ABSTRACT
The tet39 tetracycline resistance determinant and the macrolide resistance genes msrE and mphE were found in an 18.2-kb plasmid, pS30-1, recovered from a global clone 2 (GC2) Acinetobacter baumannii isolate from Singapore, that conferred resistance to tetracycline and erythromycin. pS30-1 also contains mobA and mobC genes encoding MOBQ family proteins, but attempts to mobilize pS30-1 utilizing a coresident conjugative repAci6 plasmid were unsuccessful. Eight pdif sites, consisting of inversely oriented binding sites for the XerC and XerD recombinases separated by 6 bp, were detected in pS30-1. The tet39 determinant and the msrE-mphE gene pair are each surrounded by two pdif sites in inverse orientation. Identical regions in different contexts and many previously unnoticed pdif sites were found in a number of different plasmids in GenBank, showing that the tet39 and msrE-mphE dif modules are mobile. A putative toxin/antitoxin system, a gene encoding a serine recombinase, and open reading frames of unknown function were also part of dif modules in pS30-1. In general, modules with internal XerC or XerD sites alternate. Two copies of ISAjo2-1 (94% identical to ISAjo2) in pS30-1 were inserted 5 bp from a XerC site, and this appears to be the preferred insertion site for this insertion sequence (IS) group. Apparently, Acinetobacter plasmids exploit the Acinetobacter XerC-XerD recombinases to mobilize DNA units containing resistance and other genes, via an uncharacterized mechanism. The tet39 and msrE-mphE dif modules add to the oxa24 module and the oxa58 module redefined here, bringing the total of resistance gene-containing dif modules in Acinetobacter plasmids to four.
KEYWORDS: tet39 tetracycline resistance, Acinetobacter baumannii, antibiotic resistance, dif modules, msrE-mphE macrolide resistance, plasmids
INTRODUCTION
Acinetobacter baumannii is an important nosocomial pathogen whose treatment is increasingly problematic due to high levels of extensive antibiotic resistance. In addition to resistance genes located in islands in the chromosomes of globally disseminated clones, global clone 1 and 2 (GC1 and GC2) isolates, a number of resistance genes can be carried by plasmids. Some of these genes are part of defined transposons, such as aphA6 (confers resistance to amikacin, kanamycin, and neomycin) in TnaphA6 (1) and oxa23 (carbapenem resistance) in Tn2006, Tn2008, or Tn2009 (2). These transposons have often been found in large conjugative repAci6 plasmids (1, 3, 4). However, in Acinetobacter species two other resistance genes encoding carbapenemases appear to be mobilized by a novel mechanism. The oxa24 gene and its single base pair variant encoding OXA-72 have been found flanked by inversely oriented XerC-XerD binding sites, or dif-like sites (referred to as pdif sites from here on), in plasmids from various Acinetobacter species (5–8). As this oxa24 module has several different sequences on either side (5–8), and in one case is found twice in different locations and in the opposite orientation in the pAB120 plasmid (8), it is possible to conclude that a module containing the oxa24 gene has moved to several different locations. Each pdif site is 28 bp long and contains the 11-bp binding sites for the XerC and the XerD recombinases in inverse orientation, separated by a 6-bp spacer of variable sequence (5). Re-27 elements initially identified as direct repeats in the sequence broadly surrounding another carbapenemase-encoding gene, the oxa58 gene (9), were later recognized as pdif sites (5). Several other modules surrounded by inversely oriented pdif sites (5), here referred to as dif modules, have been identified, and a putative toxin/antitoxin system in pAB120 that is also in a dif module may also be mobile (8). These modules have been found in unrelated plasmids (5, 6, 8), indicating movement, and the pdif sites are thought to facilitate the mobilization of discrete modules of DNA containing these resistance genes and other genes using the chromosomally encoded XerC-XerD recombination system.
Recently, we found the tet39 tetracycline resistance determinant in pRCH52-1, a small plasmid recovered from a non-GC1 or -GC2 clinical isolate from Brisbane, Australia, but we were unable to find clear boundaries for a module containing these genes using the two additional sequences available in GenBank at the time (10). Here, we have sequenced a GC2 isolate containing a plasmid carrying the tet39 determinant and the msrE and mphE macrolide resistance genes and shown that they are in dif modules. Several further dif modules were also identified.
RESULTS
A. baumannii isolate SGH0823.
The GC2 isolate SGH0823 (also known as S30) was sequence type 2 (ST2) using the Institut Pasteur multilocus sequence typing (MLST) scheme, and in the Oxford Scheme it was ST369 (cpn60-2, gdhB-3, gltA-1, gpi-106, gyrB-3, recA-2, and rpoD-3), a single-locus variant of ST208 varying in the gpi allele. SGH0823 was found to be extensively antibiotic resistant using the criteria for A. baumannii stated in Magiorakos et al. (11). It is resistant to carbapenems (imipenem, meropenem, and doripenem), extended-spectrum cephalosporins (cefotaxime, ceftriaxone, ceftazidime, and cefepime), penicillins, β-lactamase inhibitors (ticarcillin-clavulanic acid, ampicillin-sulbactam, and piperacillin-tazobactam), quinolones (nalidixic acid), fluoroquinolones (ciprofloxacin and levofloxacin), folate pathway inhibitors (trimethoprim and sulfamethoxazole), tetracyclines (tetracycline, minocycline, and doxycycline), and several aminoglycosides (gentamicin, amikacin, neomycin, streptomycin, and spectinomycin). SGH0823 is susceptible to tobramycin, netilmicin, and colistin.
Antibiotic resistance genes.
ResFinder identified 12 resistance determinants. The strA-strB and tet(B) genes (conferring resistance to streptomycin and to tetracycline, minocycline, and doxycycline, respectively) were assigned to AbGRI1, while the blaTEM (ampicillin resistance), aacC1 (resistance to gentamicin), aadA1 (spectinomycin resistance), sul1 (sulfonamide resistance), and aphA1b (resistance to kanamycin and neomycin) genes were assigned to AbGRI2. The structure of these islands will be described in detail elsewhere. The aphA6 gene located in TnaphA6 and the oxa23 gene located in Tn2008 were localized to a 76.9-kb repAci6 plasmid, pS30-2. The macrolide resistance genes msrE and mphE as well as the tet39 resistance determinant were detected on the same 7.9-kb contig.
pS30-1.
The sequence at both ends of the contig containing the tet39 and msrE and mphE genes matched the same end of an insertion element IS related to ISAjo2. The opposite end of the IS was found at both ends of another contig. PCR oriented the fragments and amplified single copies of this IS and the products were sequenced to complete assembly of the sequence of the plasmid pS30-1 (Fig. 1, top line). pS30-1 is 18,234 bp and encodes a replication initiation protein, Rep, that belongs to the Rep_3 superfamily (pfam01051) and contains a putative iteron sequence of four identical repeats of 22 bp adjacent to the rep gene. The Rep protein of pS30-1 is 99.7% (306/307 amino acids [aa]) identical to the Rep protein of pM131-5 (GenBank accession number JX101644) from an Acinetobacter soli (12). Thereafter, the closest Rep protein is RepAci3 (GenBank accession number GU978997), with 91% amino acid identity (278/307 aa). The copy number of pS30-1 relative to the chromosome was estimated to be 10 to 11. The plasmid was transformed into A. baumannii ATCC 17978 via electroporation using SGH0823 genomic DNA and conferred resistance to tetracycline (zone size of 6 mm compared to 20 mm for ATCC 17978) and reduced susceptibility to doxycycline (zone size of 18 mm versus 27 mm) but did not affect the zone size for minocycline. The transformants also showed increased resistance to erythromycin (>132 μg/ml) relative to that of ATCC 17978 (16 μg/ml).
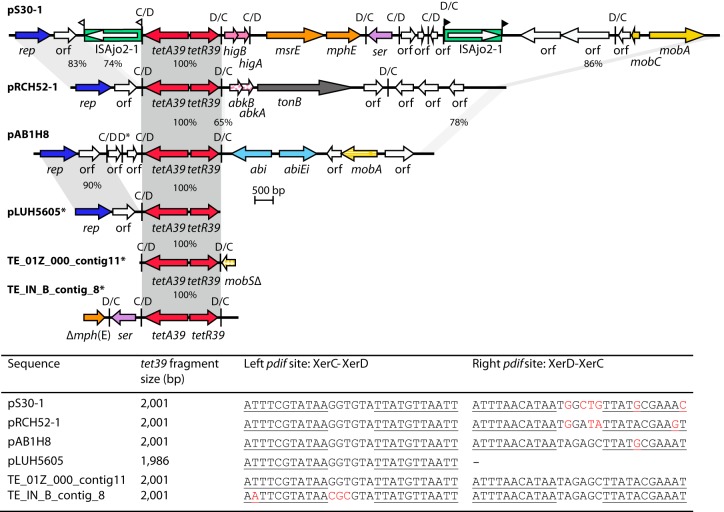
FIG 1.
Linearized map of pS30-1 compared to other regions containing the tet39 resistance determinant module. Partial sequences are marked with an asterisk. Arrows indicate the extents and directions of genes and ORFs. The tetA gene and the tetR genes of tet39 are shown in red, and rep genes are shown in dark blue. Genes encoding putative toxin/antitoxin modules are shown in solid or patterned pink. The msrE and mphE resistance genes are shown in orange, tonB is dark gray, abi genes are light blue, and ser, encoding a serine recombinase, is shown in purple. Genes encoding proteins showing homology to mobilization proteins are shown in yellow. The tonB gene encodes a TonB-dependent transporter homologue. The light green box represents ISAjo2-1, and the arrow inside represents the transposase gene. The extents of regions with significant DNA identities are shown in gray, and the numbers represent DNA identities. Vertical bars indicate pdif sites; the orientation of each site is shown above, and flags indicate target site duplications. The picture is drawn to scale according to the GenBank entries listed in Table 1. The sequences of the pdif sites that flank the tet39 module are shown in the table at the bottom of the figure. The bases highlighted in red are different.
tet39 is located between pdif sites.
Manual inspection of the region surrounding tetA39 and tetR39 in pS30-1 revealed that pdif sites flank these genes. The segment containing the tet39 resistance determinant located between the flanking pdif sites is 2,001 bp in size, and, as previously found for the oxa24 gene, the flanking sites are in inverse orientation (XerC-XerD and XerD-XerC). The same 2,001-bp segment was also found surrounded by pdif sites in plasmid pRCH52-1 although this was not noted previously (10). The original sequence for the tet39 determinant in pLUH5605 (GenBank accession number AY743590) was found to contain a pdif site but does not extend far enough to include the pdif site to the right. The tet39 module is also in pAB1H8 (see Table 1 for details of sequences), and close inspection of this sequence revealed three pdif sites that were not annotated. Previously unnoticed pdif sites were also found in the partial sequences TE_01Z_00_contig11 and TE_IN_B_contig8 (Fig. 1). As the sequences on each side of the region surrounded by the pdif sites are different in each of the structures shown in Fig. 1, it is possible to conclude that the tet39 dif module is mobile.
TABLE 1.
Sequences used during this study
| Sequence name | Sequence length (bp) | No. of pdif sitesa | Sourceb | GenBank accession no. | Reference or source |
|---|---|---|---|---|---|
| pS30-1 | 18,234 | 8 | A. baumannii | KY617771 | This study |
| pRCH52-1 | 11,164 | 3 | A. baumannii | KT346360 | 10 |
| pAB1H8 | 10,246 | 3 | A. baumannii | ANNC01000048 | 28 |
| pLU5605 | 3,727 | 1 | Acinetobacter | AY743590 | 29 |
| TE_01Z_000_contig 11 | 2,446 | 2 | Uncultured bacterium | KU545777 | 30 |
| TE_IN_B_contig_8 | 3,953 | 3 | Uncultured bacterium | KU544458 | 30 |
| EF102240c | 13,122 | 4 | A. baumannii | EF102240 | 31 |
| pABIR | 29,823 | 13 (2) | A. baumannii | EU294228 | 18 |
| pOXA58_882 | 36,862 | 16 (2) | A. pittii | CP014479 | |
| pXBB1-9 | 398,857 | 12 (9) | A. johnsonii | CP010351 | 16 |
| p255n-1 | 92,939 | 9 | A. baumannii | KT852971 | 32 |
Number of pdif sites identified in this study. If any pdif or Re-27 site was identified previously, the number found is in parentheses.
A. pittii, Acinetobacter pittii; A. johnsonii, Acinetobacter johnsonii.
This sequence is of a cloned insert from a larger plasmid from A. baumannii A1.
The msrE-mphE gene pair is surrounded by pdif sites.
Two inversely oriented pdif sites were also found flanking a 2,950-bp segment including the msrE and mphE macrolide resistance genes in pS30-1 (Fig. 2), and the presence of the pdif sites is consistent with this being a separate mobile unit. A search of the nucleotide database with this segment revealed a number of other plasmids and partial sequences that contain the same segment (Table 1), and these sequences were inspected for the presence of pdif sites (Fig. 2). A total of 41 additional pdif sites were found. However, the msrE-mphE dif module is always adjacent to another dif module carrying a toxin-antitoxin gene pair (see below) on one side and, in all but one case, adjacent to a gene encoding a serine recombinase on the other. These macrolide resistance genes are also found in Tn6180 located in AbGRI3 in some GC2 isolates (13) and in the closely related transposon Tn1548 (14) but the dif module is incomplete (Fig. 2, bar at bottom). In these transposons, the pdif site on the right is present and followed by a third sequence on this side. However, the pdif site on the left is missing, and a 120-bp segment next to it has been replaced by an ISEc29.
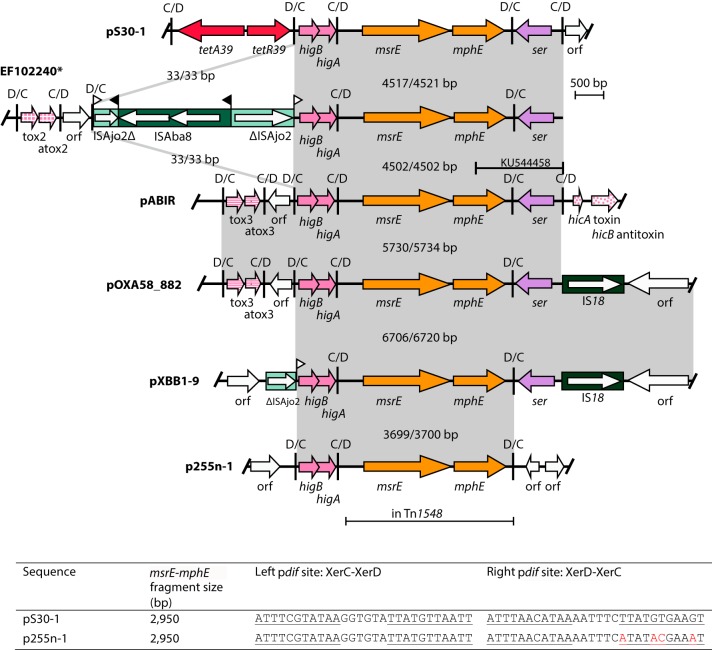
FIG 2.
Comparison of sequences containing the msrE and mphE resistance genes. Partial sequences are marked with an asterisk. Arrows indicate the extents and directions of genes and ORFs and are colored according to the scheme used in Fig. 1. The light green box indicates ISAjo2-1, while the dark green box indicates IS18; the internal arrows represent the transposase gene. Vertical bars indicate pdif sites; the direction of each site is shown above, and flags indicate target site duplications. Regions with significant DNA identity are shown in gray along with the respective base pair ratios. The picture is drawn to scale from the GenBank entries listed in Table 1. The table below the figure shows the two sequences of pdif sites that flank the msrE and mphE genes. The pdif sites of pS30-1 are identical to those of all of the plasmids shown except for those of p255n-1, for which variant bases are highlighted in red.
Additional dif modules in pS30-1.
In addition to the two modules containing antibiotic resistance genes described above, four other potential dif modules surrounded by inversely oriented pdif sites were identified in pS30-1. One, located between the tet39 and the msrE-mphE modules, contains two overlapping open reading frames (ORFs) that encode a putative toxin/antitoxin system. The first ORF produces a 107-aa protein of the HigB toxin family, while the second translates to a 91-aa protein with a helix-turn-helix DNA binding domain, likely performing the function of the counterpart antitoxin HigA (15). This module was found in the same position in all of the plasmids containing a complete msrE-mphE module (Fig. 2).
Downstream of the msrE-mphE module in pS30-1 is a region encoding a small serine recombinase (203 aa), here named ser, surrounded by inversely oriented pdif sites. The ser gene is also found following the msrE-mphE module in all available sequences except that of p255-1 (Fig. 2). However, in sequences that contain ser, there are two variants based on whether a pdif site in inverse orientation to the left-hand site of this module is present on the right. pS30-1, TE_IN_B_contig_8 (KU544458) and pABIR form one group where there is a pdif site on the right. EF102240 is also a member of this group although available sequence extends only 4 bp into the XerC binding site of the right pdif. The right pdif sequence and 29 bp of the ser module directly prior to it have been replaced by 33 bp of unrelated sequence and then an IS18 in pOXA58_882 and pXBB1-9. No site resembling a XerC-XerD binding site is found in the sequence directly following the IS18. It is possible that a recombination event has occurred in the res site associated with ser.
Another module in pS30-1, encoding a protein of no known function, is surrounded by inversely oriented pdif sites (Fig. 1, top line), and the pdif site upstream of the mobC gene and the first pdif site following the repA gene are also in inverse orientation. Two other segments that include open reading frames of unknown function are bounded by pdif sites in direct orientation, and whether these have resulted from the fusion of adjacent modules causing loss of an intervening pdif site or whether they are functional remains to be established.
ISAjo2-1.
The two copies of ISAjo2-1, the 1,482-bp insertion sequence identified in pS30-1, share 94% nucleotide identity with ISAjo2 (16), and the encoded transposase is 97% identical to that of ISAjo2. Like ISAjo2, ISAjo2-1 has 24-bp inverted repeats with 20 bp identical and produces a 5-bp target site duplication. Interestingly, both copies of this IS in pS30-1 (Fig. 1) and the ISAjo2 in EF102240 (Fig. 2) are located 5 bp from the XerC side of a pdif site, with the IS facing in the same direction with respect to the orientation of the pdif site. This indicates that these ISs may target a specific location in an orientation-specific manner. If so, their presence will point to pdif sites.
Mobilization genes in pS30-1.
pS30-1 encodes a 479-aa protein that shares 31% amino acid identity with parts of the 710-aa MobA of RSF1010 (GenBank accession number NC_001740), the prototype for the MOBQ family of mob genes (17). The reading frame upstream of mobA in pS30-1 translates to an 88-aa protein that shares 42% identity with the 95-aa MobC protein of RSF1010. The presence of mobA and mobC genes indicates that pS30-1 may be able to be mobilized if a suitable conjugative plasmid is also present in the cell. SGH0823 contains a conjugative repAci6 plasmid (pS30-2) that carries Tn2008 and was found to transfer ticarcillin resistance at a frequency of 2.3 × 10−5 transconjugants/donor. The ability of pS30-2 to mobilize pS30-1 was tested by selecting for the presence of transconjugants exhibiting tetracycline resistance in addition to rifampin and ticarcillin resistance. Only rifampin- and ticarcillin-resistant transconjugants were recovered. Though pS30-1 was not mobilized by the repAci6 plasmid, it may be mobilized by a different type of conjugative plasmid.
DISCUSSION
The fact that the tet39 module is flanked by various different sequences allowed it to be defined. However, it is likely to include only one pdif site, and which pdif site should be included is not clear as there is variation in the spacer sequence in both the pdif site on the left and in the pdif site on the right. The finding that the tet39 determinant and the msrE-mphE gene pair are each part of mobile dif modules adds to those carrying the oxa24 and oxa58 genes (5–9, 16, 18), bringing the number of antibiotic resistance determinants found in dif modules in Acinetobacter plasmids to four. In addition to the antibiotic resistance genes, several toxin-antitoxin gene combinations were found in dif modules (Fig. 1 and 2), and these likely contribute to the stability of any plasmid carrying them. Additional modules identified in this study mostly encode hypothetical proteins of unknown function.
ISAjo2-1 identified here and ISAjo2 (16) are currently placed in the IS1202 group in the ISNCY (not characterized yet) category. Our searches revealed that a relative of ISAjo2 with 90% nucleotide identity or higher is present in 10 additional sequences in GenBank. In each case, a putative XerC binding site of a pdif site was found 5 bp or, in a few cases, 6 bp away from the same end of the IS. Another IS in this group, ISAba32, has also been found in an A. baumannii plasmid, pD36-4, that is part of the D36 genome (19). ISAba32 is also 1,482 bp in size and creates a 5-bp direct duplication upon insertion; however, its inverted repeats are slightly longer at 26 bp with 20 bp identical. The sequence of ISAba32 is 73.3% identical to that of ISAjo2, and the encoded transposase is 72% identical to that of ISAjo2. Interestingly, examination of the sequence adjacent to the copy of ISAba32 in pD36-4 (GenBank accession number CP012956) revealed that it was also inserted 5 bp from an XerC binding site in a pdif site. A number of unnamed ISs with sequence identity to ISAba32 of 89% or higher were found in seven entries in GenBank. In each of these sequences, the IS is again located 5 bp away from a XerC binding site and always in the same orientation. It appears that the variants of ISAjo2 and ISAba32 both target the same sequence, a XerC binding site, although how this targeting is achieved is unclear; thus, further investigation of the ISAjo2 group of insertion sequences is warranted. Nonetheless, these ISs can serve to pinpoint pdif sites that may otherwise be difficult to find.
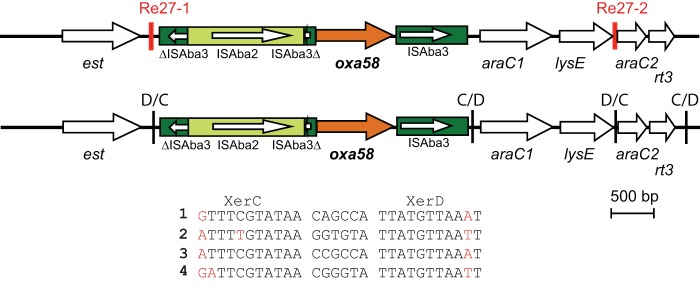
In most publications to date, only a limited number of pdif sites (or Re-27 repeats) or none of them have been recorded, and whether other sites are in the broader vicinity has not been investigated. This is likely due to the difficulty in finding them. A feature of regions containing one or more dif modules that emerged from our analysis of all pdif sites in pS30-1 and in several other plasmids is the fact that, in most cases, the pdif sites are inversely oriented. This inevitably leads to two types of dif modules, one flanked by a XerC-D and a XerD-C site (i.e., the XerD binding sites are both closer to the internal segment) and the other type flanked by a XerD-C and a XerC-D site, making the XerC sites internal. In cases where more than one dif module is present, this feature dictates that D type and C type modules should alternate, and this is usually seen (Fig. 1 and 2). As the Re-27 sites surrounding the oxa58 gene were identified as direct repeats (9, 16, 18), we examined the original sequence of the fragment from isolate MAD (GenBank accession number AY665723) for additional pdif sites. As shown in Fig. 3, two additional pdif sites were found, both in opposite orientation to the Re-27 sites. One creates a smaller dif module containing oxa58 and an adjacent module containing the genes usually annotated as araC and lysE. The second pdif site discloses an additional adjacent dif module. Similarly, inversely oriented pdif sites were found surrounding oxa58 in pOXA_58_882 and pABIR. We found 15 dif modules (8 D type and 7 C type) in pOXA_58_882 and 12 dif modules in pABIR (6 D type and 6 C type). In both cases, D type and C type modules alternate. However, two regions of pS30-1 are surrounded by directly oriented pdif sites, and searches did not reveal an additional site within them. Whether these are fusions of two dif units may become apparent as other sequences become available, but a clear fusion can be seen in the case of the ser module (Fig. 2), and the incomplete msrE-mphE gene module in Tn1548 suggests that such events do occur.
FIG 3.
Location of pdif sites in the region surrounding the oxa58 gene in a fragment from a plasmid of A. baumannii MAD. Arrows indicate the extents and directions of genes, and the oxa58 gene is shown in dark orange. The light green box indicates ISAba2, while the dark green box indicates ISAba3; the internal arrows represent the transposase gene. Vertical red and black bars indicate the Re-27 and pdif sites, respectively, and the orientation of the pdif site is shown above the bars. The figure is drawn to scale based on GenBank accession number AY665723. In the sequences of the pdif sites, the numbers 1 to 4 correspond to the sites from left to right, and the bases that vary are highlighted in red.
A pair of dif modules was recently found in Proteus mirabilis (20), and this represents the first time that dif modules have been found outside the Acinetobacter genus. One of the modules is related to the smaller oxa58 module described above, and the second is a novel module that includes an ampC gene that confers resistance to amoxicillin. In this case, the pair of dif modules, oxa58 with XerC sites internal and ampC with XerD sites internal, was present three times in a single array. This configuration clearly should preserve the alternation of an XerC-D and an XerD-C site seen in Acinetobacter plasmids and raises the possibility that dif modules may usually move in pairs.
While there is now clearly sufficient evidence to conclude that dif modules are discrete mobile elements and some of the features associated with them are emerging, further work will be needed to investigate the mechanism of movement of dif modules. A catalogue all of the dif modules present in other Acinetobacter plasmids, such as those carrying the oxa24 and oxa58 genes, compiled by careful examination of sequence surrounding other dif modules should provide additional useful insights. Experimental investigation of module movement will be needed to understand the mechanism and demonstrate that XerC and XerD are involved.
MATERIALS AND METHODS
Bacterial isolate.
SGH0823, a GC2 A. baumannii, was isolated in 2008 from Singapore General Hospital and was kindly supplied by Li Yang Hsu. The isolate was screened for resistance to 30 antibiotics using a disc diffusion method as described previously (21). Colistin susceptibility was determined by Etest (bioMérieux, Durham, NC).
DNA isolation, sequencing, and PCR amplification.
Genomic DNA for sequencing was prepared as described previously (22) and sequenced using Illumina MiSeq. Paired-end reads (read depth of 67.6) were assembled into contigs using SPAdes (version 3.5.0) (23), yielding 62 contigs ranging from 202 to 462,003 bp, with an N50 of 262,554. Primers tet39-R (10), RH2502 (5′-AACAGGGATGTTCGGGCTAT-3′), RH2503 (5′-TGTCGCTTTGAGAGTTAGGC-3′), and RH2504 (5′-TCGTATTGGTTCGCTCGGTA-3′) were used to assemble pS30-1. PCR conditions used to detect short and long amplicons were as described previously (24), and amplicons were sequenced. Sequencher, version 3.2.3 (Gene Codes Corporations, Ann Arbor, MI, USA), was used for final sequence assembly, and Gene Construction Kit, version 4 (Textco, West Lebanon, NH, USA), was used to draw figures to scale.
Sequence analysis.
Multilocus sequence typing (MLST) in the Institut Pasteur and Oxford schemes was performed via the A. baumannii MLST database (http://pubmlst.org/abaumannii). The coverage of plasmid contigs relative to the average coverage for contigs containing the genes used in both MLST schemes was used to assess copy number. Contigs containing antibiotic resistance genes were identified using ResFinder (https://cge.cbs.dtu.dk/services/ResFinder/) (25). Contigs containing fragments of IS26 and an ISAjo2-like insertion sequence were recovered using stand-alone BLAST (26). DNA and amino acid sequences were compared to those in the GenBank nucleotide and protein databases using nucleotide and protein BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi), and the sequences used for comparison are listed in Table 1. The ISFinder database (https://www-is.biotoul.fr) (27) was used to compare ISs. The pdif sites in plasmids were located by first identifying a sequence matching the less variable XerD binding site (5′-ATTTAACATAA-3′) and then examining the sequence 6 bp in either direction for a site resembling the XerC binding site (5′-TTATGCGAAAT-3′) of Acinetobacter.
Transformation and conjugation.
ATCC 17978 cells (tetracycline susceptible) were grown to an optical density at 600 nm (OD600) of 0.4 to 0.7 and made electrocompetent with a series of washes with 10% glycerol. Chromosomal DNA (2 μl of 2,940 ng/μl) was transformed by electroporation into competent cells using a 0.2-cm cuvette and the following parameters: 2.5 kV, 25 μF, and 200 Ω. Following recovery in LB medium, transformants were selected on L-agar containing 4 mg/liter of tetracycline and were screened for the presence of the tetA39 gene (10). Transformants were tested for resistance to tetracycline, doxycycline, and minocycline using a disc diffusion method as described previously (21). Resistance to erythromycin was determined by patching ATCC 17978 and transformants on Muller-Hinton agar (cation adjusted) containing various concentrations of erythromycin (2, 8, 16, 32, 64, and 128 μg/ml).
A spontaneous rifampin-resistant mutant of the sulfonamide-resistant A. baumannii strain ATCC 19606 was isolated for use as a recipient in conjugation experiments. Matings were performed as described previously (4), and transconjugants were recovered on L-agar plates containing rifampin (100 mg/liter) to select for the recipient and with ticarcillin (100 mg/liter) to select for the transfer of the repAci6 plasmid which carries the oxa23 gene. Potential transconjugants were purified and screened on L-agar containing kanamycin (20 mg/liter), to which the donor is resistant and the recipient is susceptible, to eliminate spontaneous rifampin-resistant derivatives of the donor. To detect mobilization, transconjugants were selected on L-agar containing tetracycline (4 mg/liter), ticarcillin (100 mg/liter), and rifampin (100 mg/liter).
Accession number(s).
The sequence of pS30-1 has been submitted to GenBank under accession number KY617771.
ACKNOWLEDGMENTS
We thank Li Yang Hsu from Singapore General Hospital for supplying the isolate SGH0823 used in this study. We also thank Ian Grainge for providing the predicted sequences of the XerC and XerD binding sites of Acinetobacter.
Funding for this study was received from National Health and Medical Research Council grant 1079616. G.A.B. is supported by an Australian Postgraduate Award.
REFERENCES
- 1.Nigro SJ, Hall RM. 2014. Amikacin resistance plasmids in extensively antibiotic-resistant GC2 Acinetobacter baumannii from two Australian hospitals. J Antimicrob Chemother 69:3435–3437. doi: 10.1093/jac/dku310. [DOI] [PubMed] [Google Scholar]
- 2.Nigro SJ, Hall RM. 2016. Structure and context of Acinetobacter transposons carrying the oxa23 carbapenemase gene. J Antimicrob Chemother 71:1135–1147. doi: 10.1093/jac/dkv440. [DOI] [PubMed] [Google Scholar]
- 3.Hamidian M, Kenyon JJ, Holt KE, Pickard D, Hall RM. 2014. A conjugative plasmid carrying the carbapenem resistance gene blaOXA-23 in AbaR4 in an extensively resistant GC1 Acinetobacter baumannii isolate. J Antimicrob Chemother 69:2625–2628. doi: 10.1093/jac/dku188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamidian M, Holt KE, Pickard D, Dougan G, Hall RM. 2014. A GC1 Acinetobacter baumannii isolate carrying AbaR3 and the aminoglycoside resistance transposon TnaphA6 in a conjugative plasmid. J Antimicrob Chemother 69:955–958. doi: 10.1093/jac/dkt454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Andrea MM, Giani T, D'Arezzo S, Capone A, Petrosillo N, Visca P, Luzzaro F, Rossolini GM. 2009. Characterization of pABVA01, a plasmid encoding the OXA-24 carbapenemase from Italian isolates of Acinetobacter baumannii. Antimicrob Agents Chemother 53:3528–3533. doi: 10.1128/AAC.00178-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merino M, Acosta J, Poza M, Sanz F, Beceiro A, Chaves F, Bou G. 2010. OXA-24 carbapenemase gene flanked by XerC/XerD-like recombination sites in different plasmids from different Acinetobacter species isolated during a nosocomial outbreak. Antimicrob Agents Chemother 54:2724–2727. doi: 10.1128/AAC.01674-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grosso F, Quinteira S, Poirel L, Novais A, Peixe L. 2012. Role of common blaOXA-24/OXA-40-carrying platforms and plasmids in the spread of OXA-24/OXA-40 among Acinetobacter species clinical isolates. Antimicrob Agents Chemother 56:3969–3972. doi: 10.1128/AAC.06255-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Povilonis J, Seputiene V, Krasauskas R, Juskaite R, Miskinyte M, Suziedelis K, Suziedeliene E. 2013. Spread of carbapenem-resistant Acinetobacter baumannii carrying a plasmid with two genes encoding OXA-72 carbapenemase in Lithuanian hospitals. J Antimicrob Chemother 68:1000–1006. doi: 10.1093/jac/dks499. [DOI] [PubMed] [Google Scholar]
- 9.Poirel L, Nordmann P. 2006. Genetic structures at the origin of acquisition and expression of the carbapenem-hydrolyzing oxacillinase gene blaOXA-58 in Acinetobacter baumannii. Antimicrob Agents Chemother 50:1442–1448. doi: 10.1128/AAC.50.4.1442-1448.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamidian M, Holt KE, Pickard D, Hall RM. 2016. A small Acinetobacter plasmid carrying the tet39 tetracycline resistance determinant. J Antimicrob Chemother 71:269–271. doi: 10.1093/jac/dkv293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 12.Huang TW, Lauderdale TL, Liao TL, Hsu MC, Chang FY, Chang SC, Khong WX, Ng OT, Chen YT, Kuo SC, Chen TL, Mu JJ, Tsai SF. 2015. Effective transfer of a 47 kb NDM-1-positive plasmid among Acinetobacter species. J Antimicrob Chemother 70:2734–2738. doi: 10.1093/jac/dkv191. [DOI] [PubMed] [Google Scholar]
- 13.Blackwell GA, Holt KE, Bentley SD, Hsu LY, Hall RM. 2017. Variants of AbGRI3 carrying the armA gene in extensively antibiotic-resistant Acinetobacter baumannii from Singapore. J Antimicrob Chemother 72:1031–1039. doi: 10.1093/jac/dkw542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Zorn B, Catalan A, Escudero JA, Dominguez L, Teshager T, Porrero C, Moreno MA. 2005. Genetic basis for dissemination of armA. J Antimicrob Chemother 56:583–585. doi: 10.1093/jac/dki246. [DOI] [PubMed] [Google Scholar]
- 15.Tian QB, Ohnishi M, Tabuchi A, Terawaki Y. 1996. A new plasmid-encoded proteic killer gene system: cloning, sequencing, and analyzing hig locus of plasmid Rts1. Biochem Biophys Res Commun 220:280–284. doi: 10.1006/bbrc.1996.0396. [DOI] [PubMed] [Google Scholar]
- 16.Feng Y, Yang P, Wang X, Zong Z. 2016. Characterization of Acinetobacter johnsonii isolate XBB1 carrying nine plasmids and encoding NDM-1, OXA-58 and PER-1 by genome sequencing. J Antimicrob Chemother 71:71–75. doi: 10.1093/jac/dkv324. [DOI] [PubMed] [Google Scholar]
- 17.Francia MV, Varsaki A, Garcillan-Barcia MP, Latorre A, Drainas C, de la Cruz F. 2004. A classification scheme for mobilization regions of bacterial plasmids. FEMS Microbiol Rev 28:79–100. doi: 10.1016/j.femsre.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Zarrilli R, Vitale D, Di Popolo A, Bagattini M, Daoud Z, Khan AU, Afif C, Triassi M. 2008. A plasmid-borne blaOXA-58 gene confers imipenem resistance to Acinetobacter baumannii isolates from a Lebanese hospital. Antimicrob Agents Chemother 52:4115–4120. doi: 10.1128/AAC.00366-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamidian M, Hawkey J, Holt KE, Hall RM. 2015. Genome sequence of Acinetobacter baumannii strain D36, an antibiotic-resistant isolate from lineage 2 of global clone 1. Genome Announc 3:e01478–. doi: 10.1128/genomeA.01478-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girlich D, Bonnin RA, Bogaerts P, De Laveleye M, Huang DT, Dortet L, Glaser P, Glupczynski Y, Naas T. 2017. Chromosomal amplification of the blaOXA-58 carbapenemase gene in a Proteus mirabilis clinical isolate. Antimicrob Agents Chemother 61:e01697-16. doi: 10.1128/AAC.01697-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nigro SJ, Hall RM. 2016. Loss and gain of aminoglycoside resistance in global clone 2 Acinetobacter baumannii Australia via modification of genomic resistance islands and acquisition of plasmids. J Antimicrob Chemother 71:2432–2440. doi: 10.1093/jac/dkw176. [DOI] [PubMed] [Google Scholar]
- 22.Nigro SJ, Post V, Hall RM. 2011. Aminoglycoside resistance in multiply antibiotic-resistant Acinetobacter baumannii belonging to global clone 2 from Australian hospitals. J Antimicrob Chemother 66:1504–1509. doi: 10.1093/jac/dkr163. [DOI] [PubMed] [Google Scholar]
- 23.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamidian M, Hall RM. 2011. AbaR4 replaces AbaR3 in a carbapenem-resistant Acinetobacter baumannii isolate belonging to global clone 1 from an Australian hospital. J Antimicrob Chemother 66:2484–2491. doi: 10.1093/jac/dkr356. [DOI] [PubMed] [Google Scholar]
- 25.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. 2009. BLAST+: architecture and applications. BMC Bioinformatics 10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. 2006. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res 34:D32–D36. doi: 10.1093/nar/gkj014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho AY, Chow KH, Law PY, Tse H, Ho PL. 2013. Draft genome sequences of two multidrug-resistant Acinetobacter baumannii strains of sequence type ST92 and ST96. Genome Announc 1:e00296-13. doi: 10.1128/genomeA.00296-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agerso Y, Guardabassi L. 2005. Identification of Tet 39, a novel class of tetracycline resistance determinant in Acinetobacter spp. of environmental and clinical origin. J Antimicrob Chemother 55:566–569. doi: 10.1093/jac/dki051. [DOI] [PubMed] [Google Scholar]
- 30.Pehrsson EC, Tsukayama P, Patel S, Mejia-Bautista M, Sosa-Soto G, Navarrete KM, Calderon M, Cabrera L, Hoyos-Arango W, Bertoli MT, Berg DE, Gilman RH, Dantas G. 2016. Interconnected microbiomes and resistomes in low-income human habitats. Nature 533:212–216. doi: 10.1038/nature17672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poirel L, Mansour W, Bouallegue O, Nordmann P. 2008. Carbapenem-resistant Acinetobacter baumannii isolates from Tunisia producing the OXA-58-like carbapenem-hydrolyzing oxacillinase OXA-97. Antimicrob Agents Chemother 52:1613–1617. doi: 10.1128/AAC.00978-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schultz MB, Pham Thanh D, Tran Do Hoan N, Wick RR, Ingle DJ, Hawkey J, Edwards DJ, Kenyon JJ, Phu Huong Lan N, Campbell JI, Thwaites G, Thi Khanh Nhu N, Hall RM, Fournier-Level A, Baker S, Holt KE. 2016. Repeated local emergence of carbapenem-resistant Acinetobacter baumannii in a single hospital ward. Microbial Genomics 2:e000050. doi: 10.1099/mgen.0.000050. [DOI] [PMC free article] [PubMed] [Google Scholar]