Abstract
Radiofrequency ablation (RFA) has been widely used for the treatment of various solid organ malignancies. Over the last decade, endosonographers have gradually shifted the application of RFA from porcine models to humans to treat a spectrum of diseases. RFA is performed in patients with pancreatic carcinoma who are not candidates for surgery. In this paper, we will discuss various indications for RFA, its procedural details and complications. At present, endoscopic ultrasound-guided RFA is gradually incorporated into the management of various diseases and opens a new avenue for disease treatment.
Keywords: Pancreatic carcinoma, Radiofrequency ablation
Core tip: Endoscopic ultrasound-guided radiofrequency ablation (RFA) is a rapidly emerging modality, whose application has shifted from porcine models to humans over the last decade. In this review, we provide details on the indications, thermokinetic principles and complications related to RFA, which should be judiciously applied in the management of various diseases.
INTRODUCTION
Over the last two decades, palliation techniques for pancreatic adenocarcinoma have changed significantly. New developments in endoscopic ultrasound-guided therapies have also rapidly emerged[1]. Radiofrequency ablation (RFA) utilizes high frequency alternating current and can result in coagulative necrosis[2,3], and it can be applied percutaneously, intraoperatively or in combination with endoscopic ultrasound (EUS). This modality is gradually gaining popularity among endosonographers at tertiary centers. EUS-RFA is now an established anti-tumor therapy and an alternative to surgery[4].
Pancreatic adenocarcinoma is an aggressive tumor with a dismal survival rate due to delayed diagnosis. Only 10% of patients qualify for curative surgery[5]. The majority of patients have an unresectable locally advanced disease with encasement of vessels (superior mesenteric vessels, portal vein and/or hepatic artery)[6]. One-year survival rate in these patients is less than 5% after diagnosis[7].
EUS-guided RFA was first used in a porcine model by Goldberg et al[8] in 1999. EUS is used for various therapeutic procedures as it can be precisely applied in pancreatic lesions and helps delineate the area of interest for ablation[9-11].
EUS provides real-time imaging of deeply located anatomical structures such as the pancreas which is difficult to approach via the percutaneous route[12]. RFA has been widely utilized in the treatment of liver, lung and kidney tumors[13-15].
MECHANISM OF RFA
RFA is based on the principle that high frequency alternating current is converted into thermal energy which results in coagulative necrosis of surrounding tissue[16]. Thermal exposure above 45 °C results in denaturation of cell proteins and is utilized in the treatment of various tumors[17].
There are three important components in this procedure: the generator, the needle and the tissue.
The generator utilizes alternating current and converts it into thermal energy which is transferred through the exposed part of the needle[8,18].
RFA also causes thermal damage to the epithelium with a gradual rise in temperature, which results in destruction of cyst epithelium[19].
CLINICAL APPLICATIONS OF RFA
RFA is principally utilized in various benign and malignant conditions, including intraoperative applications. Studies have suggested that RFA leads to tumor necrosis and a reduction in tumor volume[20].
RFA can also be used in patients with malignant biliary obstruction for endobiliary ablation in the self-expandable metallic stent to improve stent patency[21].
A cryothermal probe (ERBE, Elektromedizin GmbH) has been used for palliation in locally advanced pancreatic carcinoma patients, with a technical success rate of 72.8% and median survival of 6 mo post ablation with manageable complications including jaundice, duodenal stricture and cystic fluid collection[20-22].
INDICATIONS
EUS-guided RFA is indicated in various diseases including: (1) pancreatic adenocarcinoma[23]; (2) patients after chemoradiotherapy; (3) patients with progressive tumor growth causing biliary or gastric outlet obstruction[24]; (4) liver metastasis[25]; (5) intraductal papillary mucinous neoplasms (IPMN)[26,27]; and (6) insulinoma[28,29].
PROCEDURE
A 19 G needle is usually used to puncture the pancreatic tissue under EUS guidance, the stylet is removed to introduce a thin wire which is connected to the generator, and then the tissue is ablated. This principle has been applied using a Habib EUS-RFA catheter (EMcision Ltd., London, United Kingdom) where a monopolar probe with a diameter of 1 Fr and length of 220 cm is utilized with a 2 cm active electrode tip to ablate the tissue[28,30,31]. It ablates for 2 min, which is considered one ablation with a break of 60 s for cooling. Up to 10 ablations can be applied to the tissue with interspersed cooling periods (Figures 1 and 2). In the case of a cyst, the lesion is aspirated prior to ablation. This technique should not be used in patients with cardiac pacemakers or other active implants.
Figure 1.
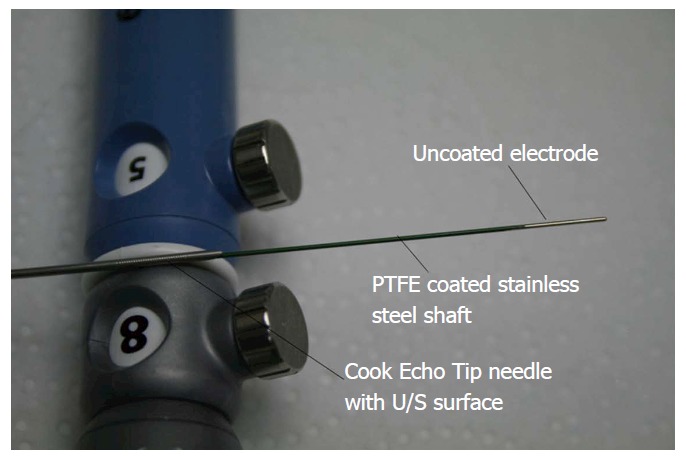
Habib RF needle with Cook Echo Tip needle. Courtesy of EMcision International Inc.
Figure 2.

Radiofrequency generator (RITA 1500 X, ANGIODYNAMICS).
Another novel 18 G RFA electrode (EUSRA RF Electrode; STARmed, Koyang, South Korea) with a total working length of 150 cm is also used. This electrode has the unique feature of two 0.8 mm diameter holes which are located 5 mm away from the tip, and can be used for aspiration and injection. The active electrode length is 7 mm while the tip exposure length is 10 mm. This RF electrode is attached to the RF generator (VIVA Combo system; STARmed) to ablate the tissue[32]. It results in the ablation of 1-3 cm of localized tissue from the needle tip[32-34].
A new flexible hybrid bipolar probe also known as the cryotherm probe (ERBE Elektromedizin, Tubingen, Germany) has recently been introduced, which combines cryotechnology with RFA[35]. This probe has an advantage over a monopolar probe in that it causes less collateral damage, but it is less efficient than a monopolar probe[36-38].
Cooling using a cryogenic gas increases the effect of RFA and interstitial devitalization[12]. It also proves that cooling does not affect the efficacy of ablation[39].
TIME AND TEMPERATURE UTILIZED IN RFA
RFA was successfully used in other organs such as the liver, intrahepatic tumors and muscle to achieve maximum coagulation within 6 min, prior to its application in the pancreas[40]. The Manchester group was among the first to validate and define the thermokinetic principles in the pancreas[41]. As the distance from the electrode increases, the temperature tends to decrease[26]. The optimal temperature for thermal ablation was demonstrated in a porcine model by Date[42] in 2005. It was concluded that optimal thermokinetics was generated at a temperature of 90 °C when applied for 5 min. This leads to ablation of pancreatic tissue without injury to adjacent organs.
A few other studies have also established the relationship between temperature and the rate of complications[43,44].
It was again established in a study by Girelli et al[45] that a decrease in temperature from 105 °C to 90 °C leads to an overall reduction in the complication rate from 24% to 8%.
Wu et al[36] showed that when a temperature of 30 °C was applied, this led to a high rate of postoperative morbidity, where complications included pancreatic fistula, portal thrombosis, septic shock and massive bleeding.
EUS-RFA OF THE PANCREAS IS DIFFERENT TO THAT OF OTHER ORGANS
EUS-RFA is better than planned palliative R2 resections in pancreatic carcinoma patients as it results in decreased morbidity, mortality and reduced hospital stay. There are certain important and significant differences in ablation of the pancreas compared with other organs: (1) the RFA protocol for other organs cannot be applied to the pancreas as the physical properties of the pancreas are entirely different from those of other organs; (2) the pancreas is surrounded by other organs (the stomach and duodenum), vessels and bile ducts and thus has an increased risk of thermal-induced injury; and (3) pancreatic cancer usually has diffuse margins, whereas hepatic carcinoma or metastasis has discrete margins; therefore, it is difficult to completely ablate pancreatic carcinoma in a single session[16].
EVALUATION OF EFFICACY OF RFA TREATMENT
Lesion size can be evaluated by imaging at repeated intervals. Tumor progression can be estimated by an improvement in symptoms (abdominal pain, back pain) or biochemical indices (CA19-9 levels)[46,47].
RATIO OF PASSES TO THE SIZE OF THE LESION
The ratio of the number of passes to the size of the lesion is extremely variable in different studies with a median value of 0.5 (range, 0.36-19). This can be explained by the application of different devices[41,46,48].
COMPLICATIONS OF RFA
The fear of adverse events related to EUS-RFA also limits its application by clinicians in pancreatic carcinoma patients.
Most complications are related to thermal injury to pancreatic parenchyma (acute pancreatitis) and surrounding structures including thermal damage to superior mesenteric vessels, bile ducts, the portal vein, stomach and duodenum[12,49-51]. Mild abdominal pain was reported by 25%-33% of patients in various studies[33]. Frequent complications were gastrointestinal hemorrhage, pancreatic fistula, bile leak, portal vein thrombosis, pseudocyst and sepsis. The overall postoperative morbidity rate was 28.3% and mortality was approximately 4%[52].
The pancreas is different to other organs such as the liver and kidney where RFA has been successfully utilized for the treatment of carcinomas. Optimal thermokinetic characteristics of the pancreas have not been completely determined, thus there is no standardized protocol for pancreatic RFA. Usually two or more sessions of RFA are required for pancreatic carcinoma ablation[12,32,33]. Retroperitoneal location, proximity to major vessels, distal bile duct crossing the head of the pancreas and closeness to the stomach and duodenum are also major hurdles[44].
CONCLUSION
Normal pancreatic tissue is thermosensitive, thus RFA can lead to an inflammatory response with fibrosis and occasionally cystic collections. A clearer understanding of the principles of thermokinetics in humans is required to effectively ablate abnormal tissues. Better ablation devices with minimal side effects and complications may ensure improved results in the future. Further studies with a large number of subjects will provide a better understanding of this novel technique.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: No potential conflicts of interest exist.
Peer-review started: February 9, 2017
First decision: April 10, 2017
Article in press: June 12, 2017
P- Reviewer: Araujo RLC, McKenna O, Seth D S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Zhang FF
References
- 1.Bhutani MS, Arora A. New developments in endoscopic ultrasound-guided therapies. Endosc Ultrasound. 2015;4:304–311. doi: 10.4103/2303-9027.170419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Figueroa-Barojas P, Bakhru MR, Habib NA, Ellen K, Millman J, Jamal-Kabani A, Gaidhane M, Kahaleh M. Safety and efficacy of radiofrequency ablation in the management of unresectable bile duct and pancreatic cancer: a novel palliation technique. J Oncol. 2013;2013:910897. doi: 10.1155/2013/910897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsui Y, Nakagawa A, Kamiyama Y, Yamamoto K, Kubo N, Nakase Y. Selective thermocoagulation of unresectable pancreatic cancers by using radiofrequency capacitive heating. Pancreas. 2000;20:14–20. doi: 10.1097/00006676-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Brugge WR. EUS-guided tumor ablation with heat, cold, microwave, or radiofrequency: will there be a winner? Gastrointest Endosc. 2009;69:S212–S216. doi: 10.1016/j.gie.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 5.Warshaw AL, Fernández-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455–465. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- 6.Verslype C, Van Cutsem E, Dicato M, Cascinu S, Cunningham D, Diaz-Rubio E, Glimelius B, Haller D, Haustermans K, Heinemann V, et al. The management of pancreatic cancer. Current expert opinion and recommendations derived from the 8th World Congress on Gastrointestinal Cancer, Barcelona, 2006. Ann Oncol. 2007;18 Suppl 7:vii1–vii10. doi: 10.1093/annonc/mdm210. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg SN, Mallery S, Gazelle GS, Brugge WR. EUS-guided radiofrequency ablation in the pancreas: results in a porcine model. Gastrointest Endosc. 1999;50:392–401. doi: 10.1053/ge.1999.v50.98847. [DOI] [PubMed] [Google Scholar]
- 9.Facciorusso A, Maso MD, Barone M, Muscatiello N. Echoendoscopic ethanol ablation of tumor combined to celiac plexus neurolysis improved pain control in a patient with pancreatic adenocarcinoma. Endosc Ultrasound. 2015;4:342–344. doi: 10.4103/2303-9027.170428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kongkam P, Benjasupattananun P, Taytawat P, Navicharoen P, Sriuranpong V, Vajragupta L, Klaikaew N, Ridtitid W, Treeprasertsuk S, Rerknimitr R, et al. Pancreatic cancer in an Asian population. Endosc Ultrasound. 2015;4:56–62. doi: 10.4103/2303-9027.151361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhutani MS. Role of endoscopic ultrasound for pancreatic cystic lesions: Past, present, and future! Endosc Ultrasound. 2015;4:273–275. doi: 10.4103/2303-9027.170400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrara S, Arcidiacono PG, Albarello L, Addis A, Enderle MD, Boemo C, Campagnol M, Ambrosi A, Doglioni C, Testoni PA. Endoscopic ultrasound-guided application of a new hybrid cryotherm probe in porcine pancreas: a preliminary study. Endoscopy. 2008;40:321–326. doi: 10.1055/s-2007-995595. [DOI] [PubMed] [Google Scholar]
- 13.Jansen MC, van Hillegersberg R, Chamuleau RA, van Delden OM, Gouma DJ, van Gulik TM. Outcome of regional and local ablative therapies for hepatocellular carcinoma: a collective review. Eur J Surg Oncol. 2005;31:331–347. doi: 10.1016/j.ejso.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Simon CJ, Dupuy DE. Current role of image-guided ablative therapies in lung cancer. Expert Rev Anticancer Ther. 2005;5:657–666. doi: 10.1586/14737140.5.4.657. [DOI] [PubMed] [Google Scholar]
- 15.Boss A, Clasen S, Kuczyk M, Anastasiadis A, Schmidt D, Graf H, Schick F, Claussen CD, Pereira PL. Magnetic resonance-guided percutaneous radiofrequency ablation of renal cell carcinomas: a pilot clinical study. Invest Radiol. 2005;40:583–590. doi: 10.1097/01.rli.0000174473.32130.28. [DOI] [PubMed] [Google Scholar]
- 16.Kim J. Endoscopic Ultrasound-Guided Treatment of Pancreatic Cystic and Solid Masses. Clin Endosc. 2015;48:308–311. doi: 10.5946/ce.2015.48.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armellini E, Crinò SF, Ballarè M, Occhipinti P. Endoscopic ultrasound-guided radiofrequency ablation of a pancreatic neuroendocrine tumor. Endoscopy. 2015;47 Suppl 1 UCTN:E600–E601. doi: 10.1055/s-0034-1393677. [DOI] [PubMed] [Google Scholar]
- 18.Cosman ER, Nashold BS, Ovelman-Levitt J. Theoretical aspects of radiofrequency lesions in the dorsal root entry zone. Neurosurgery. 1984;15:945–950. [PubMed] [Google Scholar]
- 19.Rhim H, Kim YS, Heo JN, Koh BH, Cho OK, Kim Y, Seo HS. Radiofrequency thermal ablation of hepatic cyst. J Vasc Interv Radiol. 2004;15:95–96. doi: 10.1097/01.rvi.0000106382.55825.32. [DOI] [PubMed] [Google Scholar]
- 20.Keane MG, Bramis K, Pereira SP, Fusai GK. Systematic review of novel ablative methods in locally advanced pancreatic cancer. World J Gastroenterol. 2014;20:2267–2278. doi: 10.3748/wjg.v20.i9.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steel AW, Postgate AJ, Khorsandi S, Nicholls J, Jiao L, Vlavianos P, Habib N, Westaby D. Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest Endosc. 2011;73:149–153. doi: 10.1016/j.gie.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 22.Arcidiacono PG, Carrara S, Reni M, Petrone MC, Cappio S, Balzano G, Boemo C, Cereda S, Nicoletti R, Enderle MD, et al. Feasibility and safety of EUS-guided cryothermal ablation in patients with locally advanced pancreatic cancer. Gastrointest Endosc. 2012;76:1142–1151. doi: 10.1016/j.gie.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Changela K, Patil R, Duddempudi S, Gaduputi V. Endoscopic Ultrasound-Guided Radiofrequency Ablation of the Pancreatic Tumors: A Promising Tool in Management of Pancreatic Tumors. Can J Gastroenterol Hepatol. 2016;2016:4189358. doi: 10.1155/2016/4189358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 25.Pandya GJ, Shelat VG. Radiofrequency ablation of pancreatic ductal adenocarcinoma: The past, the present and the future. World J Gastrointest Oncol. 2015;7:6–11. doi: 10.4251/wjgo.v7.i2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JS, Seo DW, Song TJ, Park do H, Lee SS, Lee SK, Kim MH. Endoscopic ultrasound-guided ablation of branch-duct intraductal papillary mucinous neoplasms: Feasibility and safety tests using porcine gallbladders. Dig Endosc. 2016;28:599–606. doi: 10.1111/den.12628. [DOI] [PubMed] [Google Scholar]
- 27.Arshad HM, Bharmal S, Duman DG, Liangpunsakul S, Turner BG. Advanced endoscopic ultrasound management techniques for preneoplastic pancreatic cystic lesions. J Investig Med. 2017;65:7–14. doi: 10.1136/jim-2016-000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waung JA, Todd JF, Keane MG, Pereira SP. Successful management of a sporadic pancreatic insulinoma by endoscopic ultrasound-guided radiofrequency ablation. Endoscopy. 2016;48 Suppl 1:E144–E145. doi: 10.1055/s-0042-104650. [DOI] [PubMed] [Google Scholar]
- 29.Lakhtakia S, Ramchandani M, Galasso D, Gupta R, Venugopal S, Kalpala R, Reddy DN. EUS-guided radiofrequency ablation for management of pancreatic insulinoma by using a novel needle electrode (with videos) Gastrointest Endosc. 2016;83:234–239. doi: 10.1016/j.gie.2015.08.085. [DOI] [PubMed] [Google Scholar]
- 30.Silviu UB, Daniel P, Claudiu M, Săndulescu L, Simona F, Ştefan P, Valeriu Ş, Adrian S. Endoscopic ultrasound-guided radiofrequency ablation of the pancreas: An experimental study with pathological correlation. Endosc Ultrasound. 2015;4:330–335. doi: 10.4103/2303-9027.170426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chapman CG, Siddiqui UD. New Scopes, New Accessories, New Stents for Interventional Endoscopic Ultrasound. Clin Endosc. 2016;49:41–46. doi: 10.5946/ce.2016.49.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim HJ, Seo DW, Hassanuddin A, Kim SH, Chae HJ, Jang JW, Park DH, Lee SS, Lee SK, Kim MH. EUS-guided radiofrequency ablation of the porcine pancreas. Gastrointest Endosc. 2012;76:1039–1043. doi: 10.1016/j.gie.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 33.Pai M, Habib N, Senturk H, Lakhtakia S, Reddy N, Cicinnati VR, Kaba I, Beckebaum S, Drymousis P, Kahaleh M, et al. Endoscopic ultrasound guided radiofrequency ablation, for pancreatic cystic neoplasms and neuroendocrine tumors. World J Gastrointest Surg. 2015;7:52–59. doi: 10.4240/wjgs.v7.i4.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song TJ, Seo DW, Lakhtakia S, Reddy N, Oh DW, Park DH, Lee SS, Lee SK, Kim MH. Initial experience of EUS-guided radiofrequency ablation of unresectable pancreatic cancer. Gastrointest Endosc. 2016;83:440–443. doi: 10.1016/j.gie.2015.08.048. [DOI] [PubMed] [Google Scholar]
- 35.Hines-Peralta A, Hollander CY, Solazzo S, Horkan C, Liu ZJ, Goldberg SN. Hybrid radiofrequency and cryoablation device: preliminary results in an animal model. J Vasc Interv Radiol. 2004;15:1111–1120. doi: 10.1097/01.RVI.0000136031.91939.EC. [DOI] [PubMed] [Google Scholar]
- 36.Wu Y, Tang Z, Fang H, Gao S, Chen J, Wang Y, Yan H. High operative risk of cool-tip radiofrequency ablation for unresectable pancreatic head cancer. J Surg Oncol. 2006;94:392–395. doi: 10.1002/jso.20580. [DOI] [PubMed] [Google Scholar]
- 37.Van Goethem BE, Rosenveldt KW, Kirpensteijn J. Monopolar versus bipolar electrocoagulation in canine laparoscopic ovariectomy: a nonrandomized, prospective, clinical trial. Vet Surg. 2003;32:464–470. doi: 10.1053/jvet.2003.50052. [DOI] [PubMed] [Google Scholar]
- 38.Lee JM, Han JK, Choi SH, Kim SH, Lee JY, Shin KS, Han CJ, Choi BI. Comparison of renal ablation with monopolar radiofrequency and hypertonic-saline-augmented bipolar radiofrequency: in vitro and in vivo experimental studies. AJR Am J Roentgenol. 2005;184:897–905. doi: 10.2214/ajr.184.3.01840897. [DOI] [PubMed] [Google Scholar]
- 39.Fegrachi S, Molenaar IQ, Klaessens JH, Besselink MG, Offerhaus JA, van Hillegersberg R. Radiofrequency ablation of the pancreas with and without intraluminal duodenal cooling in a porcine model. J Surg Res. 2013;184:867–872. doi: 10.1016/j.jss.2013.04.068. [DOI] [PubMed] [Google Scholar]
- 40.Goldberg SN, Gazelle GS, Dawson SL, Rittman WJ, Mueller PR, Rosenthal DI. Tissue ablation with radiofrequency: effect of probe size, gauge, duration, and temperature on lesion volume. Acad Radiol. 1995;2:399–404. doi: 10.1016/s1076-6332(05)80342-3. [DOI] [PubMed] [Google Scholar]
- 41.Date RS, Biggins J, Paterson I, Denton J, McMahon RF, Siriwardena AK. Development and validation of an experimental model for the assessment of radiofrequency ablation of pancreatic parenchyma. Pancreas. 2005;30:266–271. doi: 10.1097/01.mpa.0000153334.65729.a6. [DOI] [PubMed] [Google Scholar]
- 42.Date RS. Current status of local ablative techniques in the treatment of pancreatic cancer. Pancreas. 2006;33:198–199. doi: 10.1097/01.mpa.0000229006.39667.ea. [DOI] [PubMed] [Google Scholar]
- 43.Elias D, Baton O, Sideris L, Lasser P, Pocard M. Necrotizing pancreatitis after radiofrequency destruction of pancreatic tumours. Eur J Surg Oncol. 2004;30:85–87. doi: 10.1016/j.ejso.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 44.Siriwardena AK. Radiofrequency ablation for locally advanced cancer of the pancreas. JOP. 2006;7:1–4. [PubMed] [Google Scholar]
- 45.Girelli R, Frigerio I, Salvia R, Barbi E, Tinazzi Martini P, Bassi C. Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Br J Surg. 2010;97:220–225. doi: 10.1002/bjs.6800. [DOI] [PubMed] [Google Scholar]
- 46.Spiliotis JD, Datsis AC, Michalopoulos NV, Kekelos SP, Vaxevanidou A, Rogdakis AG, Christopoulou AN. Radiofrequency ablation combined with palliative surgery may prolong survival of patients with advanced cancer of the pancreas. Langenbecks Arch Surg. 2007;392:55–60. doi: 10.1007/s00423-006-0098-5. [DOI] [PubMed] [Google Scholar]
- 47.Cantore M, Girelli R, Mambrini A, Frigerio I, Boz G, Salvia R, Giardino A, Orlandi M, Auriemma A, Bassi C. Combined modality treatment for patients with locally advanced pancreatic adenocarcinoma. Br J Surg. 2012;99:1083–1088. doi: 10.1002/bjs.8789. [DOI] [PubMed] [Google Scholar]
- 48.Hadjicostas P, Malakounides N, Varianos C, Kitiris E, Lerni F, Symeonides P. Radiofrequency ablation in pancreatic cancer. HPB (Oxford) 2006;8:61–64. doi: 10.1080/13651820500466673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaidhane M, Smith I, Ellen K, Gatesman J, Habib N, Foley P, Moskaluk C, Kahaleh M. Endoscopic Ultrasound-Guided Radiofrequency Ablation (EUS-RFA) of the Pancreas in a Porcine Model. Gastroenterol Res Pract. 2012;2012:431451. doi: 10.1155/2012/431451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoon WJ, Brugge WR. Endoscopic ultrasonography-guided tumor ablation. Gastrointest Endosc Clin N Am. 2012;22:359–369, xi. doi: 10.1016/j.giec.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 51.Seo DW. EUS-Guided Antitumor Therapy for Pancreatic Tumors. Gut Liver. 2010;4 Suppl 1:S76–S81. doi: 10.5009/gnl.2010.4.S1.S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pezzilli R, Ricci C, Serra C, Casadei R, Monari F, D’Ambra M, Corinaldesi R, Minni F. The problems of radiofrequency ablation as an approach for advanced unresectable ductal pancreatic carcinoma. Cancers (Basel) 2010;2:1419–1431. doi: 10.3390/cancers2031419. [DOI] [PMC free article] [PubMed] [Google Scholar]


