12-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nodieva A, Jansone I, Broka L, Pole I, Skenders G, Baumanis V. Recent nosocomial transmission and genotypes of multidrug-resistant Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2010;14:427–33. [PubMed] [Google Scholar]
- 47.Wang W, Hu Y, Mathema B, Jiang W, Kreiswirth B, Xu B. Recent transmission of W-Beijing family Mycobacterium tuberculosis in rural eastern China. Int J Tuberc Lung Dis. 2012;16:306–11. doi: 10.5588/ijtld.11.0304. [DOI] [PubMed] [Google Scholar]
- 48.Li X, Zhang Y, Shen X, et al. Transmission of drug-resistant tuberculosis among treated patients in Shanghai, China. J Infect Dis. 2007;195:864–69. doi: 10.1086/511985. [DOI] [PubMed] [Google Scholar]
- 49.Dalton T, Cegielski P, Akksilp S, et al. the Global PETTS Investigators Prevalence of and risk factors for resistance to second-line drugs in people with multidrug-resistant tuberculosis in eight countries: a prospective cohort study. Lancet. 2012;380:1406–17. doi: 10.1016/S0140-6736(12)60734-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeon CY, Hwang SH, Min JH, et al. Extensively drug-resistant tuberculosis in South Korea: risk factors and treatment outcomes among patients at a tertiary referral hospital. Clin Infect Dis. 2008;46:42–49. doi: 10.1086/524017. [DOI] [PubMed] [Google Scholar]
- 51.Blower SM, Chou T. Modeling the emergence of the ‘hot zones’: tuberculosis and the amplification dynamics of drug resistance. Nat Med. 2004;10:1111–16. doi: 10.1038/nm1102. [DOI] [PubMed] [Google Scholar]
- 52.Cohen T, Murray M. Modeling epidemics of multidrug-resistant M. tuberculosis of heterogeneous fitness. Nat Med. 2004;10:1117–21. doi: 10.1038/nm1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gagneux S, Long CD, Small PM, Van T, Schoolnik GK, Bohannan BJ. The competitive cost of antibiotic resistance in Mycobacterium tuberculosis. Science. 2006;312:1944–46. doi: 10.1126/science.1124410. [DOI] [PubMed] [Google Scholar]
- 54.Uys PW, Warren R, van Helden PD, Murray M, Victor TC. Potential of rapid diagnosis for controlling drug-susceptible and drug-resistant tuberculosis in communities where Mycobacterium tuberculosis infections are highly prevalent. J Clin Microbiol. 2009;47:1484–90. doi: 10.1128/JCM.02289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dowdy DW, Chaisson RE, Maartens G, Corbett EL, Dorman SE. Impact of enhanced tuberculosis diagnosis in South Africa: a mathematical model of expanded culture and drug susceptibility testing. Proc Natl Acad Sci USA. 2008;105:11293–98. doi: 10.1073/pnas.0800965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin HH, Dowdy D, Dye C, Murray M, Cohen T. The impact of new tuberculosis diagnostics on transmission: why context matters. Bull World Health Organ. 2012;90:739–47A. doi: 10.2471/BLT.11.101436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Menzies NA, Cohen T, Lin HH, Murray M, Salomon JA. Population health impact and cost-effectiveness of tuberculosis diagnosis with Xpert MTB/RIF: a dynamic simulation and economic evaluation. PLoS Med. 2012;9:e1001347. doi: 10.1371/journal.pmed.1001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luria SE, Delbrück M. Mutations of bacteria from virus sensitivity to virus resistance. Genetics. 1943;28:491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.David HL. Probability distribution of drug-resistant mutants in unselected populations of Mycobacterium tuberculosis. Appl Microbiol. 1970;20:810–14. doi: 10.1128/am.20.5.810-814.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Desouza GA, Fortuin S, Aguilar D, et al. Using a label-free proteomic method to identify differentially abundant proteins in closely related hypo- and hyper-virulent clinical Mycobacterium tuberculosis Beijing isolates. Mol Cell Proteomics. 2010;9:2414–23. doi: 10.1074/mcp.M900422-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Velayati AA, Farnia P, Ibrahim TA, et al. Differences in cell wall thickness between resistant and nonresistant strains of Mycobacterium tuberculosis: using transmission electron microscopy. Chemotherapy. 2009;55:303–07. doi: 10.1159/000226425. [DOI] [PubMed] [Google Scholar]
- 62.Velayati AA, Farnia P, Masjedi MR, et al. Totally drug-resistant tuberculosis strains: evidence of adaptation at the cellular level. Eur Respir J. 2009;34:1202–03. doi: 10.1183/09031936.00081909. [DOI] [PubMed] [Google Scholar]
- 63.Mitchison DA. How drug resistance emerges as a result of poor compliance during short course chemotherapy for tuberculosis. Int J Tuberc Lung Dis. 1998;2:10–15. [PubMed] [Google Scholar]
- 64.Gumbo T, Louie A, Deziel MR, Drusano GL. Pharmacodynamic evidence that ciprofloxacin failure against tuberculosis is not due to poor microbial kill but to rapid emergence of resistance. Antimicrob Agents Chemother. 2005;49:3178–81. doi: 10.1128/AAC.49.8.3178-3181.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gumbo T, Louie A, Deziel MR, Parsons LM, Salfinger M, Drusano GL. Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling. J Infect Dis. 2004;190:1642–51. doi: 10.1086/424849. [DOI] [PubMed] [Google Scholar]
- 66.Gumbo T, Dona CS, Meek C, Leff R. Pharmacokinetics-pharmacodynamics of pyrazinamide in a novel in vitro model of tuberculosis for sterilizing effect: a paradigm for faster assessment of new antituberculosis drugs. Antimicrob Agents Chemother. 2009;53:3197–204. doi: 10.1128/AAC.01681-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gumbo T, Louie A, Deziel MR, et al. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob Agents Chemother. 2007;51:3781–88. doi: 10.1128/AAC.01533-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gumbo T, Louie A, Liu W, et al. Isoniazid’s bactericidal activity ceases because of the emergence of resistance, not depletion of Mycobacterium tuberculosis in the log phase of growth. J Infect Dis. 2007;195:194–201. doi: 10.1086/510247. [DOI] [PubMed] [Google Scholar]
- 69.Gumbo T, Louie A, Liu W, et al. Isoniazid bactericidal activity and resistance emergence: integrating pharmacodynamics and pharmacogenomics to predict efficacy in different ethnic populations. Antimicrob Agents Chemother. 2007;51:2329–36. doi: 10.1128/AAC.00185-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Srivastava S, Musuka S, Sherman C, Meek C, Leff R, Gumbo T. Efflux-pump-derived multiple drug resistance to ethambutol monotherapy in Mycobacterium tuberculosis and the pharmacokinetics and pharmacodynamics of ethambutol. J Infect Dis. 2010;201:1225–31. doi: 10.1086/651377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pasipanodya JG, Gumbo T. A new evolutionary and pharmacokinetic-pharmacodynamic scenario for rapid emergence of resistance to single and multiple anti-tuberculosis drugs. Curr Opin Pharmacol. 2011;11:457–63. doi: 10.1016/j.coph.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmalstieg AM, Srivastava S, Belkaya S, et al. The antibiotic resistance arrow of time: efflux pump induction is a general first step in the evolution of mycobacterial drug resistance. Antimicrob Agents Chemother. 2012;56:4806–15. doi: 10.1128/AAC.05546-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Louw GE, Warren RM, Gey van Pittius NC, et al. Rifampicin reduces susceptibility to ofloxacin in rifampicin-resistant Mycobacterium tuberculosis through efflux. Am J Respir Crit Care Med. 2011;184:269–76. doi: 10.1164/rccm.201011-1924OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Machado D, Couto I, Perdigão J, et al. Contribution of efflux to the emergence of isoniazid and multidrug resistance in Mycobacterium tuberculosis. PLoS One. 2012;7:e34538. doi: 10.1371/journal.pone.0034538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chatterjee A, Saranath D, Bhatter P, Mistry N. Global transcriptional profiling of longitudinal clinical isolates of Mycobacterium tuberculosis exhibiting rapid accumulation of drug resistance. PLoS One. 2013;8:e54717. doi: 10.1371/journal.pone.0054717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Srivastava S, Pasipanodya JG, Meek C, Leff R, Gumbo T. Multidrug-resistant tuberculosis not due to noncompliance but to between-patient pharmacokinetic variability. J Infect Dis. 2011;204:1951–59. doi: 10.1093/infdis/jir658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Steenwinkel JE, ten Kate MT, de Knegt GJ, et al. Consequences of noncompliance for therapy efficacy and emergence of resistance in murine tuberculosis caused by the Beijing genotype of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2012;56:4937–44. doi: 10.1128/AAC.00124-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chigutsa E, Visser ME, Swart EC, et al. The SLCO1B1 rs4149032 polymorphism is highly prevalent in South Africans and is associated with reduced rifampin concentrations: dosing implications. Antimicrob Agents Chemother. 2011;55:4122–27. doi: 10.1128/AAC.01833-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pasipanodya JG, Srivastava S, Gumbo T. Meta-analysis of clinical studies supports the pharmacokinetic variability hypothesis for acquired drug resistance and failure of antituberculosis therapy. Clin Infect Dis. 2012;55:169–77. doi: 10.1093/cid/cis353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pasipanodya JG, Gumbo T. A meta-analysis of self-administered versus directly observed therapy effect on microbiologic failure, relapse, and acquired drug resistance in tuberculosis patients. Clin Infect Dis. 2013;57:21–31. doi: 10.1093/cid/cit167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis. 2013;208:1464–73. doi: 10.1093/infdis/jit352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vadwai V, Daver G, Udwadia Z, Sadani M, Shetty A, Rodrigues C. Clonal population of Mycobacterium tuberculosis strains reside within multiple lung cavities. PLoS One. 2011;6:e24770. doi: 10.1371/journal.pone.0024770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dharmadhikari AS, Kabadi M, Gerety B, Hickey AJ, Fourie PB, Nardell E. Phase I, single-dose, dose-escalating study of inhaled dry powder capreomycin: a new approach to therapy of drug-resistant tuberculosis. Antimicrob Agents Chemother. 2013;57:2613–19. doi: 10.1128/AAC.02346-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ford CB, Shah RR, Maeda MK, et al. Mycobacterium tuberculosis mutation rate estimates from different lineages predict substantial differences in the emergence of drug-resistant tuberculosis. Nat Genet. 2013;45:784–90. doi: 10.1038/ng.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Borrell S, Gagneux S. Infectiousness, reproductive fitness and evolution of drug-resistant Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2009;13:1456–66. [PubMed] [Google Scholar]
- 86.Gumbo T. Biological variability and the emergence of multidrug-resistant tuberculosis. Nat Genet. 2013;45:720–21. doi: 10.1038/ng.2675. [DOI] [PubMed] [Google Scholar]
- 87.Comas I, Borrell S, Roetzer A, et al. Whole-genome sequencing of rifampicin-resistant Mycobacterium tuberculosis strains identifies compensatory mutations in RNA polymerase genes. Nat Genet. 2012;44:106–10. doi: 10.1038/ng.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun G, Luo T, Yang C, et al. Dynamic population changes in Mycobacterium tuberculosis during acquisition and fixation of drug resistance in patients. J Infect Dis. 2012;206:1724–33. doi: 10.1093/infdis/jis601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Basile JI, Geffner LJ, Romero MM, et al. Outbreaks of Mycobacterium tuberculosis MDR strains induce high IL-17 T-cell response in patients with MDR tuberculosis that is closely associated with high antigen load. J Infect Dis. 2011;204:1054–64. doi: 10.1093/infdis/jir460. [DOI] [PubMed] [Google Scholar]
- 90.Eum SY, Lee YJ, Min JH, et al. Association of antigen-stimulated release of tumor necrosis factor-alpha in whole blood with response to chemotherapy in patients with pulmonary multidrug-resistant tuberculosis. Respiration. 2010;80:275–84. doi: 10.1159/000283687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Geffner L, Yokobori N, Basile J, et al. Patients with multidrug-resistant tuberculosis display impaired Th1 responses and enhanced regulatory T-cell levels in response to an outbreak of multidrug-resistant Mycobacterium tuberculosis M and Ra strains. Infect Immun. 2009;77:5025–34. doi: 10.1128/IAI.00224-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Semple PL, Binder AB, Davids M, Maredza A, van Zyl-Smit RN, Dheda K. Regulatory T-cells attenuate mycobacterial stasis in alveolar and blood-derived macrophages from patients with TB. Am J Respir Crit Care Med. 2013;187:1249–58. doi: 10.1164/rccm.201210-1934OC. [DOI] [PubMed] [Google Scholar]
- 93.Skrahin A, Ahmed RK, Ferrara G, et al. Autologous mesenchymal stromal cell infusion as adjunct treatment in patients with multidrug and extensively drug-resistant tuberculosis: an open-label phase 1 safety trial. Lancet Respir Med. 2014;2:108–22. doi: 10.1016/S2213-2600(13)70234-0. [DOI] [PubMed] [Google Scholar]
- 94.Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138:105–15. doi: 10.1111/imm.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dheda K, Schwander SK, Zhu B, van Zyl-Smit RN, Zhang Y. The immunology of tuberculosis: from bench to bedside. Respirology. 2010;15:433–50. doi: 10.1111/j.1440-1843.2010.01739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Basu S, Andrews JR, Poolman EM, et al. Prevention of nosocomial transmission of extensively drug-resistant tuberculosis in rural South African district hospitals: an epidemiological modelling study. Lancet. 2007;370:1500–07. doi: 10.1016/S0140-6736(07)61636-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Basu S, Friedland GH, Medlock J, et al. Averting epidemics of extensively drug-resistant tuberculosis. Proc Natl Acad Sci USA. 2009;106:7672–77. doi: 10.1073/pnas.0812472106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jones-López EC, Namugga O, Mumbowa F, et al. Cough aerosols of Mycobacterium tuberculosis predict new infection: a household contact study. Am J Respir Crit Care Med. 2013;187:1007–15. doi: 10.1164/rccm.201208-1422OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. Superspreading and the effect of individual variation on disease emergence. Nature. 2005;438:355–59. doi: 10.1038/nature04153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Woolhouse ME, Dye C, Etard JF, et al. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc Natl Acad Sci USA. 1997;94:338–42. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jones-López EC, Namugga O, Mumbowa F, et al. Cough aerosols of Mycobacterium tuberculosis predict new infection: a household contact study. Am J Respir Crit Care Med. 2013;187:1007–15. doi: 10.1164/rccm.201208-1422OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Andersson DI. The biological cost of mutational antibiotic resistance: any practical conclusions? Curr Opin Microbiol. 2006;9:461–65. doi: 10.1016/j.mib.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 103.Müller B, Borrell S, Rose G, Gagneux S. The heterogeneous evolution of multidrug-resistant Mycobacterium tuberculosis. Trends Genet. 2013;29:160–69. doi: 10.1016/j.tig.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Borrell S, Gagneux S. Strain diversity, epistasis and the evolution of drug resistance in Mycobacterium tuberculosis. Clin Microbiol Infect. 2011;17:815–20. doi: 10.1111/j.1469-0691.2011.03556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–15. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Steingart KR. Xpert MTB/RIF test for detection of pulmonary tuberculosis and rifampicin resistance. J Evid Based Med. 2013;6:58. [Google Scholar]
- 107.Theron G, Zijenah L, Chanda D, et al. for the TB-NEAT team Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet. 2014;383:424–35. doi: 10.1016/S0140-6736(13)62073-5. [DOI] [PubMed] [Google Scholar]
- 108.Jacobson KR, Theron D, Kendall EA, et al. Implementation of genotype MTBDRplus reduces time to multidrug-resistant tuberculosis therapy initiation in South Africa. Clin Infect Dis. 2013;56:503–08. doi: 10.1093/cid/cis920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rishi S, Sinha P, Malhotra B, Pal N. A comparative study for the detection of mycobacteria by BACTEC MGIT 960, Lowenstein Jensen media and direct AFB smear examination. Indian J Med Microbiol. 2007;25:383–86. doi: 10.4103/0255-0857.37344. [DOI] [PubMed] [Google Scholar]
- 110.Smith SE, Kurbatova EV, Cavanaugh JS, Cegielski JP. Global isoniazid resistance patterns in rifampin-resistant and rifampin-susceptible tuberculosis. Int J Tuberc Lung Dis. 2012;16:203–05. doi: 10.5588/ijtld.11.0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.WHO. Towards universal access to diagnosis and treatment of multidrug-resistant and extensively drug-resistant tuberculosis by 2015. Geneva: World Health Organization; 2011. (WHO progress report 2011). [Google Scholar]
- 112.Barnard M, Albert H, Coetzee G, O’Brien R, Bosman ME. Rapid molecular screening for multidrug-resistant tuberculosis in a high-volume public health laboratory in South Africa. Am J Respir Crit Care Med. 2008;177:787–92. doi: 10.1164/rccm.200709-1436OC. [DOI] [PubMed] [Google Scholar]
- 113.Barnard M, Gey van Pittius NC, van Helden PD, Bosman M, Coetzee G, Warren RM. The diagnostic performance of the GenoType MTBDRplus version 2 line probe assay is equivalent to that of the Xpert MTB/RIF assay. J Clin Microbiol. 2012;50:3712–16. doi: 10.1128/JCM.01958-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rutledge JA, Crouch JB. The ultimate results in 1654 cases of tuberculosis treated at the modern Woodmen of America sanatorium. Am Rev Tuberc. 1919;2:755–63. [Google Scholar]
- 115.Hillemann D, Rüsch-Gerdes S, Richter E. Feasibility of the GenoType MTBDRsl assay for fluoroquinolone, amikacin-capreomycin, and ethambutol resistance testing of Mycobacterium tuberculosis strains and clinical specimens. J Clin Microbiol. 2009;47:1767–72. doi: 10.1128/JCM.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barnard M, Warren R, Gey van Pittius N, et al. Genotype MTBDRsl line probe assay shortens time to diagnosis of extensively drug-resistant tuberculosis in a high-throughput diagnostic laboratory. Am J Respir Crit Care Med. 2012;186:1298–305. doi: 10.1164/rccm.201205-0960OC. [DOI] [PubMed] [Google Scholar]
- 117.Miotto P, Cabibbe AM, Mantegani P, et al. GenoType MTBDRsl performance on clinical samples with diverse genetic background. Eur Respir J. 2012;40:690–98. doi: 10.1183/09031936.00164111. [DOI] [PubMed] [Google Scholar]
- 118.Dixit P, Singh U, Sharma P, Jain A. Evaluation of nitrate reduction assay, resazurin microtiter assay and microscopic observation drug susceptibility assay for first line antitubercular drug susceptibility testing of clinical isolates of M tuberculosis. J Microbiol Methods. 2012;88:122–26. doi: 10.1016/j.mimet.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 119.Dheda K, Warren RM, Zumla A, Grobusch MP. Extensively drug-resistant tuberculosis: epidemiology and management challenges. Infect Dis Clin North Am. 2010;24:705–25. doi: 10.1016/j.idc.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 120.Gandhi NR, Nunn P, Dheda K, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet. 2010;375:1830–43. doi: 10.1016/S0140-6736(10)60410-2. [DOI] [PubMed] [Google Scholar]
- 121.Migliori GB, Dheda K, Centis R, et al. Review of multidrug-resistant and extensively drug-resistant TB: global perspectives with a focus on sub-Saharan Africa. Trop Med Int Health. 2010;15:1052–66. doi: 10.1111/j.1365-3156.2010.02581.x. [DOI] [PubMed] [Google Scholar]
- 122.WHO. WHO updated references on management of drug-resistant tuberculosis: guidelines for the programmatic management of drug-resistant tuberculosis—2011 update. Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
- 123.Schaaf HS, Moll AP, Dheda K. Multidrug- and extensively drug-resistant tuberculosis in Africa and South America: epidemiology, diagnosis and management in adults and children. Clin Chest Med. 2009;30:667–83. vii–viii. doi: 10.1016/j.ccm.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 124.Pietersen E, Ignatius E, Streicher EM, et al. Long-term outcomes of patients with extensively drug-resistant tuberculosis in South Africa: a cohort study. Lancet. 2014 doi: 10.1016/S0140-6736(13)62675-6. published online Jan 17. http://dx.doi.org/10.1016/S0140-6736(13)62675-6. [DOI] [PubMed]
- 125.Müller B, Streicher EM, Hoek KG, et al. inhA promoter mutations: a gateway to extensively drug-resistant tuberculosis in South Africa? Int J Tuberc Lung Dis. 2011;15:344–51. [PubMed] [Google Scholar]
- 126.Jacobson KR, Tierney DB, Jeon CY, Mitnick CD, Murray MB. Treatment outcomes among patients with extensively drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis. 2010;51:6–14. doi: 10.1086/653115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.De Lorenzo S, Alffenaar JW, Sotgiu G, et al. Efficacy and safety of meropenem-clavulanate added to linezolid-containing regimens in the treatment of MDR-/XDR-TB. Eur Respir J. 2013;41:1386–92. doi: 10.1183/09031936.00124312. [DOI] [PubMed] [Google Scholar]
- 128.Sirgel FA, Warren RM, Böttger EC, Klopper M, Victor TC, van Helden PD. The rationale for using rifabutin in the treatment of MDR and XDR tuberculosis outbreaks. PLoS One. 2013;8:e59414. doi: 10.1371/journal.pone.0059414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee M, Lee J, Carroll MW, et al. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med. 2012;367:1508–18. doi: 10.1056/NEJMoa1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Voelker R. MDR-TB has new drug foe after fast-track approval. JAMA. 2013;309:430. doi: 10.1001/jama.2013.94. [DOI] [PubMed] [Google Scholar]
- 131.Gler MT, Skripconoka V, Sanchez-Garavito E, et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med. 2012;366:2151–60. doi: 10.1056/NEJMoa1112433. [DOI] [PubMed] [Google Scholar]
- 132.Skripconoka V, Danilovits M, Pehme L, et al. Delamanid improves outcomes and reduces mortality in multidrug-resistant tuberculosis. Eur Respir J. 2013;41:1393–400. doi: 10.1183/09031936.00125812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.O’Donnell MR, Padayatchi N, Master I, Osburn G, Horsburgh CR. Improved early results for patients with extensively drug-resistant tuberculosis and HIV in South Africa. Int J Tuberc Lung Dis. 2009;13:855–61. [PMC free article] [PubMed] [Google Scholar]
- 134.Gandhi NR, Shah NS, Andrews JR, et al. the Tugela Ferry Care and Research (TF CARES) Collaboration HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med. 2010;181:80–86. doi: 10.1164/rccm.200907-0989OC. [DOI] [PubMed] [Google Scholar]
- 135.Shean K, Streicher E, Pieterson E, et al. Drug-associated adverse events and their relationship with outcomes in patients receiving treatment for extensively drug-resistant tuberculosis in South Africa. PLoS One. 2013;8:e63057. doi: 10.1371/journal.pone.0063057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dubrovina I, Miskinis K, Lyepshina S, et al. Drug-resistant tuberculosis and HIV in Ukraine: a threatening convergence of two epidemics? Int J Tuberc Lung Dis. 2008;12:756–62. [PubMed] [Google Scholar]
- 137.Nelson LJ, Talbot EA, Mwasekaga MJ, et al. Antituberculosis drug resistance and anonymous HIV surveillance in tuberculosis patients in Botswana, 2002. Lancet. 2005;366:488–90. doi: 10.1016/S0140-6736(05)67062-6. [DOI] [PubMed] [Google Scholar]
- 138.Nunes EA, De Capitani EM, Coelho E, et al. Patterns of anti-tuberculosis drug resistance among HIV-infected patients in Maputo, Mozambique, 2002–2003. Int J Tuberc Lung Dis. 2005;9:494–500. [PubMed] [Google Scholar]
- 139.Wallengren K, Scano F, Nunn P, et al. Drug-resistant tuberculosis, KwaZulu-Natal, South Africa, 2001–2007. Emerg Infect Dis. 2011;17:1913–16. doi: 10.3201/eid1710.100952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Keshavjee S, Gelmanova IY, Farmer PE, et al. Treatment of extensively drug-resistant tuberculosis in Tomsk, Russia: a retrospective cohort study. Lancet. 2008;372:1403–09. doi: 10.1016/S0140-6736(08)61204-0. [DOI] [PubMed] [Google Scholar]
- 141.Mitnick C, Bayona J, Palacios E, et al. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. N Engl J Med. 2003;348:119–28. doi: 10.1056/NEJMoa022928. [DOI] [PubMed] [Google Scholar]
- 142.Mitnick CD, Shin SS, Seung KJ, et al. Comprehensive treatment of extensively drug-resistant tuberculosis. N Engl J Med. 2008;359:563–74. doi: 10.1056/NEJMoa0800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Abdool-Karim SS, Abdool-Karim Q, Friedland G, Lalloo U, El-Sadr WM, the START project Implementing antiretroviral therapy in resource-constrained settings: opportunities and challenges in integrating HIV and tuberculosis care. AIDS. 2004;18:975–79. doi: 10.1097/00002030-200404300-00004. [DOI] [PubMed] [Google Scholar]
- 144.Dartois V, Barry CE. Clinical pharmacology and lesion penetrating properties of second- and third-line antituberculous agents used in the management of multidrug-resistant (MDR) and extensively-drug resistant (XDR) tuberculosis. Curr Clin Pharmacol. 2010;5:96–114. doi: 10.2174/157488410791110797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Brust JC, Shah NS, van der Merwe TL, et al. Adverse events in an integrated home-based treatment program for MDR-TB and HIV in KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2013;62:436–40. doi: 10.1097/QAI.0b013e31828175ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Isaakidis P, Varghese B, Mansoor H, et al. Adverse events among HIV/MDR-TB co-infected patients receiving antiretroviral and second line anti-TB treatment in Mumbai, India. PLoS One. 2012;7:e40781. doi: 10.1371/journal.pone.0040781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Seung KJ, Omatayo DB, Keshavjee S, Furin JJ, Farmer PE, Satti H. Early outcomes of MDR-TB treatment in a high HIV-prevalence setting in Southern Africa. PLoS One. 2009;4:e7186. doi: 10.1371/journal.pone.0007186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Holtz TH, Sternberg M, Kammerer S, et al. Time to sputum culture conversion in multidrug-resistant tuberculosis: predictors and relationship to treatment outcome. Ann Intern Med. 2006;144:650–59. doi: 10.7326/0003-4819-144-9-200605020-00008. [DOI] [PubMed] [Google Scholar]
- 149.Skolimowska KH, Rangaka MX, Meintjes G, et al. Altered ratio of IFN-γ/IL-10 in patients with drug resistant Mycobacterium tuberculosis and HIV—tuberculosis immune reconstitution inflammatory syndrome. PLoS One. 2012;7:e46481. doi: 10.1371/journal.pone.0046481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Falzon D, Jaramillo E, Schünemann HJ, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J. 2011;38:516–28. doi: 10.1183/09031936.00073611. [DOI] [PubMed] [Google Scholar]
- 151.Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Abdool Karim SS, Naidoo K, Grobler A, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011;365:1492–501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Blanc FX, Sok T, Laureillard D, et al. the CAMELIA (ANRS 1295–CIPRA KH001) Study Team Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365:1471–81. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Havlir DV, Kendall MA, Ive P, et al. and the AIDS Clinical Trials Group Study A5221 Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365:1482–91. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gandhi NR, Moll AP, Lalloo U, et al. the Tugela Ferry Care and Research (TFCaRes) Collaboration Successful integration of tuberculosis and HIV treatment in rural South Africa: the Sizonq’oba study. J Acquir Immune Defic Syndr. 2009;50:37–43. doi: 10.1097/QAI.0b013e31818ce6c4. [DOI] [PubMed] [Google Scholar]
- 156.Manosuthi W, Chottanapand S, Thongyen S, Chaovavanich A, Sungkanuparph S. Survival rate and risk factors of mortality among HIV/tuberculosis-coinfected patients with and without antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;43:42–46. doi: 10.1097/01.qai.0000230521.86964.86. [DOI] [PubMed] [Google Scholar]
- 157.Velasco M, Castilla V, Sanz J, et al. the COMESEM Cohort Effect of simultaneous use of highly active antiretroviral therapy on survival of HIV patients with tuberculosis. J Acquir Immune Defic Syndr. 2009;50:148–52. doi: 10.1097/QAI.0b013e31819367e7. [DOI] [PubMed] [Google Scholar]
- 158.Dheda K, Lampe FC, Johnson MA, Lipman MC. Outcome of HIV-associated tuberculosis in the era of highly active antiretroviral therapy. J Infect Dis. 2004;190:1670–76. doi: 10.1086/424676. [DOI] [PubMed] [Google Scholar]
- 159.Shah NS, Pratt R, Armstrong L, Robison V, Castro KG, Cegielski JP. Extensively drug-resistant tuberculosis in the United States, 1993–2007. JAMA. 2008;300:2153–60. doi: 10.1001/jama.300.18.2153. [DOI] [PubMed] [Google Scholar]
- 160.Sotgiu G, Ferrara G, Matteelli A, et al. Epidemiology and clinical management of XDR-TB: a systematic review by TBNET. Eur Respir J. 2009;33:871–81. doi: 10.1183/09031936.00168008. [DOI] [PubMed] [Google Scholar]
- 161.Chaulk CP, Kazandjian VA. Directly observed therapy for treatment completion of pulmonary tuberculosis: consensus statement of the Public Health Tuberculosis Guidelines Panel. JAMA. 1998;279:943–48. doi: 10.1001/jama.279.12.943. [DOI] [PubMed] [Google Scholar]
- 162.Ahuja SD, Ashkin D, Avendano M, et al. the Collaborative Group for Meta-Analysis of Individual Patient Data in MDR-TB Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med. 2012;9:e1001300. doi: 10.1371/journal.pmed.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Falzon D, Gandhi N, Migliori GB, et al. Resistance to fluoroquinolones and second-line injectable drugs: impact on multidrug-resistant TB outcomes. Eur Respir J. 2013;42:156–68. doi: 10.1183/09031936.00134712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Agonafir M, Lemma E, Wolde-Meskel D, et al. Phenotypic and genotypic analysis of multidrug-resistant tuberculosis in Ethiopia. Int J Tuberc Lung Dis. 2010;14:1259–65. [PubMed] [Google Scholar]
- 165.Gandhi NR, Andrews JR, Brust JC, et al. Risk factors for mortality among MDR- and XDR-TB patients in a high HIV prevalence setting. Int J Tuberc Lung Dis. 2012;16:90–97. doi: 10.5588/ijtld.11.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Brust JC, Shah NS, Scott M, et al. Integrated, home-based treatment for MDR-TB and HIV in rural South Africa: an alternate model of care. Int J Tuberc Lung Dis. 2012;16:998–1004. doi: 10.5588/ijtld.11.0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Heller T, Lessells RJ, Wallrauch CG, et al. Community-based treatment for multidrug-resistant tuberculosis in rural KwaZulu-Natal, South Africa. Int J Tuberc Lung Dis. 2010;14:420–26. [PubMed] [Google Scholar]
- 168.Ajbani K, Rodrigues C, Shenai S, Mehta A. Mutation detection and accurate diagnosis of extensively drug-resistant tuberculosis: report from a tertiary care center in India. J Clin Microbiol. 2011;49:1588–90. doi: 10.1128/JCM.00113-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dheda K, Migliori GB. The global rise of extensively drug-resistant tuberculosis: is the time to bring back sanatoria now overdue? Lancet. 2012;379:773–75. doi: 10.1016/S0140-6736(11)61062-3. [DOI] [PubMed] [Google Scholar]
- 170.Denholm JT, Amon JJ, O’Brien R, et al. Attitudes towards involuntary incarceration for tuberculosis: a survey of Union members. Int J Tuberc Lung Dis. 2014;18:155–59. doi: 10.5588/ijtld.13.0609. [DOI] [PubMed] [Google Scholar]
- 171.Baussano I, Nunn P, Williams B, Pivetta E, Bugiani M, Scano F. Tuberculosis among health care workers. Emerg Infect Dis. 2011;17:488–94. doi: 10.3201/eid1703.100947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.O’Donnell MR, Jarand J, Loveday M, et al. High incidence of hospital admissions with multidrug-resistant and extensively drug-resistant tuberculosis among South African health care workers. Ann Intern Med. 2010;153:516–22. doi: 10.1059/0003-4819-153-8-201010190-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jarand J, Shean K, O’Donnell M, et al. Extensively drug-resistant tuberculosis (XDR-TB) among health care workers in South Africa. Trop Med Int Health. 2010;15:1179–84. doi: 10.1111/j.1365-3156.2010.02590.x. [DOI] [PubMed] [Google Scholar]
- 174.WHO. International Labour Organization The Joint WHO-ILO-UNAIDS policy guidelines on improving health workers’ access to HIV and TB prevention, treatment, care and support services. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 175.Becerra MC, Appleton SC, Franke MF, et al. Tuberculosis burden in households of patients with multidrug-resistant and extensively drug-resistant tuberculosis: a retrospective cohort study. Lancet. 2011;377:147–52. doi: 10.1016/S0140-6736(10)61972-1. [DOI] [PubMed] [Google Scholar]
- 176.Vella V, Racalbuto V, Guerra R, et al. Household contact investigation of multidrug-resistant and extensively drug-resistant tuberculosis in a high HIV prevalence setting. Int J Tuberc Lung Dis. 2011;15:1170–75. i. doi: 10.5588/ijtld.10.0781. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


