Abstract
Background
Median historical time to kidney transplant is misleading because it does not convey the competing risks of death or removal from the waiting list. We developed and validated a competing risk model to calculate likelihood of outcomes for kidney transplant candidates and demonstrate how this information differs from median time to transplant.
Methods
Data were obtained from the US Scientific Registry of Transplant Recipients. The retrospective cohort included 163 636 adults listed for kidney transplant before December 31, 2011. Predictors were age, sex, blood type, calculated panel-reactive antibodies, donation service area, dialysis duration, comorbid conditions, and body mass index. Outcomes were deceased or living donor transplant, death or removal from the list due to deteriorating medical condition, or removal due to other reasons. We calculated hazards for the possible outcomes, then the cumulative incidence function for a given candidate using competing risk methodology. Discrimination and calibration were assessed through C statistics and calibration plots for each cause-specific Cox proportional hazard model.
Results
C statistics ranged from 0.64 to 0.73. Calibration plots showed good calibration. The competing risk model shows probability of all possible outcomes for up to 12 years given a candidate's characteristics, contrasted with the median waiting time for that candidate's donation service area.
Conclusions
A competing risk model conveys more relevant information than the median waiting time for a given transplant center. This model will be updated to create a calculator reflecting the most recent outcomes and changes in allocation policy. It illustrates the conversations that should be initiated with transplant candidates.
“How long will it take to get a kidney?” This is one of the questions most commonly asked by kidney transplant candidates. The answer given is generally based on the time that previous deceased donor recipients spent on the waiting list, typically expressed as the median waiting time, or the time to when half of candidates underwent transplant. Most often, Kaplan-Meier plots are used to determine median waiting times. Kaplan-Meier plots indicate the proportion of patients who are still waiting at a given time after listing. Patients removed from the waiting list without undergoing transplant are “censored,” or not included in the calculation of waiting time. However, giving candidates only this median time information is disingenuous, because it obscures the important fact that most candidates will not undergo deceased donor transplant. In 2012 alone, of the nearly 89 000 candidates waitlisted for kidney transplant, more than 5000 died before undergoing transplant, and an additional 2000 were removed from the list due to deteriorating medical conditions.1 Half of kidney transplant candidates older than 60 years when listed died before undergoing deceased donor transplant,2 but this information is generally not conveyed to patients at the time of their transplant evaluation.
Four outcomes are possible for a kidney transplant candidate after listing: (1) deceased donor transplant, (2) living donor transplant, (3) death or removal from the list due to deteriorating medical condition, or (4) removal from the list due to other reasons. Because removal from the waiting list due to reasons other than deceased donor transplant precludes deceased donor transplant from occurring, these other reasons are considered competing risks for receiving a deceased donor kidney. Important determinants of waiting time include donation service area (DSA), blood type, and number of human leukocyte antigens that preclude transplant (unacceptable antigens).3 Characteristics that influence the chances of undergoing deceased donor transplant may affect the chances of being removed from the waiting list for other reasons differently. Important considerations in reporting “waiting time” include how these characteristics affect the chances of deceased donor transplant, and also how they affect the chances of removal from the waiting list for other reasons, best done using a competing risk analysis.
To illustrated how calculated median time to transplant differs from likely outcomes on the waiting list, we used a competing risk model to develop and validate a region-specific waitlist outcome calculator that demonstrates what the chances are of undergoing deceased donor transplant and what the chances are of an outcome that would prevent deceased donor transplant, such as death or deteriorating medical condition.
MATERIALS AND METHODS
Source of Data
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes listing and outcome data on all donors, waitlisted candidates, and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere.4 The Health Resources and Services Administration, US Department of Health and Human Services, provides oversight of the activities of the OPTN and SRTR contractors. Analyses were performed in SAS. SAS 9.1.3 (SAS Institute, Cary NC) and R 3.2.2 (R Core Team [2015]. R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/) was used.
Study Population
We included all 163636 adult (aged ≥ 18 years) candidates in the United States whose first listing was for a kidney-alone transplant and who were on the list between January 1, 2007, and December 31, 2011. Candidates who were removed from the list for any reason including transplant before January 1, 2007, were excluded to allow for a more contemporary sample. Baseline characteristics at the time of listing were retrospectively ascertained. This sampling method maximized follow-up time, which ranged from 1 day to 12 years or longer, while still including a more contemporary sample of transplant candidates. We excluded additional listings for the same person; if a candidate was listed at 1 center and subsequently listed at another, only the first listing was counted. However, if a candidate maintained listings at multiple sites without discontinuing active status at the program of first listing, no censoring occurred. Listings were censored 12 years after the listing date due to the exceedingly small number of candidates (2%) still listed after 12 years, with last date of follow-up December 31, 2011. Therefore, the earliest listing date for this cohort was 1994, but 99% of candidates were listed after 2000 (Fig. S1, SDC, http://links.lww.com/TP/B266).
Outcomes
In a competing risk analysis, only 1 of the possible outcomes can be observed. For example, a candidate who undergoes living donor transplant cannot also undergo deceased donor transplant. The probability of remaining on the waiting list is a function of all the hazards (deceased donor transplant, living donor transplant, death or deteriorating condition, remaining on the list, and other removal). These objective outcomes are reported by OPTN for every transplant candidate listed in the United States. Other reasons for removal include the following: refused transplant; candidate listed in error; candidate listed for unacceptable antigens only; candidate condition improved, transplant not needed; candidate removed in error; changed to kidney-pancreas transplant; program inactive for 2 or more years; underwent transplant in another country; unable to contact candidate; or “other.”
Predictors
The most parsimonious models possible while maintaining predictive ability were selected for this analysis. DSA was included in each model as a random effect along with its standard error reflecting the heterogeneity in time to event between DSAs. Candidate predictor variables at the time of listing included age, sex, blood type, calculated panel-reactive antibodies (CPRAs), DSA, dialysis duration, diabetes status, angina, cerebrovascular disease, peripheral vascular disease, hypertension, previous malignancy, chronic obstructive pulmonary disease, and body mass index (BMI). The panel-reactive antibodies (PRAs)/CPRA value used in the models was the value obtained closest to 90 days postlisting to allow time for newly listed candidates to declare unacceptable antigens. For the continuous variables (age, CPRA, dialysis duration, and BMI), penalized spline curves were fit to identify potential knots for an unpenalized linear spline curve for use in the models, resulting in a PRA knot at 80%, age knots at 40 years and 65 years, a dialysis duration knot at 5 years, and a BMI knot at 25 kg/m2.
Analytical Approach
The waitlist survival model was developed to estimate the probability of outcomes on the waiting list. Each outcome (deceased donor transplant, living donor transplant, removal from the list due to death or deteriorating condition, removal from the list due to other reasons) was modeled separately using a cause-specific Cox proportional hazards analysis to estimate its probability at any time. Separate models were created for standard criteria donor (SCD) and expanded criteria donor (ECD) deceased donor transplants, and additional models were created for each possible donor blood type for these deceased donor transplants to avoid violating the proportional hazards assumption, because this method allows for disproportional hazards. All models were further stratified by recipient blood type, except death or deteriorating condition because blood type was not a significant predictor for this model. DSA was included as a random effect in each model. Variables included in each of the final models were chosen using backward selection. Plots of scaled Schoenfeld residuals were used to check the proportionality assumption. Approximate proportional effects were found in all cases. We then calculated the cumulative incidence function, or the probability of experiencing 1 possible outcome over time, using the hazard functions for all the competing risks. The cumulative incidence is a function of the probability of remaining on the waiting list and the hazard for any 1 event; the sum of the cumulative incidences for all competing events and the probability of remaining on the waiting list is equal to 1.5
The study population was randomly split into a model development set (90% of the study population), which was used to select predictor variables, and a validation set (10%). C statistics were used to assess the accuracy of prediction of these models; a C statistic of 0.5 indicates that the discrimination of the prediction model performs no better than a coin toss, while a C statistic of 1.0 indicates that the model perfectly predicts that the predicted risk for a case is higher than for a noncase. In addition, we assessed calibration for each model using calibration plots.
RESULTS
Population Characteristics
The characterisics of the development and validation populations were similar (data not shown). Mean age at listing was 52.5 years, and 60.7% of candidates were men (Table 1). Nearly half (49.6%) of candidates were blood type O, and 43.2% had diabetes.
TABLE 1.
Characteristics of the cohort of patients on the kidney transplant waiting list at any time between January 1, 2007, and December 31, 2011
| Transplant | Waitlist removal | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Characteristics | All | Deceased donor | Living donor | Other | Died or too sick | Still waiting |
| Total | 163636 (100) | 32114 (19.6) | 18711 (11.4) | 10643 (6.5) | 23947 (14.6) | 78221 (47.8) |
| Female | 64301 (39.3) | 12547 (39.1) | 7086 (37.9) | 4,299 (40.4) | 9360 (39.1) | 31009 (39.6) |
| Blood type | ||||||
| A | 51787 (31.6) | 13018 (40.5) | 7146 (38.2) | 3106 (29.2) | 6936 (29) | 21581 (27.6) |
| AB | 5886 (3.6) | 2068 (6.4) | 742 (4) | 292 (2.7) | 669 (2.8) | 2115 (2.7) |
| B | 24778 (15.1) | 4712 (14.7) | 2479 (13.2) | 1759 (16.5) | 3840 (16) | 11988 (15.3) |
| O | 81185 (49.6) | 12316 (38.4) | 8344 (44.6) | 5486 (51.5) | 12502 (52.2) | 42537 (54.4) |
| Diabetes | 70646 (43.2) | 11934 (37.2) | 5040 (26.9) | 4986 (46.8) | 14233 (59.4) | 34453 (44) |
| PRA/CPRA, mean (SD) | 5.98 (18.55) | 7.33 (19.81) | 3.84 (13.56) | 8.36 (21.78) | 9.23 (23.10) | 4.63 (16.70) |
| Age at listing: mean (SD), y | 52.45 (12.95) | 51.51 (12.80) | 48.46 (13.88) | 52.10 (13.74) | 57.19 (11.16) | 52.39 (12.76) |
Characteristics are shown both at the time of listing and by outcome at up to 12 years of follow-up. Unless otherwise indicated, values are n (%).
Outcome Predictors
Table 2 shows the hazard ratios for predictors of deceased donor transplant for SCD and ECD kidneys for each blood type, and the C statistic for each model. After inclusion of DSA as a random effect and stratification by blood type, PRA/CPRA was the only significant predictor for deceased donor transplant; higher PRA was associated with lower hazard of deceased donor transplant. C statistics for these models ranged from 0.67 to 0.73, and concordance plots were acceptable (not shown). Table 3 shows the hazard ratios for predictors for living donor transplant, removal from the list due to death or deteriorating condition, and removal from the list due to other reasons. After inclusion of DSA and stratification by blood type, higher PRA/CPRA, older age, and presence of diabetes were associated with lower probability of living donor transplant. Age older than 65 years and male sex were associated with higher probability of removal from the waiting list due to other reasons. The model for mortality on the waiting list was more complex with more variables included to improve predictive accuracy (Table 3), and it was not stratified by recipient blood type. C statistics for these models were lower, ranging from 0.64 to 0.66. In all cases, the C statistics of the model were similar in the development set and the validation set, and no model recalibration was undertaken.
TABLE 2.
Hazard ratios (95% confidence intervals) for predictors of standard criteria and expanded criteria deceased donor transplant
| Donor blood type | ||||
|---|---|---|---|---|
|
|
||||
| Predictor | A | AB | B | O |
| SCD | ||||
| CPRA | — | 0.98 (0.98–0.99) | — | — |
| Spline CPRA > 80 | 0.97 (0.96–0.98) | — | 0.94 (0.93–0.96) | 0.98 (0.97–0.98) |
| DSA SE | 0.71 | 0.55 | 0.69 | 0.60 |
| Concordance (SE) | 0.71 (0.004) | 0.71 (0.013) | 0.72 (0.006) | 0.67 (0.004) |
| ECD | ||||
| CPRA | 0.99 (0.99-0.99) | 0.98 (0.97–0.99) | 0.98 (0.97–0.98) | 0.98 (0.98–0.99) |
| DSA SE | 0.79 | 0.43 | 0.70 | 0.75 |
| Concordance (SE) | 0.73 (0.008) | 0.67 (0.022) | 0.73 (0.012) | 0.72 (0.007) |
All models are stratified by recipient blood type and include DSA as a random effect. Spline CPRA hazard ratio accounts for the different effect noted when CPRA is > 80. DSA is included in the model as a random effect, and the SE demonstrates the heterogeneity in time-to-event between DSAs.
TABLE 3.
Hazard ratios (95% confidence intervals) for predictors of living donor transplant, death or removal from the waiting list due to deteriorating condition, and removal for other reasons
| Reasons for removal from the waiting list | |||
|---|---|---|---|
|
|
|||
| Predictor | Living donor transplant | Death or deteriorating condition | Other |
| CPRA | 0.99 (0.99-0.99) | — | — |
| Age at listing | 0.98 (0.98-0.98) | 1.04 (1.04-1.04) | 1.00 (1.00-1.00) |
| Spline: age > 65 y | — | — | 1.10 (1.09–1.11) |
| Dialysis duration at listing | — | 1.18 (1.17–1.19) | — |
| Spline: dialysis time > 5 y | — | 0.83 (0.82–0.85) | — |
| Male | — | 1.06 (1.03–1.09) | 1.04 (1.00–1.08) |
| Diabetes | 0.54 (0.52–0.56) | 1.69 (1.64–1.73) | — |
| Angina/CAD | — | 1.33 (1.23–1.44) | — |
| Symptomatic CVD | — | 1.21 (1.14–1.29) | — |
| Drug-treated COPD | — | 1.58 (1.45–1.73) | — |
| Drug-treated systemic HTN | — | 0.91 (0.88–0.93) | — |
| Any previous malignancy | — | 1.11 (1.05–1.17) | — |
| Symptomatic PVD | — | 1.38 (1.32–1.45) | — |
| BMI | — | 0.95 (0.94–0.95) | — |
| Spline: BMI > 25 kg/m2 | — | 1.05 (1.04–1.06) | — |
| DSA SE | 0.41 | — | 0.53 |
| Concordance (SE) | 0.66 (0.004) | 0.67 (0.003) | 0.64 (0.005) |
All models include DSA as a random effect. Models for living donor transplant and removal from the list due to other reasons are stratified by recipient blood type. All candidate covariates are shown. Spline hazard ratios account for the different effect noted when age is > 65 years, dialysis time is > 5 years, and BMI is greater than 25 kg/m2. DSA is included in the model as a random effect, and the SE demonstrates the heterogeneity in time-to-event between DSAs.
CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; HTN, hypertension; PVD, peripheral vascular disease.
Outcomes on the Deceased Donor Waiting List
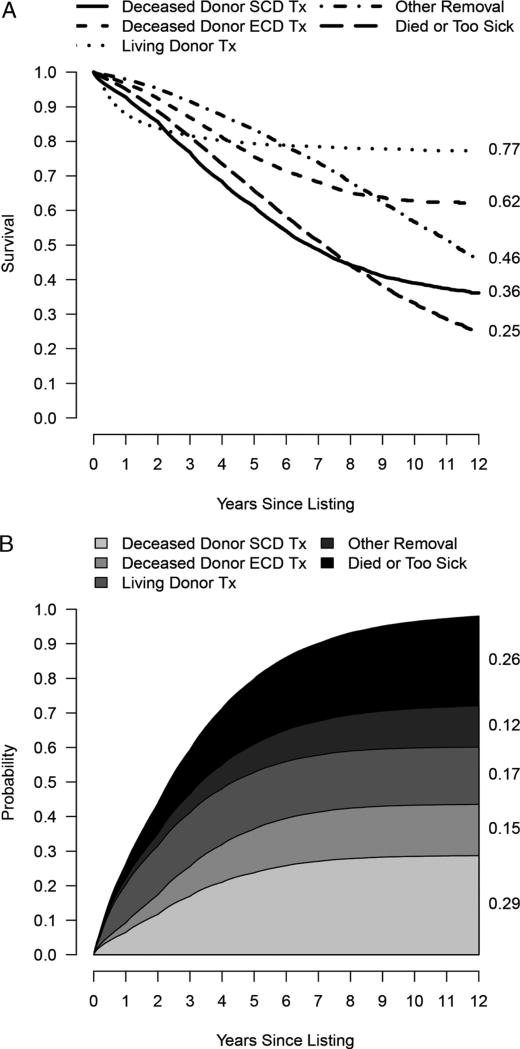
Figure 1A shows Kaplan-Meier survival curves for each of the possible outcomes on the waiting list for the 163 636 patients in the cohort. Figure 1B shows the competing risk cumulative incidence curve for the same cohort, illustrating the different information communicated by these 2 methods. As demonstrated in Figure 1A, the SCD transplant Kaplan-Meier curve censors all other outcomes and reaches 0.5 at 6.7 years for the cohort, whereas the cumulative incidence curve shows a probability of SCD transplant of only 27% at 6.7 years, and a 22% probability of death or removal due to deteriorating medical condition (Figure 1B). The cumulative incidence curve also demonstrates that by 5 years, most candidates have either undergone transplant or have been removed from the waiting list, with very few remaining on the list. Waitlist outcomes varied by blood type, CPRA, and region of the country where the candidate was listed.
FIGURE 1.
For the 163 636 patients in the cohort: A, Kaplan-Meier survival curves for each of the possible outcomes on the waiting list; and B, cumulative incidence curve for each of the competing outcomes on the waiting list, contrasting the information provided by each method.
Waitlist Outcomes Calculator
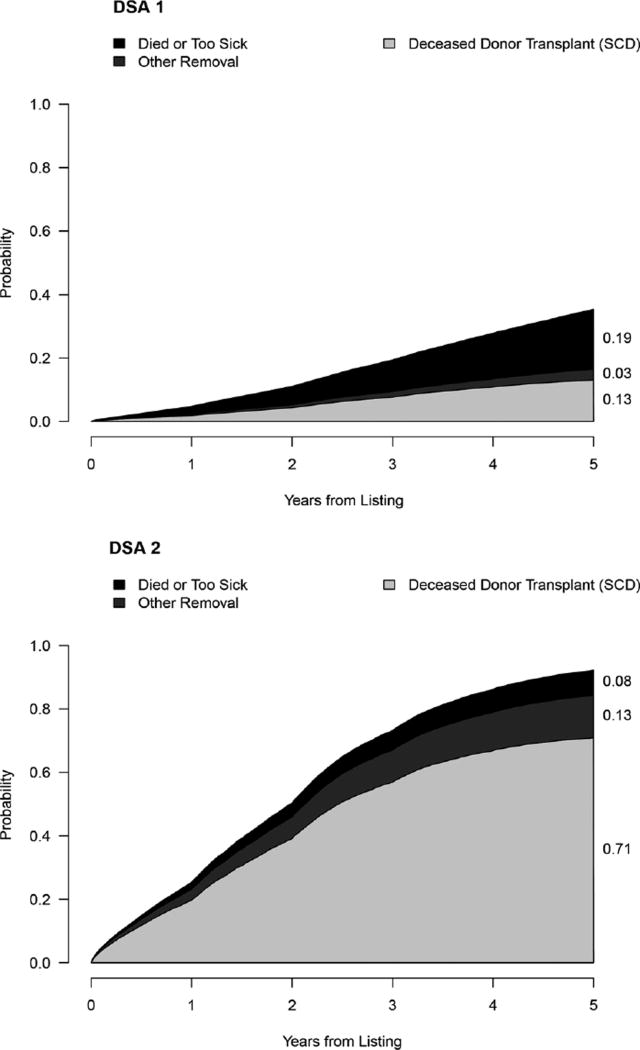
Using the competing risk model, we can estimate the likelihood of any 1 of the 4 outcomes for a given candidate at 5 years. For example, a 50-year-old man with a BMI of 28 kg/m2, PRA/CPRA of 0, blood type A, not on dialysis, with no comorbid conditions, and with no living donor, who is not willing to accept an ECD kidney, would have a 13% probability of undergoing deceased donor transplant at 5 years in DSA 1, where the median time to transplant is 5.1 years, and a 70.8% probability in DSA 2, where the median time to transplant is 1 year. Table 4 illustrates how the competing risks of transplant and death or removal from the list due to deteriorating medical condition are altered as the clinical characteristics of the candidate are changed to increase the risk of death. In this example, the same 50-year-old man's risk of death or removal from the list due to deteriorating medical condition during that time would be 18.9%and 7.9% in DSAs 1 and 2, respectively. If the same candidate had received dialysis for an additional 2 years at listing, his probability of deceased donor kidney transplant would be 12.6%at 5 years in DSA 1 and 25.1% in DSA 2; risk of death would be 25.1% and 10.7% in DSAs 1 and 2, respectively. If he had the additional comorbid conditions of diabetes, hypertension, peripheral vascular disease, angina, previous malignancy, cerebrovascular disease, and chronic obstructive pulmonary disease, his 5-year probability of undergoing transplant would decrease further to 7.1% and 47.2% in DSAs 1 and 2, respectively. Graphical output for the patient described above is shown in Figure 2. An online version of a waitlist calculator for the SRTR website is in development as data on the new kidney allocation system are accumulated.
TABLE 4.
Sample output using the competing risk model calculator for 5-y probability of deceased donor transplant and death or removal from the waiting list in 2 donation service areas, with increasing risk factors for death or removal from the list
| Probabilitya | ||||
|---|---|---|---|---|
|
|
||||
| Deceased donor transplant | Death or waitlist removal due to deteriorating medical condition |
|||
|
|
|
|||
| Candidate characteristics | DSA 1: (Median time to transplant 5.1 y) |
DSA 2: (Median time to transplant 1.0 y) |
DSA 1: (Median time to transplant 5.1 y) |
DSA 2: (Median time to transplant 1.0 y) |
| Age: 50 y | 13.0% | 70.8% | 18.9% | 7.9% |
| Sex: Male | ||||
| CPRA: 0 | ||||
| Blood type: A | ||||
| BMI: 28 kg/m2 | ||||
| Medical history: none | ||||
| With 2 y dialysis duration | 12.6% | 69.1% | 25.1% | 10.7% |
| With history of diabetes, HTN, PVD, angina, previous malignancy, CVD, and COPD | 7.1% | 47.2% | 79.5% | 42.8% |
Probability estimates at 5 years after listing.
FIGURE 2.
Sample waitlist calculator output in 2 different DSAs for a 50-year-old man with a BMI of 28 kg/m2, PRA/CPRA of 0, blood type A, not on dialysis, with no comorbid conditions, with no living donors, who is not willing to accept an ECD kidney, the same theoretical patient shown in Table 4.
DISCUSSION
This analysis illustrates the dramatically different information conveyed by considering competing risks and likelihood of outcomes, information that is not reflected in Kaplan-Meier survival analyses and median time to transplant. To our knowledge, only 1 other study has described use of competing risk models to determine outcomes after listing for deceased donor kidney transplant. Smits et al6 examined waitlist outcomes for patients listed for first kidney transplant between January 1, 1980, and December 31, 1993, in Eurotransplant. In their analysis, they assumed that hazards were constant in intervals of time after listing and analyzed aggregated data within each interval using Poisson regression. They found that the chance of undergoing transplant estimated by Kaplan-Meier methods was 84%, the chance of dying on the waiting list was 45%, and the chance of removal from the list 10 years after listing was 29%. By the competing risk method, however, the chance of undergoing transplant was 74%, the chance of dying was 12%, and the chance of removal from the list within 10 years was 8%. Thus, the Kaplan-Meier method overestimated the chance of transplant at 10 years. Waiting times are no doubt much longer now than when this study was conducted, and older patients with more comorbid conditions are being listed, changing the risk profile and likely outcomes.
Several investigators have used competing risk models to examine waitlist outcomes for liver transplant candidates.7–10 For example, in a single-center study, Kim et al7 found that the Kaplan-Meier method estimated the risk of death among waitlist candidates to be 15% at 1 year and 26% at 3 years; corresponding estimates using a competing risk method were 8% at 1 year and 10% at 3 years when the probability of transplant was included in the analytical competing risk method. In pediatric heart transplant candidates, McGiffin et al11 also reported that the Kaplan-Meier method overestimated risk of death compared with a competing risk method.
When a candidate asks, “How long will I wait for a deceased donor kidney transplant at your center?” giving the median waiting time for kidney recipients at the center does not communicate the risk of death or removal from the list and may leave the impression that the candidate will receive a kidney in that amount of time. Using Kaplan-Meier estimates that treat competing risks, such as death, as mere censoring events produces estimates of waiting time in a counterfactual world where no candidates died. The competing risk method gives a more balanced interpretation of outcomes after listing for a deceased donor transplant, but does not obviate the difficulty caregivers may have in understanding and describing waiting times and the risk of other outcomes while on the waiting list. The best answer to our candidates may be that among 100 patients “like you,” X% will have undergone deceased donor transplant by time t, Y% will have undergone living donor transplant, Z% will have died or been removed from the waiting list due to deteriorating condition, ZZ% will have been removed from the list due to other reasons, and YY% will still be waiting.
The competing risk methodology described here takes into account major characteristics that affect the outcome predictions, such as region, blood type, and number of unacceptable antigens, and is determined using separate cause-specific Cox proportional hazards models for each major outcome. A covariate in a cause-specific Cox proportional hazards regression model explains an effect on the event rate or hazard. With no competing risks, a higher rate associated with a covariate implies that the probability of the event increases, and a lower rate implies that the probability decreases. With competing risks, however, a higher or lower rate may or may not be associated with increasing or decreasing event probability; if a covariate increases the rate of transplant by a small amount but also increases the rate of death by a large amount, the probability of transplant may decrease. If a covariate decreases the rate of transplant by a small amount and the rate of death by a large amount, the probability of transplant may increase.
Developing a calculator to predict risk is a first step toward improving the ability to counsel kidney transplant candidates. Wachterman et al12 recently reported that patients with end-stage kidney disease on hemodialysis significantly overestimated their likelihood of survival. Although nephrologists' survival estimates were somewhat more accurate, they too overestimated their patients' likelihood of survival, suggesting that the knowledge base of most clinicians is not sufficient to accurately counsel patients.12 These findings are consistent with a systematic review showing that patients consistently overestimate benefits and underestimate harms for all medical interventions.13 Whether giving information about risk would change patient or provider practices regarding medical decision making is unknown, but Davison14 reported that 90% of surveyed patients with end-stage kidney disease reported wanting but not receiving prognostic information. Similarly, in focus groups of kidney transplant candidates, patients reported wanting more information that not only is presented concisely but is also relevant to their particular disease state.15,16
Our competing risk model has several important limitations. The models shown here demonstrate the need to change the way information is presented to kidney transplant candidates. However, these models will need to be updated once more data are available on the new kidney allocation system, implemented in December 2014 with the goal of improving overall graft and patient survival by matching kidneys with longer expected graft survival to recipients with longer expected survival. Despite its projected improvements in overall allograft survival, the new allocation policy may disadvantage older candidates with shorter survival expectations,17,18 which only increases the need to change the way information is presented to these candidates. However, a simulation of the distribution of kidneys under the new policy showed that overall deaths on the waiting list will not change substantially19 given the ongoing organ shortage. Early analyses of the new allocation system have shown an initial bolus effect and list reshuffling in the first 3 to 6 months. These rates have begun to settle near previous rates (OPTN November 2015 kidney allocation system monitoring report: http://optn.transplant.hrsa.gov/news/new-data-shows-kidneyallocation-system-continues-to-achieve-goals/). Given the shifts experienced during the early months of the new kidney allocation system, this time period will likely need to be excluded in the creation of new prediction models, because it represents a nonsteady state that will not be predictive of future events. Until more data on the new system are available, a publicly available calculator would be at best rudimentary if not misleading. While we await additional data for an update of the models and creation of a publicly available online calculator, these competing risk analyses demonstrate the need to change the way we counsel kidney transplant candidates and maintain open discussions while moving the field of risk communication in transplantation forward.
Second, this approach is applicable only at the time of registration on the waiting list for deceased donor kidney transplant; it is not applicable to candidates already on the waiting list and provides no information about likely outcomes after transplant. A predictive model for prevalent patients on the waiting list is particularly challenging for kidney transplant candidates due to changing health status and the variable outcomes after starting dialysis. The approach is also not a substitute for the program-specific reports that are case-mix adjusted in evaluating a program's outcomes after kidney transplant. Third, the use of a large data base such as the SRTR limits the ability to include more granular-level data, such as frailty status. However, more parsimonious models using readily available data also make a calculator more clinically useful, the predictive accuracy of any model will always be limited, and adding numerous additional factors is unlikely to improve the predictive accuracy enough to change the fundamental information about likely outcomes that this competing risk calculator provides. In addition, the mandatory reporting and 100% follow-up is a significant strength of the SRTR database.
Finally, a limitation of this type of study is difficulty in reporting the accuracy of the waitlist predictions. The models were developed on a random set of data and validated on a separate set of data. The C statistics for models predicting deceased donor transplant, death or removal due to deteriorating condition, and removal due to other reasons were all only approximately 0.64. The C statistic for the model predicting living donor transplant was higher, 0.72. These C statistics indicate that the outcomes estimate for individual patients are very approximate, and the concordance statistics do not take into account different censoring probabilities due to competing risks. Unfortunately, there is no readily available and understandable method to express the accuracy of individual prediction estimates. Many clinicians are accustomed to looking for confidence intervals; for this competing risk model, the hazards are fit into a cumulative incidence model that forces the total incidence of all outcomes to add up to 1. Therefore, if the probability of 1 outcome increases, the probability of another must decrease, and confidence intervals are not used in this situation. In addition, the Agency for Healthcare and Research Quality has recommended against this type of error information, as it increases confusion and does not add to clinical utility.20 The ability of any model to predict events for an individual patient will always be limited; even perfectly calibrated models for complex disease can only achieve values for the C statistic well below 1 and are necessarily the most relevant measure of a prediction model's accuracy.21 Our calibration plots appeared acceptable and suggest that the estimates are within clinically useful ranges. Although imperfect, a model that demonstrates the general probability of outcomes is infinitely better than the current method of reporting median time to transplant, a metric that obscures actual events and outcomes after listing. The additional information provided by risk calculators may assist providers in counseling kidney transplant candidates and assist candidates in understanding the risk.
In conclusion, it is possible to create a risk calculator to convey the probability of outcomes on the waiting list for kidney transplant candidates, and a competing risk methodology provides important information beyond the median waiting time that is most often reported. The models will require updating to reflect the experience under the new kidney allocation system, but preliminary models demonstrate the very different information about risk on the transplant waiting list that median time to transplant does not convey. More research is needed regarding the most effective method of conveying these risks to patients and regarding the impact of using risk calculators in counseling kidney transplant candidates.
Acknowledgments
The authors thank Scientific Registry of Transplant Recipients colleagues Delaney Berrini, BS, for article preparation, and Nan Booth, MSW, MPH, ELS, for article editing.
This work was conducted under the auspices of the Minneapolis Medical Research Foundation, contractor for the Scientific Registry of Transplant Recipients, as a deliverable under contract no. HHSH250201000018C (US Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation). As a US Government-sponsored work, there are no restrictions on its use. The views expressed herein are those of the authors and not necessarily those of the US Government.
Footnotes
The authors have no conflicts of interest to report.
The authors declare no conflicts of interest.
A.H., N.S., J.J.S., A.K.I., B.L.K. participated in research design. A.H., N.S., J.J.S., A. K.I., B.L.K. participated in the writing of the article. A.H., N.S., J.J.S., A.K.I., B.L.K. participated in the performance of the research. A.H., N.S., A.K.I., B.L.K. participated in data analysis.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
References
- 1.Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2012 annual data report: kidney. Am J Transplant. 2014;14:11–44. doi: 10.1111/ajt.12579. [DOI] [PubMed] [Google Scholar]
- 2.Schold JD, Srinivas TR, Sehgal AR, et al. Half of kidney transplant candidates who are older than 60 years now placed on the waiting list will die before receiving a deceased-donor transplant. Clin J Am Soc Nephrol. 2009;4:1239–1245. doi: 10.2215/CJN.01280209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaston RS, Danovitch GM, Adams PL, et al. The report of a national conference on the wait list for kidney transplantation. Am J Transplant. 2003;3:775–785. doi: 10.1034/j.1600-6143.2003.00146.x. [DOI] [PubMed] [Google Scholar]
- 4.Leppke S, Leighton T, Zaun D, et al. Scientific Registry of Transplant Recipients: collecting, analyzing and reporting data on transplantation in the United States. Transplant Rev (Orlando) 2013;27:50–56. doi: 10.1016/j.trre.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Andersen P, Geskus R, de Witte T, et al. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol. 2012;41:861–870. doi: 10.1093/ije/dyr213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smits JM, van Houwelingen HC, De Meester J, et al. Analysis of the renal transplant waiting list: application of a parametric competing risk method. Transplantation. 1998;66:1146–1153. doi: 10.1097/00007890-199811150-00006. [DOI] [PubMed] [Google Scholar]
- 7.Kim WR, Therneau TM, Benson JT, et al. Deaths on the liver transplant waiting list: an analysis of competing risks. Hepatology. 2006;43:345–351. doi: 10.1002/hep.21025. [DOI] [PubMed] [Google Scholar]
- 8.Washburn K, Harper A, Klintmalm G, et al. Regional sharing for adult status 1 candidates: reduction in waitlist mortality. Liver Transpl. 2006;12:470–474. doi: 10.1002/lt.20768. [DOI] [PubMed] [Google Scholar]
- 9.Ravaioli M, Masetti M, Ridolfi L, et al. Laboratory test variability and model for end-stage liver disease score calculation: effect on liver allocation and proposal for adjustment. Transplantation. 2007;83:919–924. doi: 10.1097/01.tp.0000259251.92398.2a. [DOI] [PubMed] [Google Scholar]
- 10.Cucchetti A1, Cescon M, Bigonzi E, et al. Priority of candidates with hepatocellular carcinoma awaiting liver transplantation can be reduced after successful bridge therapy. Liver Transpl. 2011;17:1344–1354. doi: 10.1002/lt.22397. [DOI] [PubMed] [Google Scholar]
- 11.McGiffin DC, Naftel DC, Kirklin JK, et al. Predicting outcome after listing for heart transplantation in children: comparison of Kaplan-Meier and parametric competing risk analysis. Pediatric Heart Transplant Study Group. J Heart Lung Transplant. 1997;16:713–722. [PubMed] [Google Scholar]
- 12.Wachterman MW, Marcantonio ER, Davis RB, et al. Relationship between the prognostic expectations of seriously ill patients undergoing hemodialysis and their nephrologists. JAMA Intern Med. 2013;173:1206–1214. doi: 10.1001/jamainternmed.2013.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffmann TC, Del Mar C. Patients' expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2015;175:274–286. doi: 10.1001/jamainternmed.2014.6016. [DOI] [PubMed] [Google Scholar]
- 14.Davison SN. End-of-life care preferences and needs: perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:195–204. doi: 10.2215/CJN.05960809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calestani M, Tonkin-Crine S, Pruthi R, et al. Patient attitudes towards kidney transplant listing: qualitative findings from the ATTOM study. Nephrol Dial Transplant. 2014;29:2144–2150. doi: 10.1093/ndt/gfu188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Veer SN, Bekker HL, van Biesen W. How to enhance patient experiences of decision-making about kidney transplantation? Nephrol Dial Transplant. 2014;29:1991–1993. doi: 10.1093/ndt/gfu271. [DOI] [PubMed] [Google Scholar]
- 17.Stegall MD. The right kidney for the right recipient: the status of deceased donor kidney allocation reform. Semin Dial. 2010;23:248–252. doi: 10.1111/j.1525-139X.2010.00723.x. [DOI] [PubMed] [Google Scholar]
- 18.Hippen BE, Thistlethwaite JR, Jr, Ross LF. Risk, prognosis, and unintended consequences in kidney allocation. N Engl J Med. 2011;364:1285–1287. doi: 10.1056/NEJMp1102583. [DOI] [PubMed] [Google Scholar]
- 19.Israni AK, Salkowski N, Gustafson S, et al. New national allocation policy for deceased donor kidneys in the United States and possible effect on patient outcomes. J Am Soc Nephrol. 2014;25:1842–1848. doi: 10.1681/ASN.2013070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hibbard JH, Perters EM. Supporting informed consumer health care decisions: data presentation approaches that facilitate the use of information in choice. Annu Rev Public Health. 2003;24:413–433. doi: 10.1146/annurev.publhealth.24.100901.141005. [DOI] [PubMed] [Google Scholar]
- 21.Cook N. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–935. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]