Abstract
BACKGROUND & AIMS
Depletion of interstitial cells of Cajal (ICCs) is common in diabetic gastroparesis. However, in approximately 20% of patients with diabetes, gastric emptying (GE) is accelerated. GE is also faster in obese individuals, and is associated with increased blood levels of glucose in patients with type 2 diabetes. To understand the fate of ICCs in hyperinsulinemic, hyperglycemic states characterized by rapid GE, we studied mice with mutation of the leptin receptor (Leprdb/db), which in our colony had accelerated GE. We also investigated hyperglycemia-induced signaling in the ICC lineage and ICC dependence on glucose oxidative metabolism in mice with disruption of the succinate dehydrogenase complex, subunit C gene (Sdhc).
METHODS
Mice were given breath tests to analyze GE of solids. ICCs were studied by flow cytometry, intracellular electrophysiology, isometric contractility measurement, reverse transcription PCR, immunoblot, immunohistochemistry, ELISAs, and metabolite assays; cells and tissues were manipulated pharmacologically and by RNA interference. Viable cell counts, proliferation, and apoptosis were determined by methyltetrazolium, Ki-67, proliferating cell nuclear antigen, bromodeoxyuridine, and caspase-Glo 3/7 assays. Sdhc was disrupted in 2 different strains of mice via cre recombinase.
RESULTS
In obese, hyperglycemic, hyperinsulinemic female Leprdb/db mice, GE was accelerated and gastric ICC and phasic cholinergic responses were increased. Female KitK641E/+ mice, which have genetically induced hyperplasia of ICCs, also had accelerated GE. In isolated cells of the ICC lineage and gastric organotypic cultures, hyperglycemia stimulated proliferation by mitogen-activated protein kinase 1 (MAPK1)- and MAPK3-dependent stabilization of ets variant 1 (ETV1)—a master transcription factor for ICCs—and consequent upregulation of KIT proto-oncogene receptor tyrosine kinase (KIT). Opposite changes occurred in mice with disruption of Sdhc.
CONCLUSIONS
Hyperglycemia increases ICCs via oxidative metabolism-dependent, MAPK1- and MAKP3-mediated stabilization of ETV1 and increased expression of KIT, causing rapid gastric emptying. Increases in ICCs might contribute to the acceleration in GE observed in some patients with diabetes.
Keywords: ERK, mesenchymal cells, signal transduction, gastrointestinal motility
INTRODUCTION
Interstitial cells of Cajal (ICCs) are evolutionarily preserved mesenchymal cells located throughout the gastrointestinal tract.1 ICCs play a central role in gastrointestinal motility by generating electrical slow waves underlying phasic contractile activity, mediating nerve-muscle interactions and by setting smooth muscle membrane potential and tone.1,2 ICCs differentiate from embryonic3 and adult stem cells (ICC-SCs)4,5 in response to the interdependent actions of the ets family transcription factor ETV1, a master regulator of ICC fate,6–8 and KIT ligand (KITL, also known as stem cell factor) signaling via the receptor tyrosine kinase KIT.3,9
Gastroparesis is a chronic disorder of delayed gastric emptying (GE) without mechanical obstruction.10 Among patients and animal models of diabetic gastroparesis, depletion of gastric ICCs is more common than loss of nerves.11,12 Gastroparesis worsens glycemic control and causes significant morbidity, reduced quality-of-life and frequent hospitalizations and thus represents a significant health care burden.10,13
The mechanisms of diabetes-associated ICC loss are incompletely understood and likely complex. We discovered that reduced insulin and insulin-like growth factor 1 (IGF1) signaling14,15 inhibits ICC-SC self-renewal5 and differentiation4 in part via epigenetic repression of Kitl expression.16 Reduced Kitl expression has also been linked to depletion of mature ICCs.15,17 Furthermore, ICC damage may arise from imbalance of anti- and pro-inflammatory macrophage actions due to oxidative stress.18,19
While most studies focus on delayed GE, accelerated gastric emptying was observed in approximately ~20% of symptomatic patients with type 1 or type 2 diabetes in one study and a similar proportion of poorly controlled type 2 diabetic patients in another study.20,21 Moreover, in poorly controlled type 2 diabetes, higher blood glucose was associated with faster GE, which contrasts with the acute hyperglycemia-induced delayed GE observed in healthy individuals and type 1 diabetes.13,21 Of interest, accelerated GE of solids precedes ICC loss and delayed GE in non-obese diabetic (NOD) mice, a type 1 diabetes model.22 Remarkably, our previous in vitro study indicated that even long-term hyperglycemia per se may not be detrimental to ICCs.14 Together, these data suggest that hyperglycemia may regulate ICCs and gastric function independent of other consequences of diabetes such as oxidative stress, macrophage action and reduced trophic effects of insulin and IGF1. Furthermore, the effects of hyperglycemia may depend on the endocrine and metabolic context such as hyperinsulinemia and, particularly, obesity, which itself is consistently associated with rapid GE.23
To understand the fate and function of ICCs in hyperglycemic, hyperinsulinemic states associated with obesity, we studied leptin receptor-mutant (Leprdb/db) female mice, which in our colony had accelerated solid GE. We also investigated hyperglycemia-induced signaling in primary and cultured ICCs and ICC-SCs and the dependence of the ICC lineage on glucose oxidative metabolism in conditional succinate dehydrogenase C (Sdhc)-deficient mice.
METHODS
Standard methods and additional details are described in the Supplementary Methods.
Ethics Statement
De-identified gastric tissues were collected from nondiabetic patients undergoing bariatric surgery with the approval of the Mayo Clinic Institutional Review Board (IRB 13-008138). Animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The protocols were approved by the Mayo Clinic Institutional Animal Care and Use Committee (A64812, A48315, A59014).
Human tissue preparation and culturing
Pieces of gastric tunica muscularis approximately 2 cm × 2 cm in size were prepared by cutting away the mucosa and submucosa and cultured as described for mouse tissues.4,14,15
Animals and tissue preparation
Female mice homozygous for the leptin receptor loss-of-function mutation db (Leprdb/db from the strain BKS.Cg-Dock7m+/+Leprdb/J)24 (n=51 in 6 cohorts) and age- sex- and strain-matched wild-type (WT) controls (C57BLKS/J) (n=49) were from The Jackson Laboratory (Bar Harbor, ME). We used female mice because gastroparesis is approximately 4-fold more frequent in women than in men.25 Female KitK641E/+ mice carrying the activating Kit mutation encoding KITK641E found in gastrointestinal stromal tumors (GIST) (n=22),5 and age-, sex- and strain-matched WT littermates (n=17) were from our breeding programs. B6.Cg-Gt(ROSA)26Sortm1(rtTA*M2)Jae/J;B6.Cg-Tg(tetO-cre)1Jaw/J;Sdhcfl/fl (R26M2rtTA/+;TgtetO-cre;Sdhcfl/fl) mice and R26M2rtTA/+;Sdhcfl/fl controls (4 males and 4 females per genotype) carrying loxP sites flanking exon 4 of Sdhc encoding subunit C of the tricarboxylic acid (TCA) cycle enzyme and electron transfer chain complex succinate dehydrogenase, were generated as described in ref.26 and the Supplementary Methods. Deletion of Sdhc exon 4 was induced after weaning by inclusion of 2 mg/mL doxycycline hyclate (Sigma-Aldrich, St. Louis, MO) and 5 mg/mL sucrose in the drinking water for 20 days. B6.Cg-Tg(CAG-cre/Esr1*)5Amc/J;Sdhcfl/− (TgCAG-creERTM/+;Sdhcfl/−) and Sdhcfl/− mice (2 males and 2 females per genotype) used as an additional model have been described previously.26 For Sdhc inactivation, tamoxifen diet (Harlan Laboratories, Madison, WI) was started at 2–5 mo of age and maintained for 14–18 mo until time of sacrifice (see also Supplementary Methods). Homozygous H-2Kb-tsA58 transgenic mice (Immortomice®; CBA;B10-Tg(H2Kb-tsA58)6Kio/Crl) harboring the temperature-sensitive simian virus 40 tsA58 mutant large T antigen (tsTAg)27 were from Charles River Laboratories (Wilmington, MA). BALB/c and C57BL/6J breeders were from Harlan Laboratories and The Jackson Laboratory, respectively. Mice were killed by decapitation performed under deep isoflurane (Baxter Healthcare) inhalation anesthesia. Intact gastric tunica muscularis tissues were isolated as described4,15 and used immediately or as organotypic cultures prepared according to an established protocol.4,14,15
Physiological methods
Gastric emptying of solids (200 mg baked egg yolk) was measured by a [13C]-octanoic acid breath test.22 Electrical slow waves were recorded by intracellular techniques.4,11,14,28 Spontaneous and carbachol-stimulated phasic contractile activity in circular antral muscles was studied by isometric force measurements using standard techniques (Supplementary Methods).
Cell cultures
Primary cultures containing ICCs were obtained from the gastric corpus+antrum tunica muscularis of 3-day-old BALB/c mice. Cells were co-cultured with Sl/Sl4 murine stromal cells modified to express full-length murine KITL (Sl/Sl4-KITL248) as previously described.29 For glucose starvation, tissues and cells were cultured in glucose-free Dulbecco’s Modified Eagle’s Medium (Thermo Fisher, Waltham, MA) containing 1% antibiotic-antimycotic supplemented with 1% fetal bovine serum (cells only).
Conditionally immortalized ICCs were isolated from gastric corpus+antrum tunica muscularis tissues of two homozygous, 12-day-old Immortomice as KIThigh and hematopoietic marker (F4/80, CD11b, CD11c, CD45)-negative cells by combined immunomagnetic and fluorescence-activated cell sorting using a previously published and validated approach (Approach 4 in ref.30). The purified, KIT+CD45−CD11b−CD11c−F4/80− ICCs (56,000 cells) were plated at cloning density and cultured in Clonetics Smooth Muscle Growth Medium-2 (Lonza, Allendale, NJ) under conditions permissive for the tsTAg (33°C, 100 U/mL interferon-γ;27 Sigma-Aldrich, St. Louis, MO). A verified single-cell-derived cluster with ICC-like morphology was ring-cloned and propagated as ICL2A cells.
2XSCS2F10, a wild-type ICC-SC line, was established as previously described.5,31 In this study, ICL2A and 2XSCS2F10 cells were cultured in Medium 199 with phenol red supplemented with 1% antibiotic-antimycotic, 1% L-glutamine (Thermo Fisher) and 10% fetal bovine serum (Lonza) unless indicated otherwise.
Multi-parameter flow cytometry (FCM)
Murine gastric KIT+CD44+CD34− ICCs and KITlowCD44+CD34+ ICC-SCs were enumerated using previously published labeling and gating protocols with modifications4,5,15 (see Supplementary Methods, Supplementary Tables S1–S2 and Supplementary Figure S1).
Statistical analyses
Data are expressed as means±SEM and analyzed by nonparametric methods including Mann-Whitney rank sum test and Kruskal-Wallis one-way analysis of variance (ANOVA) on ranks followed by appropriate post-hoc tests. P<0.05 was considered significant.
RESULTS
Type 2 diabetic mice have oxidative stress and accelerated gastric emptying of solids
As anticipated,24 female Leprdb/db mice aged 11–73 weeks had dramatically increased body weights, blood glucose and serum insulin levels, whereas serum IGF1 remained unchanged (Supplementary Figure S2A). We also determined oxidative stress, a hallmark of diabetes mellitus, which contributes to ICC depletion in type 1 diabetic NOD mice.28 Serum malondialdehyde (MDA), a product of polyunsaturated fatty acid peroxidation,28 and levels in the gastric musculature of 8-hydroxy-2'-deoxyguanosine (8-OHdG), a marker of oxidative DNA damage, were also significantly increased (Supplementary Figure S2B). These results indicate that Leprdb/db mice are a suitable model to study the ICC phenotype and gastric functions in hyperinsulinemic type 2 diabetes.
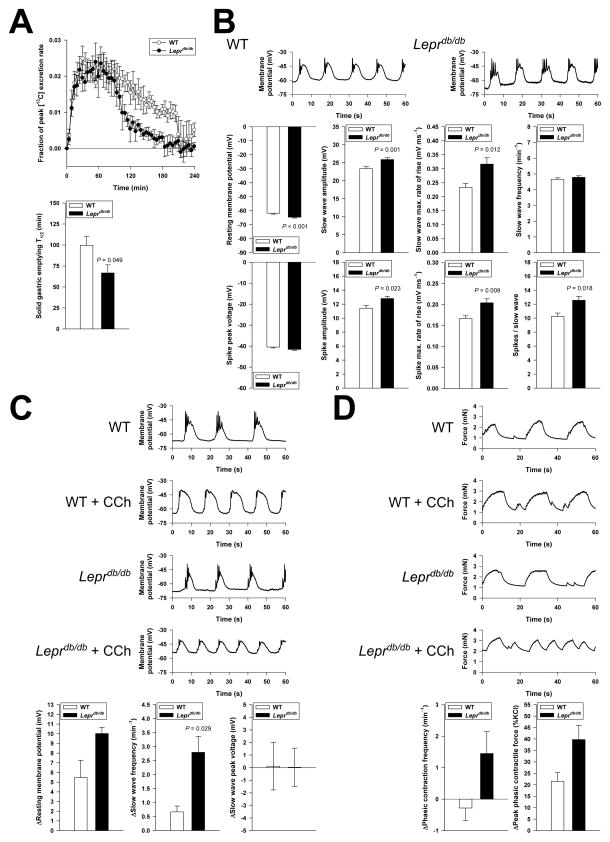
At 17 weeks of age, half-times (T1/2) of solid GE22 were significantly shorter in Leprdb/db mice whereas controls had T1/2 values highly similar to results previously obtained in nondiabetic NOD mice28 (Figure 1A). In Leprdb/db mice, GE remained accelerated at least until 50 weeks of age (T1/2= 60±6 min; mean±S.E.M.; n=5; average T1/2 of normal mice of similar age: 88–100 min22). Thus, obese, hyperinsulinemic and hyperglycemic mice in our colony showed rapid rather than delayed gastric emptying of solids.
Figure 1.
Accelerated gastric emptying and increased phasic responses to cholinergic stimulation in Leprdb/db mice. (A–B) Time course (top) and half-times (T1/2; bottom) of solid gastric emptying measured by [13C]-octanoic acid breath test in female Leprdb/db and WT mice (n=5–7/genotype). Note rapid emptying in the Leprdb/db animals. (B) Characteristics of electrical slow waves and spike potentials recorded from circular smooth muscle cells of Leprdb/db mice (70 cells, 6 animals) and WT mice (62 cells, 5 animals). Note modest gains in slow wave and spike amplitudes and rates-of-rise, and in spike frequency in Leprdb/db tissues. (C–D) Electrical (C; n=4/genotype) and contractile responses (D; n=4–6/genotype) to the cholinergic agonist carbachol (CCh; 100 nM). Slow waves were recorded in the presence of the L-type Ca2+ channel blocker nicardipine (1 μM following pretreatment with 2 μM). Spikes were incompletely inhibited by nicardipine but were not analyzed. Small (<0.5-mN), irregular contractile events were also not analyzed. CCh elicited greater increases in slow wave and contractile frequencies and peak contractile force in Leprdb/db tissues.
Increased ICCs contribute to accelerated gastric emptying
We investigated the physiological mechanisms underlying accelerated gastric emptying by intracellular electrophysiological and isometric force recordings. In isolated, pharmacologically unmanipulated antral tissues of Leprdb/db mice, we detected slight hyperpolarization of circular smooth muscle cells and modest but significant increases in the amplitudes and maximum rates-of-rise of both slow waves (events driven by ICC pacemaker activity) and action potentials (“spikes” reflecting the activity of smooth muscle L-type Ca2+ channels),1 as well as an increased number of spikes per slow wave (Figure 1B). There were no significant differences between Leprdb/db and control mice in peak voltages and slow wave frequency or in the frequency and amplitude of phasic contractions (2.77±0.25 vs. 3.10±0.55 min−1 and 1.04±0.45 vs. 1.71±0.66 mN, respectively; n=6/group). However, in response to the cholinergic agonist carbachol (100 nM), Leprdb/db tissues showed a greater increase in slow wave and contractile frequency than controls (Figure 1C–D). While peak voltages did not increase, peak phasic contractile responses were greater in the Leprdb/db antrums. Leprdb/db tissues also became more depolarized but this effect probably reflected an aphysiological action of carbachol.32 Consistent with a previous report,33 these results indicate that increased cholinergic signaling induces greater responses in Leprdb/db antrums than in WT mice. Since cholinergic input is a key determinant of gastric emptying,10 the observed changes, which involve functions of both smooth muscle cells (electromechanical coupling) and ICCs (electrical pacemaking),1,2 likely contribute to the accelerated gastric emptying seen in the Leprdb/db mice.
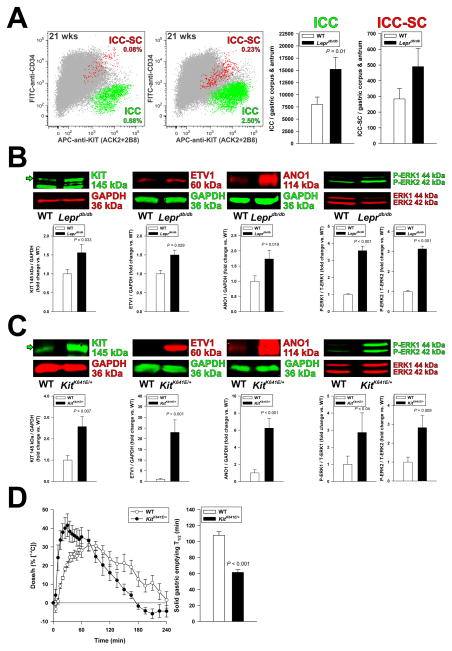
Insulin-IGF1 signaling stimulates ICC-SC proliferation directly and ICC differentiation and maintenance indirectly by regulating Kitl transcription in smooth muscle cells.4,5,14–16 To determine whether the functional changes in the hyperinsulinemic Leprdb/db mice reflected altered ICC numbers, we quantified gastric ICC and ICC-SC by multi-parameter FCM, which we extensively validated in previous studies,4,5,8,15,30 and by Western blotting (WB). FCM indicated a significant increase in gastric corpus+antrum ICCs and a trend toward increased ICC-SCs in Leprdb/db mice (Figure 2A). Consistent with these results, we detected significant increases in KIT, ETV1 and ANO1 (a calcium-activated chloride channel and ICC marker34) protein expression and in the phosphorylation of extracellular signal-regulated kinase (ERK) 1 and 2 (mitogen-activated protein kinase {MAPK} 3 and 1) in Leprdb/db stomachs (Figure 2B). KIT and ANO1 immunohistochemistry indicated a moderate increase in ICC network densities without an obvious bias toward myenteric or intramuscular ICCs (Supplementary Figure S3). These findings indicate that ICC and ICC-SC populations are increased in Leprdb/db mice.
Figure 2.
Increased ICCs contribute to accelerated gastric emptying. (A) Increased ICC (KIT+CD34− cells; green) and ICC-SC (KITlowCD34+cells; red) numbers detected by FCM in the non-hematopoietic, CD44+ fraction (gray) of gastric muscles of Leprdb/db mice vs. WT controls (n=9/group). (B) Expression of the key ICC proteins KIT, ETV1, and ANO1 and ERK1-ERK2 phosphorylation (WB; n=4–15/group) were significantly increased in Leprdb/db mice. (C–D) ICC hyperplasia associates with accelerated gastric emptying of solids in KitK641E/+ mice. (C) Consistent with the ICC hyperplasia previously reported in this strain,5 KIT, ETV1 and ANO1 expression and ERK1-ERK2 phosphorylation were elevated by WB in gastric corpus+antrum tunica muscularis tissues of female KitK641E/+ mice vs. controls (n=8–12/group). (D) Time course (left) and half-times (T1/2; right) of solid gastric emptying measured by [13C]-octanoic acid breath test in female KitK641E/+ mice (n=5–6/group). Note rapid emptying in the KitK641E/+ animals. Green arrows in B and C indicate mature, 145-kDa KIT protein.
To determine the specific contribution of increased ICC numbers to rapid GE independent of diabetes, we studied mice harboring the oncogenic KitK641E mutation. We previously demonstrated that KitK641E/+ mice lacking the neomycin resistance cassette have generalized ICC and ICC-SC hyperplasia without aberrant ICC distribution or obstructive GIST.5 Similarly to Leprdb/db mice, KIT, ETV1 and ANO1 protein expression and ERK1-ERK2 phosphorylation were upregulated in gastric muscles of female KitK641E/+ mice, albeit to a greater extent, as anticipated5 (Figure 2C). GE of solids was significantly accelerated (Figure 2D). Thus, expansion of ICCs regardless of its cause and independent of other cell types native to the tunica muscularis can lead to rapid GE, and also contribute, possibly together with accentuated cholinergic signaling, to accelerated GE in type 2 diabetes.
ICC numbers increase despite reduced KITL levels in type 2 diabetes
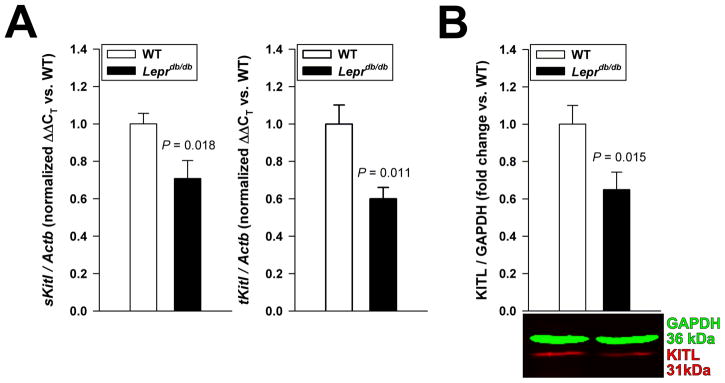
ICC hyperplasia is due to constitutive KIT activation in the KitK641E/+ mice,5 whereas in Leprdb/db animals it may reflect increased ligand-induced KIT signaling arising from Kitl transcriptional activation by insulin-IGF1 signaling.15,16 However, in the hyperinsulinemic Leprdb/db mice, expression of mRNA encoding both the “soluble” KITL isoform (a 248-aminoacid transmembrane polypeptide subject to rapid cleavage) and total KITL (which also includes the “membrane-bound” isoform, a 220-amino-acid peptide that is cleaved at a slow rate) was reduced (Figure 3A), and KITL protein was downregulated (Figure 3B). These data suggest that gastric smooth muscles of Leprdb/db mice display insulin resistance resulting in blunted Kitl transcription, and that increased ICC numbers in these tissues likely reflect mechanisms other than increased KITL-induced KIT signaling.
Figure 3.
ICC expansion occurs despite reduced Kitl expression in Leprdb/db mice. (A) Reduced expression of mRNA encoding the 248-amino-acid, “soluble” KITL isoform subject to rapid cleavage (sKitl) and mRNA common to both sKITL and the 220-amino-acid “membrane-bound” isoform (total KITL; tKitl) in the gastric corpus+antrum tunica muscularis of Leprdb/db mice vs. WT mice (n=11–13/group). (B) Reduced expression of 31-kDa KITL protein (corresponding to uncleaved, cell-associated KITL220 and secreted KITL produced from the KITL248 isoform16) in Leprdb/db mice (n=20/group).
ICCs are resistant to hyperglycemia-induced apoptosis
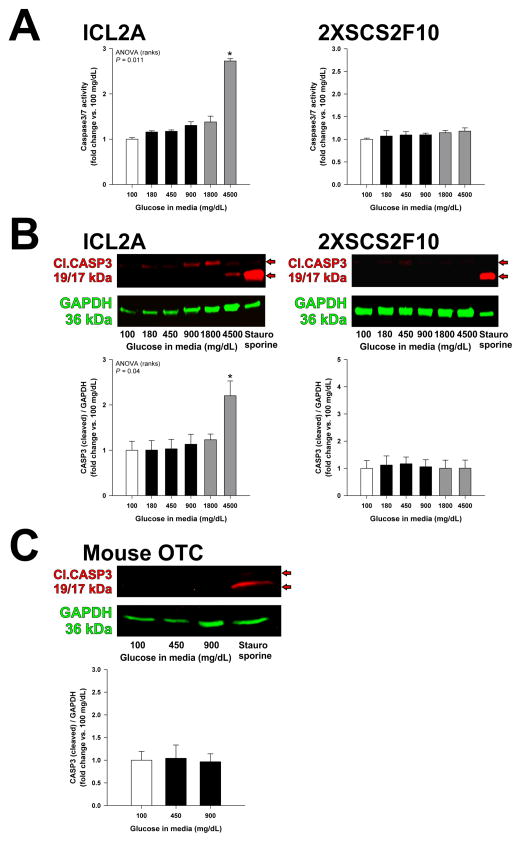
We previously reported that exposure to 600–1000 mg/dL glucose for ~10 weeks did not affect ICC networks in gastric organotypic cultures maintained with insulin, and severe hyperglycemia partially rescued ICCs even in the absence of insulin or IGF1.14 Therefore, we hypothesized that high glucose per se could stimulate the survival and/or proliferation of cells of the ICC lineage. We first examined the effect of high glucose on apoptosis in ICL2A cells, a clonal, conditionally immortalized gastric ICC line; in 2XSCS2F10 cells, a clonally-derived ICC-SC line described and characterized previously;5,31 and in organotypic cultures of intact gastric corpus+antrum tunica muscularis tissues from 14–16-day-old C57BL/6J mice. Cells and tissues were maintained for 72 h in the presence of 100–4,500 mg/dL glucose. In control cultures, glucose above 100 mg/dL was replaced with equimolar concentrations of the metabolically inert sugar alcohol mannitol. Exposure to glucose (400 mg/dL) for 24 h causes apoptosis in enteric neurons35. However, by detecting caspase 3/7 activity by Caspase-Glo 3/7 assay and/or cleaved caspase 3 by WB, we found both 2XSCS2F10 cells and organotypic cultures to be resistant to hyperglycemia-induced apoptosis, while in ICL2A cells, the apoptosis induced by extremely high glucose was due to osmotic stress (Figure 4A–C, Supplementary Figure S4A–B). These findings indicate that ICCs and gastric tunica muscularis cells in general are resistant to hyperglycemia-induced apoptosis.
Figure 4.
ICCs, ICC-SCs and gastric muscles are resistant to hyperglycemia-induced apoptosis. (A–C) Apoptosis was quantified by measuring activation of the effector caspases 3 and 7 using Caspase-Glo 3/7 assay (A) or by detecting cleaved caspase 3 (Cl.CASP3) by WB (B–C) in the conditionally immortalized ICC line ICL2A, the wild-type ICC-SC line 2XSCS2F10 and in gastric organotypic cultures (OTC) prepared from postnatal day (PND) 14–16 murine tunica muscularis. Experiments were performed after 72 h of culturing in the presence of various concentrations of glucose. Positive controls were exposed to staurosporine (3 μM; n=3–7/group). Apoptosis was only induced by 4,500 mg/dL glucose in ICL2A cells (*, P<0.05 vs. 100 mg/dL glucose). Red arrows indicate 17- and 19-kDa cleaved caspase 3.
Hyperglycemia stimulates ICC growth via the ERK-ETV1-KIT pathway
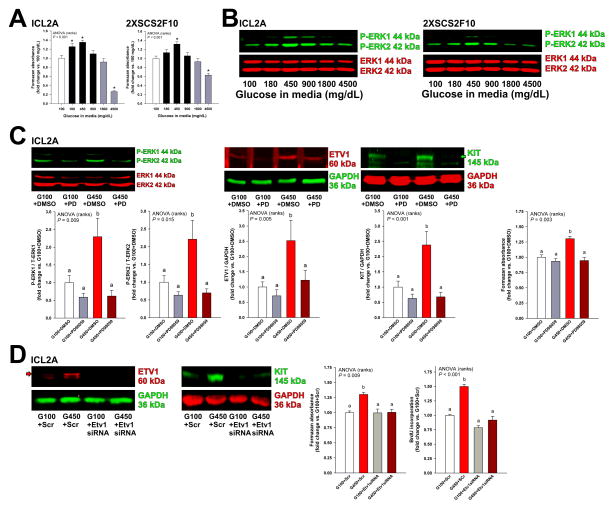
Next, we investigated whether high glucose could stimulate ICC and ICC-SC growth. In both ICL2A and 2XSCS2F10 cells, glucose applied for 72 h had concentration-dependent effect on viable cell counts determined by methyltetrazolium salt (MTS) assay with maximum stimulation occurring in response to 450 mg/dL (Figure 5A). This effect was not reproduced by mannitol; whereas the cell loss observed in response to more than 900 mg/dL glucose reflected osmotic stress (Figure 5A and Supplementary Figure S5A). 450 mg/dL glucose also promoted the proliferation of ICL2A cells detected by Ki-67 staining and 5-bromo-2’-deoxyuridine (BrdU) incorporation (Supplementary Figure S6A–B). Because ERK MAPK activation is central to ICC hyperplasia and GIST oncogenesis,6–8 we assessed ERK1-ERK2 phosphorylation in response to hyperglycemia by WB. Glucose applied for 72 h concentration-dependently activated ERK1-ERK2 phosphorylation in both ICL2A and 2XSCS2F10 cells with maximum effect occurring between 450 and 900 mg/dL glucose (Figure 5B). Again, mannitol had no effect (Supplementary Figure S5B–C).
Figure 5.
Hyperglycemia stimulates the growth of ICC and ICC-SC cell lines via the ERK-ETV1-KIT pathway. (A–B) Effect of high glucose on cell counts determined by MTS assay (A; n=3–9/cell line/group) and ERK1-ERK2 phosphorylation analyzed by WB (B; n=6/cell line/group) in ICL2A and 2XSCS2F10 cells. Cell numbers and ERK1-ERK2 phosphorylation were significantly increased by high glucose levels typically seen in Leprdb/db mice (*, P<0.05 vs. 100 mg/dL glucose) and were only reduced by extremely high concentrations. (C) In ICL2A cells, the MAPK kinase inhibitor PD98059 (PD; 20 μM) inhibited hyperglycemia-induced ERK1-ERK2 phosphorylation and upregulation of ETV1 and KIT proteins (n=8–12/group) and cell counts (n=6/group). DMSO, dimethyl sulfoxide vehicle. G100 and G450, 100 and 450 mg/dL glucose, respectively. (D) siRNA-mediated knock-down of ETV1 inhibited the hyperglycemia-induced increase in ETV1 and KIT protein levels (WB), cell numbers (MTS) and proliferation (BrdU incorporation) detected in ICL2A cells (n=6/group). Scr, scrambled-sequence control. Green and red arrows indicate mature, 145-kDa KIT and 60-kDa ETV1 proteins, respectively. Groups in B and D not sharing the same superscript are different by post-hoc tests (P<0.05).
ERK1-ERK2 activation regulates cells of the ICC-GIST lineage by inhibiting the proteasomal degradation of ETV1, a key transcription factor for Kit.6–8 Since activation of ERK1-ERK2 was accompanied by upregulation of ETV1 protein in hyperglycemic Leprdb/db mice with increased ICCs and ICC-SCs, we hypothesized that hyperglycemia could upregulate ETV1 and KIT proteins in ICC-related cells via ERK1-ERK2 phosphorylation. Indeed, increased ERK1-ERK2 phosphorylation in response to 450 mg/dL glucose was accompanied by upregulation of both ETV1 and KIT in ICL2A cells (Figure 5C). PD98059 (20 μM), a selective inhibitor of MAPK kinase, significantly inhibited the upregulation of ETV1 and KIT and inhibited the cell growth induced by high glucose with only minor effects in the presence of 100 mg/dL glucose (Figure 5C). Furthermore, the hyperglycemia-induced increase in KIT protein, cell counts and proliferation could also be blocked by small interfering RNA (siRNA)-mediated Etv1 knock-down (Figure 5D). These results indicate that hyperglycemia upregulates KIT and stimulates the proliferation of ICC and ICC-SC cell lines via ERK1-ERK2-dependent ETV1 stabilization.
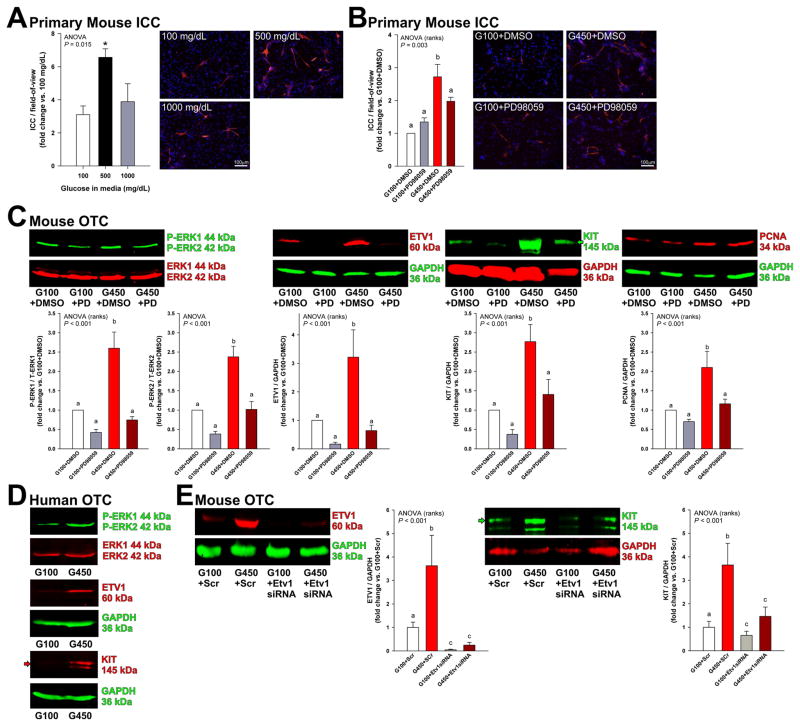
To extend the validity of our findings to physiologically more relevant systems, we next examined the effects and mechanisms of hyperglycemia in primary ICCs and organotypic cultures of gastric corpus+antrum muscles. Similarly to ICC-related cell lines, 500 mg/dL glucose for 48 h robustly increased ICC numbers in primary cultures prepared from neonatal mouse gastric smooth muscles (Figure 6A), and this effect was significantly inhibited by MAPK kinase inhibition with PD98059 (Figure 6B). Mannitol had no effect (Supplementary Figure S7A). High glucose (450 mg/dL) also activated ERK1-ERK2 phosphorylation and upregulated ETV1, KIT, and proliferating cell nuclear antigen (PCNA) proteins in an ERK1-ERK2-dependent manner in gastric organotypic cultures derived from 11-week-old C57BL/6J mice (Figure 6C) or 14–16-day-old mice (not shown). Mannitol had no effect (Supplementary Figure S7B). To extend our findings to human tissues, we prepared organotypic cultures from 3 individual human gastric tissues. Relative to 100 mg/dL glucose, 450 mg/dL glucose activated ERK1-ERK2 phophorylation and upregulated both ETV1 and KIT proteins (Figure 6D and Supplementary Figure S8). Finally, in mouse organotypic cultures, siRNA treatment targeting Etv1 (72 h) blocked the upregulation of KIT protein occurring in response to 450 mg/dL glucose (Figure 6E). Together, these results indicate that hyperglycemia-induced proliferation of cells of the ICC lineage is mediated by ERK MAPK-mediated stabilization of ETV1 and consequent upregulation of KIT expression.
Figure 6.
Hyperglycemia stimulates the growth of primary ICCs and ETV1 and KIT protein levels in gastric OTCs via the ERK-ETV1-KIT pathway. (A–B) Effect of high glucose and PD98059 (20 μM) on primary ICCs. Representative images of freshly dissociated ICCs from PND 2-3 BALB/c mice immunostained for KIT (red) after 48 h of culturing. Nuclei were counterstained with 4',6-diamidino-2-phenylindole (DAPI; blue). 450–500 mg/dL glucose increased KIT+ ICC/high-powered field (400×; n=4/group; *, P<0.05 vs. 100 mg/dL glucose), and this effect was blocked by MAPK kinase inhibition (n=3–5/group). (C–D) Effects of high glucose on ERK1-ERK2 phosphorylation and ETV1 and KIT protein levels in adult mouse and human gastric tunica muscularis OTCs. 450 mg/dL glucose applied for 48 h activated ERK1-ERK2 phosphorylaton and upregulated ETV1 and KIT protein levels in OTCs derived from gastric muscles of 11-week-old C57BL/6J mice (C; n=10–12/group) and from a patient aged 40 years (D; see two additional patients in Supplementary Figure S8). PCNA levels were also increased. PD98059 (PD; 20 μM) inhibited hyperglycemia-induced ERK1-ERK2 phosphorylation and upregulation of ETV1, KIT, and PCNA levels in mouse OTCs. (E) siRNA-mediated Etv1 knock-down blocked the upregulation of KIT and ETV1 proteins by 450 mg/dL glucose in OTCs from gastric muscles of PND 10–12 C57BL/6J mice (n=10–15/group). Green and red arrows indicate mature, 145-kDa KIT protein. Groups not sharing the same superscript are different by post-hoc tests (P<0.05).
Glucose oxidative metabolism regulates ICCs through the ERK-ETV1-KIT pathway
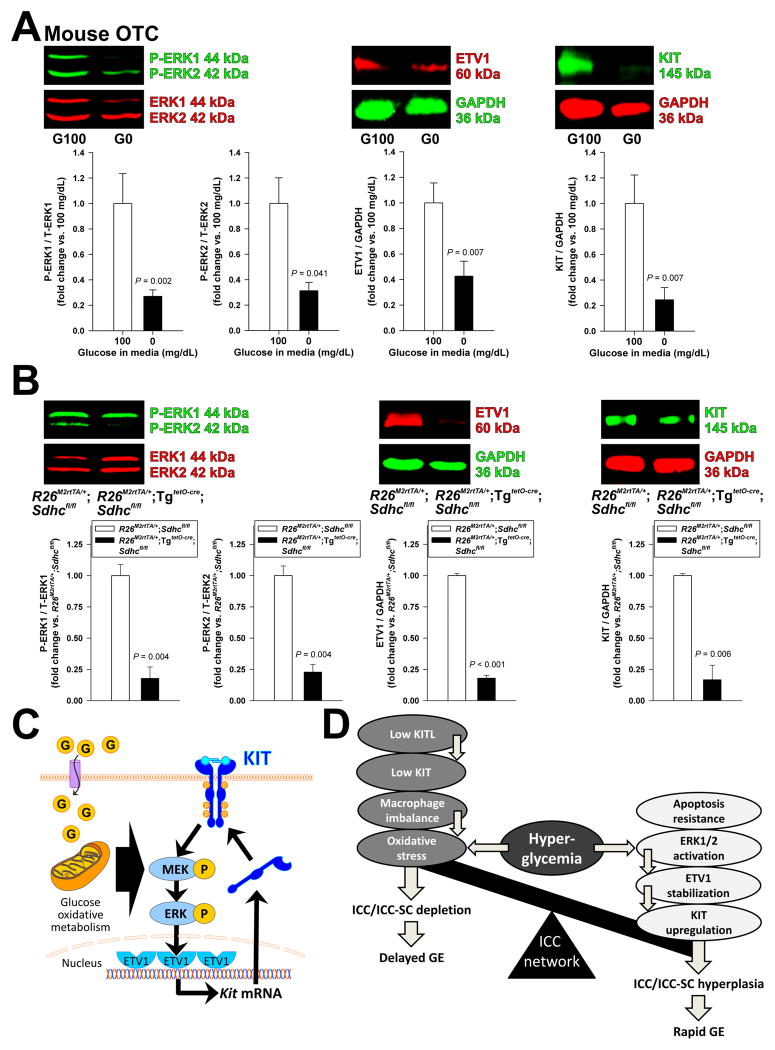
Initial activation of the ERK-ETV1-KIT regulatory loop6–8 in hyperinsulinemic, hyperglycemic mice likely occurs in a ligand-independent manner because these animals have reduced KITL levels (Figure 3). Since ICCs have an abundance of mitochondria,1,36 and ICC functions,36 as well as proliferation, KIT expression and KIT signaling in GIST depend on aerobic glycolysis,37 we hypothesized that glucose oxidative metabolism may regulate ICC populations via ERK1-ERK2 phosphorylation and consequent upregulation of ETV1 and KIT. First, we examined the effects of 48-h glucose starvation in mouse organotypic cultures and in ICL2A cells. Glucose withdrawal inhibited ERK1-ERK2 phosphorylation and downregulated both ETV1 and KIT protein levels in murine gastric smooth muscles (Figure 7A) and reduced ERK1-ERK2 phosphorylation, cell counts (by MTS assay) and cell proliferation (by BrdU incorporation and Ki-67 immunofluorescence) in ICL2A cells (Supplementary Figure S9) without inducing apoptosis or oxidative stress in either model (Supplementary Figure S10). These data indicate that glucose is specifically required for the maintenance of the ERK-ETV1-KIT pathway.
Figure 7.
Glucose oxidative metabolism regulates ICCs through the ERK-ETV1-KIT pathway. (A) Glucose deprivation (0 mg/dL; G0) for 48 h inhibited ERK1-ERK2 phosphorylation and reduced ETV1 and KIT protein expression in OTCs from gastric tunica muscularis of PND 14–16 C57BL/6J mice compared to 100 mg/dL glucose (G100) (n=8/group). (B) Effects of conditional genomic deletion of Sdhc on ERK1-ERK2 phosphorylation and ETV1 and KIT protein levels analyzed by WB in the gastric corpus+antrum tunica muscularis of doxycyclin-treated R26M2rtTA/+;TgtetO-cre;Sdhcfl/fl and R26M2rtTA/+;Sdhcfl/fl controls. Sdhc deletion reduced ERK1-ERK2 phosphorylation and ETV1 and KIT protein levels (n=5–6/group). (C) Proposed mechanism of glucose-induced KIT upregulation. Glucose (G) activates ERK1-ERK2 phosphorylation (P) in a manner dependent on oxidative metabolism. ERK1-ERK2 activation increases ETV1 protein by preventing its proteasomal degradation.6,8 ETV1 then activates KIT transcription.7,8 Increased KIT protein levels reinforce the signaling loop. (D) Proposed mechanisms regulating ICC numbers and gastric emptying. ICCs are reduced and gastric emptying is delayed by reduced KITL and KIT levels and macrophage imbalance facilitating oxidative stress from hyperglycemia. ICCs are increased and gastric emptying is accelerated by hyperglycemia-induced ERK1-ERK2 phosphorylation, ETV1 stabilization and KIT upregulation. These changes are facilitated by the ICCs’ resistance to hyperglycemia-induced apoptosis. The balance of the opposing outcomes of hyperglycemia determines ICC numbers and gastric emptying in diabetes.
To establish the in-vivo relevance of this finding, we studied R26M2rtTA/+;TgtetO-cre;Sdhcfl/fl mice wherein subunit C of the TCA cycle and electron transfer chain enzyme complex succinate dehydrogenase had been inactivated by 20-day oral tetracyclin treatment. Sdhc inactivation by genome editing reduced gastric SDHC protein to nearly undetectable levels as expected26 (Supplementary Figure S11A) and downregulated ERK1-ERK2 phosphorylation and KIT and ETV1 protein levels (Figure 7B). KITL levels were unaffected, and oxidative stress only increased modestly and comparably to the levels seen in Leprdb/db mice (Supplementary Figure S11A–B), reflecting a minor and somewhat controversial role of dysfunctional succinate dehydrogenase complex in reactive oxygen species formation.38 Finally, in tamoxifen-treated TgCAG-creERTM/+;Sdhcfl/− mice26 used as an alternative model, we detected reduced gastric ICCs and ICC-SCs by FCM (Supplementary Figure S12). Together, these results indicate that glucose oxidative metabolism is required for ICC maintenance via the ERK-ETV1-KIT pathway (Figure 7C) and underlies ICC expansion in hyperglycemic, hyperinsulinemic diabetes even in the presence of mild oxidative stress and reduced KITL levels (Figure 7D).
DISCUSSION
In this study we found type 2 diabetic Leprdb/db mice24 to have accelerated GE of solids, accentuated increases in slow wave frequency, contractile frequency and peak contractile force responses to cholinergic stimulation, as well as increased gastric ICCs and ICC-SCs. GE was similarly accelerated in KitK641E/+ mice with genetic ICC hyperplasia.5 These findings establish a direct link between ICC numbers and GE. In Leprdb/db mice, ICCs increased despite reduced KITL and increased oxidative stress, i.e., factors contributing to ICC depletion in type 1 diabetes.15,28 Both Leprdb/db and KitK641E/+ mice showed increased ERK1-ERK2 phosphorylation and upregulated ETV1 and KIT proteins. The increase in ICCs and ICC-SCs and the activation of the ERK-ETV1-KIT pathway were faithfully reproduced by in-vitro exposure of immortalized and primary ICCs, cultured ICC-SCs, as well as mouse and human gastric tunica muscularis organotypic cultures to glucose concentrations occurring in Leprdb/db mice, demonstrating a stimulatory effect of elevated glucose on ICCs. Pharmacological MAPK kinase inhibition indicated a key role for ERK1-ERK2 activation in the hyperglycemia-associated ETV1 and KIT upregulation; and siRNA-mediated Etv1 knockdown revealed that the hyperglycemia-induced increase in KIT required Etv1. Our studies also demonstrated resistance of ICCs to hyperglycemia-induced apoptosis. Experiments employing in-vitro glucose starvation and mice with genomic deletion of Sdhc revealed that the ERK-ETV1-KIT-mediated increase in ICCs involved oxidative glucose metabolism. This finding is consistent with the established dependence of ICC functions and GIST proliferation on aerobic glycolysis.36,37 Together, our results indicate that blood glucose is an important determinant of ICC populations. ICC numbers, likely in concert with other mechanisms, influence GE across the spectrum of delayed, normal and accelerated emptying. These concepts, summarized in Figure 7D, provide a new paradigm for future studies aimed at mechanistic interpretation of highly variable clinical findings.
Gastroparesis is associated with significant morbidity and health care utilization but lacks effective therapy.10,13 Delayed GE arising from diabetes involves ICC depletion.11,12 However, ~20% of diabetic patients have accelerated GE and 30–40% display normal GE.20,21 Accelerated GE may also precede gastroparesis in diabetic NOD mice.19,22 In humans, accelerated GE is associated with increased gastric motility39 but the exact mechanisms remain unclear. This study demonstrates that rapid GE may be due to ICC expansion occurring when diabetes-associated factors favoring ICC differentiation, survival, proliferation, and function outweigh the impact of factors detrimental to these cells (Figure 7D). Furthermore, increased ICC numbers arising from constitutively active KIT signaling in a genetic model of ICC hyperplasia and GIST were also associated with fast GE. These data strongly suggest that expanded gastric ICC populations and rapid emptying are causally related. It remains to be investigated whether ICC are also increased in patients with rapid GE—including diabetic patients20,21 and obese, nondiabetic individuals.23 Future studies should also address how functions of the gastroduodenal junction contribute to accelerated GE in diabetes.
To understand the mechanism of ICC gain, we first examined endocrine and metabolic factors known to impact ICCs. Surprisingly, we found that KITL, an established survival/differentiation factor for ICCs1,4,9 that is reduced in diabetic mice with ICC loss,15,17 was downregulated in Leprdb/db mice despite increased serum insulin and unchanged IGF1, which stimulate Kitl transcription.15,16 This result indicates that gastric smooth muscles are likely resistant to insulin and that ICC expansion can occur in the presence of reduced KITL levels in Leprdb/db mice. Since KIT in ICCs was upregulated by hyperglycemia via ERK1-ERK2 activation-induced ETV1 stabilization, increased KIT levels were probably able to compensate for the reduced availability of KITL.
Oxidative stress is another factor known to damage ICCs and cause delayed GE of solids.28 Although oxidative stress determined by measuring gastric 8-OHdG and circulating MDA was elevated in Leprdb/db mice, MDA concentrations were lower than the levels reported in the presence of ICC depletion and delayed GE.28 Thus, the oxidative stress observed in our cohort of Leprdb/db mice was likely insufficient to precipitate ICC loss. By the same token, the similarly modest increases seen in the Sdhc-deficient mice were unlikely to underlie the ICC loss in that model.
Limited impact of reduced KITL and oxidative stress in our BKS.Cg-Dock7m+/+Leprdb/J model may underlie differences between results in the present study and two previous reports in type 2 diabetic mice. In C57BL/KsJ-db/db mice, Yamamoto et al. reported reduced ICCs (assessed by quantifying KIT+ tissue area in histological sections) accompanying reduced Kitl mRNA and reduced GE (measured 1.5 h after spontaneous intake, over 1 h, of measured but uncontrolled amount of food).17 Significant differences in the experimental approaches aside, higher oxidative stress or lower KIT expression acting on a different genetic background could have precipitated ICC loss and delayed GE. In KitcopGFP/+;Lepob/ob mice expressing green fluorescent protein from the first exon of Kit and homozygous for the ob mutation in leptin, Ro et al. described ICC loss in the small and large intestines.40 It is notable that these mice are haploinsufficient for KIT as indicated by pigmentation defects. In 6 adult KitCreERT2/+ mice with a similarly inactivated Kit allele and pigmentation defects and 7 Kit+/+ mice from the same colony we assessed cell-surface KIT protein by FCM and found that mice with one active Kit allele, median KIT fluorescence was reduced to 32±1% of control (P=0.001). Reduction below 50% is expected in light of the positive feedback between KIT and ETV1 as reduced KIT protein would result in further reduction in Kit transcription due to increased ETV1 degradation.6–8 Reduced KIT levels would, in turn, shift the balance toward factors detrimental to ICCs leading to ICC loss even in the presence of hyperglycemia (Figure 7D). Finally, it is important to point out that this balance may also be altered by environmental factors, since in a previous study we found 4 of 18 (22%) BKS.Cg-Dock7m+/+Leprdb/J mice to have gastroparesis.18 Unfortunately, the status of the non-delayed mice at ages comparable to the ages covered in our current study (17–50 wks) is unknown because they were not tested after 10 weeks of diabetes.
Previously, we found that ICCs maintained in the presence of insulin for 68–72 days were resistant to 1000 mg/dL glucose, and in the absence of insulin, ICC networks were better preserved in the presence of high glucose.14 Consistent with these results, hyperglycemia at levels observed in Leprdb/db mice increased both ICC and ICC-SC numbers without inducing apoptosis in any of the cell models and also in intact gastric tunica muscularis tissues. Resistance to hyperglycemia-induced apoptosis may be characteristic of smooth muscle and mesenchymal cells as vascular smooth muscle cells from diabetic patients also showed resistance to apoptosis.41
ERK1-ERK2 activation stabilizes ETV1 protein, a master transcription factor for the ICC-GIST lineage.6 ETV1, in turn, stimulates Kit transcription via enhancer binding, completing a positive feedback loop.7,8 In gastric muscles of Leprdb/db mice, ERK1-ERK2 were activated and ETV1 and KIT proteins were increased. Furthermore, both MAPK kinase blockade and Etv1 knockdown robustly inhibited the upregulation of KIT induced by hyperglycemia. Collectively, these findings indicate that hyperglycemia induces ERK1-ERK2 activation, leading to upregulation of KIT via ETV1 stabilization (Figure 7C). In Leprdb/db mice in our study, this mechanism was able to override the effects of reduced Kitl expression and moderately increased oxidative stress and lead to ICC hyperplasia and accelerated GE (Figure 7D).
To get insight into the mechanism of hyperglycemia-induced ERK MAPK activation, we studied glucose-deprived cells and tissues and mice with genomic deletion of Sdhc. These experiments indicated that glucose oxidative metabolism is required for ERK MAPK activation and downstream effects on ETV1 and KIT proteins, as well as ICC and ICC-SC numbers. Furthermore, they revealed that glycemic control of ERK-ETV1-KIT signaling prevails across a broad range of glucose levels including hypoglycemia. It remains unclear how glucose oxidation regulates ERK1-ERK2 signaling. The possibility that altered succinate: α-ketoglutarate ratios may influence expression of signaling molecules regulating ERK MAPK via epigenetic mechanisms42 requires further investigation.
In conclusion, we demonstrate that Leprdb/db mice can have increased gastric ICCs despite reduced KITL signaling and increased oxidative stress. Hyperglycemia-induced ERK1-ERK2 activation causes ICC growth via ETV1 and KIT upregulation. Increased ICCs can in turn lead to accelerated GE. This mechanism may contribute to the accelerated GE seen in a significant subset of diabetic patients.
Supplementary Material
Acknowledgments
Grant support: This work was supported in part by National Institutes of Health Grants R01DK058185, P01DK068055, R01CA166025, and the NIDDK Diabetic Complications Consortium (DiaComp, www.diacomp.org) grant U24DK076169; the Life Raft Group, the Rebecca E. Saunders Fund in Anorexia Nervosa and Bulimia Research, the Paradifference Foundation and the Mayo Clinic Center for Individualized Medicine (http://mayoresearch.mayo.edu/center-for-individualized-medicine). The funding agencies had no role in the study analysis or writing of the manuscript. Its contents are solely the responsibility of the authors.
We thank Laura N. Popko for contributing to the analysis of the electrophysiological recordings, Jessica E. Mason for her help with the animal studies, Dr. Gregory J. Gores (Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota) for granting us access to the LI-COR Odyssey Scanner and Dr. David Williams (Indiana University School of Medicine, Indianapolis, IN) for providing the Sl/Sl4 murine stromal cells genetically modified to express full-length murine KITL (Sl/Sl4-KITL248). We also thank the Mayo Clinic Immunochemical Core Laboratory (director: Dr. Ravinder J. Singh; Development Lead: Dr. Roy B. Dyer) for the serum insulin and insulin-like growth factor 1 assays.
Abbreviations
- 3D
3-dimensional
- 8-OHdG
8-hydroxyl-2'-deoxyguanosine
- ANO1
anoctamin-1 (also known as Tmem16a)
- AF
Alexa Fluor
- APC
allophycocyanin
- C+A
corpus plus antrum
- CCh
carbachol
- DAPI
4',6-diamidino-2-phenylindole
- DMSO
dimethyl sulfoxide
- FCM
flow cytometry
- FITC
fluorescein isothiocyanate
- ERK
extracellular signal-regulated kinase
- ETV1
ets variant 1
- GE
gastric emptying
- GIST
gastrointestinal stromal tumor(s)
- ICC
interstitial cell of Cajal
- IGF1
insulin-like growth factor 1
- IGF1R
IGF1 receptor
- INSR
insulin receptor
- KIT
v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog
- KITL
KIT ligand, also known as stem cell factor
- LEPR
leptin receptor
- MAPK
mitogen-activated protein kinase
- MDA
malondialdehyde
- MTS
methyl-tetrazolium salt
- NOD
nonobese diabetic
- OTC
organotypic culture
- PCNA
proliferating cell nuclear antigen
- PE
phycoerythrin
- PND
postnatal day
- R26M2rtTA
B6.Cg-Gt(ROSA)26Sortm1(rtTA*M2)Jae/J
- RTK
receptor tyrosine kinase
- SC
stem cell(s)
- SDH
succinate dehydrogenase
- siRNA
small interfering RNA
- T1/2
half-time
- TCA
tricarboxylic acid cycle
- TgCAG-creERTM
B6.Cg-Tg(CAG-cre/Esr1*)5Amc/J
- TgtetO-cre
B6.Cg-Tg(tetO-cre)1Jaw/J
- tsTAg
temperature-sensitive simian virus 40 tsA58 mutant large T antigen
- WB
western blotting
- WT
wild-type
Footnotes
Disclosures: These authors disclose the following: Brian P. Rubin was a member of the Speakers Bureau and the Advisory Board of Novartis Pharmaceuticals Corp. The remaining authors disclose no conflicts.
Author contributions: Study concept and design: Y.H., T.O. Acquisition of data: Y.H., Y.T., S.A. Saravanaperumal, M.R.B., J.A.S., A.L., S.T.E., G.C., M.N.H., F.J. Al K., S.A. Syed, G.B.G., K.M.C., G.J.S., K.E.M., S.J.G. Analysis and interpretation of data: Y.H., S.A. Saravanaperumal, D.R.L., A.E.B, G.F., T.O. Material support: M.L.K., B.P.R., L.J.M. Drafting of the manuscript: Y.H., T.O. Critical revision of the manuscript for important intellectual content: Y.H., A,E.B., G.F., T.O. Obtained funding: L.J.M., G.F., T.O. Study supervision: T.O.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Resource sharing: Animal models and cell lines are available from the authors.
References
- 1.Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. Physiol Rev. 2014;94:859–907. doi: 10.1152/physrev.00037.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil. 2008;20(Suppl 1):54–63. doi: 10.1111/j.1365-2982.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- 3.Torihashi S, Ward SM, Sanders KM. Development of c-Kit-positive cells and the onset of electrical rhythmicity in murine small intestine. Gastroenterology. 1997;112:144–155. doi: 10.1016/s0016-5085(97)70229-4. [DOI] [PubMed] [Google Scholar]
- 4.Lorincz A, Redelman D, Horvath VJ, et al. Progenitors of interstitial cells of Cajal in the postnatal murine stomach. Gastroenterology. 2008;134:1083–1093. doi: 10.1053/j.gastro.2008.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardsley MR, Horvath VJ, Asuzu DT, et al. Kitlow stem cells cause resistance to Kit/platelet-derived growth factor alpha inhibitors in murine gastrointestinal stromal tumors. Gastroenterology. 2010;139:942–952. doi: 10.1053/j.gastro.2010.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chi P, Chen Y, Zhang L, et al. ETV1 is a lineage survival factor that cooperates with KIT in gastrointestinal stromal tumours. Nature. 2010;467:849–853. doi: 10.1038/nature09409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ran L, Sirota I, Cao Z, et al. Combined inhibition of MAP kinase and KIT signaling synergistically destabilizes ETV1 and suppresses GIST tumor growth. Cancer Discov. 2015;5:304–315. doi: 10.1158/2159-8290.CD-14-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashi Y, Bardsley MR, Toyomasu Y, et al. Platelet-Derived Growth Factor Receptor-alpha Regulates Proliferation of Gastrointestinal Stromal Tumor Cells With Mutations in KIT by Stabilizing ETV1. Gastroenterology. 2015;149:420–432. e416. doi: 10.1053/j.gastro.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huizinga JD, Thuneberg L, Kluppel M, et al. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 10.Camilleri M, Bharucha AE, Farrugia G. Epidemiology, mechanisms, and management of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2011;9:5–12. doi: 10.1016/j.cgh.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ordog T, Takayama I, Cheung WK, et al. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731–1739. doi: 10.2337/diabetes.49.10.1731. [DOI] [PubMed] [Google Scholar]
- 12.Grover M, Farrugia G, Lurken MS, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140:1575–1585. e1578. doi: 10.1053/j.gastro.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halland M, Bharucha AE. Relationship Between Control of Glycemia and Gastric Emptying Disturbances in Diabetes Mellitus. Clin Gastroenterol Hepatol. 2016;14:929–936. doi: 10.1016/j.cgh.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horvath VJ, Vittal H, Ordog T. Reduced insulin and IGF-I signaling, not hyperglycemia, underlies the diabetes-associated depletion of interstitial cells of Cajal in the murine stomach. Diabetes. 2005;54:1528–1533. doi: 10.2337/diabetes.54.5.1528. [DOI] [PubMed] [Google Scholar]
- 15.Horvath VJ, Vittal H, Lorincz A, et al. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of Cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130:759–770. doi: 10.1053/j.gastro.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi Y, Asuzu DT, Gibbons SJ, et al. Membrane-to-nucleus signaling links insulin-like growth factor-1- and stem cell factor-activated pathways. PLoS One. 2013;8:e76822. doi: 10.1371/journal.pone.0076822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto T, Watabe K, Nakahara M, et al. Disturbed gastrointestinal motility and decreased interstitial cells of Cajal in diabetic db/db mice. J Gastroenterol Hepatol. 2008;23:660–667. doi: 10.1111/j.1440-1746.2008.05326.x. [DOI] [PubMed] [Google Scholar]
- 18.Choi KM, Kashyap PC, Dutta N, et al. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology. 2010;138:2399–2409. 2409e2391. doi: 10.1053/j.gastro.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cipriani G, Gibbons SJ, Kashyap PC, et al. Intrinsic Gastrointestinal Macrophages: Their Phenotype and Role in Gastrointestinal Motility. Cell Mol Gastroenterol Hepatol. 2016;2:120–130. e121. doi: 10.1016/j.jcmgh.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bharucha AE, Camilleri M, Forstrom LA, et al. Relationship between clinical features and gastric emptying disturbances in diabetes mellitus. Clin Endocrinol (Oxf) 2009;70:415–420. doi: 10.1111/j.1365-2265.2008.03351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bharucha AE, Kudva Y, Basu A, et al. Relationship between glycemic control and gastric emptying in poorly controlled type 2 diabetes. Clin Gastroenterol Hepatol. 2015;13:466–476. e461. doi: 10.1016/j.cgh.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi KM, Zhu J, Stoltz GJ, et al. Determination of gastric emptying in nonobese diabetic mice. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1039–1045. doi: 10.1152/ajpgi.00317.2007. [DOI] [PubMed] [Google Scholar]
- 23.Acosta A, Camilleri M, Shin A, et al. Quantitative gastrointestinal and psychological traits associated with obesity and response to weight-loss therapy. Gastroenterology. 2015;148:537–546. e534. doi: 10.1053/j.gastro.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chua SC, Jr, Chung WK, Wu-Peng XS, et al. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271:994–996. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- 25.Jung HK, Choung RS, Locke GR, 3rd, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225–1233. doi: 10.1053/j.gastro.2008.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Her YF, Nelson-Holte M, Maher LJ., 3rd Oxygen concentration controls epigenetic effects in models of familial paraganglioma. PLoS One. 2015;10:e0127471. doi: 10.1371/journal.pone.0127471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jat PS, Noble MD, Ataliotis P, et al. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci U S A. 1991;88:5096–5100. doi: 10.1073/pnas.88.12.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi KM, Gibbons SJ, Sha L, et al. Interleukin 10 Restores Gastric Emptying, Electrical Activity, and Interstitial Cells of Cajal Networks in Diabetic Mice. Cell Mol Gastroenterol Hepatol. 2016;2:454–467. doi: 10.1016/j.jcmgh.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rich A, Miller SM, Gibbons SJ, et al. Local presentation of Steel factor increases expression of c-kit immunoreactive interstitial cells of Cajal in culture. Am J Physiol Gastrointest Liver Physiol. 2003;284:G313–320. doi: 10.1152/ajpgi.00093.2002. [DOI] [PubMed] [Google Scholar]
- 30.Ordog T, Redelman D, Miller LJ, et al. Purification of interstitial cells of Cajal by fluorescence-activated cell sorting. Am J Physiol Cell Physiol. 2004;286:C448–456. doi: 10.1152/ajpcell.00273.2003. [DOI] [PubMed] [Google Scholar]
- 31.Dave M, Hayashi Y, Gajdos GB, et al. Stem cells for murine interstitial cells of Cajal suppress cellular immunity and colitis via prostaglandin E2 secretion. Gastroenterology. 2015;148:978–990. doi: 10.1053/j.gastro.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Worth AA, Forrest AS, Peri LE, et al. Regulation of gastric electrical and mechanical activity by cholinesterases in mice. J Neurogastroenterol Motil. 2015;21:200–216. doi: 10.5056/jnm14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.James AN, Ryan JP, Crowell MD, et al. Regional gastric contractility alterations in a diabetic gastroparesis mouse model: effects of cholinergic and serotoninergic stimulation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G612–619. doi: 10.1152/ajpgi.00431.2003. [DOI] [PubMed] [Google Scholar]
- 34.Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, et al. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1370–1381. doi: 10.1152/ajpgi.00074.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anitha M, Gondha C, Sutliff R, et al. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006;116:344–356. doi: 10.1172/JCI26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ward SM, Ordog T, Koh SD, et al. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000;525(Pt 2):355–361. doi: 10.1111/j.1469-7793.2000.t01-1-00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muhlenberg T, Grunewald S, Treckmann J, et al. Inhibition of KIT-glycosylation by 2-deoxyglucose abrogates KIT-signaling and combination with ABT-263 synergistically induces apoptosis in gastrointestinal stromal tumor. PLoS One. 2015;10:e0120531. doi: 10.1371/journal.pone.0120531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gottlieb E, Tomlinson IP. Mitochondrial tumour suppressors: a genetic and biochemical update. Nat Rev Cancer. 2005;5:857–866. doi: 10.1038/nrc1737. [DOI] [PubMed] [Google Scholar]
- 39.Bharucha AE, Manduca A, Lake DS, et al. Gastric motor disturbances in patients with idiopathic rapid gastric emptying. Neurogastroenterol Motil. 2011;23:617–e252. doi: 10.1111/j.1365-2982.2011.01710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ro S, Park C, Jin J, et al. A model to study the phenotypic changes of interstitial cells of Cajal in gastrointestinal diseases. Gastroenterology. 2010;138:1068–1078. e1061–1062. doi: 10.1053/j.gastro.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruiz E, Gordillo-Moscoso A, Padilla E, et al. Human vascular smooth muscle cells from diabetic patients are resistant to induced apoptosis due to high Bcl-2 expression. Diabetes. 2006;55:1243–1251. doi: 10.2337/db05-0949. [DOI] [PubMed] [Google Scholar]
- 42.Xiao M, Yang H, Xu W, et al. Inhibition of alpha-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 2012;26:1326–1338. doi: 10.1101/gad.191056.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.