Abstract
A great proportion of nitrate taken up by plants is stored in vacuoles. Vacuolar nitrate accumulation and release is of great importance to nitrate reallocation and efficient utilization. However, how plants mediate nitrate efflux from vacuoles to cytoplasm is largely unknown. The current study identified NPF5.11, NPF5.12 and NPF5.16 as vacuolar nitrate efflux transporters in Arabidopsis. Histochemical analysis showed that NPF5.11, NPF5.12 and NPF5.16 were expressed preferentially in root pericycle cells and xylem parenchyma cells, and further analysis showed that these proteins were tonoplast-localized. Functional characterization using cRNA-injected Xenopus laevis oocytes showed that NPF5.11, NPF5.12 and NPF5.16 were low-affinity, pH-dependent nitrate uptake transporters. In npf5.11 npf5.12 npf5.16 triple mutant lines, more root-fed 15NO3 − was translocated to shoots compared to the wild type control. In the NPF5.12 overexpression lines, proportionally less nitrate was maintained in roots. These data together suggested that NPF5.11, NPF5.12 and NPF5.16 might function to uptake nitrate from vacuoles into cytosol, thus serving as important players to modulate nitrate allocation between roots and shoots.
Introduction
Nitrate is the major nitrogen source for most plants, especially those grown in aerobic soil conditions1. Once taken up from soil, nitrate is either assimilated or stored in vacuoles. As the largest organelle in fully expanded plant cells, vacuoles are identified as the major nitrate storage pools and contain up to 90% of the total cellular nitrate2, 3. However, vacuolar nitrate is not readily accessible to NR (nitrate reductase), thus it has to be reallocated for metabolic use when necessary4, 5. Vacuolar nitrate release helps to maintain the relative steady level of cytosolic nitrate when external nitrogen supply was limited6, 7. During the dark-to-light transition, nitrate remobilization from vacuoles was also observed to comply with the new steady state caused by the increased NR activity4. Therefore, vacuolar nitrate and its remobilization are important for the regulation of nitrogen assimilation and nitrogen use efficiency5, 8, 9.
Transport across the tonoplast is energized by the vacuolar H+-ATPase (V-ATPase) and the vacuolar H+-pyrophosphatase (V-PPase), which create the proton gradient and the membrane potential10–12. For nitrate, its accumulation in vacuoles is probably mediated by the nitrate/proton antiport machinery13–16 and the nitrate/proton symport system may serve to remobilize vacuolar nitrate16–18. However, only a few tonoplast localized nitrate transporters have been identified up to date. AtCLCa and AtNRT2.7 are two transporters responsible for nitrate accumulation in vacuoles19–21. AtCLCa was a tonoplast localized 2NO3 −/1 H+ antiporter expressed in both shoots and roots20, 22. Disruption of AtCLCa led to approximately 50% decrease of vacuolar nitrate, suggesting an important role for AtCLCa in vacuolar nitrate accumulation19, 20. AtNRT2.7, however, was a tonoplast localized transporter expressed exclusively in seeds, which regulated the kinetics of seed germination by affecting nitrate storage in seed vacuoles21. AtCLCc was also supposed to be involved in vacuolar nitrate accumulation, because it was tonoplast localized and the related mutants showed lower nitrate contents22, 23. Regarding nitrate efflux from vacuoles, however, indirect evidences imply that AtCLCb and OsNPF7.2 might get involved, as they both were tonoplast-localized, and heterologous expression in Xenopus laevis oocytes indicated that they mediated nitrate uptake, but no evidence showed that functional disruption of these genes led to nitrate accumulation in vacuoles24, 25. AtCLCa was also implied to get involved in vacuolar nitrate efflux, because it mediated anion homeostasis in stomata movement26, while nitrate is one of the anions contributing to stomatal movement27, 28.
In the current study, three tonoplast-localized NRT1/NPF family members NPF5.11, NPF5.12 and NPF5.16 were identified by bioinformatics analysis, and functional characterization was performed. Our data suggested that these three transporters were all tonoplast localized, and mediated nitrate uptake in a pH-dependent low-affinity manner when heterologously expressed in oocytes. Further analysis indicated that they possibly modulated nitrate allocation between roots and shoots via vacuolar nitrate release.
Results
Tonoplast Localization of NPF5.11, NPF5.12 and NPF5.16
Based on previous studies about vacuole proteome29, we targeted NPF5.12 and its close homologs NPF5.11 and NPF5.16, members of NRT1/NPF family30, as candidate transporters for vacuolar nitrate transport. NPF5.11, NPF5.12 and NPF5.16 were predicted to contain 11, 12, 10 transmembrane domains (http://www.cbs.dtu.dk/services/TMHMM/), respectively. To investigate the subcellular localization of NPF5.11, NPF5.12 and NPF5.16, NPF5.11-EYFP, NPF5.12-EYFP and NPF5.16-EYFP driven by the cauliflower mosaic virus 35 S promoter were transiently expressed in Arabidopsis mesophyll protoplast. The yellow fluorescence signals of NPF5.11-EYFP (Fig. 1a–c), NPF5.12-EYFP (Fig. 1d–f) and NPF5.16-EYFP (Fig. 1g–i) were detected in the membrane around the large central vacuole. Similar results were obtained by transiently expressing these fusion proteins in onion epidermal cells, verifying that NPF5.11, NPF5.12 and NPF5.16 were tonoplast localized (Supplementary Fig. S1).
Figure 1.
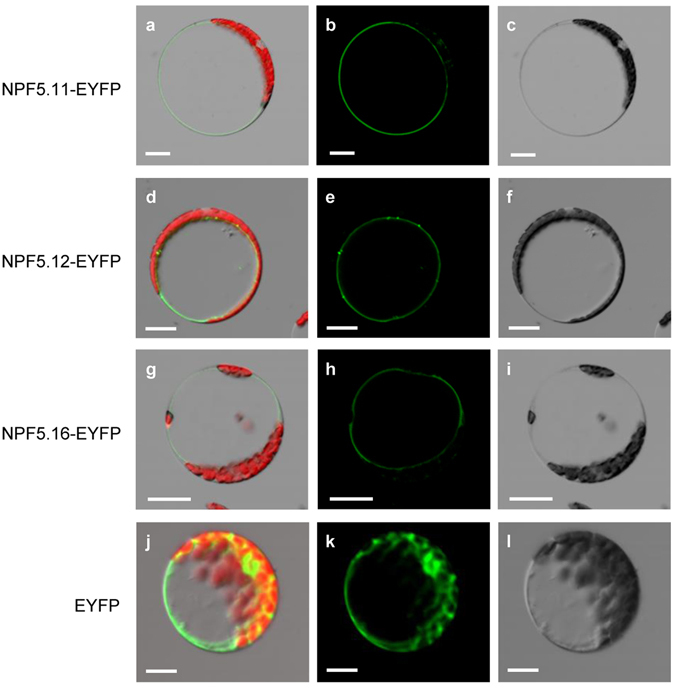
Subcellular localization of NPF5.11, NPF5.12 and NPF5.16. NPF5.11-EYFP (a–c), NPF5.12-EYFP (d–f), NPF5.16-EYFP (g–i) or EYFP (j–l) was driven by the cauliflower mosaic virus 35 S promoter and transiently expressed in Arabidopsis mesophyll protoplasts. Overlap images of EYFP (green) and chlorophyll (red) fluorescence (a,d,g,j), EYFP fluorescence (b,e,h,k), and bright-field (c,f,i,l) images are shown. Bars = 20 μm.
NPF5.11, NPF5.12 and NPF5.16 are pH-dependent Low-Affinity Nitrate Transporters
Given that Xenopus laevis oocytes did not contain vacuoles, we firstly tested the expression and localization of NPF5.11, NPF5.12 and NPF5.16 in oocytes. As a well-documented nitrate transporter31, NRT1.8 fused with GFP was used as a positive control and its fluorescence was detected at the rim of oocytes (Supplementary Fig. S2). Likewise, NPF5.11-EYFP, NPF5.12-EYFP and NPF5.16-EYFP fusion proteins could express in plasma membrane of oocytes though they were tonoplast localized transporters in Arabidopsis, indicating that we could use oocytes to explore the function of NPF5.11, NPF5.12 and NPF5.16 (Supplementary Fig. S2).
Electrophysiological analysis using cRNA-injected oocytes were performed to test whether NPF5.11, NPF5.12 and NPF5.16 could use nitrate as substrate. After 2 days of incubation, oocytes were voltage clamped at −60 mV and perfused with 10 mM nitrate at pH 5.5. Compared with water-injected oocytes (Fig. 2a), a larger inward current was induced by CHL1-injected oocytes (Fig. 2b), as reported before32. NPF5.11-, NPF5.12- or NPF5.16-injected oocytes also induced inward currents (Fig. 2c–e), indicating that they were electrogenic transporters using nitrate as the substrate.
Figure 2.
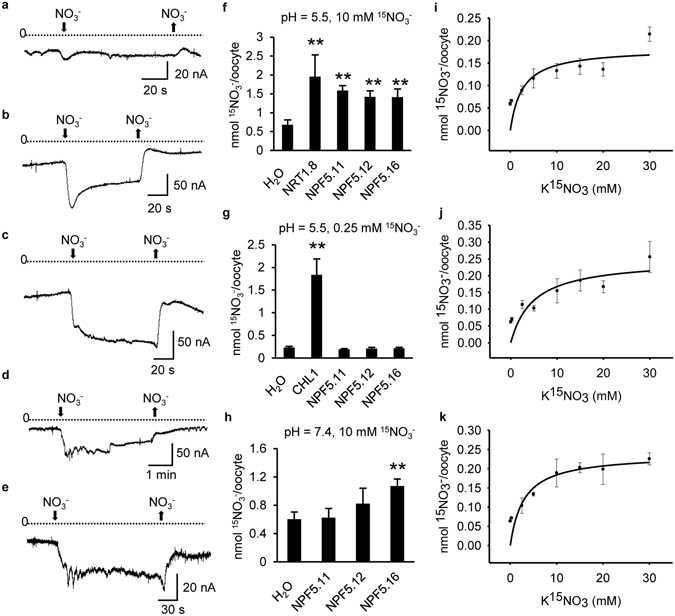
Functional characterization of NPF5.11, NPF5.12 and NPF5.16 in oocytes. (a–e) Currents elicited in oocytes injected with H2O (a), CHL1 cRNA (b), NPF5.11 cRNA (c), NPF5.12 cRNA (d) or NPF5.16 cRNA (e). Oocytes were voltage clamped at −60 mV and representative inward currents elicited by 10 mM NO3 − at pH 5.5 were recorded. (f–h) Nitrate uptake activity in oocytes injected with H2O, NPF5.11 cRNA, NPF5.12 cRNA, NPF5.16 cRNA, NRT1.8 cRNA or CHL1 cRNA. Oocytes were incubated with 10 mM 15NO3 − at pH 5.5 (f), 0.25 mM 15NO3 − at pH 5.5 (g) or 10 mM 15NO3 − at pH 7.4 (h) for 12 h. Values are means ± SD (n = 8–12). Asterisks indicate difference at P < 0.01 (**) compared with the H2O-injected oocytes by Student’s t-test. (i–k) Uptake kinetics of NPF5.11 (i), NPF5.12 (j) and NPF5.16 (k). Oocytes injected with NPF5.11 cRNA (i), NPF5.12 cRNA (j) or NPF5.16 cRNA (k) were incubated with indicated concentrations of 15NO3 − at pH 5.5 for 1.5 h, and the 15N contents were determined. Values are means ± SD (n = 6–12). The Km was calculated by fitting to the Michaelis-Menten equation using a nonlinear least squares method in the SigmaPlot program. The Km was 2.57 mM, 4.84 mM, or 2.91 mM for NPF5.11, NPF5.12 or NPF5.16, respectively.
Nitrate transport activities of NPF5.11, NPF5.12 and NPF5.16 were further confirmed by analyzing 15NO3 − uptake activity. NPF5.11-, NPF5.12-, NPF5.16- or NRT1.8-injected oocytes showed enhanced 15NO3 − uptake activity when incubated with 10 mM 15NO3 − at pH 5.5, compared with water-injected oocytes (Fig. 2f). However, NPF5.11-, NPF5.12- or NPF5.16-injected oocytes almost did not uptake 15NO3 − when assayed with 0.25 mM 15NO3 − at pH 5.5, while CHL1-injected oocytes still showed high uptake activity (Fig. 2g). In addition, as expected for proton-coupled transporters, 15NO3 − uptake activities of NPF5.11-, NPF5.12- or NPF5.16-injected oocytes at pH 7.4 were much lower than those at pH 5.5, comparable with the negative control (Fig. 2h). It is worth mentioning that NPF5.11, NPF5.12 and NPF5.16 did not efflux nitrate from oocytes under pH 5.5 or pH 7.4 (Supplementary Fig. S3).
To further determine the uptake affinity of NPF5.11, NPF5.12 and NPF5.16, uptake activity of NPF5.11-, NPF5.12- or NPF5.16-injected oocytes at pH 5.5 was measured using different concentrations of 15NO3 − ranging from 0.25 mM to 30 mM as substrates. The K m for nitrate was calculated by fitting to the Michaelis-Menten equation, and was estimated as 2.57 mM, 4.84 mM, 2.91 mM respectively for NPF5.11, NPF5.12 and NPF5.16 (Fig. 2i–k). Taken together, these results suggested that NPF5.11, NPF5.12 and NPF5.16 were pH-dependent low-affinity nitrate transporters.
NPF5.11, NPF5.12 and NPF5.16 are mainly expressed in vascular stele of roots and leaves
The tissue localization of genes could provide hint for their physiological role. To elucidate the expression pattern of NPF5.11, NPF5.12 and NPF5.16, promoter-GUS (β-glucuronidase) reporter analysis was performed. The promoter region of NPF5.11, NPF5.12 and NPF5.16 were used for driving the expression of GUS in Columbia (Clo-0). As shown in Fig. 3, NPF5.11, NPF5.12 and NPF5.16 had a similar expression pattern, expressing in both shoots and roots. In shoots, they were mainly expressed in leaf veins while the mesophyll cells were also stained (Fig. 3a,e,i). In roots, GUS activity was detected in root vascular stele (Fig. 3b,f,j). Cross-sections of young seedling roots showed that NPF5.11 pro::GUS, NPF5.12 pro::GUS and NPF5.16 pro::GUS were expressed in pericycle cells and parenchyma cells, and NPF5.11 pro::GUS was also expressed in the phloem (Fig. 3c,d,g,h,k,l).
Figure 3.

NPF5.11, NPF5.12 and NPF5.16 are preferentially expressed in vascular tissues. Histochemical localization of GUS activity in NPF5.11 pro::GUS transgenic plants (a–d), NPF5.12 pro::GUS transgenic plants (e–h) and NPF5.16 pro::GUS transgenic plants (i–l). The expression patterns of NPF5.11, NPF5.12 and NPF5.16 were determined in whole-mount seedlings (a,e,i), seedling roots (b,f,j) or cross-sectioned seedling roots (c,d,g,h,k,l). (m,n,o) Transcript expression of NPF5.11 (m), NPF5.12 (n) and NPF5.16 (o) in 28 d old plants. 1–8 indicated leaf positions arranged according to leaf ages (old to young); R, root; F, flower; S, stem. Data were normalized to that of SAND. Values are means ± SD, n = 3. Bars = 10 μm.
The expression patterns of these three genes in adult plant were further investigated by qRT-PCR analysis. NPF5.11, NPF5.12 and NPF5.16 all showed high expression in root while the expression in flower and stem was quite low (Fig. 3m,n,o). The expression of NPF5.12 in old leaves was higher than that in young leaves, while NPF5.16 was preferentially expressed in young leaves (Fig. 3n,o).
More nitrate is translocated to shoots in triple mutant
Considering the tonoplast localization (Fig. 1) and pH-dependent nitrate uptake (Fig. 2), we proposed that NPF5.11, NPF5.12 and NPF5.16 might be responsible for uptaking nitrate from vacuole (pH 5.533) to cytoplasm in Arabidopsis. To test this hypothesis, we generated several lines of their single, double, and even triple mutants (Supplementary Figs S4,S6,S7). Note that only double mutant lines of npf5.12 npf5.16 were generated because NPF5.11 and NPF5.12 were tightly linked in Arabidopsis genome and npf5.11 mutant lines were in Ws background. Given vacuolar nitrate efflux is supposed to be enhanced when nitrogen is limited, we firstly analyzed the nitrate contents in leaves and roots in these mutants under both control condition and nitrogen-starved condition. As shown in Supplementary Figs S5, S6 and S7, no obvious difference was observed between the wild type control and all the mutants. However, when they were fed with 15NO3 −, the ratio of 15N concentration in shoots against that in roots (shoot/root) was higher in triple mutant lines than in the wild type (Fig. 4a, Supplementary Fig. S8), while no significant difference was observed between the single mutant lines and the wild type (Supplementary Fig. S9). These results suggested that more 15NO3 − was translocated to shoots in triple mutants, while our data also indicated that the root uptake capacity of triple mutant lines was not affected (Fig. 4b).
Figure 4.
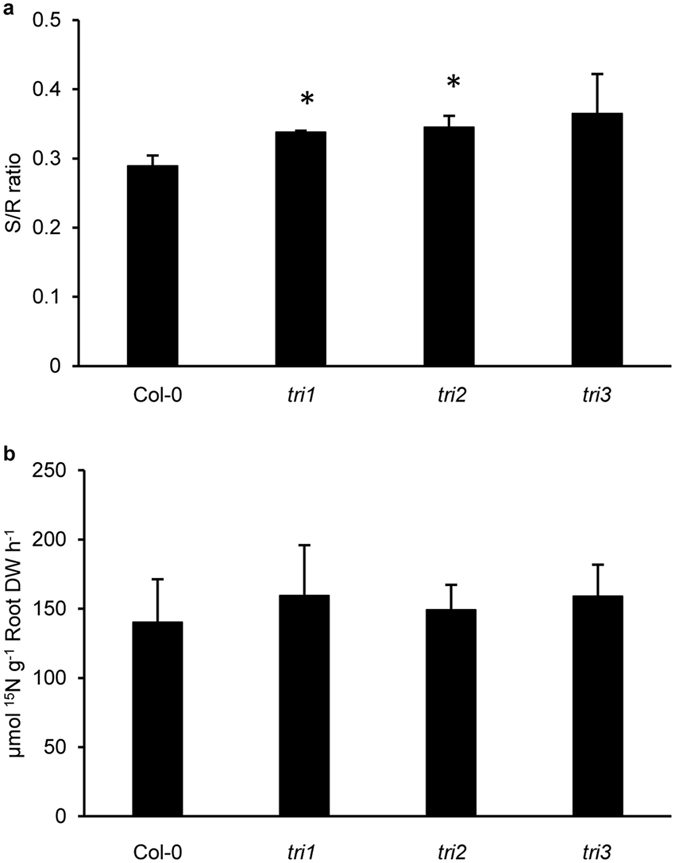
Root-to-shoot nitrate transport enhanced in the triple mutant plants npf5.11 npf5.12 npf5.16. Plants were grown in hydroponics for 28 days and treated with 2.25 mM K15NO3 for 30 min. 15N contents in shoots and roots were analyzed. 15N concentration ratio between shoots and roots (S/R ratio, a) and root uptake activity (b) were determined. Values are means ± SD, n = 3. Asterisks indicate difference between wild type and triple mutant lines at P < 0.05 (*) by Student’s t-test.
Root nitrate content is reduced in NPF5.12 overexpression lines
To further investigate the function of NPF5.11, NPF5.12 and NPF5.16, the overexpression lines of NPF5.11, NPF5.12 and NPF5.16 under the control of 35 S promoter were generated (Fig. 5a,b) and the nitrate content was analyzed (Fig. 5c, Supplementary Figs S10, S11). The result showed that nitrate contents in roots of NPF5.12 overexpression lines were lower than that of wild type under nitrogen-starved condition (Fig. 5c). This observation was not found in NPF5.11 and NPF5.16 overexpression lines (Supplementary Fig. S11). One explanation could be that NPF5.11 and NPF5.16 might require other components to function properly in planta.
Figure 5.
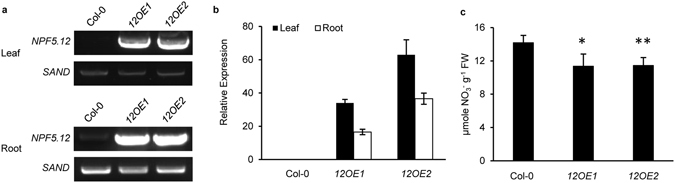
Decreased nitrate accumulation in roots of NPF5.12 overexpression lines. (a,b) Identification of NPF5.12 overexpression lines by RT-PCR (a) and quantitative PCR analysis (b). (c) 24 days old hydroponically grown plants were subjected to nitrogen-starvation for 30 h, then roots were sampled and nitrate contents were determined by HPLC. 12OE1 and 12OE2 were two independent NPF5.12 overexpression lines. Values are means ± SD, n = 5–7. Asterisks indicate difference between wild type and overexpression lines at P < 0.05 (*) and P < 0.01 (**) by Student’s t-test.
Discussion
Significant progresses have been made in clarifying the nitrate uptake and transport in Arabidopsis by the characterization of the transporters in NRT1/NPF, NRT2, CLC and SLAC/SLAH families34. However, our current knowledge about nitrate transport across the tonoplast is quite limited though the significance of this process is widely recognized.
The transporters responsible for vacuolar nitrate efflux should be tonoplast-localized and uptake nitrate toward cytoplasm. Our data suggested that NPF5.11, NPF5.12 and NPF5.16 were localized in tonoplast in Arabidopsis (Fig. 1) and plasma membrane in Xenopus laevis oocytes (Supplementary Fig. S2), and in oocytes they could elicit inward currents by external nitrate and uptake nitrate in a pH dependent way (Fig. 2). When these results were assigned to the topology of the plant tonoplast, the inward currents might represent NO3 −/H+ efflux from the vacuole to the cytoplasm24, because the external medium for oocytes corresponds to the vacuole in planta35, 36 and the pH of vacuole in Arabidopsis is about 5.533, 37. Thus we speculated that NPF5.11, NPF5.12 and NPF5.16 were responsible for vacuolar nitrate release in Arabidopsis.
Considering that NPF5.11, NPF5.12 and NPF5.16 are predominantly expressed in vacuole membrane of pericycle cells and xylem parenchyma cells in roots (Figs 1, 3). We proposed that they might be involved in the regulation of nitrate long-distance transport by modulating the vacuolar sequestration capacity (VSC) of nitrate in roots. Relationship between VSC and long-distance transport of metals in plant have been well documented38, and accumulating evidences indicated that VSC of essential anions including sulfate and nitrate also regulated their long-distance transport39, 40. The vacuolar sulfate efflux transporters SULTR4;1 and SULTR4;2 played an essential role in delivering sulfate to the xylem vessels by balancing storage and turnover of sulfate in the root vacuoles39. While Han et al., found that the decreased VSC of nitrate in roots would enhance nitrate transport to shoots and contribute to a higher nitrogen use efficiency (NUE)40. Our hypothesis about the physiological role of NPF5.11, NPF5.12 and NPF5.16 was supported by the observation that more proportion of 15NO3 − was translocated to shoots in triple mutant lines (Fig. 4) and overexpression of NPF5.12 resulted in a lower nitrate contents in roots (Fig. 5c). In the triple mutant lines, root VSC increased but not too much was available to newly absorbed nitrate due to the impaired nitrate efflux from vacuoles, resulting in less vacuolar nitrate sequestration and the consequent enhancement of 15NO3 − long-distance transport to shoots when fed with 15NO3 − for a short time (30 min). In NPF5.12 overexpression lines, the overall nitrate contents in roots decreased because of the lower VSC of nitrate, thus leading to the higher S/R ratios in the overexpression lines (Fig. 5c, Supplementary Fig. S10b).
No significant difference of nitrate contents was detected between all the mutants and wild type under various growth conditions we tested (Supplementary Figs S5, S6, S7). We proposed that there might be other transporters or channels that function redundantly with NPF5.11, NPF5.12 and NPF5.16, as the reutilization of vacuolar nitrate is crucial to environmental adaption for plants. The speculation is according with the observation that more 15NO3 − was translocated to shoots in triple mutant but not in single mutant. Similarly, no obvious changes in nitrate allocation was observed in mutants of AtCLCb or OsNPF7.2 24, 25. In addition, considering their specific tissue localization in roots (Fig. 3), we speculated that physiological effect of NPF5.11, NPF5.12 and NPF5.16 in nitrate allocation might be more noticeable specifically in pericycle cells and parenchyma cells. Thus more definitive evidences are needed in the future to demonstrate the working model for vacuolar nitrate efflux.
Methods
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Col-0 or Ws was used as the wild-type control. The Arabidopsis T-DNA mutant lines npf5.11-1 (FLAG_493A07) and npf5.11-2 (FLAG_592C02) were ordered from INRA (National Institute for Agricultural Research)41; npf5.12-1 (GABI_810C10) was ordered from NASC (European Arabidopsis Stock Centre)42; npf5.12-2 (CS871745), npf5.16-1 (SALK_152449 C) and npf5.16-2 (SALK_200474 C) were ordered from ABRC (Arabidopsis Biological Resource Center)43, 44. Homozygous mutant plants were screened by PCR45. The npf5.12 npf5.16 double mutant lines of were generated by crossing npf5.12 and npf5.16 and identified by PCR. The double mutant npf5.12 npf5.16 was further transformed with CRISPR/Cas9 system to generate the triple mutant npf5.11 npf5.12 npf5.16, using two different target sequences46. The double mutant lines used for transformation were: tri1, npf5.12-1 npf5.16-2; tri2, npf5.12-1 npf5.16-1; tri3, npf5.12-2 npf5.16-1. The CRISPR-Cas9 T-DNA was not existent in triple mutants by Cas9 PCR confirming47. The primers used in these assays are listed in Supplemental Table S1.
Arabidopsis plants were grown in quarter-strength hydroponic solution at 22 °C with 16-h-light/8-h-dark cycles as described48. Plants were grown to 3-4 weeks old and then were treated with nitrogen-starved nutrient solution by replacing KNO3 and Ca(NO3)2 with KCl and CaCl2 as indicated time.
Functional Analysis of NPF5.11, NPF5.12 and NPF5.16 in Xenopus laevis Oocytes
cDNA fragments of targeted genes were recovered by restriction digestion and then subcloned into the oocyte expression vector pOO249. cRNA was synthesized using the Ambion mMessage mMachine kit according to the manufacturer’s manual. Oocytes were isolated and injected with 50 ng cRNA as described previously50. CHL1 cRNA or NRT1.8 cRNA injected oocytes were used as positive control and water-injected oocytes were used as negative control. Oocytes were incubated in a ND-96 Ringer solution for 2 days as described31. Voltage clamp recordings were initiated in a bath solution containing 230 mM mannitol, 0.15 mM CaCl2, and 10 mM MES/Tris, pH 5.551. Nitrate uptake or efflux assays with 15NO3 − were performed as described50, 52, 53 using a continuous-flow isotope ratio mass spectrometer coupled to a carbon nitrogen elemental analyzer (Vario EL III/Isoprime; Elementar). Uptake kinetics assays were performed as described54.
EYFP Fusion and Subcellular Localization
The amplified NPF5.11, NPF5.12 and NPF5.16 cDNA fragments were cloned in frame in front of EYFP in the vector 35 S::EYFP/PA7, resulting in the NPF5.11-EYFP, NPF5.12-EYFP and NPF5.16-EYFP constructs under the control of the 35 S promoter. The resulted constructs were transiently expressed in Arabidopsis protoplast using the polyethylene glycol-mediated transformation method55. Alternatively, these constructs were also transiently expressed in onion epidermal cells using a particle gun–mediated system (PDS-1000/He; Bio-Rad). The transformed protoplasts and bombarded cells were held in the dark at 22 °C for more than 30 h followed by EYFP imaging using confocal microscopy (Olympus-FV1000).
For the GFP- or EYFP- fusion proteins expression in oocytes assay, the constructs were generated by introducing NRT1.8-GFP, NPF5.11-EYFP, NPF5.12-EYFP or NPF5.16-EYFP into the vector pOO2. The cRNA was synthesized and injected into oocytes. After cultivating 2 days, fluorescence was observed using confocal microscope (Olympus-FV1000).
Histochemical Analysis and Tissue Sectioning
A 1679-bp, a 1314-bp or a 1695-bp genomic fragment immediately upstream from the NPF5.11, NPF5.12 or NPF5.16 start codon, respectively, was amplified using primers listed in Supplemental Table S1. After sequencing, the fragments were cloned into the binary vector GUS /pCambias1300 and were then transformed into Col-0 as described31. GUS staining was performed overnight as described31. Semithin sections (4μm) were cut, mounted on glass slides, and visualized on Leica-DM6000.
RT-PCR and Quantitative RT-PCR
Plants were grown to 28 days old in hydroponics, and then were sampled as indicated. Total RNA was extracted using TRIzol reagent (Invitrogen). First-strand cDNA synthesis and RT-PCR were performed as described31. Quantitative RT-PCR was performed on a Corbett Research Rotor-Gene 3000 thermal cycler using SYBR Premix Ex-Taq (TaKaRa) according to the manufacturer’s protocol. The primers used in these assays are listed in Supplemental Table S1, and the expression levels were normalized to those of the SAND or Actin2 control.
Nitrate Content Determination by HPLC
Plants were grown to 3–4 weeks old and were treated with nitrogen-starved nutrient solution for indicated time. Leaves and roots were harvested and washed at least three times by ultrapure water for 5 min and then extracted nitrate as described56.
Analysis of Root-to-Shoot Nitrate Transport Using 15NO3−
Wild type and triple mutant plants were grown in hydroponics for 28 days old and then were transferred to 0.1 mM CaSO4 for 1 min, labeled in quarter-strength hydroponics medium with 2.25 mM K15NO3 with 99% atom excess of 15N for 30 min. At the end of labeling, plants were washed in 0.1 mM CaSO4 for 1 min and the shoots and roots were separated. The shoots and roots were sampled and detected as described57.
Statistical Analysis
Two-tailed Student’s t tests were performed. Differences were deemed significant (*) at P < 0.05 and extremely significant (**) at P < 0.01.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
We thank Dr. Yi-Fang Tsay (Institute of Molecular Biology, Academia Sinica) for helping with electrophysiological analysis; Li Zhang (Instrumental Analysis Center, Shanghai Jiao Tong University) for helping with 15N analysis; and Xiao-Shu Gao (Core Facility, Shanghai Institute of Plant Physiology and Ecology, Shanghai Institutes for Biological Sciences) for helping with confocal microscopy. This work was supported by the National Science Foundation of China (Grants 31421093, 31325003) and the XDPB0402 of Chinese Academy of Sciences.
Author Contributions
J.G. and Y.H. designed the research. Y.H., Y.C., D.L., Y.G. and H.Y. performed the experiments. J.G., Y.H. and J.P. analyzed the data. J.G., Y.H. and J.P. wrote the paper.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-06744-5
Accession Codes: Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: NPF5.11 (At1g72130), NPF5.12 (At1g72140), NPF5.16 (At1g22550), NRT1.8 (At4g21680), CHL1 (At1g12110), SAND (At2g28390), Actin2 (At3g18780).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Crawford NM. Nitrate: nutrient and signal for plant growth. Plant Cell. 1995;7:859–868. doi: 10.1105/tpc.7.7.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Granstedt RC, Huffaker RC. Identification of the leaf vacuole as a major nitrate storage pool. Plant Physiol. 1982;70:410–413. doi: 10.1104/pp.70.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinoia E, Heck U, Wiemken A. Vacuoles as storage compartments for nitrate in barley leaves. Nature. 1981;289:292–294. doi: 10.1038/289292a0. [DOI] [Google Scholar]
- 4.Cookson SJ, Williams LE, Miller AJ. Light-dark changes in cytosolic nitrate pools depend on nitrate reductase activity in Arabidopsis leaf cells. Plant Physiol. 2005;138:1097–1105. doi: 10.1104/pp.105.062349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lea US, et al. Mutation of the regulatory phosphorylation site of tobacco nitrate reductase results in high nitrite excretion and NO emission from leaf and root tissue. Planta. 2004;219:59–65. doi: 10.1007/s00425-004-1209-6. [DOI] [PubMed] [Google Scholar]
- 6.Izmailov SF. Saturation and utilization of nitrate pools in pea and sugar beet leaves. Russ J Plant Physiol. 2004;51:189–193. doi: 10.1023/B:RUPP.0000019212.20774.c7. [DOI] [Google Scholar]
- 7.van der Leij M, Smith SJ, Miller AJ. Remobilisation of vacuolar stored nitrate in barley root cells. Planta. 1998;205:64–72. doi: 10.1007/s004250050297. [DOI] [Google Scholar]
- 8.Martinoia E, Maeshima M, Neuhaus HE. Vacuolar transporters and their essential role in plant metabolism. J Exp Bot. 2007;58:83–102. doi: 10.1093/jxb/erl183. [DOI] [PubMed] [Google Scholar]
- 9.Krebs M, et al. Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation. Proc Natl Acad Sci USA. 2010;107:3251–3256. doi: 10.1073/pnas.0913035107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeshima M. Tonoplast transporters: organization and function. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:469–497. doi: 10.1146/annurev.arplant.52.1.469. [DOI] [PubMed] [Google Scholar]
- 11.Gaxiola RA, Palmgren MG, Schumacher K. Plant proton pumps. FEBS Lett. 2007;581:2204–2214. doi: 10.1016/j.febslet.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 12.Schumacher K, Krebs M. The V-ATPase: small cargo, large effects. Curr Opin Plant Biol. 2010;13:724–730. doi: 10.1016/j.pbi.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Miller AJ, Smith SJ. Nitrate transport and compartmentation in cereal root cells. J Exp Bot. 1996;47:843–854. doi: 10.1093/jxb/47.7.843. [DOI] [Google Scholar]
- 14.Kabała K, Kłobus G, Janicka-Russak M. Nitrate transport across the tonoplast of Cucumis sativus L. root cells. J Plant Physiol. 2003;160:523–530. doi: 10.1078/0176-1617-00787. [DOI] [PubMed] [Google Scholar]
- 15.Miller AJ, Smith SJ. The mechanism of nitrate transport across the tonoplast of barley root cells. Planta. 1992;187:554–557. doi: 10.1007/BF00199977. [DOI] [PubMed] [Google Scholar]
- 16.Schumaker KS, Sze H. Decrease of pH gradients in tonoplast vesicles by NO(3) and Cl: evidence for H-coupled anion transport. Plant Physiol. 1987;83:490–496. doi: 10.1104/pp.83.3.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sze H. H+-translocating ATPases: advances using membrane vesicles. Plant Biology. 1985;36:175–208. [Google Scholar]
- 18.Blumwald E, Poole RJ. Nitrate storage and retrieval in Beta vulgaris: Effects of nitrate and chloride on proton gradients in tonoplast vesicles. Proc Natl Acad Sci USA. 1985;82:3683–3687. doi: 10.1073/pnas.82.11.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geelen D, et al. Disruption of putative anion channel gene AtCLC-a in Arabidopsis suggests a role in the regulation of nitrate content. Plant J. 2000;21:259–267. doi: 10.1046/j.1365-313x.2000.00680.x. [DOI] [PubMed] [Google Scholar]
- 20.De Angeli A, et al. The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature. 2006;442:939–942. doi: 10.1038/nature05013. [DOI] [PubMed] [Google Scholar]
- 21.Chopin F, et al. The Arabidopsis ATNRT2.7 nitrate transporter controls nitrate content in seeds. Plant Cell. 2007;19:1590–1602. doi: 10.1105/tpc.107.050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lv Q, et al. Cloning and molecular analyses of the Arabidopsis thaliana chloride channel gene family. Plant Science. 2009;176:650–661. doi: 10.1016/j.plantsci.2009.02.006. [DOI] [Google Scholar]
- 23.Harada H, Kuromori T, Hirayama T, Shinozaki K, Leigh RA. Quantitative trait loci analysis of nitrate storage in Arabidopsis leading to an investigation of the contribution of the anion channel gene, AtCLC-c, to variation in nitrate levels. J Exp Bot. 2004;55:2005–2014. doi: 10.1093/jxb/erh224. [DOI] [PubMed] [Google Scholar]
- 24.von der Fecht-Bartenbach J, Bogner M, Dynowski M, Ludewig U. CLC-b-mediated NO-3/H+ exchange across the tonoplast of Arabidopsis vacuoles. Plant Cell Physiol. 2010;51:960–968. doi: 10.1093/pcp/pcq062. [DOI] [PubMed] [Google Scholar]
- 25.Hu R, et al. Knock-down of a tonoplast localized low-affinity nitrate transporter OsNPF7.2 affects rice growth under high nitrate supply. Front Plant Sci. 2016;7:1529. doi: 10.3389/fpls.2016.01529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wege S, et al. Phosphorylation of the vacuolar anion exchanger AtCLCa is required for the stomatal response to abscisic acid. Sci Signal. 2014;7:ra65. doi: 10.1126/scisignal.2005140. [DOI] [PubMed] [Google Scholar]
- 27.Humble GD, Hsiao TC. Specific requirement of potassium for light-activated opening of stomata in epidermal strips. Plant Physiol. 1969;44:230–234. doi: 10.1104/pp.44.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo FQ, Young J, Crawford NM. The nitrate transporter AtNRT1.1 (CHL1) functions in stomatal opening and contributes to drought susceptibility in Arabidopsis. Plant cell. 2003;15:107–117. doi: 10.1105/tpc.006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaquinod M, et al. A proteomics dissection of Arabidopsis thaliana vacuoles isolated from cell culture. Mol Cell Proteomics. 2007;6:394–412. doi: 10.1074/mcp.M600250-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsay YF, Chiu CC, Tsai CB, Ho CH, Hsu PK. Nitrate transporters and peptide transporters. FEBS Lett. 2007;581:2290–2300. doi: 10.1016/j.febslet.2007.04.047. [DOI] [PubMed] [Google Scholar]
- 31.Li JY, et al. The Arabidopsis nitrate transporter NRT1.8 functions in nitrate removal from the xylem sap and mediates cadmium tolerance. Plant Cell. 2010;22:1633–1646. doi: 10.1105/tpc.110.075242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsay YF, Schroeder JI, Feldmann KA, Crawford NM. The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell. 1993;72:705–713. doi: 10.1016/0092-8674(93)90399-B. [DOI] [PubMed] [Google Scholar]
- 33.Schroeder JI. Physiology: nitrate at the ion exchange. Nature. 2006;442:877–878. doi: 10.1038/nature04999. [DOI] [PubMed] [Google Scholar]
- 34.O’Brien JA, et al. Nitrate transport, sensing, and responses in plants. Mol Plant. 2016;9:837–856. doi: 10.1016/j.molp.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Wang C, et al. Rice SPX-Major Facility Superfamily3, a vacuolar phosphate efflux transporter, is involved in maintaining phosphate homeostasis in rice. Plant Physiol. 2015;169:2822–2831. doi: 10.1104/pp.15.01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergsdorf EY, Zdebik AA, Jentsch TJ. Residues important for nitrate/proton coupling in plant and mammalian CLC transporters. J Biol Chem. 2009;284:11184–11193. doi: 10.1074/jbc.M901170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen J, et al. Organelle pH in the Arabidopsis endomembrane system. Mol Plant. 2013;6:1419–1437. doi: 10.1093/mp/sst079. [DOI] [PubMed] [Google Scholar]
- 38.Peng JS, Gong JM. Vacuolar sequestration capacity and long-distance metal transport in plants. Front Plant Sci. 2014;5:19. doi: 10.3389/fpls.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kataoka T, et al. Vacuolar sulfate transporters are essential determinants controlling internal distribution of sulfate in Arabidopsis. Plant Cell. 2004;16:2693–2704. doi: 10.1105/tpc.104.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han YL, et al. Nitrogen use efficiency is mediated by vacuolar nitrate sequestration capacity in roots of Brassica napus. Plant Physiol. 2016;170:1684–1698. doi: 10.1104/pp.15.01377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brunaud V, et al. T-DNA integration into the Arabidopsis genome depends on sequences of pre-insertion sites. EMBO Rep. 2002;3:1152–1157. doi: 10.1093/embo-reports/kvf237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scholl RL, May ST, Ware DH. Seed and molecular resources for Arabidopsis. Plant Physiol. 2000;124:1477–1480. doi: 10.1104/pp.124.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alonso JM, et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301:653–657. doi: 10.1126/science.1086391. [DOI] [PubMed] [Google Scholar]
- 44.Sessions A, et al. A high-throughput Arabidopsis reverse genetics system. Plant Cell. 2002;14:2985–2994. doi: 10.1105/tpc.004630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krysan PJ, Young JC, Sussman MR. T-DNA as an insertional mutagen in Arabidopsis. Plant Cell. 1999;11:2283–2290. doi: 10.1105/tpc.11.12.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mao Y, et al. Application of the CRISPR-Cas system for efficient genome engineering in plants. Mol Plant. 2013;6:2008–2011. doi: 10.1093/mp/sst121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feng Z, et al. Multigeneration analysis reveals the inheritance, specificity, and patterns of CRISPR/Cas-induced gene modifications in Arabidopsis. Proc Natl Acad Sci USA. 2014;111:4632–4637. doi: 10.1073/pnas.1400822111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gong JM, Lee DA, Schroeder JI. Long-distance root-to-shoot transport of phytochelatins and cadmium in Arabidopsis. Proc Natl Acad Sci USA. 2003;100:10118–10123. doi: 10.1073/pnas.1734072100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ludewig U, von Wiren N, Frommer WB. Uniport of NH4+ by the root hair plasma membrane ammonium transporter LeAMT1;1. J Biol Chem. 2002;277:13548–13555. doi: 10.1074/jbc.M200739200. [DOI] [PubMed] [Google Scholar]
- 50.Hsu PK, Tsay YF. Two phloem nitrate transporters, NRT1.11 and NRT1.12, are important for redistributing xylem-borne nitrate to enhance plant growth. Plant Physiol. 2013;163:844–856. doi: 10.1104/pp.113.226563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang NC, Liu KH, Lo HJ, Tsay YF. Cloning and functional characterization of an Arabidopsis nitrate transporter gene that encodes a constitutive component of low-affinity uptake. Plant Cell. 1999;11:1381–1392. doi: 10.1105/tpc.11.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Léran S, et al. Arabidopsis NRT1.1 is a bidirectional transporter involved in root-to-shoot nitrate translocation. Mol Plant. 2013;6:1984–1987. doi: 10.1093/mp/sst068. [DOI] [PubMed] [Google Scholar]
- 53.Lin SH, et al. Mutation of the Arabidopsis NRT1.5 nitrate transporter causes defective root-to-shoot nitrate transport. Plant Cell. 2008;20:2514–2528. doi: 10.1105/tpc.108.060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang YY, Tsay YF. Arabidopsis nitrate transporter NRT1.9 is important in phloem nitrate transport. Plant Cell. 2011;23:1945–1957. doi: 10.1105/tpc.111.083618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Xu YH, Yi HY, Gong JM. Vacuolar membrane transporters OsVIT1 and OsVIT2 modulate iron translocation between flag leaves and seeds in rice. Plant J. 2012;72:400–410. doi: 10.1111/j.1365-313X.2012.05088.x. [DOI] [PubMed] [Google Scholar]
- 56.Meng S, et al. Arabidopsis NRT1.5 mediates the suppression of nitrate starvation-induced leaf senescence by modulating foliar potassium level. Mol Plant. 2016;9:461–470. doi: 10.1016/j.molp.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 57.Zhang GB, Yi HY, Gong JM. The Arabidopsis ethylene/jasmonic acid-NRT signaling module coordinates nitrate reallocation and the trade-off between growth and environmental adaptation. Plant Cell. 2014;26:3984–3998. doi: 10.1105/tpc.114.129296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


