Abstract
Dengue virus (DENV), the causative agent of dengue disease, is among the most important mosquito-borne pathogens worldwide. DENV is composed of four closely related serotypes and belongs to the Flaviviridae family alongside other important arthropod-borne viral pathogens such as Zika virus (ZIKV), West Nile virus (WNV) and Yellow Fever virus (YFV). After infection, the antibody response is mostly directed to the viral E glycoprotein which is composed of three structural domains named DI, DII and DIII that share variable degrees of homology among different viruses. Recent evidence supports a close serological interaction between ZIKV and DENV. The possibility of worse clinical outcomes as a consequence of antibody-dependent enhancement of infection (ADE) due to cross-reactive antibodies with poor neutralisation activity is a matter of concern. We tested polyclonal sera from groups of female Balb/C mice vaccinated with DNA constructs expressing DI/DII, DIII or the whole sE from different DENV serotypes and compared their activity in terms of cross-reactivity, neutralisation of virus infection and ADE. Our results indicate that the polyclonal antibody responses against the whole sE protein are highly cross-reactive with strong ADE and poor neutralisation activities due to DI/DII immunodominance. Conversely, anti-DIII polyclonal antibodies are type-specific, with no ADE towards ZIKV, WNV and YFV, and strong neutralisation activity restricted only to DENV.
Introduction
Dengue virus (DENV) is one of the most important human viral pathogens worldwide [1]. Dengue infections are a major public health concern with significant socioeconomic consequences, particularly in developing countries. The disease is endemic in tropical regions around the world, with more than 3.9 billion people at primary risk of infection; current estimates indicate that around 390 million people have DENV infections each year which result in more than 96 million symptomatic cases [2].
DENV is composed of 4 closely related serotypes (DENV1-4) and belongs to the Flaviviridae family that includes other important and closely related arthropod-borne human viral pathogens such as Zika virus (ZIKV), West Nile virus (WNV), Yellow Fever virus (YFV), Japanese Encephalitis virus (JEV) and Tick-Borne Encephalitis virus (TBEV). Like all flaviviruses, DENV is an enveloped virus with a single-stranded, positive sense RNA genome of ≈11 Kb encoding a single viral polyprotein which renders 10 mature viral proteins: 3 structural (Capsid (C), pre-membrane (PrM) and envelope glycoprotein (E)) and 7 non-structural (NS) proteins (NS1,-2A,-2B,-3,-4A,-4B and -5) [3]. E protein is the principal constituent of the viral particle, playing key roles during viral assembly and endosomal-mediated virus internalisation [4]. The E ectodomain, also termed soluble E (sE), comprises approximately the first 400 aminoacids and is formed by three different structural domains named DI, DII and DIII. DI is a β-barrel structure located at the centre of the E monomer while DII forms an elongated finger-like structure that carries the internal fusion loop. DIII is an Ig-like domain and has been implicated in binding to cellular receptors [5, 6].
E protein is also the main antigenic target of the human antibody response [7]. Neutralising epitopes have been described on all three domains, with domain-specific antibodies displaying different degrees of cross-reactivity among DENV serotypes and other flaviviruses [8–10]. Antibodies against DIII are generally described to be highly neutralising; coincidently, this region shows also the highest variability between serotypes inducing antibodies that are usually highly specific [8, 9, 11, 12]. Recently, new types of potent cross-neutralising antibodies against complex quaternary epitopes, conserved among different viruses and restricted to the dimeric conformation of E or the surface of the whole viral particle have been described and are thought to be the main drivers of viral neutralisation after infection in humans [13–17]. On the other hand, antibodies against epitopes on DI and DII (DI/DII) are, generally, poorly neutralising and highly cross-reactive with other flaviviruses [18]. Interestingly, antibody responses from flavivirus-infected individuals are dominated by highly cross-reactive antibodies mainly focused to antigenic determinants around DII, especially the fusion loop, with relatively reduced contribution of antibodies against DIII or the more complex quaternary epitopes [19–21].
Development of an efficient vaccine against DENV has been a high priority. CYD-TVD, a tetravalent live YF17D-based chimeric vaccine, is the first licensed candidate available worldwide. Its effectiveness, however, has been questioned due to the lack of homogenous protection against all 4 DENV serotypes and a signal of increased risk of severe dengue manifestations in young children [22–24]. In dengue disease, one of the main challenges is the need to develop a single vaccine formulation that confers a balanced, long-lasting and highly neutralising response against all 4 serotypes. This is mainly due to the risk of enhancing infection through a phenomenon known as antibody-dependent enhancement of infection (ADE). In ADE, recognition of viral particles by cross-reacting antibodies with non-neutralising activity or at sub-neutralising concentrations leads to an increased Fcγ receptor-mediated uptake of opsonized particles by monocytes, macrophages and dendritic cells, which results in an increased and more severe infection [25].
Until recently, ZIKV infections were sporadic, mostly asymptomatic and restricted to small geographical areas. However, the virus has rapidly spread to a global scale similar to that of DENV, and is now associated with severe neurological and developmental conditions in humans [26, 27]. DENV and ZIKV are closely related: while the aminoacid sequence of E among DENV serotypes differs by 30–35%, DENV and ZIKV E sequences differs by 41–46%, which explains the significant antigenic overlap between them [7, 28]. Even though previous studies have indicated the possibility of ADE among heterologous flaviviruses [29, 30], it was only recently shown that poorly-neutralising cross-reactive antibodies against DENV are able to enhance ZIKV infection and vice versa [31, 32]. In contrast to previous reports based on the study of monoclonal antibodies [32, 33], here we show the activities of polyclonal sera against DENV E protein obtained by domain-based DNA-immunisations in mice. Immunocompetent mouse models, like Balb/c, have been historically used to study the antibody response against DENV vaccines [34, 35]. We used this model to describe how the immune response towards different E domains contributes to virus enhancement and neutralisation of DENV, ZIKV, WNV and YFV, thus providing valuable information for proper antigen design.
Materials and methods
Cell lines and viruses
Vero cells (ATCC CCL-81, Rockville, MD, USA) HEK293 cells (ATCC, CRL-1573) and HEK293T/17 cells (HEK293-T, ATCC CRL-11268) were cultured in Dulbecco's modified Eagle's medium (DMEM, Life Technologies, Paisley, UK) supplemented with 10% heat-inactivated foetal calf serum (FCS) (Life Technologies), 50 μg/ml gentamycin and 2 mM L-glutamine. K562 cells (ATCC CCL-243) were maintained in RPMI 1640 medium (Life Technologies) supplemented with 10% FCS and 50 μg/ml gentamycin. Cell cultures were grown at 37°C with 5% of CO2.
ZIKV 976 Uganda strain, WNV New York 1999 strain and YFV French neurotropic virus strain (provided by Alessandro Marcello, ICGEB, Trieste, Italy) and DENV1 Hawaii A strain, DENV2 NGC strain, DENV3 isolate 3140/09 and DENV4 TC25 strain, were used. All viral strains were propagated in Vero cells in DMEM containing 2% FCS.
Plasmid DNA constructs
DENV E glycoprotein sequences were obtained from DENV1 Nauru Island strain (GenBank accession number U88535.1), DENV2 New Guinea C strain (AF038403), DENV3 3H87 strain (M93130), and DENV4 Dominica strain (AF326573.1). Codon-optimised DIII sequences of all DENV serotypes (E protein codons 297–416 for DENV1, DENV2 and DENV4; and 295–414 for DENV3), and sE from DENV3 (codons 1–414) and DENV4 (codons 1–416) were obtained as synthetic fragments (GenScript, Piscataway, NJ, USA). Each DIII and sE sequence was fused to an amino-terminal immunoglobulin leader sequence (sec) [36] and to a carboxy-terminal SV5 tag (GKPIPNPLLGLD) [37]. DIII-SV5 constructs also contained the human IgG heavy chain constant domain 3 (γCH3) downstream of the SV5 tag [38]. The DI/DII-SV5 constructs (codons 1–294 for DENV3 and 1–296 for DENV4) were obtained by removing the DIII coding regions from the sE-SV5 plasmids of DENV3 and DENV4 [39]. All constructs were cloned in pVAX (Thermo Fisher Scientific, Waltham, MA, USA) vector. To produce mono-biotinylated DIII-εCH4 recombinant proteins for ELISA, DIII segments were cloned upstream of the human IgE heavy chain constant domain 4 (εCH4), followed by the biotin acceptor peptide (BAP) sequence (GLNDIFEAQKIEWHE) [40, 41], into a bigenic vector containing the gene for a secretory E. coli biotin ligase [41]. Likewise, mono-biotinylated versions of DENV3 and DENV4 sE and DI/DII were fused upstream of the BAP tag into the same bigenic vector.
Animal immunisations
5–6 weeks old, female, Balb/c mice were purchased from Harlan (Milan, Italy) and kept in an isolated hygienic facility, housed in 8 groups of 8 animals within IVC cages (Allentown, Tecniplast Sealsafe Blue series, 1500 cm2) under a 12 h light/dark cycle at 22±5°C, with unrestricted access to bedding (Tapvei, 4HP, Paekna, Estonia), food (Envigo, 2018S, Madison, WI, USA) and water, and monitored three times a week throughout the course of the study. Animals were immunised three times at two weeks intervals (Days 1, 15 and 30) by biolistic delivery of 1 μm gold particles coated with 1 μg of plasmid DNA using Gene Gun technology (Bio-Rad, Hercules, CA, USA); in the case of the DIII-tetravalent formulation, animals were vaccinated with two-1 μg DNA shots as previously described [38]. No signs of illness or distress were found following immunisation. Blood samples were collected at day 90 by sub-mandibular puncture and mice were finally euthanized following the CO2 inhalation method. De-complemented pooled sera samples (30 min. at 56°C) were stored at -20°C until use.
Expression of recombinant dengue proteins
Transfections of HEK293T/17 cells were performed with standard calcium phosphate method as previously described [42]. Cellular extracts were prepared in 100 μl of TNN lysis buffer (100 mM Tris-HCl, pH 8, 250 mM NaCl, 0.5% NP-40) at 4°C, supplemented with Protease Inhibitor Cocktail (Sigma-Aldrich, St. Louis, MO, USA).
Western blot
Samples were separated by 10% SDS-PAGE and then transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Temecula, CA, USA). After blocking with 5% Milk in PBS, membranes were incubated for 1h with anti-SV5 mAb (1:10,000 dilution) [37], washed, and probed for 1h with HRP-linked anti-mouse IgG goat antibodies, (KPL, Gaithersburg, MA, USA, 074–1809). Mouse HRP-conjugated anti-actin mAb (clone AC-15, Sigma-Aldrich) was used as loading control. Signals were developed by ECL (ThermoFisher-Pierce, Rockford, IL, USA).
ELISA
Recombinant mono-biotinylated proteins were obtained from dialyzed supernatants of stably transfected HEK293 cells clones as previously reported [38]. The relative concentrations of biotinylated proteins were normalized by western blot and ELISA, and comparable amounts of mono-biotinylated DIII-εCH4-BAP, DI/DII-BAP and sE-BAP proteins were captured on Nunc Maxi Sorp Immuno-Plates (ThermoFisher-Nunc, Roskilde, Denmark) previously coated with 100 μl/well of 5 μg/ml avidin (Sigma-Aldrich) in 50mM Na2CO3/NaHCO3 buffer. ELISA assays were performed using different dilutions of sera from immunised mice and revealed using HRP-linked anti-mouse IgG γ-chain goat antibodies (Jackson ImmunoResearch, Newmarket, UK, 115-035-071) and TMB substrate (Sigma), as previously described [38]. Antigen-specific IgG titres are expressed as the reciprocal dilution at which the optical density of sample at a 450 nm wave length (OD450), was 3 times higher than that obtained for the pre-immune control serum. Pre-immune sera showed the same performance as negative control sera from animals DNA-immunised with an irrelevant protein fused to γCH3. Avidity indexes were determined as previously described [38] using a modified ELISA protocol to determine the relative change between OD450 values obtained with and without washings in 6M urea, expressed as percentages. In agreement with previous reports, avidity indexes above 30% were considered high [43].
Immunofluorescence
Vero cells were infected with virus preparations at a MOI of 0.5 for 36h (24h for WNV), fixed with 3.7% paraformaldehyde (PFA) in PBS for 20 min, quenched with 150 mM glycine in PBS, permeabilised with 1% Triton in PBS for 15 min, blocked with 0.1% BSA in PBS for 1h and then incubated with anti-DIII, anti-DI/DII or anti-sE sera (1:100 dilution), mAb 4G2 (Millipore, Temecula, CA, USA; 50 ng/ul) or pre-immune control sera, followed by Alexa488-conjugated goat anti-mouse IgG (Jackson ImmunoResearch, Newmarket, UK, 1:1000). Labelled cells were mounted with ProLong supplemented with DAPI (Thermo Fisher Scientific). Images were acquired using a Nikon Eclipse Ti-E microscope.
Foci reduction neutralisation test (FRNT)
FRNT was carried out in 48 multi-well plates, on Vero cells seeded at a density of 6.5x104 cells/well 24h before infection. Sera samples were 2-fold serially diluted and incubated for 1.5h at 36°C with an equal volume of DMEM serum-free medium containing 50 foci-forming units (FFU) of each virus. Vero cells were then infected in duplicate for 1h at 36°C, the inoculum was then removed and cells overlaid with 0.5 ml of DMEM containing 2% FCS and 3% carboxymethylcellulose (Sigma). After 3 days incubation at 36°C, cell monolayers were washed and fixed for 20min with PFA 3.7%, permeabilised with a 1% Triton solution in PBS for 10 min and treated with 0.3% H2O2 in methanol for 30 min. Infection foci were developed incubating with mAb 4G2 (1 ng/μl) for 1h at room temperature (RT), followed by incubation with HRP-conjugated goat anti-mouse IgG (Jackson ImmunoResearch, 1:1000). Foci were then stained with 100 μl True-Blue reagent (KPL, Gaithersburg, MA, USA) and counted. Neutralising antibody titres were expressed as the serum dilution yielding a 50% foci reduction when compared against pre-immune sera (FRNT50).
Antibody-dependent viral infection in K562 cells
10-fold dilutions of sera or mAb 4G2 were incubated with an equal volume of RPMI 1640 serum-free medium containing 4x103 FFUs of each virus and incubated for 1.5h at 36°C in round-bottom 96 multi-well plates (Corning-Costar, Corning, NY, USA). 4x104 K562 cells (0.1 MOI) were added to the sera-virus mixture and incubated for 72 h (48 h for WNV) at 36°C. Cells were then fixed for 30 min with 2% PFA on ice, blocked in permeabilisation buffer (0.1% saponin (Sigma-Aldrich), 2% FBS and 0.1% NaN3 in PBS) for 30 min at 4°C and incubated with mAb 4G2 (1 ng/ul in permeabilisation buffer) followed by Alexa 488-conjugated goat anti-mouse IgG (Jackson ImmunoResearch, 1:1000 in permeabilisation buffer), both for 1h at RT. After washing, cells were resuspended in PBS containing 2% FBS and 0.1% NaN3 and analysed by cytofluorimetry in a FACSCalibur (BD Biosciences, San Jose, CA, USA).
Statistical analysis
In all cases, data were obtained from a minimum of three independent experiments (n) done in triplicate. Serological experiments were performed using pooled sera from groups of immunised mice. Unless indicated otherwise, arithmetic means ± standard deviations were calculated and analysed using unpaired two-tailed t test when needed (GraphPad Prism 6.0, GraphPad Software Inc., La Jolla, CA, USA); p values <0.05 were considered significant and variances between compared groups were not significantly different. Sample size was not statistically assessed and data distribution was assumed to be normal. Data collection or analysis was not blinded.
Ethical statement
All animal procedures described in this study were approved by the ICGEB Animal Welfare Board and the Italian Ministry of Health (Ministero della Salute) (approved protocol DGSAF0024706) and conducted adhering to institutional and international guidelines for animal experimentation, and in compliance to laws and policies established in the legislation D. L.vo 26/2014 of the Italian Government.
Results
Immunological dominance of DENV E protein DI/DII epitopes
We have previously reported antibody responses in mice following DNA immunisations with DENV sE protein (anti-sE) or with only domain III (anti-DIII). DIII of all four DENV serotypes were engineered as a fusion with the human IgG CH3 dimerising domain to allow optimised expression and secretion from mammalian cells [38]. Upon immunisations, these constructs induced anti-DIII responses with strong neutralising activities for the homologous DENV serotype and reduced cross-reactivity and ADE for the non-homologous serotypes [38]. In contrast, anti-sE antibodies from mice immunised with DENV3 sE, showed low anti-DIII activity with most of the antibody response directed against domains I and II (DI/DII) [38], in agreement with previous studies [19, 44].
To further confirm DI/DII immunodominance, we tested sera from mice immunised with constructs encoding the whole sE protein or only DI/DII or DIII (schematically shown in Fig 1A). While DIII from all 4 DENV serotypes were studied, only sE and DI/DII constructs of DENV3 and DENV4 were included as the encoded proteins were previously shown to be properly folded and efficiently secreted from transfected mammalian cells, contrary to those of DENV1 and DENV2 that are poorly secreted, thus inducing significantly low immune responses upon DNA vaccination [39].
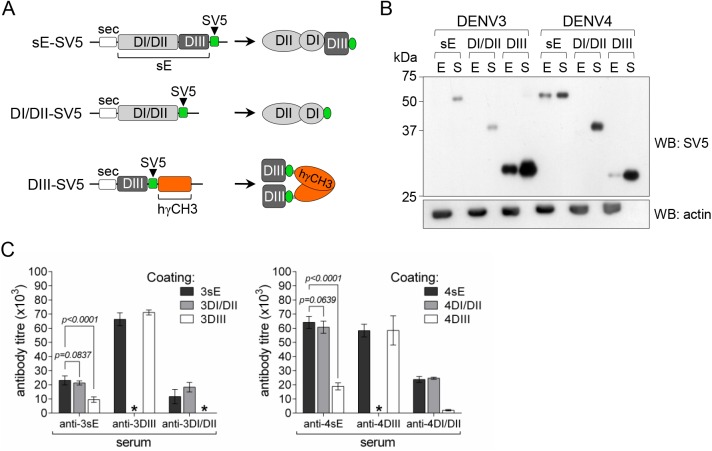
Fig 1. Antibody responses against DENV sE antigens are highly dominated by DI/DII epitopes.
A) Scheme of DNA constructs and the expected products used for immunisations. B) Western blot profile of expression and secretion from transfected HEK293T cells (E, cellular extracts; S, culture supernatants) of sE-SV5, DI/DII-SV5 and DIII-SV5 constructs from DENV3 and DENV4; anti-actin was used as loading control. C) ELISA antibody titres of pooled sera from mice immunised with the indicated constructs determined on plates coated with homologous sE, DIII or DI/DII antigens from DENV3 (left panel) and DENV4 (right panel) as indicated (in each case, n = 4. Comparisons of anti-sE reactivity against sE and DI/DII antigens (t = 1.172 df = 6 for DENV3, t = 1.541 df = 6 for DENV4), and against sE and DIII antigens (t = 8.160 df = 6 for DENV3, t = 28.22 df = 6 for DENV4) are shown. *, indicates antibody titre below pre-immune control.
Immune responses against sE, DI/DII and DIII of DENV3 and DENV4 were compared by ELISA on plates coated with normalised amounts of the different homologous antigens. In agreement with previous studies [38, 39], antibody titres obtained from DNA-immunised animals were directly dependent on the relative amount of antigen secreted from transfected mammalian cells (Fig 1B). As shown in Fig 1C, anti-DI/DII and anti-DIII sera showed high specificity against their respective domains while anti-sE antibodies were mostly directed against DI/DII, thus confirming DI/DII immunodominance in relation to DIII. In both cases, there was no significant difference between anti-sE antibody titres determined on plates coated with sE or with DI/DII. Conversely, the difference was highly significant when comparing anti-sE titres on sE- or DIII-coated plates. As expected, antibodies directed against the human γCH3 domain did not cross-react with the εCH4 thus confirming specificity of the assay (S1 Fig). The reactivity curves of each pooled sera against the different antigens are shown in panel A of S2 Fig; in all cases anti-DI/DII responses showed a significantly reduced avidity index (Panel B of S2 Fig).
Antibodies against DI/DII, but not against DIII are highly cross-reactive
We have previously reported a detailed analysis on the cross-reactive properties of the different anti-DIII responses against all four DENV viral serotypes [38]. Based on the specificity of anti-DIII responses, we first investigated cross-reactivity of antibodies specifically induced with DIII (of all 4 DENV serotypes), and with DI/DII and sE proteins (of DENV3 and DENV4) by immunofluorescence in cells infected with the four DENV serotypes (Fig 2) and with three other flaviviruses: ZIKV, WNV, and YFV (Fig 3). Reactivity of each anti-DIII sera against its homologous DENV serotype was included to show the presence of reactive antibodies in the monovalent formulation. Since vaccine candidates against DENV are required to elicit a protective response against all 4 serotypes, we also included sera from animals immunised with a tetravalent formulation of the DIII-based vaccine to properly assess the reactivity of the potential candidate (Tetra-DIII) [38].
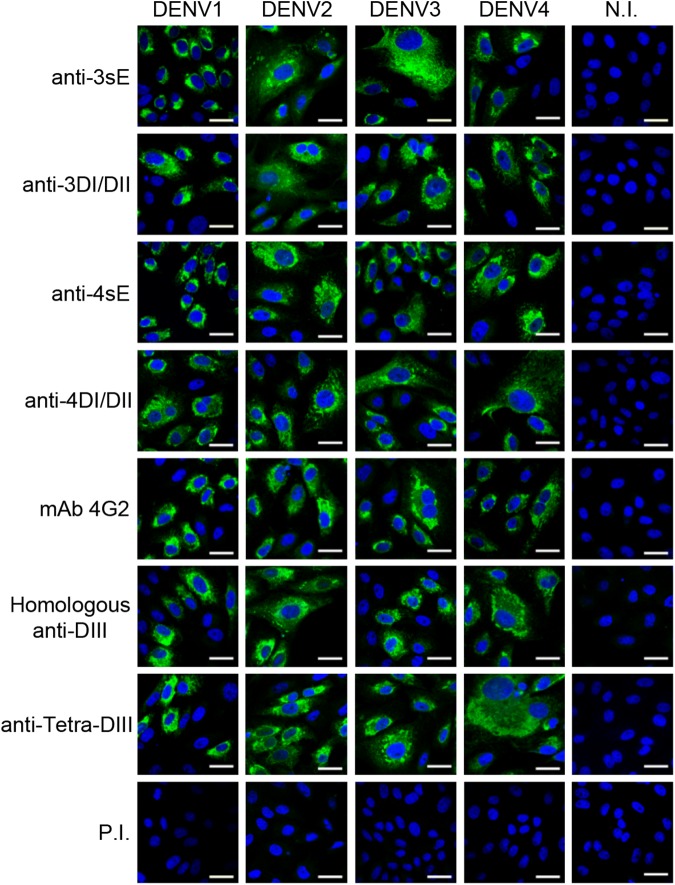
Fig 2. Reactivity profiles of anti-DENV sera in DENV-infected cells.
Immunofluorescence of Vero cells infected with all four DENV serotypes and reacted with antibodies elicited with 3sE and 4sE or the corresponding 3DI/DII and 4DI/DII. mAb 4G2, the homologous anti-DIII and the tetravalent anti-DIII sera were included as controls to show reactivity towards each serotype. N.I., non-infected cells; P.I., pre-immune serum. Bar, 30 μm. Representative images from independent experiments are shown.
Fig 3. DENV anti-DIII sera do not cross-react with ZIKV, WNV and YFV.
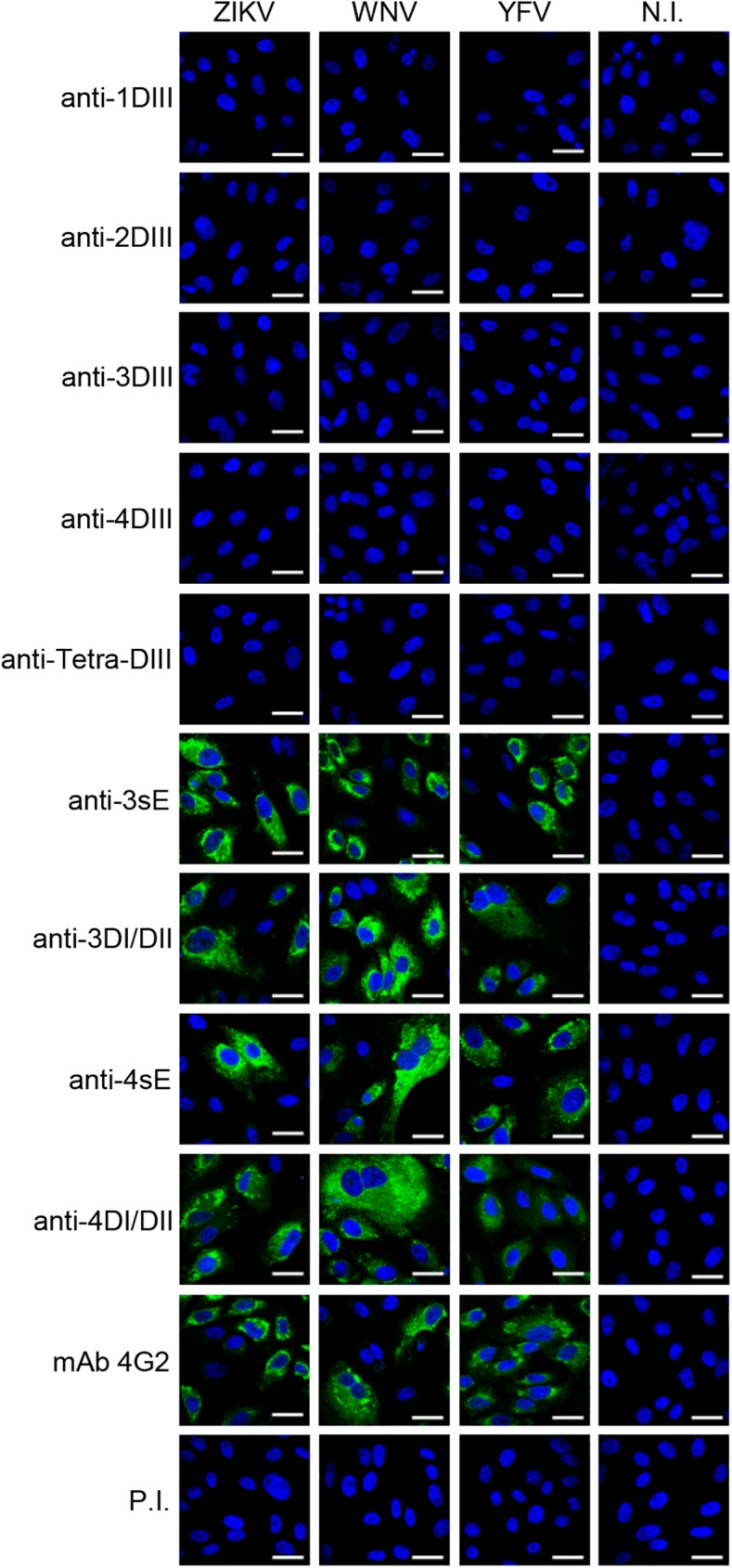
Immunofluorescence of Vero cells infected with ZIKV, WNV and YFV reacted with antibodies elicited against each of the four DENV DIII antigens (1DIII, 2DIII, 3DIII and 4DIII), the Tetra-DIII formulation, 3sE, 3DI/IDII, 4sE and 4DI/DII. mAb 4G2 was included as a control. N.I., non-infected; P.I., pre-immune serum. Bar, 30 μm. Representative images from independent experiments are shown.
As expected, monovalent anti-DIII antibodies showed high reactivity towards their homologous serotypes. Likewise, sera from mice immunised with Tetra-DIII clearly recognised all four DENV serotypes (Fig 2). In addition, both anti-sE and anti-DI/DII sera were highly cross-reactive against all DENV serotypes (Fig 2). Interestingly, when tested on cells infected with the three other flaviviruses (ZIKV, WNV and YFV), anti-DIII antibodies (either serotype-specific or Tetra-DIII) were completely negative, whereas antibodies induced with sE or DI/DII were strongly positive in all cases, further supporting not only the immunodominance of DI/DII epitopes, but also the high degree of cross-reactivity of these antibodies (Fig 3). For all viruses, mAb 4G2, which recognizes the fusion loop of all flaviviruses [45], and pre-immune sera were used as positive and negative controls, respectively.
ADE and neutralisation activities of anti-DI/DII and anti-DIII sera
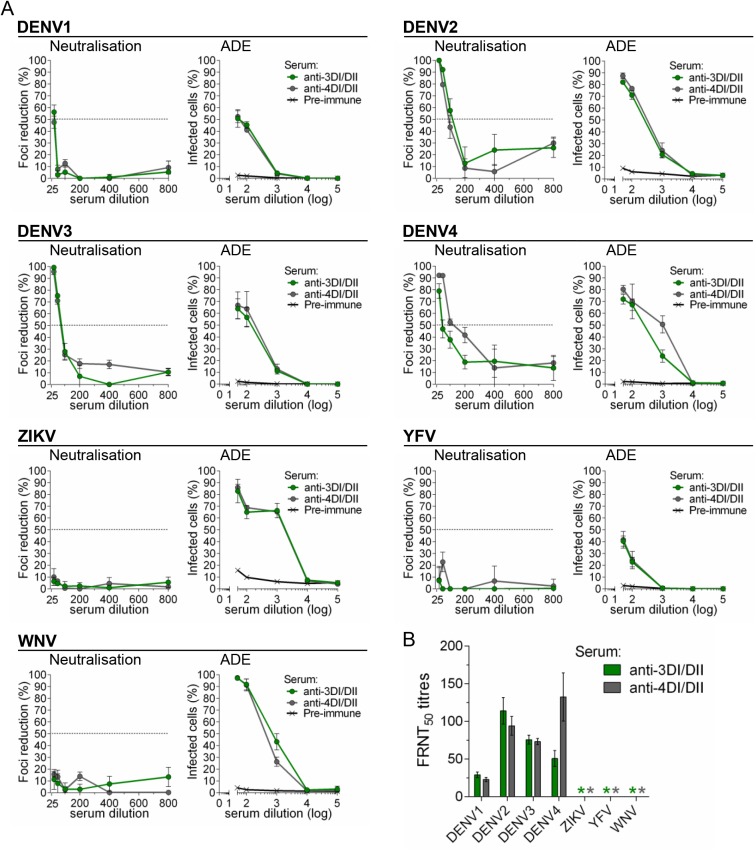
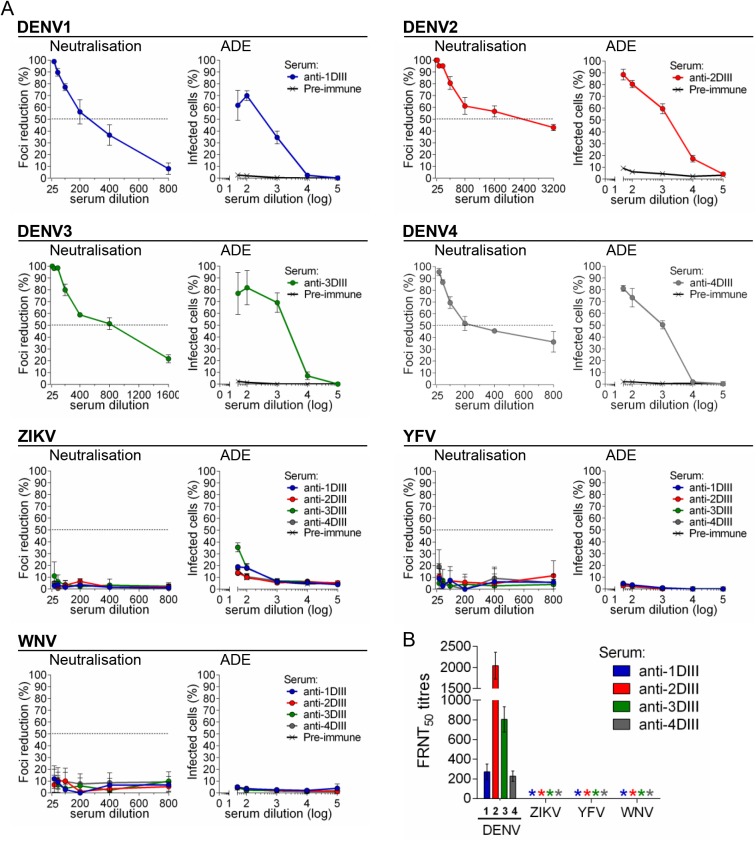
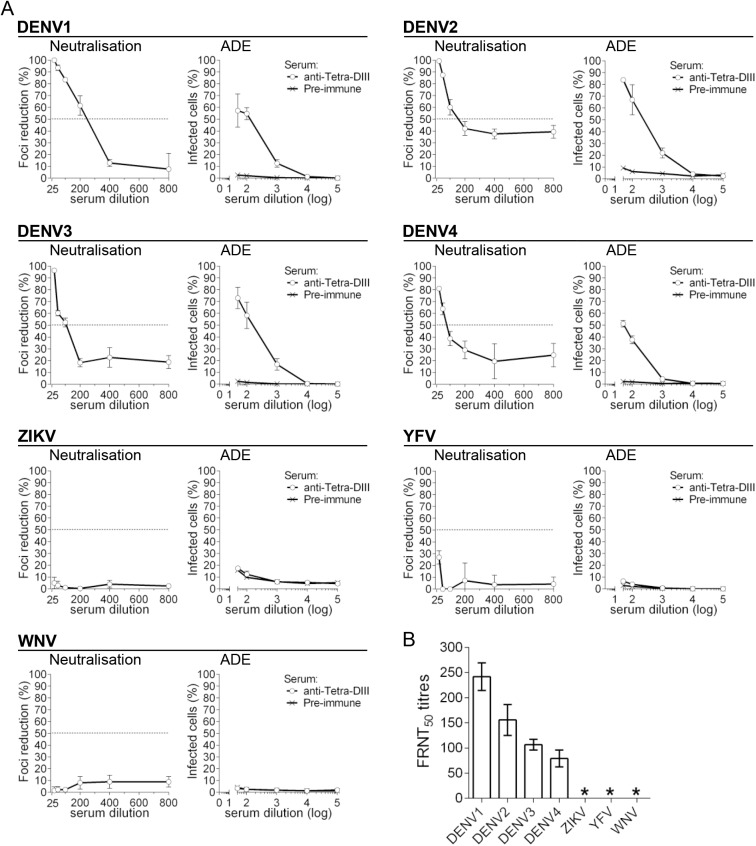
In order to dissect the properties of polyclonal antibody responses elicited by the different E structural domains, and because of the broad cross-reactivity of anti-DI/DII (and anti-sE) sera in contrast to the relatively high specificity of anti-DIII, we subsequently tested ADE and neutralisation activities of the different sera in K562 and Vero cells, respectively. Since anti-DI/DII and anti-sE responses (for both DENV3 and DENV4) showed the same reactivity profiles, we only compared anti-DI/DII with anti-DIII specific sera. Antibodies elicited against DI/DII of DENV3 and DENV4 (anti-3DI/DII and anti-4DI/DII, respectively) showed strong ADE on all four DENV serotypes as well as on ZIKV, WNV and YFV (Fig 4A). Neutralisation activity, however, was only relevant for DENV serotypes and negative on all other viruses (Fig 4A and 4B). In contrast, anti-DIII antibodies showed no ADE (Fig 5A) and no neutralisation (Fig 5A and 5B) on ZIKV, WNV and YFV. As expected, each anti-DIII serum was able to neutralise and induce ADE on the homologous DENV serotype. As a control of the ADE assay, all tested flaviviruses showed ADE when incubated with mAb 4G2 (S3 Fig).
Fig 4. DENV DI/DII induces poorly-neutralising, highly-enhancing cross-reactive antibodies.
A) Neutralisation and ADE of all four DENV serotypes, ZIKV, WNV and YFV, with DENV anti-3DI/DII and anti-4DI/DII pooled sera (in all cases, n = 3 for each virus-sera combination). B) FRNT50 titres, as determined in (A), * indicates FRNT50 titres below detection limit of the assay (≥25).
Fig 5. DIII vaccination induces DENV-specific neutralising antibodies.
A) Neutralisation and ADE of all four DENV serotypes, ZIKV, WNV and YFV, with DENV anti-DIII sera. To prove anti-DIII reactivity, DENV viruses were tested using pooled sera against the homologous serotypes, while non-DENV viruses were tested against all four anti-DIII sera (in all cases, n = 3 for each virus-sera combination). B) FRNT50 titres, as determined in (A), * indicates FRNT50 titres below detection limit of the assay (≥25).
We then determined ADE and neutralisation activities of sera from mice vaccinated with Tetra-DIII. Not surprisingly, Tetra-DIII sera showed neutralisation activity for all four DENV serotypes but not for the other flaviviruses (Fig 6A). More importantly, ADE was completely negative on the non-DENV flaviviruses, and positive on the four DENV serotypes, as expected (Fig 6A). Neutralisation titres are shown in Fig 6B.
Fig 6. DENV-DIII tetravalent vaccine formulation elicits DENV-specific neutralising antibodies.
A) Neutralisation and ADE of all four DENV serotypes, ZIKV, WNV and YFV, with pooled sera from mice vaccinated with the Tetra-DIII formulation (in all cases, for each virus-sera combination n = 3). B) FRNT50, as determined in (A), * indicates FRNT50 titres below detection limit of the assay (≥25).
Thus, our data strongly support the specificity of anti-DIII polyclonal responses, and further highlight the importance of inducing anti-DIII antibodies as a source of effective DENV-specific neutralisation activity without promoting ADE of other closely related flaviviruses such as ZIKV, WNV and YFV.
Discussion
Patients with a secondary DENV infection are at a higher risk of suffering the more severe haemorrhagic manifestations of the disease. In fact, severe symptoms following DENV infections are 15–80 times more frequent in secondary infections, and pre-existing heterotypic DENV antibodies are found in almost all patients with severe disease [25]. ADE was initially proposed to explain this phenomenon and concern over its role in DHF/DSS has justified the requirement for all DENV vaccines candidates to induce a balanced and long-lasting protective response against all serotypes at the same time. Given that the current ZIKV outbreak overlaps many DENV-endemic areas and the high degree of homology between DENV and ZIKV, there is great concern on how the outcome of ZIKV infections might be altered in DENV immune patients [46, 47]. Recent studies have demonstrated that pre-existing anti-DENV antibodies enhance ZIKV infection in vitro and in vivo, suggesting that DENV immunity could represent a risk to develop more severe symptoms upon ZIKV infection [31, 48]. The complex serological relationship between DENV and ZIKV was further confirmed by showing reciprocal in vitro and in vivo ADE following isolation of monoclonal antibodies from infected patients [28, 32]. Moreover, due to significant antigen overlapping, this could also apply to infections by other flaviviruses like WNV and YFV [30, 48].
Together, these results indicate a complex serological interaction between flaviviruses, with serious implications regarding the design and evaluation of future DENV and ZIKV vaccines. Most notably, these findings introduce the need to consider the relative activity of antibodies in terms of ADE among closely related flaviviruses, particularly when cross-reactive antibodies are present.
We have previously reported an efficient way to induce strong antibody responses against all four DENV serotypes, based on a DNA-vaccine with DIII, properly engineered to significantly increase expression and secretion in mammalian cells as immunising antigen [38]. The antibody responses showed high neutralisation titres and reduced ADE among the different DENV serotypes in THP-1 cells that express high levels of FcγRI, FcγRIIA and FcγRIIB [49].
Here, instead, we used K562 cells, which are FcγRI negative and express high levels of FcγRIIA that significantly favours FcγR-mediated infection, thus allowing us to evaluate the ADE potential of the different sera with higher sensitivity as these cells are particularly prone for ADE-mediated infection [49]. In this context, sera unable to induce ADE in K562 cells are highly unlikely to do so in other less susceptible cells. Our new data indicate that only anti-DIII antibodies show high type-specificity as they did not recognize other non-DENV flaviviruses. As a consequence, DENV anti-DIII antibodies, even in the context of a tetravalent vaccine formulation, do not promote ADE of WNV, ZIKV and YFV. In contrast, anti-DI/DII antibodies are highly cross-reactive with strong ADE activity for WNV, ZIKV and YFV and without significant neutralisation activity. This is an important point to consider as ADE is always present, even with antibodies with strong neutralising activity particularly at non-neutralising concentrations, as a consequence of the natural waning of antibody levels in time after infection or vaccination [50].
The development of a vaccine efficiently preventing DENV infection while avoiding ADE of the different serotypes and other closely related flaviviruses remains an unmet challenge. Our data indicate that DIII-based vaccines could avoid the risk of inducing ADE of other closely related flaviviruses. They also stress the DI/DII immunodominance with respect to DIII when using the whole sE as immunising antigen, resulting in poorly-neutralising, highly cross-reactive and ADE promoting antibodies. In addition, anti-DI/DII responses showed significantly low avidity, a feature that has been shown to negatively correlate with the neutralising capacity of antibodies [51, 52]. These results are in agreement with published data indicating that cross-reactive antibodies against the virion are the main drivers of heterotypic DENV enhancement of infection [13, 44]. Recently, highly cross-reactive monoclonal antibodies against DI/DII from DENV- and ZIKV-infected patients were shown to be poorly neutralising but potently infection-enhancing [32]. Furthermore, a recent study with monoclonal antibodies obtained from ZIKV-immunised mice, showed that type-specific protective and neutralising antibodies bound mainly to DIII, identifying ZIKV DIII as a potentially safe immunogen for vaccines [33].
Preliminary data on new vaccine candidates against ZIKV rely mostly on a variety of genetic immunisation approaches based on the expression of the E protein together with PrM [53–58]. In some of these cases, however, ADE activities of the induced antibodies against other flaviviruses were not assessed [53–56, 58]. Furthermore, attempts of reducing the risk of ADE in mRNA-based ZIKV vaccine, diminished the efficacy of the vaccine when compared to the original construct, highlighting the complexity involved in proper antigen design [57]. Finally, the introduction of PrM in some of these formulations is a matter of concern, as anti-Pr antibodies have been shown to be also highly cross-reactive with strong ADE activity and almost no neutralising capacity [59].
Despite several studies on responses against DENV and ZIKV after infection, deep understanding of the natural polyclonal antibody response following immunisation with vaccine candidates is lacking. Our study describes the first domain-based characterization of the polyclonal immune response induced against DENV sE protein, by comparing cross-reactivity, neutralisation and ADE activities of antibodies elicited by DNA immunization with constructs expressing DIII or DI/DII separately. By analyzing polyclonal responses, our results confirm previously reported data on DENV and ZIKV using monoclonal antibodies and further highlight DIII as a strong candidate to develop DENV-specific vaccines without the concerns regarding cross-reactivity to other flaviviruses, particularly ZIKV.
As previously reported [38], our tetravalent DIII-based DNA vaccine formulation elicited neutralizing activity against all four DENV virus serotypes. However, in contrast to other DIII-based vaccines [60, 61], there was interference between the different antigens as the FRNT50 titres were reduced when compared against the monovalent formulations, thus highlighting the need for further development of the Tetra-DIII formulation in order to achieve balanced, long-lasting and strong neutralising responses.
The description of strong neutralising antibodies against conserved dimer-dependent epitopes has opened the opportunity for the development of a universal vaccine for DENV and ZIKV [17, 28]. However, our data raise questions as to their potential use due to the large involvement of other immunodominant DI/DII epitopes. In this scenario, effective modulation of antigenicity to favour responses against cross-neutralising epitopes would be required. The recent descriptions of covalently-stabilised E dimers from DENV and ZIKV represent an important step towards achieving this goal [62, 63].
Although the true impact of ADE in DENV infection has not been fully elucidated and no animal model has been able to predict the full repertoire of the antibody response in humans [64], this phenomena introduces significant concerns for the development of efficient vaccine candidates. There is abundant evidence supporting the effect of antibody-mediated enhancement and disease between DENV serotypes; however, there are no reports confirming a correlation between in vitro ADE assays and increased risk of infection by other flaviviruses; moreover, there is no clinical evidence of increased disease severity from WNV, ZIKV and YFV in DENV-immune patients or vice versa. Nonetheless, the risk for haemorrhagic complications found in (CYD-TVD)-vaccinated children, suggests that these concerns are relevant and should be taken under consideration during preclinical and clinical evaluation of potential vaccines. In addition, recent data indicate that host-related factors are also involved in severe dengue associated to ADE [65].
Based on these complex serological interactions, the choice of antigen for the design of vaccine candidates is of paramount importance, as it should be able to induce highly neutralising antibodies with negative or much reduced ADE of other related viruses. These requirements are fulfilled by DENV DIII in the format we have described. Moreover, DNA-vaccine platforms, and genetic vaccines in general, have an excellent safety profile, are highly stable and can be manufactured in a short period of time [66]. Our data support further development of DIII-based DNA-vaccines to induce type-specific neutralising antibodies that circumvent the risk of ADE among flaviviruses.
Supporting information
Sera from animals immunised with 3DIII-CH3, or with an irrelevant protein fused to gCH3 (Irr-gCH3) tested on plates coated with mono-biotinylated 3DIII-eCH4 (left panel), human gCH2-CH3 (centre panel) and Irr-eCH4 (right panel). PI: pre-immune sera.
(TIF)
A) ELISA profiles of antibody responses from mice immunised with DIII, DI/DII and sE form DENV3 and DENV4, tested on plates coated with the homologous sE, DIII or DI/DII antigens. DENV3, top panels; DENV4, bottom panels (in all cases, n = 12). B) Avidity index of sera from animals immunised with DIII, DI/DII and sE constructs from DENV3 (left) and DENV4 (right) (in all cases n = 18), mean±s.d. are shown.
(TIF)
ADE of all four DENV serotypes, ZIKV, WNV and YFV on K562 cells, using the control mAb 4G2 (in all cases n = 3).
(TIF)
(PDF)
Acknowledgments
We would like to thank Dr. Alessandro Marcello (ICGEB, Trieste, Italy) for kindly providing us valuable materials and reagents. We express our thanks to the personnel of ICGEB Animal Experimentation Facility for their assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
JLSC and JR were supported by Arturo Falaschi ICGEB pre-doctoral and postdoctoral fellowships, respectively. FA was supported by FIRB-Futuro in Ricerca grant (RBFR13209E), Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR), Italy. The funders played no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Murray NE, Quam MB, Wilder-Smith A. Epidemiology of dengue: past, present and future prospects. Clinical epidemiology. 2013;5:299–309. Epub 2013/08/31. PubMed Central PMCID: PMCPMC3753061. doi: 10.2147/CLEP.S34440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–7. Epub 2013/04/09. doi: 10.1038/nature12060 ; PubMed Central PMCID: PMCPMC3651993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simmons CP, Farrar JJ, Nguyen v V, Wills B. Dengue. The New England journal of medicine. 2012;366(15):1423–32. Epub 2012/04/13. doi: 10.1056/NEJMra1110265 . [DOI] [PubMed] [Google Scholar]
- 4.Lindenbach BD, Heinz-Jurgen T, Rice CM. Flaviviridae: The Viruses and Their Replication In: Knipe DM, Howley PM, editors. Fields' Virology. 5th ed: Lippincott-Raven; 2007. [Google Scholar]
- 5.Modis Y, Ogata S, Clements D, Harrison SC. Variable surface epitopes in the crystal structure of dengue virus type 3 envelope glycoprotein. Journal of virology. 2005;79(2):1223–31. Epub 2004/12/23. doi: 10.1128/JVI.79.2.1223-1231.2005 ; PubMed Central PMCID: PMCPMC538574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, et al. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell. 2002;108(5):717–25. Epub 2002/03/15. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flipse J, Smit JM. The Complexity of a Dengue Vaccine: A Review of the Human Antibody Response. PLoS neglected tropical diseases. 2015;9(6):e0003749 Epub 2015/06/13. doi: 10.1371/journal.pntd.0003749 ; PubMed Central PMCID: PMCPMC4465930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seif SA, Morita K, Matsuo S, Hasebe F, Igarashi A. Finer mapping of neutralizing epitope(s) on the C-terminal of Japanese encephalitis virus E-protein expressed in recombinant Escherichia coli system. Vaccine. 1995;13(16):1515–21. Epub 1995/11/01. . [DOI] [PubMed] [Google Scholar]
- 9.Sanchez MD, Pierson TC, McAllister D, Hanna SL, Puffer BA, Valentine LE, et al. Characterization of neutralizing antibodies to West Nile virus. Virology. 2005;336(1):70–82. Epub 2005/05/04. doi: 10.1016/j.virol.2005.02.020 . [DOI] [PubMed] [Google Scholar]
- 10.Sukupolvi-Petty S, Brien JD, Austin SK, Shrestha B, Swayne S, Kahle K, et al. Functional analysis of antibodies against dengue virus type 4 reveals strain-dependent epitope exposure that impacts neutralization and protection. Journal of virology. 2013;87(16):8826–42. Epub 2013/06/21. PubMed Central PMCID: PMCPMC3754038. doi: 10.1128/JVI.01314-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crill WD, Roehrig JT. Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. Journal of virology. 2001;75(16):7769–73. Epub 2001/07/20. PubMed Central PMCID: PMCPMC115016. doi: 10.1128/JVI.75.16.7769-7773.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gromowski GD, Barrett ND, Barrett AD. Characterization of dengue virus complex-specific neutralizing epitopes on envelope protein domain III of dengue 2 virus. Journal of virology. 2008;82(17):8828–37. Epub 2008/06/20. doi: 10.1128/JVI.00606-08 ; PubMed Central PMCID: PMCPMC2519678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Alwis R, Smith SA, Olivarez NP, Messer WB, Huynh JP, Wahala WM, et al. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(19):7439–44. Epub 2012/04/14. doi: 10.1073/pnas.1200566109 ; PubMed Central PMCID: PMCPMC3358852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dejnirattisai W, Wongwiwat W, Supasa S, Zhang X, Dai X, Rouvinsky A, et al. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nature immunology. 2015;16(2):170–7. Epub 2014/12/17. doi: 10.1038/ni.3058 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rouvinski A, Guardado-Calvo P, Barba-Spaeth G, Duquerroy S, Vaney MC, Kikuti CM, et al. Recognition determinants of broadly neutralizing human antibodies against dengue viruses. Nature. 2015;520(7545):109–13. Epub 2015/01/13. doi: 10.1038/nature14130 . [DOI] [PubMed] [Google Scholar]
- 16.Barba-Spaeth G, Dejnirattisai W, Rouvinski A, Vaney MC, Medits I, Sharma A, et al. Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature. 2016;536(7614):48–53. Epub 2016/06/25. doi: 10.1038/nature18938 . [DOI] [PubMed] [Google Scholar]
- 17.Swanstrom JA, Plante JA, Plante KS, Young EF, McGowan E, Gallichotte EN, et al. Dengue Virus Envelope Dimer Epitope Monoclonal Antibodies Isolated from Dengue Patients Are Protective against Zika Virus. mBio. 2016;7(4). Epub 2016/07/21. doi: 10.1128/mBio.01123-16 ; PubMed Central PMCID: PMCPMC4958264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliphant T, Nybakken GE, Engle M, Xu Q, Nelson CA, Sukupolvi-Petty S, et al. Antibody recognition and neutralization determinants on domains I and II of West Nile Virus envelope protein. Journal of virology. 2006;80(24):12149–59. Epub 2006/10/13. doi: 10.1128/JVI.01732-06 ; PubMed Central PMCID: PMCPMC1676294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wahala WM, Kraus AA, Haymore LB, Accavitti-Loper MA, de Silva AM. Dengue virus neutralization by human immune sera: role of envelope protein domain III-reactive antibody. Virology. 2009;392(1):103–13. Epub 2009/07/28. PubMed Central PMCID: PMCPMC2746956. doi: 10.1016/j.virol.2009.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vratskikh O, Stiasny K, Zlatkovic J, Tsouchnikas G, Jarmer J, Karrer U, et al. Dissection of antibody specificities induced by yellow fever vaccination. PLoS pathogens. 2013;9(6):e1003458 Epub 2013/07/03. doi: 10.1371/journal.ppat.1003458 ; PubMed Central PMCID: PMCPMC3688551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, Quyen NT, et al. The human immune response to Dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell host & microbe. 2010;8(3):271–83. Epub 2010/09/14. doi: 10.1016/j.chom.2010.08.007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capeding MR, Tran NH, Hadinegoro SR, Ismail HI, Chotpitayasunondh T, Chua MN, et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet. 2014;384(9951):1358–65. Epub 2014/07/16. doi: 10.1016/S0140-6736(14)61060-6 . [DOI] [PubMed] [Google Scholar]
- 23.Villar L, Dayan GH, Arredondo-Garcia JL, Rivera DM, Cunha R, Deseda C, et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. The New England journal of medicine. 2015;372(2):113–23. Epub 2014/11/05. doi: 10.1056/NEJMoa1411037 . [DOI] [PubMed] [Google Scholar]
- 24.Hadinegoro SR, Arredondo-Garcia JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. The New England journal of medicine. 2015;373(13):1195–206. Epub 2015/07/28. doi: 10.1056/NEJMoa1506223 . [DOI] [PubMed] [Google Scholar]
- 25.Guzman MG, Vazquez S. The complexity of antibody-dependent enhancement of dengue virus infection. Viruses. 2010;2(12):2649–62. Epub 2011/10/14. doi: 10.3390/v2122649 ; PubMed Central PMCID: PMCPMC3185591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campos GS, Bandeira AC, Sardi SI. Zika Virus Outbreak, Bahia, Brazil. Emerging infectious diseases. 2015;21(10):1885–6. Epub 2015/09/25. doi: 10.3201/eid2110.150847 ; PubMed Central PMCID: PMCPMC4593454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musso D, Gubler DJ. Zika Virus. Clinical microbiology reviews. 2016;29(3):487–524. Epub 2016/04/01. doi: 10.1128/CMR.00072-15 ; PubMed Central PMCID: PMCPMC4861986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, Duangchinda T, et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nature immunology. 2016;17(9):1102–8. Epub 2016/06/25. doi: 10.1038/ni.3515 ; PubMed Central PMCID: PMCPMC4994874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fagbami A, Halstead SB, Marchette N, Larsen K. Heterologous flavivirus infection-enhancing antibodies in sera of Nigerians. The American journal of tropical medicine and hygiene. 1988;38(1):205–7. Epub 1988/01/01. . [DOI] [PubMed] [Google Scholar]
- 30.Chan KR, Wang X, Saron WA, Gan ES, Tan HC, Mok DZ, et al. Cross-reactive antibodies enhance live attenuated virus infection for increased immunogenicity. Nature microbiology. 2016;1:16164 Epub 2016/09/20. doi: 10.1038/nmicrobiol.2016.164 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Priyamvada L, Quicke KM, Hudson WH, Onlamoon N, Sewatanon J, Edupuganti S, et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(28):7852–7. Epub 2016/06/30. doi: 10.1073/pnas.1607931113 ; PubMed Central PMCID: PMCPMC4948328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science (New York, NY). 2016;353(6301):823–6. Epub 2016/07/16. doi: 10.1126/science.aaf8505 . [DOI] [PubMed] [Google Scholar]
- 33.Zhao H, Fernandez E, Dowd KA, Speer SD, Platt DJ, Gorman MJ, et al. Structural Basis of Zika Virus-Specific Antibody Protection. Cell. 2016;166(4):1016–27. Epub 2016/08/01. doi: 10.1016/j.cell.2016.07.020 ; PubMed Central PMCID: PMCPMC4983199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zellweger RM, Shresta S. Mouse models to study dengue virus immunology and pathogenesis. Frontiers in immunology. 2014;5:151 Epub 2014/05/02. doi: 10.3389/fimmu.2014.00151 ; PubMed Central PMCID: PMCPMC3989707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zompi S, Harris E. Animal models of dengue virus infection. Viruses. 2012;4(1):62–82. Epub 2012/02/23. doi: 10.3390/v4010062 ; PubMed Central PMCID: PMCPMC3280519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li E, Pedraza A, Bestagno M, Mancardi S, Sanchez R, Burrone O. Mammalian cell expression of dimeric small immune proteins (SIP). Protein engineering. 1997;10(6):731–6. Epub 1997/06/01. . [DOI] [PubMed] [Google Scholar]
- 37.Hanke T, Szawlowski P, Randall RE. Construction of solid matrix-antibody-antigen complexes containing simian immunodeficiency virus p27 using tag-specific monoclonal antibody and tag-linked antigen. The Journal of general virology. 1992;73 (Pt 3):653–60. Epub 1992/03/01. doi: 10.1099/0022-1317-73-3-653 . [DOI] [PubMed] [Google Scholar]
- 38.Poggianella M, Slon Campos JL, Chan KR, Tan HC, Bestagno M, Ooi EE, et al. Dengue E Protein Domain III-Based DNA Immunisation Induces Strong Antibody Responses to All Four Viral Serotypes. PLoS neglected tropical diseases. 2015;9(7):e0003947 Epub 2015/07/29. doi: 10.1371/journal.pntd.0003947 ; PubMed Central PMCID: PMCPMC4517776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slon Campos JL, Poggianella M, Marchese S, Bestagno M, Burrone OR. Secretion of dengue virus envelope protein ectodomain from mammalian cells is dependent on domain II serotype and affects the immune response upon DNA vaccination. The Journal of general virology. 2015;96(11):3265–79. Epub 2015/09/12. doi: 10.1099/jgv.0.000278 . [DOI] [PubMed] [Google Scholar]
- 40.Beckett D, Kovaleva E, Schatz PJ. A minimal peptide substrate in biotin holoenzyme synthetase-catalyzed biotinylation. Protein science: a publication of the Protein Society. 1999;8(4):921–9. Epub 1999/04/22. PubMed Central PMCID: PMCPMC2144313. doi: 10.1110/ps.8.4.921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Predonzani A, Arnoldi F, Lopez-Requena A, Burrone OR. In vivo site-specific biotinylation of proteins within the secretory pathway using a single vector system. BMC biotechnology. 2008;8:41 Epub 2008/04/22. PubMed Central PMCID: PMCPMC2373293. doi: 10.1186/1472-6750-8-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual 2nd ed. Sambrook J, editor. New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 43.de Souza VA, Fernandes S, Araujo ES, Tateno AF, Oliveira OM, Oliveira RR, et al. Use of an immunoglobulin G avidity test to discriminate between primary and secondary dengue virus infections. Journal of clinical microbiology. 2004;42(4):1782–4. Epub 2004/04/09. PubMed Central PMCID: PMCPMC387572. doi: 10.1128/JCM.42.4.1782-1784.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Alwis R, Williams KL, Schmid MA, Lai CY, Patel B, Smith SA, et al. Dengue viruses are enhanced by distinct populations of serotype cross-reactive antibodies in human immune sera. PLoS pathogens. 2014;10(10):e1004386 Epub 2014/10/03. doi: 10.1371/journal.ppat.1004386 ; PubMed Central PMCID: PMCPMC4183589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Costin JM, Zaitseva E, Kahle KM, Nicholson CO, Rowe DK, Graham AS, et al. Mechanistic study of broadly neutralizing human monoclonal antibodies against dengue virus that target the fusion loop. Journal of virology. 2013;87(1):52–66. Epub 2012/10/19. PubMed Central PMCID: PMCPMC3536401. doi: 10.1128/JVI.02273-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sirohi D, Chen Z, Sun L, Klose T, Pierson TC, Rossmann MG, et al. The 3.8 A resolution cryo-EM structure of Zika virus. Science (New York, NY). 2016;352(6284):467–70. Epub 2016/04/02. doi: 10.1126/science.aaf5316 ; PubMed Central PMCID: PMCPMC4845755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kostyuchenko VA, Lim EX, Zhang S, Fibriansah G, Ng TS, Ooi JS, et al. Structure of the thermally stable Zika virus. Nature. 2016;533(7603):425–8. Epub 2016/04/20. doi: 10.1038/nature17994 . [DOI] [PubMed] [Google Scholar]
- 48.Bardina SV, Bunduc P, Tripathi S, Duehr J, Frere JJ, Brown JA, et al. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science (New York, NY). 2017;356(6334):175–80. Epub 2017/04/01. doi: 10.1126/science.aal4365 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chawla T, Chan KR, Zhang SL, Tan HC, Lim AP, Hanson BJ, et al. Dengue virus neutralization in cells expressing Fc gamma receptors. PloS one. 2013;8(5):e65231 Epub 2013/05/30. doi: 10.1371/journal.pone.0065231 ; PubMed Central PMCID: PMCPMC3661447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pierson TC, Xu Q, Nelson S, Oliphant T, Nybakken GE, Fremont Daved H, et al. The Stoichiometry of Antibody-Mediated Neutralization and Enhancement of West Nile Virus Infection. Cell host & microbe. 2007;1(2):135–45. doi: 10.1016/j.chom.2007.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dowd KA, Pierson TC. Antibody-mediated neutralization of flaviviruses: a reductionist view. Virology. 2011;411(2):306–15. Epub 2011/01/25. PubMed Central PMCID: PMCPMC3100196. doi: 10.1016/j.virol.2010.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Puschnik A, Lau L, Cromwell EA, Balmaseda A, Zompi S, Harris E. Correlation between dengue-specific neutralizing antibodies and serum avidity in primary and secondary dengue virus 3 natural infections in humans. PLoS neglected tropical diseases. 2013;7(6):e2274 Epub 2013/06/21. PubMed Central PMCID: PMCPMC3681624. doi: 10.1371/journal.pntd.0002274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abbink P, Larocca RA, De La Barrera RA, Bricault CA, Moseley ET, Boyd M, et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science (New York, NY). 2016;353(6304):1129–32. Epub 2016/08/06. doi: 10.1126/science.aah6157 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Larocca RA, Abbink P, Peron JP, Zanotto PM, Iampietro MJ, Badamchi-Zadeh A, et al. Vaccine protection against Zika virus from Brazil. Nature. 2016;536(7617):474–8. Epub 2016/06/30. doi: 10.1038/nature18952 ; PubMed Central PMCID: PMCPMC5003703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dowd KA, Ko SY, Morabito KM, Yang ES, Pelc RS, DeMaso CR, et al. Rapid development of a DNA vaccine for Zika virus. Science (New York, NY). 2016;354(6309):237–40. Epub 2016/10/07. doi: 10.1126/science.aai9137 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017;543(7644):248–51. Epub 2017/02/06. doi: 10.1038/nature21428 ; PubMed Central PMCID: PMCPMC5344708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell. 2017;168(6):1114–25 e10. Epub 2017/02/23. doi: 10.1016/j.cell.2017.02.017 ; PubMed Central PMCID: PMCPMC5388441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim E, Erdos G, Huang S, Kenniston T, Falo LD Jr., Gambotto A. Preventative Vaccines for Zika Virus Outbreak: Preliminary Evaluation. EBioMedicine. 2016;13:315–20. Epub 2016/10/09. doi: 10.1016/j.ebiom.2016.09.028 ; PubMed Central PMCID: PMCPMC5264651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science (New York, NY). 2010;328(5979):745–8. Epub 2010/05/08. doi: 10.1126/science.1185181 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Block OK, Rodrigo WW, Quinn M, Jin X, Rose RC, Schlesinger JJ. A tetravalent recombinant dengue domain III protein vaccine stimulates neutralizing and enhancing antibodies in mice. Vaccine. 2010;28(51):8085–94. Epub 2010/10/21. doi: 10.1016/j.vaccine.2010.10.004 . [DOI] [PubMed] [Google Scholar]
- 61.Chiang CY, Pan CH, Chen MY, Hsieh CH, Tsai JP, Liu HH, et al. Immunogenicity of a novel tetravalent vaccine formulation with four recombinant lipidated dengue envelope protein domain IIIs in mice. Scientific reports. 2016;6:30648 Epub 2016/07/30. doi: 10.1038/srep30648 ; PubMed Central PMCID: PMCPMC4965760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Slon Campos JL, Marchese S, Rana J, Mossenta M, Poggianella M, Bestagno M, et al. Temperature-dependent folding allows stable dimerization of secretory and virus-associated E proteins of Dengue and Zika viruses in mammalian cells. Scientific reports. 2017;7(1):966 Epub 2017/04/21. doi: 10.1038/s41598-017-01097-5 ; PubMed Central PMCID: PMCPMC5430527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rouvinski A, Dejnirattisai W, Guardado-Calvo P, Vaney MC, Sharma A, Duquerroy S, et al. Covalently linked dengue virus envelope glycoprotein dimers reduce exposure of the immunodominant fusion loop epitope. Nature communications. 2017;8:15411 Epub 2017/05/24. doi: 10.1038/ncomms15411 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cassetti MC, Durbin A, Harris E, Rico-Hesse R, Roehrig J, Rothman A, et al. Report of an NIAID workshop on dengue animal models. Vaccine. 2010;28(26):4229–34. Epub 2010/05/04. doi: 10.1016/j.vaccine.2010.04.045 ; PubMed Central PMCID: PMCPMC2919823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang TT, Sewatanon J, Memoli MJ, Wrammert J, Bournazos S, Bhaumik SK, et al. IgG antibodies to dengue enhanced for FcgammaRIIIA binding determine disease severity. Science (New York, NY). 2017;355(6323):395–8. Epub 2017/01/28. doi: 10.1126/science.aai8128 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Porter KR, Raviprakash K. Nucleic acid (DNA) immunization as a platform for dengue vaccine development. Vaccine. 2015;33(50):7135–40. Epub 2015/10/16. doi: 10.1016/j.vaccine.2015.09.102 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sera from animals immunised with 3DIII-CH3, or with an irrelevant protein fused to gCH3 (Irr-gCH3) tested on plates coated with mono-biotinylated 3DIII-eCH4 (left panel), human gCH2-CH3 (centre panel) and Irr-eCH4 (right panel). PI: pre-immune sera.
(TIF)
A) ELISA profiles of antibody responses from mice immunised with DIII, DI/DII and sE form DENV3 and DENV4, tested on plates coated with the homologous sE, DIII or DI/DII antigens. DENV3, top panels; DENV4, bottom panels (in all cases, n = 12). B) Avidity index of sera from animals immunised with DIII, DI/DII and sE constructs from DENV3 (left) and DENV4 (right) (in all cases n = 18), mean±s.d. are shown.
(TIF)
ADE of all four DENV serotypes, ZIKV, WNV and YFV on K562 cells, using the control mAb 4G2 (in all cases n = 3).
(TIF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.