Abstract
The usage of essential oils as antimicrobial agents is gaining attention. Besides, pet turtles were known to harbor a range of pathogenic bacteria while the turtle keeping is a growing trend worldwide.The current study examined the antimicrobial activity of lemon grass oil (LGO) against seven species of Gram negative bacteria namely; Aeromonas hydrophila, A. caviae, Citrobacter freundii, Salmonella enterica, Edwardsiella tarda, Pseudomonas aeruginosa, and Proteus mirabilis isolated from three popular species of pet turtles. Along with the results of disc diffusion, minimum inhibitory and minimum bactericidal concentration (MIC and MBC) tests, LGO was detected as effective against 6 species of bacteria excluding P. aeruginosa. MIC of LGO for the strains except P. aeruginosa ranged from 0.016 to 0.5% (V/V). The lowest MIC recorded in the E. tarda strain followed by A. hydrophilla, C. freundii, P. mirabilis, and S. enterica. Interestingly, all the bacterial species except E. tarda were showing high multiple antimicrobial resistance (MAR) index values ranging from 0.36 to 0.91 upon the 11 antibiotics tested although they were sensitive to LGO.
Keywords: Lemongrass oil, antimicrobial property, pet turtles, pathogenic bacteria
Down the ages, numerous essential oils (EOs) extracted from plant materials have been used for their aroma, flavor, bactericidal, preservative and medicinal properties [1]. Particularly, essential oils have been tested for their potential implications as alternative remedies to treat many infectious diseases [2]. Since EOs are a rich source of biologically active compounds, investigating the antimicrobial properties of EOs extracted from aromatic plants is a growing interest [3].
Lemongrass (Cymbopogon citratus), a tall perennial grass comprising of about 55 species, grows in tropical and subtropical habitats [4]. The key active constituents of lemongrass oil (LGO) giving its distinct aroma are citral (65-86%), neral and geraniol [5]. It was identified that lemongrass oil bears antidepressant, antioxidant, antiseptic, sedative, nervine, bactericidal, and fungicidal properties [6]. As a bactericidal agent, the LGO was found to be effective against many bacterial species including Acinetobacter baumanii, Aeromonas veronii, Enterococcus faecalis, Escherichia coli, Klebsiella pneumonia, Salmonella enterica, Serratia marcesens, Proteus vulgaris, Enterobacter aerogenes, Corynebacterium equii, Staphylococcus aureus and so on [3,7,8,9].
Turtles are now gaining attention worldwide owing to their commercial value, particularly as exotic pets. Specifically, South Korea was among the top highest buyers of pet turtles from USA [10]. However, the popularity of pet turtles has not been accompanied by dissemination of the public health concerns that arise when raising them. In the meantime, pet turtle have been known to harbor a variety of microbes either opportunistic or readily pathogenic [11,12,13]. Besides, bacterial strains showing virulence and antimicrobial resistance with the aid of genetic determinants advocates their medical importance [14,15,16,17,18]. Common expressions of affection towards pets involve physical contact, yet turtle owners may not be aware of the risk of contracting a pathogen when handling their pet without adequate countermeasures.
Usage of antibiotics in controlling bacterial growth or treating the infections is a common medical practice, but the emergence of drug resistant bacteria is one of the most serious threats constraining successful treatment of microbial diseases [19]. Therefore, the applicability of natural products instead of synthetic drugs against bacteria has become attentive. Since, some of the key constituents of EOs act in a parallel way to the synthesized antibiotics [20], their utility towards the therapeutic application in controlling pathogenic bacteria owns significance. Particularly, the application of EOs to prevent turtle-borne zoonoses is noteworthy.
Therefore, our study sought to investigate the antimicrobial properties of LGO against seven species of pathogenic bacteria isolated from three popular pet turtle species by detecting the susceptibility by disc diffusion, minimum inhibitory concentration (MIC), and minimum bactericidal concentration (MBC) tests.
Materials and Methods
Turtles and bacteria
All the studied strains of bacteria were isolated from three popular turtle species namely; yellow-bellied slider (Trachemys scripta scripta), Chinese stripe-necked turtle (Ocadia sinensis) and river cooter (Pseudemys concinna concinna) which were reared under the laboratory conditions. A total of 40 bacterial strains belonging into seven species comprising, Aeromonas hydrophila, A. caviae, Citrobacter freundii, Salmonella enterica, Edwardsiella tarda, Pseudomonas aeruginosa, Proteus mirabilis were selected for the study. All the strains of bacteria had been previously identified up to their species level by 16s rRNA sequencing and BLAST compatibility with GenBank database.
EO and the source
Professional grade LGO (EO extracted from Cymbopogon citratus) manufactured by EuroAroma®, Germany was purchased from a Korean local merchant. According to the manufacturer, the LGO has been isolated by distillation from the leaves of lemongrass grown in China and the product has been tested for 100% purity by chiral method.
Disc diffusion test
Target bacterial strains were cultured in tryptic soy agar (TSA) (MBcell, Seoul, Korea) one night at 37℃ then diluted to a concentration of 0.5 MacFarland units (1.5×106 CFU/mL) in sterile saline which were used as the inocula. The Mueller Hinton agar (MHA) (MBcell, Seoul, Korea) plates were prepared in 90 mm diameter Petri plates where the thickness of the agar was 4 mm. One hundred microliters (100 µL) of each bacterial solution was inoculated onto the plates and uniformly distributed by sterile cotton swabs. Inoculated plates were allowed to stand for 15 minutes. At the same time, 6 mm diameter sterile disks (ADVANTEC®, Japan) were soaked with 20 µL of the LGO with different dilutions; 1:1 (pure LGO) and 1:2, 1:5, 1:10; 1 part of the LGO inrespective parts of the methanolic solution. Then the disks soaked with LGO were symmetrically placed onto the inoculated medium immediately by means of sterile tweezers and the plates were further sealed by thin plastic wrap in order to prevent evaporation. One of the disks was soaked with sterile distilled water as a control. Test was conducted in triplicates and the plates were incubated for 24±2 h at 37℃ under aerobic conditions. The effect of LGO was evaluated by measuring the diameter of the areas with no bacterial growth.
The susceptibility of 11 selected antibiotics namely; ampicillin (10 g), amoxicillin (30 g), cefoxitin (30 g), cephalothin (30 g), ceftriaxone (30 g), imipenem (10 g), gentamycin (10 g), amikacin (30 g), streptomycin (10 g), nalidixic acid (30 g), and ciprofloxacin (5 g) was examined on MHA (MBcell, Seoul, Korea) following the recommendations of Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute [21].
Calculating the Multiple Antibiotic Resistance (MAR) index
Following the disc diffusion test results of antibiotics, one strain from each species showing the strongest resistance with in the species was selected for calculating the MAR index. MAR index was calculated as the ratio of number of antibiotics to which bacteria was resistant to total number of antibiotics to which the bacteria was exposed.
MIC and MBC
The MIC of LGO was determined by broth microdilution method according to the National Committee for Clinical Laboratory Standards (NCCLS). All strains were cultured on TSA and incubated at 37℃ for 24 h prior to MIC determination. The inoculum density of each test organism was adjusted equivalent to 0.5 McFarland units prepared in sterile saline. One hundred microliters (100 µL) of double strength Mueller Hinton broth containing 5% dimethyl sulfoxide (DMSO) was dispensed into wells of 96-well micro titer plates. In the first column of wells, LGO was added with a final concentration of 2% (V/V) and then serially diluted by two-fold across the plate until a final concentration of 0.004% (V/V). One hundred microliters (100 µL) of each bacterial suspension was added to each well and the plates were incubated at 37℃ for 16 h. The assay for each of the pathogens was conducted in triplicates.
In order to determine the MBC, the culture medium from wells which have LGO concentration higher than MIC were smeared on separate TSA plates and incubated at 37℃ for 24 h. MBC was determined as the lowest concentration of LGO which gave no growth of bacteria on the TSA plate.
Results
Disc diffusion test
The susceptibility pattern of LGO against turtle-borne bacteria is given in Table 1. The results revealed that the LGO showed antibacterial activity against the majority of tested Gram negative bacteria (85%) except P. aeruginosa with varying magnitudes. The sensitivity was found gradually increasing with the increase in concentration of oil. The highest resistance was observed in P. aeruginosa in which all the tested strains showing no any growth inhibition to any of the dilutions of LGO. In contrast, E. tarda was observed as the most susceptible species where the highest inhibition zone (44 mm) was observed. A. hydrophila was comparatively more sensitive to LGO showing inhibition even in the 1:10 dilution where A. caviae was showing a reduced sensitivity than A. hydrophila. The highest inhibition recorded for A. hydrophila was 32 mm for 1:1 dilution while the least was 10 mm for 1:10. In the case of A. caviae the highest inhibition observed was 18 mm in 1:1 and 7 mm was the least for 1:5. The susceptibility of C. freundii, P. mirabilis, and S. enterica was observed to be more or less similar where the inhibition was not pronounced after 1:2 dilution.
Table 1. Susceptibility pattern of LGO against pet turtle-borne bacteria.
| Bacterial strain* | Inhibition zonea (mm) with different LGO dilutionsb added on disc | MIC % (V/V) |
MBCc % (V/V) |
MIC : MBC | |||
|---|---|---|---|---|---|---|---|
| 1:1 | 1:2 | 1:5 | 1:10 | ||||
| PA1 | NA | NA | NA | NA | >2 | ND | - |
| PA2 | NA | NA | NA | NA | >2 | ND | - |
| PA3 | NA | NA | NA | NA | >2 | ND | - |
| PA4 | NA | NA | NA | NA | >2 | ND | - |
| PA5 | NA | NA | NA | NA | >2 | ND | - |
| PA6 | NA | NA | NA | NA | >2 | ND | - |
| CF1 | 12 | 8 | NA | NA | 0.125 | 0.125 | 1:1 |
| CF2 | 10 | 8 | NA | NA | 0.063 | 0.25 | 1:4 |
| CF3 | 11 | 8 | NA | NA | 0.063 | 0.125 | 1:2 |
| CF4 | 10 | 8 | NA | NA | 0.063 | 0.25 | 1:4 |
| CF5 | 10 | 8 | NA | NA | 0.125 | 0.25 | 1:4 |
| CF6 | 10 | 8 | NA | NA | 0.125 | 0.25 | 1:2 |
| CF7 | 8 | 7 | NA | NA | 0.125 | 0.25 | 1:2 |
| CF8 | 9 | 8 | NA | NA | 0.125 | 0.25 | 1:2 |
| CF9 | 9 | 8 | NA | NA | 0.125 | 0.25 | 1:2 |
| CF10 | 10 | 8 | NA | NA | 0.063 | 0.125 | 1:2 |
| PM1 | 24 | 20 | 8 | NA | 0.063 | 0.063 | 1:1 |
| PM2 | 10 | 8 | NA | NA | 0.25 | 0.25 | 1:1 |
| PM3 | 18 | 13 | 9 | NA | 0.125 | 0.5 | 1:4 |
| PM4 | 18 | 10 | NA | NA | 0.125 | 0.5 | 1:4 |
| PM5 | 20 | 18 | NA | NA | 0.125 | 0.5 | 1:4 |
| PM6 | 18 | 17 | NA | NA | 0.063 | 0.125 | 1:2 |
| SE1 | 11 | 9 | NA | NA | 0.5 | 0.5 | 1:1 |
| SE2 | 12 | 10 | NA | NA | 0.063 | 0.063 | 1:1 |
| SE3 | 13 | 11 | NA | NA | 0.063 | 0.063 | 1:1 |
| SE4 | 12 | 10 | 9 | NA | 0.063 | 0.063 | 1:1 |
| SE5 | 12 | 11 | NA | NA | 0.5 | 0.5 | 1:1 |
| SE6 | 10 | 10 | NA | NA | 0.031 | 0.031 | 1:1 |
| SE7 | 10 | 9 | NA | NA | 0.125 | 0.125 | 1:1 |
| SE8 | 8 | 8 | NA | NA | 0.125 | 0.125 | 1:1 |
| AC1 | 18 | 12 | 7 | NA | 0.063 | 0.125 | 1:2 |
| AC2 | 12 | 10 | 7 | NA | 0.063 | 0.125 | 1:2 |
| AH1 | 32 | 24 | 11 | 8 | 0.031 | 0.125 | 1:4 |
| AH2 | 30 | 22 | 12 | 9 | 0.063 | 0.063 | 1:1 |
| AH3 | 22 | 20 | 15 | 10 | 0.031 | 0.031 | 1:1 |
| AH4 | 32 | 24 | 14 | 8 | 0.031 | 0.125 | 1:4 |
| AH5 | 22 | 16 | 14 | 10 | 0.031 | 0.031 | 1:1 |
| AH6 | 24 | 20 | 10 | 8 | 0.031 | 0.125 | 1:4 |
| AH7 | 22 | 20 | 14 | 10 | 0.031 | 0.125 | 1:4 |
| ET1 | 44 | 30 | 19 | 12 | 0.016 | 0.25 | 1:16 |
*Strain number was given according to the species; PA=Pseudomonas aeruginosa, CF=Citrobacter freundii, PM=Proteus mirabilis, SE=Salmonella enterica, AC=Aeromonas caviae, AH=A. hydrophila, ET=Edwardsiella tarda
aInhibition zone; NA=No growth inhibition.
bConcentration added on disc; 1:1=pure oil, 1:2, 1:5, 1:10 = 1 part of LGO in respective parts of the dilution.
cMBC; ND=Not Done.
The antibiotic resistance profile of tested bacterial species is summarized in Table 2. A. caviae was resistant to all tested antibiotics except streptomycin but, the E. tarda strain was sensitive to all tested antibiotics. In addition, P. aeruginosa strongly resisted 10 antibiotics being sensitive only to ciprofloxacin. Both A. hydrophila and C. freundii also showed resistance to 7 antibiotics while S. enterica could resist only amoxicillin, ampicillin, cefoxitin and cephalothin.
Table 2. Antimicrobial resistance patterns of pet turtle-borne bacteria in disc diffusion test.
| Bacterial species | Percentage susceptibility* of antimicrobials | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ampicillin (10 µg) | Amoxicillin (30 µg) | Cefoxitin (30 µg) | Cephalothin (30 µg) | Ceftriaxone (30 µg) | Imipenem (10 µg) | Gentamicin (10 µg) | Amikacin (30 µg) | Streptomycin (10 µg) | Nalidixic acid (30 µg) | Ciprofloxacin (5 µg) | ||
| Aeromonas caviae (n=2) | R | 2 (100%) | 2 (100%) | 1 (50%) | 2 (100%) | 2 (100%) | 1 (50%) | 2 (100%) | 2 (100%) | - | 1 (50%) | 2 (100%) |
| I | - | - | - | - | - | - | - | - | - | - | - | |
| S | - | - | 1 (50%) | - | - | 1 (50%) | - | - | 2 (100%) | 1 (50%) | - | |
| A. hydrophila (n=7) | R | 6 (86%) | 6 (86%) | 1 (14%) | 2 (29%) | 2 (28%) | - | - | - | 1 (14%) | 4 (57%) | - |
| I | - | - | 1 (14%) | - | - | - | - | - | - | 1 (14%) | - | |
| S | 1 (14%) | 1 (14%) | 5 (72%) | 5 (71%) | 5 (72%) | 7 (100%) | 7 (100%) | 7 (100%) | 6 (86%) | 2 (29%) | 7 (100%) | |
| Citrobacter freundii (n=10) | R | 10 (100%) | 10 (100%) | 9 (90%) | 10 (100%) | - | - | 3 (30%) | - | 5 (50%) | - | 2 (20%) |
| I | - | - | 1 (10%) | - | - | 1 (10%) | - | - | - | - | 2 (20%) | |
| S | - | - | - | - | 10 (100%) | 9 (90%) | 7 (70%) | 10 (100%) | 5 (50%) | 10 (100%) | 6 (60%) | |
| Salmonella enterica (n=8) | R | 6 (75%) | 7 (87.5%) | 7 (87.5%) | 7 (87.5%) | - | - | - | - | - | - | - |
| I | 1 (12.5%) | - | - | - | - | - | - | - | - | 1 (12.5%) | - | |
| S | 1 (12.5%) | 1 (12.5%) | 1 (12.5%) | 1 (12.5%) | 8 (100%) | 8 (100%) | 8 (100%) | 8 (100%) | 8 (100%) | 7 (87.5%) | 8 (100%) | |
| Proteus mirabilis (n=6) | R | 2 (33%) | 3 (50%) | - | 1 (16.5%) | - | - | - | - | - | 1 (16.5%) | 2 (33%) |
| I | - | - | - | 1 (16.5%) | - | - | - | - | 1 (17%) | 1 (16.5%) | - | |
| S | 4 (67%) | 3 (50%) | 6 (100%) | 4 (67%) | 6 (100%) | 6 (100%) | 6 (100%) | 6 (100%) | 5 (83%) | 4 (67%) | 4 (67%) | |
| Pseudomonas aeruginosa (n=6) | R | 6 (100%) | 6 (100%) | 6 (100%) | 6 (100%) | 6 (100%) | 5 (83%) | 6 (100%) | 5 (83%) | 6 (100%) | 6 (100%) | - |
| I | - | - | - | - | - | - | - | 1 (17%) | - | - | - | |
| S | - | - | - | - | - | 1 (17%) | - | - | - | - | 6 (100%) | |
| Edwardsiella tarda (n=1) | R | - | - | - | - | - | - | - | - | - | - | - |
| I | - | - | - | - | - | - | - | - | - | 1 (100%) | - | |
| S | 1 (100%) | 1 (100%) | 1 (100%) | 1 (100%) | 1 (100%) | 1 (100%) | 1 (100%) | 1 (100%) | 1 (100%) | - | 1 (100%) | |
*Susceptibility pattern; R=resistant, I=intermediate, S=susceptible were designated using breakpoints described by the Clinical Laboratory Standards Institute [21].
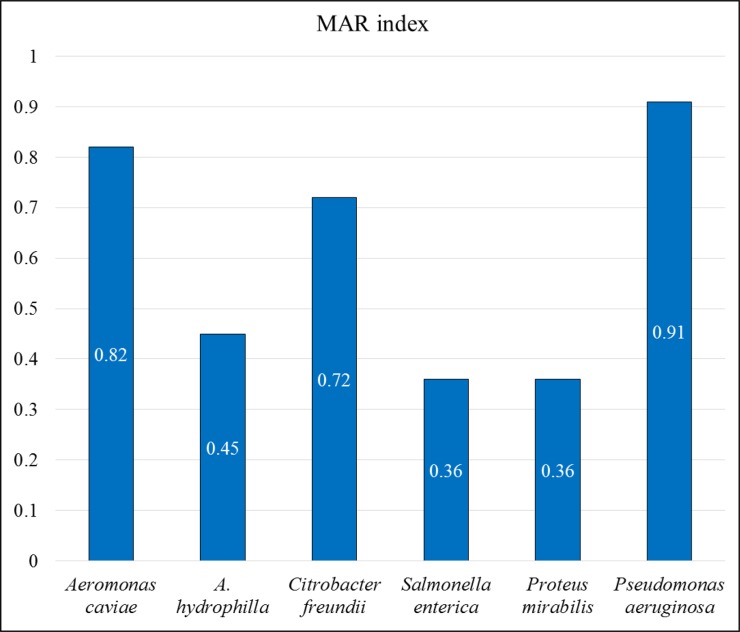
According to the MAR index calculated for each species, the highest value (0.91) was obtained for P. aeruginosa (Figure 1). P. mirabilis, and S. enterica were calculated to have the lowest MAR index (0.36). MAR index values of other bacterial species are 0.82, 0.72, and 0.45 for A. caviae, C. freundii, and A. hydrophila respectively. More importantly, all the species were detected to have MAR index higher than 0.2 which is the breakpoint to detect the high risk of infection.
Figure 1. Multiple Antibiotic Resistance (MAR) index values of pet turtle-borne bacterial species (The index was calculated by dividing the no. of resistant antimicrobials by 11; the total antimicrobials tested).
MIC and MBC
MIC and MBC values of each strain were given in Table 1. MIC of LGO tested for turtle-borne bacteria ranged from 0.016 to 0.5% (V/V) among the species other than P. aeruginosa and the majority of the strains were having MIC 0.125%. P. aeruginosa was detected to be the most resistant where the MIC was >2%. In MIC test, 0.5% was observed in two strains of S. enterica while the following (0.125%) was detected in 2 S. enterica, 3 P. mirabilis and 6 C. freundii isolates. Interestingly, E. tarda was found to be the species bearing the least MIC (0.016%).
With respect to MBC, the values ranged from being identical or greater than MIC as shown in Table 1. In the case of E. tarda the MIC: MBC ratio was detected the highest as 1:16 and the rest did not exceed 1:4. In addition, 3 P. mirabilis, 4 A. hydrophila and 6 C. freundii showed the 1:4 ratio where 1:2 was more pronounced in A. caviae, and C. freundii. Noticeably, MIC and MBC of all S. enterica isolates remained alike.
Discussion
It has been proven that plant EOs are agents advocating the killing or inhibiting the growth of pathogenic bacteria with the aid of many antibacterial properties. Besides, LGO has been reported to have good antibacterial properties [3,7,8,9]. Evidently, the infection of Gram negative bacteria is hard to treat than Gram positives owing to their intrinsic and acquired resistance mechanisms [22]. Since, pet turtle keeping is a growing trend but, reported to harbor many Gram negative bacteria [11,12,13,14,15,16,17,18], efficacy of EOs as antibacterial agents for turtle-borne bacteria worth examining. Moreover, to our knowledge, this is the first to study the antibacterial properties of LGO against pet-turtle borne Gram negative bacteria.
Along with the susceptibility results, LGO successfully inhibited the growth of six species of bacteria except P. aeruginosa. As the disc diffusion and MIC outcomes revealed, P. aeruginosa was observed to have a MIC higher than 2% LGO. Similar observation was reported in previous studies where the inhibition was zero for several different LGO concentrations [22,23]. On the other hand, P. aeruginosa was observed to resist 10 out of 11 antibiotics tested, being susceptible only to ciprofloxacin, while bearing the highest MAR index value; 0.9. The reason could be because, the P. aeruginosa demonstrates almost all the practically known enzymatic and mutational mechanisms of bacterial resistance. Often these mechanisms occur simultaneously, thus conferring combined resistance to many treatment agents [24].
More importantly, majority of the tested gram negative bacteria were sensitive to LGO even in lower concentrations. In this study, E. tarda was identified as the most sensitive species which showed the minimum MIC; 0.016%. Markedly, the ratio of MIC: MBC of E. tarda was detected as 1:16 revealing that the E. tarda can survive even at bit higher LGO strengths although its growth is inhibited in a very lower LGO concentration. A similar kind of observation was reported for eucalyptus oil against E. tarda isolated from fish [25]. Another study encountered 77% of the E. tarda isolates exhibiting sensitivity to LGO [23]. In the case of Aeromonas spp., which showed a strong sensitivity to LGO, A. hydrophila showed a comparatively higher sensitivity compared to A. caviae. Average MICs of A. hydrophila and A. caviae were 0.03 and 0.06% respectively. In a previous study in which fish isolated Aeromonas spp. were tested, zone of inhibition for 1:5 dilution was 44.7 mm and MBC value was around 0.31% (V/V) [26]. LGO has been reported to be effective against 78% of the Aeromonas spp. isolates in another similar study [23]. Among the 10 C. freundii, 6 P. mirabilis and 8 S. enterica isolates tested, average MIC values were around 0.12% together with comparable disc diffusion zone inhibitions. In contrary, a previous study reported LGO having a minor effect on C. freundii and P. mirabilis where only 7.7 and 33% of the isolates were susceptible [23]. However, S. enterica tested on leafy greens and from food origin were discovered more sensitive to LGO although the bacteria were multi drug resistant [27,28]. Moreover, in our study, the similar MIC and MBC values of S. enterica seems significant since, most of the other bacterial strains gave MIC: MBC ratio higher than 1.
Meanwhile, the antibiotic resistance profile indicated all the species excluding E. tarda showing resistance to 4 or more antibiotics. This was facilitated by MAR index values where all the species except E. tarda have been calculated as ≥0.36. More importantly, P. aeruginosa (0.91), A. caviae (0.82), and C. freundii (0.72) were noted as comparatively high risk species towards the public health. Bacteria bearing MAR index 0.2 is suggested to originate from a high risk source of contamination where several antibiotics are used. MAR in bacteria is most commonly associated with the presence of plasmids containing several resistance genes, each encoding an antibiotic resistance phenotype. High level of MAR indicates a serious need for broad-based antimicrobial resistance surveillance and planning of effective interventions to reduce the multidrug resistance in those pathogens [29]. More significantly, our study could successfully control the growth of such MAR bacteria in vitro using LGO. Accordingly, the possible application of LGO in controlling and treating infections of these Gram negative bacteria can be recommended. It might be due to the reason that, a major component of LGO, citral, was known to elucidate the antimicrobial activity via changes in intracellular pH, membrane potential, intracellular ATP concentration, and membrane integrity [30].
Considered as a whole, it is reasonable to conclude that LGO is an effective antimicrobial agent in controlling the infection of turtle-borne pathogenic Gram negative bacteria with exception of P. aeruginosa. In addition, the high MAR index values of most of the isolates should not be undervalued because of the potential public health risk of pet turtle keepers. Being consistent with these outcomes, it is recommended to investigate the applicability of different EOs against more bacterial species of turtle origin.
Footnotes
Conflict of interests: The authors declare that there is no financial conflict of interests to publish these results.
References
- 1.Burt S. Essential oils: their antibacterial properties and potential applications in foods--a review. Int J Food Microbiol. 2004;94(3):223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Tepe B, Daferera D, Sökmen M, Polissiou M, Sökmen A. In vitro antimicrobial and antioxidant activities of the essential oils and various extracts of Thymus eigii M. Zohary et P.H. Davis. J Agric Food Chem. 2004;52(5):1132–1137. doi: 10.1021/jf035094l. [DOI] [PubMed] [Google Scholar]
- 3.Hammer KA, Carson CF, Riley TV. Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol. 1999;86(6):985–990. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 4.Cheel J, Theoduloz C, Rodríguez J, Schmeda-Hirschmann G. Free radical scavengers and antioxidants from Lemongrass (Cymbopogon citratus (DC.) Stapf.) J Agric Food Chem. 2005;53(7):2511–2517. doi: 10.1021/jf0479766. [DOI] [PubMed] [Google Scholar]
- 5.Teuscher E. Medicinal Spices-A Handbook of Culinary Herbs, Spices, Spice Mixtures and Their Essential Oils. Stuttgart, Germany: Medpharm Scientific Publishers; 2006. [Google Scholar]
- 6.Gardner ZE, McGuffin M. American Herbal Products Association's botanical safety handbook. 2nd ed. Boca Raton: CRC Press, American Herbal Products Association; 2013. [Google Scholar]
- 7.Aiemsaard J, Aiumlamai S, Aromdee C, Taweechaisupapong S, Khunkitti W. The effect of lemongrass oil and its major components on clinical isolate mastitis pathogens and their mechanisms of action on Staphylococcus aureus DMST 4745. Res Vet Sci. 2011;91(3):e31–e37. doi: 10.1016/j.rvsc.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Friedman M, Henika PR, Mandrell RE. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J Food Prot. 2002;65(10):1545–1560. doi: 10.4315/0362-028x-65.10.1545. [DOI] [PubMed] [Google Scholar]
- 9.Maizura M, Fazilah A, Norziah MH, Karim AA. Antibacterial activity and mechanical properties of partially hydrolyzed sago starch-alginate edible film containing lemongrass oil. J Food Sci. 2007;72(6):C324–C330. doi: 10.1111/j.1750-3841.2007.00427.x. [DOI] [PubMed] [Google Scholar]
- 10.HSUS. The Trade in Live Reptiles: Exports from the United States. Washington, DC: The Humane Society of the United States; 2001. [Google Scholar]
- 11.Back DS, Shin GW, Wendt M, Heo GJ. Prevalence of Salmonella spp. in pet turtles and their environment. Lab Anim Res. 2016;32(3):166–170. doi: 10.5625/lar.2016.32.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutton CS, Revan F, Wang C, Xu C, Norton TM, Stewart KM, Kaltenboeck B, Soto E. Salmonella enterica prevalence in leatherback sea turtles (Dermochelys coriacea) in St. Kitts, West Indies. J Zoo Wildl Med. 2013;44(3):765–768. doi: 10.1638/2012-0216R1.1. [DOI] [PubMed] [Google Scholar]
- 13.Wendt M, Heo GJ. Multilocus sequence typing analysis of Pseudomonas aeruginosa isolated from pet Chinese stripe-necked turtles (Ocadia sinensis) Lab Anim Res. 2016;32(4):208–216. doi: 10.5625/lar.2016.32.4.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Díaz MA, Cooper RK, Cloeckaert A, Siebeling RJ. Plasmid-mediated high-level gentamicin resistance among enteric bacteria isolated from pet turtles in Louisiana. Appl Environ Microbiol. 2006;72(1):306–312. doi: 10.1128/AEM.72.1.306-312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hossain S, Wimalasena SHMP, Heo GJ. Virulence factors and antimicrobial resistance pattern of Citrobacter freundii isolated from healthy pet turtles and their environment. Asian J Anim Vet Adv. 2017;12(1):10–16. [Google Scholar]
- 16.Nowakiewicz A, Ziółkowska G, Ziba P, Stpniewska K, Tokarzewski S. Russian tortoises (Agrionemys horsfieldi) as a potential reservoir for Salmonella spp. Res Vet Sci. 2012;92(2):187–190. doi: 10.1016/j.rvsc.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Shin DM, Hossain S, Wimalasena S, Heo G-J. Antimicrobial resistance and virulence factors of Edwardsiella tarda isolated from pet turtles. Pak Vet J. 2017;37(1):85–89. [Google Scholar]
- 18.De Silva BCJ, Wimalasena SHMP, Hossain S, Pathirana HNKS, Heo G-J. Characterization of quinolone resistance of Pseudomonas aeruginosa isolated from pet chinese stripe-necked turtles (Ocadia sinensis) Asian J Anim Vet Adv. 2017;12(3):152–160. [Google Scholar]
- 19.Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T. 2015;40(4):277–283. [PMC free article] [PubMed] [Google Scholar]
- 20.Langeveld WT, Veldhuizen EJ, Burt SA. Synergy between essential oil components and antibiotics: a review. Crit Rev Microbiol. 2014;40(1):76–94. doi: 10.3109/1040841X.2013.763219. [DOI] [PubMed] [Google Scholar]
- 21.CLSI. Performance standards for antimicrobial susceptibility testing: Twenty-fourth informational supplement: CLSI M100-S24. Wayne, USA: Clinical and Laboratory Standards Institute (CLSI); 2014. [Google Scholar]
- 22.Naik MI, Fomda BA, Jaykumar E, Bhat JA. Antibacterial activity of lemongrass (Cymbopogon citratus) oil against some selected pathogenic bacteria. Asian Pac J Trop Med. 2010;3(7):535–538. [Google Scholar]
- 23.Singh BR, Singh V, Singh RK, Ebibeni N. Antimicrobial activity of lemongrass (Cymbopogon citratus) oil against microbes of environmental, clinical and food origin. Int Res J Pharm Pharmacol. 2011;1(9):228–236. [Google Scholar]
- 24.Strateva T, Yordanov D. Pseudomonas aeruginosa-a phenomenon of bacterial resistance. J Med Microbiol. 2009;58(Pt 9):1133–1148. doi: 10.1099/jmm.0.009142-0. [DOI] [PubMed] [Google Scholar]
- 25.Park JW, Wendt M, Heo GJ. Antimicrobial activity of essential oil of Eucalyptus globulus against fish pathogenic bacteria. Lab Anim Res. 2016;32(2):87–90. doi: 10.5625/lar.2016.32.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Starliper CE, Ketola HG, Noyes AD, Schill WB, Henson FG, Chalupnicki MA, Dittman DE. An investigation of the bactericidal activity of selected essential oils to Aeromonas spp. J Adv Res. 2015;6(1):89–97. doi: 10.1016/j.jare.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore-Neibel K, Gerber C, Patel J, Friedman M, Ravishankar S. Antimicrobial activity of lemongrass oil against Salmonella enterica on organic leafy greens. J Appl Microbiol. 2012;112(3):485–492. doi: 10.1111/j.1365-2672.2011.05222.x. [DOI] [PubMed] [Google Scholar]
- 28.Vazirian M, Kashani ST, Ardekani MRS, Khanavi M, Jamalifar H, Fazeli MR. Antimicrobial activity of lemongrass (Cymbopogon citratus (DC) Stapf.) essential oil against food-borne pathogens added to cream-filled cakes and pastries. J Essent Oil Res. 2012;24(6):579–582. [Google Scholar]
- 29.Osundiya OO, Oladele RO, Oduyebo OO. Multiple antibiotic resistance (MAR) indices of Pseudomonas and Klebsiella species isolates in Lagos University Teaching Hospital. Afr J Cln Exper Microbiol. 2013;14(3):164–168. [Google Scholar]
- 30.Shi C, Song K, Zhang X, Sun Y, Sui Y, Chen Y, Jia Z, Sun H, Sun Z, Xia X. Antimicrobial Activity and Possible Mechanism of Action of Citral against Cronobacter sakazakii. PLoS One. 2016;11(7):e0159006. doi: 10.1371/journal.pone.0159006. [DOI] [PMC free article] [PubMed] [Google Scholar]