Abstract
Angiogenesis is a pivotal process for growth, invasion and spread of the majority of solid tumors including melanoma. Anti‐angiogenic agents have not been systematically tested in patients with advanced melanoma. Clinical efficacy of angiogenesis inhibitors targeting endothelial cells has not been as affirmative as initially hoped and improved clinical outcomes have been observed in combination with chemotherapy or additional drugs for many types of human cancer. However, angiogenesis is not only dependent on endothelial cell invasion and proliferation, it also requires pericyte coverage of vascular sprouts for stabilization and maturation of vascular walls. Recent data suggest that pericytes might be able to confer resistance to anti‐vascular endothelial growth factor (VEGF) therapy. This review will focus on the significance of the vascular phenotype but also on the impact of pericyte‐mediated vessel maturation for the susceptibility to anti‐angiogenic therapy, including malignant melanoma, which we identified as crucial factor regarding therapeutic efficacy.
Keywords: Melanoma, Angiogenesis, Pericytes, Vessel maturation, anti-Angiogenic therapy
Abbreviations
- Ang-2
angiopoietin-2
- bFGF
basic fibroblast growth factor
- Flt-1
VEGF receptor 1
- Hif
hypoxia-inducible factor
- IL-8
Interleukin-8
- KDR
VEGF receptor 2
- MMP
matrix metalloproteinases
- MVD
microvessel density
- NG-2
chondroitin sulfate proteoglycan
- NO
nitric oxide
- PHD
Prolyl hydroxylase
- PDGF-B
platelet-derived growth factor B
- PDGFR-β
PDGF receptor β
- PlGF
placental growth factor
- PTK/ZK
PTK787/ZK222584
- RGS-5
regulator of G protein signaling 5
- SD
standard deviation
- vSMC
vascular smooth muscle cells
- VEGF
vascular endothelial growth factor
- VEGFR
VEGF receptor
1. Introduction
Angiogenesis, the formation of new vessels from pre‐existing blood vessels and the remodeling of the newly formed vascular network, are widely considered as essential procedures to nourish the growing tumor and to remove metabolic waste products (Folkman, 1971). It was first described by Judah Folkman in 1971 to occur as a consequence of tumor hypoxia, which is a ubiquitous phenomenon in invasive cancer when they reach approximately 1 mm3 in size or if the distance between vessels is larger than 100–250 μm (Folkman, 1971). Intratumoral hypoxia leads to the stabilization and expression of the pro‐tumorigenic hypoxia‐inducible factor (Hif)α transcription factors. Under normal oxygen levels, Hif‐1α and Hif‐2α are short‐lived because prolyl hydroxylase (PHD) proteins mediate their degradation (Fong and Takeda, 2008). During the process of hypoxia, PHD proteins show reduced activity, which results in the formation of heterodimerization by Hif‐1α and Hif‐2α with Hif‐1β. This complex is able to trigger the expression of genes containing hypoxia‐responsive elements, including the gene encoding vascular endothelial growth factor (VEGF) (Qing and Simon, 2009). Hypoxia is able to initiate a cascade of cellular signaling leading to vasodilation via release of nitric oxide (NO) (Kim et al., 1998), increase of permeability of the vascular network by VEGF and angiopoietin‐2 (Ang‐2), and degradation of the vascular basement membrane via extracellular matrix via matrix metalloproteinases (MMPs) (Greenberg et al., 2008). Under hypoxic conditions, pericytes are able to secrete VEGF which can act in a paracrine manner on endothelial cell proliferation (Reynolds et al., 2000). In consequence, the quiescent vascular network is getting disturbed and activated.
Whereas blood vessel growth is tightly controlled under physiological conditions, tumor progression is highly associated with the acquisition of an angiogenic phenotype based on a transition from the avascular to the vascular phase (Folkman et al., 1989). This process is called the “angiogenic‐switch”, in which the balance of pro‐ and anti‐angiogenic molecules leads toward angiogenic inducers (Folkman and Hanahan, 1991; Hanahan and Weinberg, 2000). Multiple signals are able to trigger the angiogenic switch, including metabolic and mechanical stress, inflammatory processes or genetic mutations (Carmeliet and Jain, 2000). Tumor vessels are formed by the recruitment of circulating endothelial progenitor cells, which are derived form haemangioblasts that undergo a process of aberrant differentiation within the tumor milieu (Peters et al., 2005). Tumor vasculature is often characterized by the presence of poorly developed so called “immature” vessels with multiple branch‐points and deregulated vessel spouting which contributes to tumor hypoxia. Neovascularization enhances tumor cell proliferation, local invasion and hematogenous metastasis. However, the abnormalities of tumor vessels provide the potential for targeting these vessels without destroying the normal vasculature (Arap et al., 2002).
Tumor vascularization is a dynamic process in terms of new vessel formation by angiogenesis and remodeling of existing vessels (Folkman, 2006; Gilead and Neeman, 1999). Tumor vessels express novel molecules which can be serve as selective therapeutic targets (St et al., 2000) and show structural and functional abnormalities like destabilization, which results in enhanced vascular leakage, important for the accessibility of drugs to tumor compartments (Hashizume et al., 2000; Hobbs et al., 1998). The vascular stabilization process is mediated by mural cells, which are getting subdivided in pericytes and vascular smooth muscle cells (vSMCs) (Benjamin et al., 1998; Hirschi et al., 1998). Pericytes and pericyte‐like cells have been implicated as mediators of several processes associated with tumor angiogenesis and metastasis. Although the role of pericytes and their impact on anti‐angiogenic drug resistance, is still not clear and controversial discussed. The benefit of targeting both, pericytes and endothelial cells in tumor vessels, has been shown in several tumor models (Bergers et al., 2003; Erber et al., 2004; Nisancioglu et al., 2010) and therefore receptor tyrosine kinase inhibitors that block both VEGFRs and plateled‐derived growth factor receptors (PDGFR)s have been presented to be more efficacious in combination than single used (Bergers et al., 2003; Erber et al., 2004).
In the case of human cutaneous melanoma, the impact on tumor‐induced angiogenesis for disease progression and metastasis remains controversial (Fallowfield and Cook, 1991; Folkman et al., 1989; Ilmonen et al., 1999). It is currently not known whether the angiogenic phenotype of melanomas changes between the transition from primary to metastatic disease, but this seems to be an important area of investigation in further studies, because it appears that it is predominantly immature tumor vasculature that shows sensitivity to angiogenesis inhibitors (Feron, 2004; Helfrich et al., 2010). In addition, alternative growth factor‐independent mechanisms have been reported, both experimentally and in human tumors, including melanoma (Dome et al., 2002; Paku, 1998) which will be discussed in more detail in the following article. These aspects lead to the assumption, that compounds that may be efficient inhibitors of angiogenesis and tumor growth in angiogenesis‐dependent tumors its effects may be limited in growth factor‐independent tumors utilizing a quiescent and mature vascular network. Because clinical experience using anti‐angiogenic drugs without targeting additional pathways is still limited in case of melanoma, we will briefly review novel data concerning first hints for the biological significance of the vascular bed and the impact of pericyte‐mediated vessel maturation for the outcome of anti‐VEGF therapy in human melanoma.
2. Impact of tumor angiogenesis for melanoma disease
Malignant melanoma is a neoplastic disease of increasing incidence with poor prognosis and limited therapeutic options, particularly in advanced tumors. The 5‐year‐survival rate of advanced tumors with distant metastases is still as low as 5–10% (Balch et al., 2009). Early detection and surgical excision therefore continue to be the key variables for long‐term survival and eventual cure. The identification of early stage III disease is still challenging and consequently among the primary objectives of cancer follow‐up programs. Induction of tumor angiogenesis on melanoma was first described after transplantation of human melanoma fragments into a hamster check pouch (Hillen et al., 2006). However, the clinical and prognostic significance of tumor angiogenesis for melanoma progression and metastasis, as evidenced by the determination of intratumoral microvessel densities and detection of VEGF expression has remained controversial. Various studies have established an association between microvessel density (MVD) and the progression of malignant melanoma from the radial to the vertical growth phase, which signals a change to the potentially metastatic phenotype of malignant melanoma (Erhard et al., 1997; Marcoval et al., 1997), although heterogeneity was observed between individual cases (Barnhill et al., 1992). In addition, the onset of angiogenesis in thin melanomas was associated with inflammatory regression and induction of the vertical growth phase (Barnhill and Levy, 1993). In contrast, a number of retrospective histological studies have reported an inverse correlation between tumor microvessel density and disease‐free and overall survival of melanoma patients (Erhard et al., 1997; Graham et al., 1994; Hillen et al., 2006; Srivastava et al., 1988; Straume et al., 1999). Several other investigators, in contrast, failed to detect any correlation between melanoma vascularization and prognosis (Barnhill et al., 1994; Kiss et al., 2007). Thus, the potential prognostic value of tumor vascularization in human cutaneous melanomas remains unresolved. Reasons for these contradictory results might be explained by non‐standardized assessment of tumor vascularity, such as using ‘hot spot’ or ‘representative field’ analysis. In addition, usage of various histological markers for the detection of tumor‐associated blood vessels (e.g. CD31, CD34, CD105) might also be a reason for the diversity of results in existing reports, e.g. CD31 is also expressed by lymphatic vessels. Taking these observations together, it is evident that, although there is a strong argument in favor of a positive relationship between angiogenesis, tumor progression and metastasis, a large study examining MVD across all thicknesses of melanoma, and how it correlates with prognosis and rate of relapses, is still missing.
3. Angiogenic cytokines in melanoma
The growth of solid primary tumors, such as melanoma, and the process of lymph node and organ metastases beyond a size of 1–2 mm3 are always accompanied by the initiation of angiogenesis for maintenance of expanding malignant cell population with oxygen and nutrients (Folkman, 1971). Angiogenesis in melanoma is stimulated by a variety of growth factors such as VEGF and basic fibroblast growth factor (bFGF) (Ribatti et al., 2003). Sprouting of capillaries from pre‐existing blood vessels is accomplished by a hypoxia‐driven mechanism within which VEGF‐A has been identified as the most potent inducer of the angiogenic cascade (Neufeld et al., 1999). Although VEGF has been described as an autocrine endothelial cell regulator of angiogenesis and vascular homeostasis (Lee et al., 2007), VEGF is produced by most tumor cells and acts paracrine on tumor‐associated endothelial cells (Kerbel, 2008). Tumor cell VEGF production results as a consequence of oncogenic activation and the increasing hypoxia of a growing tumor nodule with an avascular or hypovascular center (Carmeliet, 2005b; Mazure et al., 1996). Consequently, most tumors, including melanomas, express high levels of VEGF (Einspahr et al., 2007; Ugurel et al., 2001). Enhanced secretion of VEGF‐A by melanoma cells has already been detected in dysplastic lesions (Einspahr et al., 2007) and correlated to the transition from the radial to the vertical growth phase and development of metastases (Erhard et al., 1997; Salven et al., 1997). It is shown that increased microvascular density, strong VEGF‐A tumor immunoreactivity, increased vascular diameter and high number of vascular pillars are correlated with a high Breslow index (>3.6 mm) (Ribatti et al., 2005).
Another important stimulator of melanoma angiogenesis is the placental growth factor (PlGF) (Lacal et al., 2000). PlGF‐1 and ‐2 are expressed by melanoma cells and known to bind neuropillin‐1 and ‐2 receptors expressed on endothelial cells (Odorisio et al., 2006). Neuropilin‐1 is able to act as a co‐receptor for VEGF‐A, resulting in increased binding affinity of VEGF‐A to the VEGFR receptor (VEGFR)‐2 (Whitaker et al., 2001). In addition, PlGF acts through its binding to VEGFR‐1 inducing the mobilization and recruitment of VEGFR‐1+ hematopoietic precursors from bone marrow and enhancing blood vessel maturation by acting on VEGFR‐1‐expressing smooth muscle cells/pericytes (Donnini et al., 1999). PlGF is known to form heterodimers with VEGF‐A and enhances melanoma angiogenesis by activating VEGFR‐2 on endothelial cells (Donnini et al., 1999; Luttun et al., 2004).
Expression of bFGF (FGF‐2) has been detected in metastatic and primary invasive melanoma whereas no expression was found in melanocytes of benign nevi (Reed et al., 1994). In addition, a significant correlation between melanoma progression, percentage of FGF‐2 expressing tumor cells and the number of mast cells which, in turns, secrete other angiogenic factors, such as VEGF‐A, was reported (Salven et al., 1997).
Interleukin‐8 (IL‐8) expression was found to be dramatically increased in a majority of cutaneous melanoma compared to normal epidermis and benign lesions (Bar‐Eli, 1999). Melanoma‐derived IL‐8 is able to induce endothelial cell migration, modulate vascular permeability and enhances stress fiber formation. These activities resulted in enhanced angiogenesis, rapid tumor growth and increased metastatic potential (Bar‐Eli, 1999; Mahler et al., 2004; Melnikova and Bar‐Eli, 2006). Several studies have reported that increased serum level of VEGF, bFGF and several other angiogenic factors such as IL‐8, Ang‐2, angiogenin and endostatin are correlated with disease progression, poor progression‐free and overall survival of melanoma patients (Bar‐Eli, 1999; Birck et al., 1999; Donnini et al., 1999; Helfrich et al., 2009; Kurschat et al., 2007; Ugurel et al., 2001; Westphal et al., 2000). Yet, all hitherto analyzed angiogenesis biomarkers have little prognostic impact for individual prognosis of tumor patients.
4. Pericyte‐mediated blood vessel maturation
Blood vessels are composed of two distinct cell types: Endothelial cells and mural cells. Depending on their density, morphology, location and marker expression, mural cells can be subdivided into pericytes and vSMCs that form the outer layers of the vascular wall (Sims, 2000). vSMCs are associated with arteries and veins where they form multiple concentric layers, whereas pericytes are embedded within the basement membrane of capillaries, either as solitary cells or a single‐cell layer and share their basal membrane with the endothelial cell (Gerhardt and Betsholtz, 2003). Based on the multiple cytoplasmic processes, distinctive cytoskeletal elements and envelopment of endothelial cells, pericytes are generally considered to be contractile cells and participate in the regulation of blood flow in the microcirculation (Shepro and Morel, 1993). Mural cells presenting phenotypes between pericytes and vSMA are associated with arterioles and venules (Gerhardt and Betsholtz, 2003). The marker profile of pericytes varies depending on the tissue of origin, whereas the most common markers being desmin, alpha‐smooth muscle actin (SMA), PDGFR (Bergers and Song, 2005), chondroitin sulfate proteoglycan (NG‐2) (Bondjers et al., 2003; Schlingemann et al., 1991) and the regulator of G protein signaling 5 (RGS‐5) (Bondjers et al., 2003). Only little is known about the marker expression variability of pericytes in tumors because most studies have used only a single marker, usually α‐SMA or desmin, and have therefore equated lack of immunoreactivity with lack of pericytes. Published reports suggested that the amount of pericyte coverage on vessels in different tumors ranges from extensive to little or none (Benjamin et al., 1999; Eberhard et al., 2000). One possible explanation for these controversial reports might be therefore based on the marker expression among tumors.
Pericytes and endothelial‐pericyte signaling mechanisms have been implicated as mediators of several cancer associated processes like tumor angiogenesis and metastasis. Tumor pericytes appear to play a critical role in regulating vessel maturation, stabilization, quiescence and function, even where they are less abundant and more loosely attached to vessels compared to healthy tissue (Benjamin et al., 1998; Hellstrom et al., 1999, 2001; Hirschi and D'Amore, 1996).
Compared to quiescent pericytes, activated pericytes can change their expression profile (Benjamin et al., 1998), leading to phenotypes that are highly proliferative with the pluripotent ability to differentiate into other pericytes, matrix‐forming cell, vSMCs, or adipocytes. They are thought to be at least partially responsible for the irregular, tortuous and leaky blood vessels found within tumors (Abramsson et al., 2003; Morikawa et al., 2002). Preclinical and clinical studies have therefore focused on endothelial/mural cell‐interaction as putative targets in tumor therapy (Jain, 1998). Although the role of pericytes in tumor pathophysiology is still not identified and controversial findings by targeting pericyte‐endothelial cell interactions have been reported.
The interplay of PDGF‐B, secreted by endothelial cells, and PDGFR‐β, expressed on pericytes, is an essential process for mural cell recruitment and prevention of vessel leakage during development (Andrae et al., 2008; Armulik et al., 2005; Betsholtz et al., 2005; Carmeliet, 2005a; Hellstrom et al., 1999). PDGF‐B is thereby secreted as a homodimer from the endothelium of angiogenic sprouts where it serves as an attractant for co‐migrating pericytes, expressing PDGFR‐beta (‐β). In addition, PDGF‐B stimulates proliferation of vSMCs and induces mural cell fate in undifferentiated mesenchymal cells (Gaengel et al., 2009). PDGF‐B binds to heparan sulfate proteoglycans on the cell surface or in the extracellular matrix through its C‐terminal retention motif. Extracellular retention limits the range of action of PDGF‐B, which is important for the tight adhesion formation between pericytes and the vessel wall (Gaengel et al., 2009). It has been shown in murine models, that ablation of PDGF‐B or PDGFR‐β results in mural cell deficiency leading to widespread vascular leakage and pericyte lethality (Lindahl and Betsholtz, 1998). Although mutant mice lacking the C‐terminal retention motif in PDGF‐B are viable but have fewer pericytes, which are poorly integrated into the vascular wall, especially in the retina and kidney (Lindblom et al., 2003). It has recently been shown that PDGF‐BB increases tumor pericyte coverage by activation of stromal‐derived‐factor‐1A (SDF‐1A) based on the observation, that the ensuing SDF‐1A chemotaxis gradient corresponds to PDGF‐BB induced pericyte recruitment, partially because of increased pericyte motility (Song et al., 2009). Inhibition of PDGFR signaling by using a receptor tyrosine kinase inhibitor SU6668 causes pericyte detachment and vessel regression, followed by diminished tumor growth in several tumor (Bergers et al., 2003; Reinmuth et al., 2001; Shaheen et al., 2001a). Increased expression levels of PDGF‐AA, ‐BB and its receptor PDGFR‐β could also be detected in human melanoma tissue by using immunohistochemistry (Barnhill et al., 1996).
A second important mediator for vascular maturation and endothelial cell survival is the angiopoietin (Ang)/Tie system. The Ang family of secreted growth factors consists of four members Ang‐1 and Ang‐2, and the orthologes Ang‐3 (murine) and Ang‐4 (human) and two corresponding tyrosine kinase receptors (Tie1 and Tie2). The angiopoietins Ang‐1 and Ang‐2 have been identified as agonistic and antagonistic ligands of Tie2 (also known as TEK) (Fiedler and Augustin, 2006; Suri et al., 1996). Constitutive Ang‐1/Tie2 signaling is required to maintain the quiescent phenotype of the vascular endothelium. Ang‐1 binds to the Tie2 receptor on endothelial cells to establish pericyte‐endothelial interactions by inhibition of proliferation and stabilization of newly formed blood vessels, promoting vascular maturation. Ang‐1 is predominantly expressed by perivascular and mural cells (Davis et al., 1996; Sundberg et al., 2002; Wakui et al., 2006) suggesting a paracrine mode of action. In addition, Tie2 signaling induces endothelial cells to express and secrete endothelial‐derived heparin binding epidermal‐like growth factor (HB‐EGF), which binds to epidermal growth factor receptors and promote migration of pericytes (Iivanainen et al., 2003).
Ang‐2 acts as a context specific antagonist of Ang‐1/Tie2 signaling. During the angiogenic activation of the endothelium by cytokines, Ang‐2 is getting rapidly released from Weibel‐Palade bodies of the endothelial cells, where it is stored in the quiescent vessel situation (Fiedler et al., 2004). As such, it destabilizes the quiescent EC phenotype through its binding to Tie2 in competition with Ang‐1, reduces pericyte coverage and destabilization of the vascular wall, even in the presence of VEGF stimulation (Cao et al., 2007; Imhof and urrand‐Lions, 2006). Inhibition of Tie2 blocks mural cell recruitment to developing vessels and leads to increased expression of MT1‐MMP by endothelial cells (Yana et al., 2007). Yet, Ang‐2 has also been reported to be capable of acting as an agonist of Tie2 (Teichert‐Kuliszewska et al., 2001). The major expression site for Ang‐2 is the endothelium itself, suggesting an autocrine function (Fiedler et al., 2006), although expression in perivascular cells including vSMCs and pericytes has been reported as well (Maisonpierre et al., 1997; Wakui et al., 2006). In human umbilical vein endothelial cells (HUVECs), VEGF induces Tie2 shedding in an Akt/Pl3K‐dependent process. It has been hypothesized that this contributes to Ang‐2 mediated destabilization of the quiescent endothelial cell phenotype, priming the cells for pro‐angiogenic ligand stimulation and vessel development (Findley et al., 2007).
Ang‐2 thereby acts in tumors primarily as promoter of angiogenesis which has made it an attractive target for therapeutic intervention (Oliner et al., 2004). Endothelial cell secretion of Ang‐2 is therefore readily detectable in the circulation which has stimulated the exploitation of circulating soluble Ang‐2 (sAng‐2) in the blood as biomarker of endothelial cell activation (Orfanos et al., 2007; Wang et al., 2007). In recent years, significant upregulation of circulating soluble Ang‐2 (sAng‐2) was found in tumor patients e.g., in esophageal squamous cell cancer (Zhou et al., 2007), hepatocellular carcinoma (Kuboki et al., 2008), lung cancer (Park et al., 2007) and also in patients of malignant melanoma (Helfrich et al., 2009). Sera from progressing melanoma patients over time identified increasing concentrations of sAng‐2, suggesting that Ang‐2 might be functionally involved in melanoma angiogenesis, and therefore in melanoma progression and metastasis. It has been shown in vivo as well as in vitro that Ang‐2 expression by melanoma cells significantly contributed to the detected levels of circulating sAng‐2, whereas high levels of sAng‐2 were associated with stage of disease (Figure 1A) and poor patient overall survival (Figure 1B). (Helfrich et al., 2009). In addition, silencing of Ang‐2 in melanoma cells has been shown to reduce the invasive and migratory capacity of the tumor cells (Helfrich et al., 2009). Tie2 expression by melanoma cells, in combination with the preliminary characterization of a functional autocrine Ang‐2/Tie2 loop in primary human tumor‐derived melanoma cells, has been made the Ang‐2/Tie2 signaling system as a putative therapeutic target for human melanomas. Intense efforts are presently made to generate and validate ligand neutralizing Ang‐2 antibodies (Oliner et al., 2004), soluble Tie receptor traps and small molecular weight Tie2 receptor tyrosine kinase inhibitors to therapeutically interfere with Ang/Tie signaling (Lin et al., 1997). First clinical trials targeting the vessel destabilization argent Ang‐2 in patients of malignant melanoma will be initiated in the future, however, detailed and careful analyses in large cohorts of melanoma patients with different disease progress are needed for validating the Ang/Tie system for its anti‐angiogenic and anti‐tumor therapeutic impact.
Figure 1.
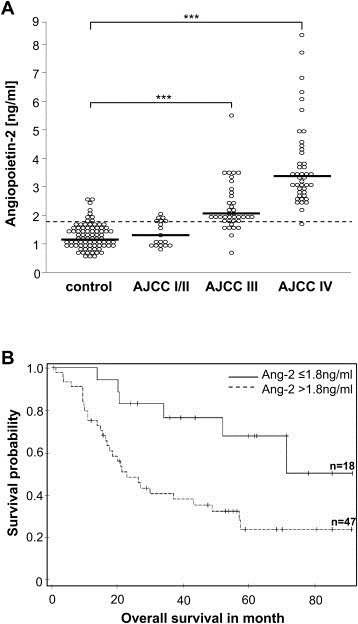
Soluble Ang‐2 levels in patients of malignant melanoma. (A) Detection of circulating Ang‐2 levels (sAng‐2) in different stages of melanoma patients (AJCC I/II to AJCC IV) compared to healthy control patients (ctrl). Soluble Ang‐2 levels in patients with malignant melanoma stage III (n = 37) and IV (n = 43) were significantly elevated compared to the control population (n = 82) (Wilcoxon rank sum test ctrl vs. stage III p < 0.0001; ctrl vs. stage IV p < 0.0001). Soluble Ang‐2 was not elevated in serum of stage I/II melanoma patients (p = 0.08). The dotted line indicates cut‐off for Ang‐2 (1.8 ng/ml) determined as 90% quantile of the control population. Median values of the experimental groups are visualized by the horizontal lines. (B) Correlation of sAng‐2 levels with overall survival in patients of malignant melanoma. Kaplan–Meier curves of melanoma patients with sAng‐2 concentrations in serum exceeding the cut‐off value at1.8 ng/ml (dotted line) or sAng‐2 concentrations of less than 1.8 ng/ml (solid line).
5. Angiogenesis‐independent growth mechanisms in melanoma
Neo‐vascularization has been considered as synonymous with directed vessel ingrowth in almost all of these studies, but alternative, growth factor independent, mechanisms have been reported, both experimentally and in human tumors (Holash et al., 1999). It has been shown for some human cancers, including non‐small cell lung carcinomas (Pezzella et al., 1997) and human glioma (Holash et al., 1999), that tumors in more natural settings do not always originate with vascular involvement, particularly when they arise within or metastasize to vascularized tissue. In such settings, tumor cells have the ability to incorporate ‘co‐opt’ host vessels (Leenders et al., 2002b), which has also been shown as an important mechanism during development of melanoma of the brain (Kusters et al., 2002) and cutaneous melanoma (Dome et al., 2002). It has been shown in both, human cutaneous melanoma but also in murine melanoma models, that the peritumoral vascular plexus presented at the melanoma base was continuously being incorporated into the growing tumor mass during progression. In addition, ultrastructural analyzes presented a pericyte‐mediated stabilization of the mature vascular network of the incorporated vessels (Dome et al., 2002). The interplay between co‐option of existing vessels and subsequent tumor‐induced angiogenesis has still not been extensively examined nor the role of the angiogenic factors during this processes.
Although increased tumor angiogenesis has been generally associated with increased metastasis, it has been demonstrated that tumor vascularization is not a marker of metastasis in the case of malignant melanoma (Barnhill et al., 1994; Kiss et al., 2007). Experimental studies have been shown, that cancer cells have the ability to create “mosaic” vessels by localization into the vascular wall of tumors where both, endothelial cells and tumor cells, form the luminal surface (Chang et al., 2000).
Recent observations have also suggested that aggressive melanoma cells may be able to generate vascular channels independent of tumor angiogenesis. This phenomenon is called “vasculogenic mimicry” in which some melanoma cells appear to acquire the capability to form blood channels in the absence of endothelial cells (Hendrix et al., 2001; Maniotis et al., 1999).
Parallel with progression, melanoma acquires a vascular network, whereas an increasing number of tumor cells express the laminin receptor, which enables their adhesion to the vascular wall, favoring tumor cell extravasation and metastases (Mahabeleshwar and Byzova, 2007). The process of tumor cell extravasation have been described for human melanoma and mouse models, where melanoma cells occupy a “pericyte‐like” location on the abluminal surface of the endothelium and migrate along these structure which allows the development of distant metastases without induction of angiogenesis (Barnhill and Lugassy, 2004; Lugassy et al., 2004).
6. Vascular phenotype and its impact on anti‐VEGF therapy in melanoma
VEGF is the main angiogenic ligand targeted to date. Several strategies against VEGF‐A (Ferrara et al., 2004; Leenders and Lubsen, 2002a; Siemeister et al., 1998) or its receptors, VEGF receptor (VEGFR)‐1 (Flt‐1) and its major signaling receptor, VEGFR‐2 (KDR/Flk‐1) (Kunkel et al., 2001; Sweeney et al., 2002) have been developed including neutralizing humanized antibodies. Another way to efficiently perturb VEGF‐A signaling is to block the kinase activity of VEGF‐receptors by small‐molecule inhibitors, such as sorafenib, sunitinib or PTK787/ZK222584 (Escudier et al., 2007; Hess‐Stumpp et al., 2005; Thomas et al., 2007). They have been shown to potently prevent tumor neovascularization and progression of many tumor types. By using human melanoma xenograft models, the inhibition of VEGF via neutralizing antibodies, VEGF antisense and RNA interference resulted in decreased tumor growth and metastases (Li et al., 2002; Oku et al., 1998). Bevacizumab, a humanized monoclonal IgG antibody targeting VEGF, has been shown anti‐angiogenic effects and consequent clinical benefits in a number of solid tumors, including colorectal, breast and non‐small‐lung cancer.
However, targeting VEGF signaling alone is often not sufficiently effective at inducing vessel regression and does not prevent re‐growth of tumor vessels (Baluk et al., 2005). Because pericytes are discussed to protect the vascular network against anti‐VEGF therapies, studies have been initiated targeting the pericyte‐endothelial cell association or pericytes alone which should result in enhanced vessel destabilization. It has recently been shown in a murine tumor model, developing spontaneously multiple malignant melanomas, but also for human melanoma metastases taken at clinical relapse in patients undergoing adjuvant treatment using bevacizumab, that tumor vessels resistant to anti‐VEGF therapy were characterized by enhanced vessel diameter and normalization of the vascular bed by coverage of mature pericytes, and immunoreactivity for desmin, NG‐2, PDGFR‐β and the late stage maturity marker α‐SMA (Figure 2A–D) (Helfrich et al., 2010). In addition, tumor vessels resistant to anti‐VEGF therapy showed only minor expression of pro‐angiogenic factors and its corresponding receptors (e.g. Ang‐2/Tie2, VEGF‐A/VEGFR‐2), resulting in low‐angiogenic potential and resistance to the traditional anti‐angiogenic approaches (Helfrich et al., 2010). Therefore, it is highly desirable to identify alternative ways to modulate tumor vasculature that do not interfere with the complex regulatory network of pro‐angiogenic factors.
Figure 2.
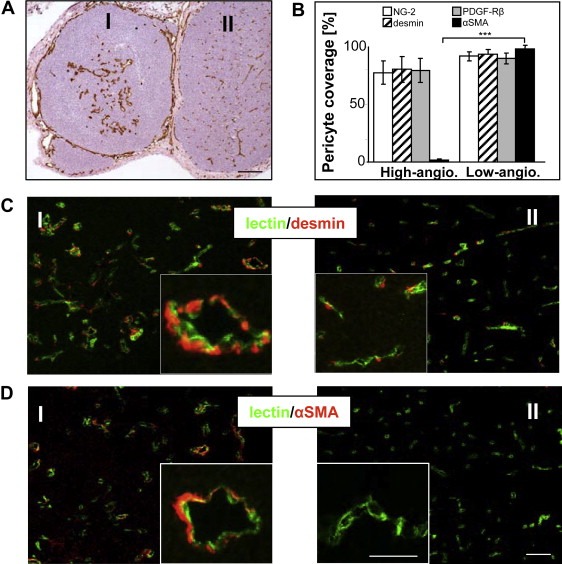
Immunohistological analyses of mural cell maturation and stabilization of the vascular network in melanoma of MT/ret‐transgenic mice. (A) Representative image for immunoperoxidase detection of blood vessels by using the endothelial marker CD31 in melanoma of low‐ (I) and high‐ (II) angiogenic potential. (B) Quantification of vessel coverage, calculated as the percentage of NG‐2‐, desmin‐, PDGFR‐β‐ or α‐SMA‐positive cells compared to the number of CD31‐positive vessels, ∗∗∗p ≤ 0.001. (C) Immunohistochemical double‐staining for the endothelial marker CD31 (green) and the early pericytic marker desmin (red) in the corresponding low‐(I) and high‐vascularized (II) tumor, as well as (D) for the late differentiation marker α‐SMA (red). Representative images of more than 10 independently performed experiments were presented. Error bars, mean ± SD. Scale bar A: 50 μm, C, D: 100 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
This has lead to the speculation that targeting tumor pericytes might be a suitable strategy for overcoming pericyte‐mediated resistance to VEGF pathway inhibition. It has been shown that vascular regression could result in vessel normalization and therefore in re‐opening of previously collapsed vessels (Padera et al., 2004). Based on this knowledge, this could explain, how PDGF pathway inhibition leads to improved drug delivery (Baranowska‐Kortylewicz et al., 2005; Jayson et al., 2005; Pietras et al., 2002). PDGF inhibition has been shown to disrupt pericyte support and sensitized endothelial cells to anti‐angiogenic chemotherapy, which resulted in regression of pre‐existing tumor vasculature in a model for malignant melanoma (Pietras et al., 2002; Pietras and Hanahan, 2005). But based on the fact that pericyte targeting therapies are resulting in tumor vessel normalization followed by re‐opening of pre‐collapsed vessels, this could also result in increased tumor expansion, progression and metastases based on enhanced tumor supply with oxygen and nutrients, and would therefore result in a poor patient outcome.
Several studies have reported controversial results by combining VEGF and PDGF signaling pathway inhibition. It have been shown that inhibition of PDFR‐β and VEGF resulted in enhanced blockage of tumor angiogenesis compared to VEGF inhibition alone (Erber et al., 2004; Farhadi et al., 2005; Shaheen et al., 2001a, 2001b). In contract, it has recently been published that the absence of pericytes does not increase the sensitivity of tumor vessels to VEGF‐blockage in a pericyte deficient mouse model and also by using transplantation experiments (Nisancioglu et al., 2010). However, a human clinical trial for renal carcinoma showed that inhibition of VEGF and PDGF pathways resulted in no therapeutic benefit compared to single anti‐VEGF treatment, in fact, the combined regimen exhibited increased toxicity (Hainsworth et al., 2005). In addition, in melanoma patients, a phase II trial investigating both targets PDGFR and c‐kit, was disappointing in its outcome (Ugurel et al., 2005).
Thus, the fundamental question about whether targeting of pericytes enhances the anti‐tumor effect elicited by anti‐VEGF‐A therapies remains unanswered. Additional clinical studies are needed to clarify the potential benefits and risks associated with anti‐pericyte cancer therapy.
7. Clinical trials targeting angiogenesis in melanoma
The different biological aspects need to be considered and are being addressed in the clinical setting separately. This leads to approaches trying to neutralize pro‐angiogenic ligands, blockade of cell receptor‐dependent signaling, inhibition of critical pathways involved in survival and proliferation of endothelial cells and/or pericytes and attempts to positively modulate the tumor‐matrix interaction.
8. Neutralization of pro‐angiogenic ligands
The major pro‐angiogenic player which can be targeted at the moment is VEGF. Its critical role in preclinical models in vitro as well as in vivo has been described above. Neutralization of VEGF is being attempted by various approaches including neutralizing antibodies, and VEGF‐trap. Bevacizumab is a humanized monoclonal antibody designed to bind to VEGF leading to clinical beneficial effects in various solid tumors including breast, renal and colorectal cancers which led to its global registration for this diseases. Clinical efficacy data in melanoma using bevacizumab are accumulating but are not sufficiently convincing for the company to go for registration yet. Clinical efficacy of bevacizumab as single‐agent was quite limited with a median overall survival and PFS comparable to DTIC. One of the earlier studies included 32 patients with metastatic melanoma who were randomized to receive bevacizumab (15 mg/kg intravenously every 2 weeks) with or without low‐dose IFN‐alpha2b (1 MU/m2 subcutaneously daily). Prolonged disease stabilization was achieved in one‐quarter of metastatic melanoma patients. Low‐dose IFN‐alpha2b did not augment the activity of bevacizumab (Varker et al., 2007). Similarly, bevacizumab was combined with carboplatin and paclitaxel in 53 pretreated patients achieving a 17% partial response (PR) (Perez et al., 2009). In a second clinical phase II study in melanoma treatment‐naïve patients were included in a randomized study design receiving carboplation/paclitaxel plus/minus bevacizumab (O'Day et al., 2009). Interestingly, adding bevacizumab led to an improved PFS by 22% and and prolonged survival by 22%, however, because of the size of the study failed to reach statistical significance although a positive trend was obvious. Currently (September 2010), 27 clinical trials are listed in http://clinicaltrials.gov with 8 still actively recruiting patients. Furthermore, UK Melanoma group is recruiting melanoma patients in clinical stage III into their AVAST‐M trial in order to investigate whether adjuvant VEGF‐blockade for 1 year with bevacizumab is having a clinical impact onto disease progression compared to untreated controls.
Previous studies have found that one of the most effective ways to block the VEGF‐signaling pathway is to prevent VEGF from binding to its normal receptors by administering decoy‐soluble receptors. VEGF‐trap is recombinant fusion protein that is a compositedecoy receptorbased onVEGFreceptorsVEGFR1and VEGFR2,fusedto an Fc segment of IgG1 (Holash et al., 2002). This VEGF‐Trap effectively suppresses tumor growth and vascularization in vivo, resulting in stunted and almost completely avascular tumors. VEGF‐Trap‐(aflibercept) mediated blockade may be superior to that achieved by other agents, such monoclonal antibodies and is also currently been tested in early clinical trials which do allow also recruitment of melanoma patients.
9. Blockade of cell‐receptor‐dependent signaling
At least 3 tyrosine kinase inhibitors (TKI) directly affecting the VEGF signaling have been tested in human melanoma. Sorafenib is the most intensively tested compound because it was believed that sorafenib would also block increased signaling trough mutant braf. Sorafenib has been registered for treatment of advanced renal and hepatic cellular carcinomas in the meantime based probably on its inhibitory of the VEGFR signaling pathway. Despite disappointing effects of sorafenib as a single agent in metastatic melanoma (Eisen et al., 2006), sorafenib was tested in the first‐line and second‐line setting in a randomized controlled fashion in combination with carboplation/paclitaxel in 800 and 270 eligible patients, respectively. In both trials no clinical benefit was detectable by adding sorafenib (Hauschild et al., 2009; Flaherty et al., 2010b). Other combinations of sorafenib including temozolomide, DTIC or interferon failed also to demonstrate any survival benefit.
Axitinib (AGO13736) is a small‐molecule TKI with selective inhibitory activity for VEGFR‐1, ‐2, ‐3, PDGR‐α and PDGR‐β, which is also at an early stage of evaluation in melanoma, and recent results from a phase II study demonstrated its single‐agent activity in a subgroup of melanoma patients (Fruehauf et al., 2008). Results are still not published as a full paper and follow‐up activities in melanoma are missing, despite promising activity also in renal cell carcinoma (Rixe et al., 2007) which has led to a clinical phase III trial in that disease.
Valatinib (PTK787) is another small‐molecule which infers with VEGFR‐1, VEGFR‐2, PDGFR and c‐kit signaling which was tested in the UK as single agent in 29 patients (reviewed in Basu, 2009). Disease control rate was limited by 40% and final results are awaited.
Other approaches which have been pioneered in small trials aimed at disrupting a potential synergy between EGFR and VEGFR signaling or trying to inhibit PDGFR have not resulted in any positive signal. However, one has to keep in mind that several of the agents employed might not be the best to test such a hypothesis and translational research is missing in most of such studies, so that firm conclusion can not be drawn at this moment.
10. Inhibition of critical pathways involved in survival and proliferation of endothelial cells and/or pericytes
Inhibition of signaling pathway has gained immediate attention in treating melanoma patients with the clinical success of PLX4032 blocking mutant braf (Flaherty et al., 2010a). The RAS/PAF/MEK pathway is believed to be also of crucial importance through its regulation of survival and proliferation of endothelial cells and pericytes. Whether targeting mutant braf is also associated with any anti‐angiogenic effect has not been studied in detail so far. Several small molecules were designed to inhibit the RAF/RAS pathway downstream including the MEK‐inhibitors AZD6422, CI‐1040 which have generated only very limited clinical benefits. Whether those agents have a profound effect on endothelial cell survival or proliferation is currently unclear. The same is true with mTOR‐blocking agents such as rapamycin, everolimus or temsirolimus although around 20 clinical trials are listed in http://clinicaltrials.gov, same in combination with other anti‐angiogenic compounds (e.g. valtinib, bevacizumab etc). Clinical study as well as translational research results are awaited.
11. Modulation of tumor‐matrix interaction
This is an area still in its infancy in view of translating preclinical data into successful clinical trials in melanoma. This in part caused by insufficient insights in certain areas or in broad (and too unspecific) blockade of targeted molecules (such as MMPs). Newer generations of more specific inhibitors might change this scenario.
Adhesion molecules and particularly integrins have been in focus of researchers interested in the metastatic process. Integrins αvβ3 and αvβ5 are known to upregulate on endothelial cells during tumor neovascularisation (Eskens et al., 2003). Several αvβ3 integrin ligands including vitronectin, osteopontin and bone sialoprotein are known to modulate both VEGF as well as FGF‐2‐induced tumor angiogenesis in mouse models (Stupack and Cheresh, 2004). Studies from several groups indicate that αvβ3 integrin plays a very crucial role in the progression of cutaneous melanomas from the benign radial growth phase to the metastatic vertical growth phase (Seftor, 1998). Studies utilizing αvβ3 integrin inhibitory peptides, antibodies or small molecular compounds indicate that αvβ3 integrin indeed is required for tumor endothelial cell survival, therefore its inhibition is believed to led to the regression of tumor vasculature. As integrins are known to positively regulate angiogenesis, tumor growth and metastasis, several inhibitors of integrins are currently being tested in clinical studies. These include cilengitide, ATN‐161 (both peptide integrin inhibitors), CNTO‐95 and vitaxin (the last two are humanized monoclonal antibodies) focusing on inhibition of the αvβ3 integrin complex or αv integrin alone. Results of phase 1 clinical trials of vitaxin (etaracizumab, MEDI‐522, Abegrin) indicated that it stabilized disease and reduced the risk of metastases. However, recent results of phase clinical 2 trials indicated a very modest response and were not very encouraging also in combination with DTIC (reviewed in Schadendorf et al., 2009).
12. Conclusion
Up to now, the impact of angiogenesis for the process of melanoma progression and metastasis in still not clarified. Based on the reported alternative angiogenic‐independent growth mechanisms in melanoma, targeting tumor angiogenesis by using anti‐angiogenic drugs will be not or only limited efficient in patients, where tumor growth occurs by utilizing mature vessels of the host. In addition, it has been demonstrated that tumor‐associated blood vessels exist in melanoma which show only minor expression of pro‐angiogenic factors and therefore failed angiogenic potential, it is not surprising that anti‐VEGF therapy is not able to target this type of vascular network.
Thus, detailed analyses of the vascular network in primary tumors, metastases but also relapses of melanoma patients are of high importance for the understanding of melanoma disease progression. Only an understanding of the importance and the impact of tumor angiogenesis as well as the relevance of angiogenic factors involved during disease progression in melanoma can help us to overcome the problem of drug resistance and may be useful to pave the way toward a more rational development of second generation anti‐angiogenic combination therapies.
Helfrich Iris and Schadendorf Dirk, (2011), Blood vessel maturation, vascular phenotype and angiogenic potential in malignant melanoma: One step forward for overcoming anti‐angiogenic drug resistance?, Molecular Oncology, 5, doi: 10.1016/j.molonc.2011.01.003.
References
- Abramsson, A. , Lindblom, P. , Betsholtz, C. , 2003. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J. Clin. Invest.. 112, 1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrae, J. , Gallini, R. , Betsholtz, C. , 2008. Role of platelet-derived growth factors in physiology and medicine. Genes Dev.. 22, 1276–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arap, W. , Haedicke, W. , Bernasconi, M. , Kain, R. , Rajotte, D. , Krajewski, S. , Ellerby, H.M. , Bredesen, D.E. , Pasqualini, R. , Ruoslahti, E. , 2002. Targeting the prostate for destruction through a vascular address. Proc. Natl. Acad. Sci. U. S. A.. 99, 1527–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik, A. , Abramsson, A. , Betsholtz, C. , 2005. Endothelial/pericyte interactions. Circ. Res.. 97, 512–523. [DOI] [PubMed] [Google Scholar]
- Balch, C.M. , Gershenwald, J.E. , Soong, S.J. , Thompson, J.F. , Atkins, M.B. , Byrd, D.R. , 2009. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol.. 27, 6199–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk, P. , Hashizume, H. , McDonald, D.M. , 2005. Cellular abnormalities of blood vessels as targets in cancer. Curr. Opin. Genet. Dev.. 15, 102–111. [DOI] [PubMed] [Google Scholar]
- Baranowska-Kortylewicz, J. , Abe, M. , Pietras, K. , Kortylewicz, Z.P. , Kurizaki, T. , Nearman, J. , Paulsson, J. , Mosley, R.L. , Enke, C.A. , Ostman, A. , 2005. Effect of platelet-derived growth factor receptor-beta inhibition with STI571 on radioimmunotherapy. Cancer Res.. 65, 7824–7831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Eli, M. , 1999. Role of interleukin-8 in tumor growth and metastasis of human melanoma. Pathobiology. 67, 12–18. [DOI] [PubMed] [Google Scholar]
- Barnhill, R.L. , Levy, M.A. , 1993. Regressing thin cutaneous malignant melanomas (< or = 1.0 mm) are associated with angiogenesis. Am. J. Pathol.. 143, 99–104. [PMC free article] [PubMed] [Google Scholar]
- Barnhill, R.L. , Lugassy, C. , 2004. Angiotropic malignant melanoma and extravascular migratory metastasis: description of 36 cases with emphasis on a new mechanism of tumour spread. Pathology. 36, 485–490. [DOI] [PubMed] [Google Scholar]
- Barnhill, R.L. , Fandrey, K. , Levy, M.A. , Mihm, M.C. , Hyman, B. , 1992. Angiogenesis and tumor progression of melanoma. Quantification of vascularity in melanocytic nevi and cutaneous malignant melanoma. Lab. Invest.. 67, 331–337. [PubMed] [Google Scholar]
- Barnhill, R.L. , Busam, K.J. , Berwick, M. , Blessing, K. , Cochran, A.J. , Elder, D.E. , Fandrey, K. , Karaoli, T. , White, W.L. , 1994. Tumour vascularity is not a prognostic factor for cutaneous melanoma. Lancet. 344, 1237–1238. [DOI] [PubMed] [Google Scholar]
- Barnhill, R.L. , Xiao, M. , Graves, D. , Antoniades, H.N. , 1996. Expression of platelet-derived growth factor (PDGF)-A, PDGF-B and the PDGF-alpha receptor, but not the PDGF-beta receptor, in human malignant melanoma in vivo. Br. J. Dermatol.. 135, 898–904. [DOI] [PubMed] [Google Scholar]
- Basu, B. , Biswas, S. , Wrigley, J. , Sirohi, B. , Corrie, P. , 2009. Angiogenesis in cutaneous malignant melanoma and potential therapeutic strategies. Expert Rev. Anticancer Ther.. 9, 1583–1598. [DOI] [PubMed] [Google Scholar]
- Benjamin, L.E. , Hemo, I. , Keshet, E. , 1998. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 125, 1591–1598. [DOI] [PubMed] [Google Scholar]
- Benjamin, L.E. , Golijanin, D. , Itin, A. , Pode, D. , Keshet, E. , 1999. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J. Clin. Invest.. 103, 159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers, G. , Song, S. , 2005. The role of pericytes in blood-vessel formation and maintenance. Neuro. Oncol.. 7, 452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers, G. , Song, S. , Meyer-Morse, N. , Bergsland, E. , Hanahan, D. , 2003. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J. Clin. Invest.. 111, 1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsholtz, C. , Lindblom, P. , Gerhardt, H. , 2005. Role of pericytes in vascular morphogenesis. EXS. 115–125. [DOI] [PubMed] [Google Scholar]
- Birck, A. , Kirkin, A.F. , Zeuthen, J. , Hou-Jensen, K. , 1999. Expression of basic fibroblast growth factor and vascular endothelial growth factor in primary and metastatic melanoma from the same patients. Melanoma Res.. 9, 375–381. [DOI] [PubMed] [Google Scholar]
- Bondjers, C. , Kalen, M. , Hellstrom, M. , Scheidl, S.J. , Abramsson, A. , Renner, O. , Lindahl, P. , Cho, H. , Kehrl, J. , Betsholtz, C. , 2003. Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. Am. J. Pathol.. 162, 721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, Y. , Sonveaux, P. , Liu, S. , Zhao, Y. , Mi, J. , Clary, B.M. , Li, C.Y. , Kontos, C.D. , Dewhirst, M.W. , 2007. Systemic overexpression of angiopoietin-2 promotes tumor microvessel regression and inhibits angiogenesis and tumor growth. Cancer Res.. 67, 3835–3844. [DOI] [PubMed] [Google Scholar]
- Carmeliet, P. , Jain, R.K. , 2000. Angiogenesis in cancer and other diseases. Nature. 407, 249–257. [DOI] [PubMed] [Google Scholar]
- Carmeliet, P. , 2005. Angiogenesis in life, disease and medicine. Nature. 438, 932–936. [DOI] [PubMed] [Google Scholar]
- Carmeliet, P. , 2005. VEGF as a key mediator of angiogenesis in cancer. Oncology. 69, (Suppl. 3) 4–10. [DOI] [PubMed] [Google Scholar]
- Chang, Y.S. , di, T.E. , McDonald, D.M. , Jones, R. , Jain, R.K. , Munn, L.L. , 2000. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc. Natl. Acad. Sci. U. S. A.. 97, 14608–14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, S. , Aldrich, T.H. , Jones, P.F. , Acheson, A. , Compton, D.L. , Jain, V. , Ryan, T.E. , Bruno, J. , Radziejewski, C. , Maisonpierre, P.C. , 1996. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 87, 1161–1169. [DOI] [PubMed] [Google Scholar]
- Dome, B. , Paku, S. , Somlai, B. , Timar, J. , 2002. Vascularization of cutaneous melanoma involves vessel co-option and has clinical significance. J. Pathol.. 197, 355–362. [DOI] [PubMed] [Google Scholar]
- Donnini, S. , Machein, M.R. , Plate, K.H. , Weich, H.A. , 1999. Expression and localization of placenta growth factor and PlGF receptors in human meningiomas. J. Pathol.. 189, 66–71. [DOI] [PubMed] [Google Scholar]
- Eberhard, A. , Kahlert, S. , Goede, V. , Hemmerlein, B. , Plate, K.H. , Augustin, H.G. , 2000. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res.. 60, 1388–1393. [PubMed] [Google Scholar]
- Einspahr, J.G. , Thomas, T.L. , Saboda, K. , Nickolof, B.J. , Warneke, J. , Curiel-Lewandrowski, C. , Ranger-Moore, J. , Duckett, L. , Bangert, J. , Fruehauf, J.P. , 2007. Expression of vascular endothelial growth factor in early cutaneous melanocytic lesion progression. Cancer. 110, 2519–2527. [DOI] [PubMed] [Google Scholar]
- Eisen, T. , Ahmad, T. , Flaherty, K.T. , Gore, M. , Kaye, S. , Marais, R. , Gibbens, I. , Hackett, S. , James, M. , Schuchter, L.M. , Nathanson, K.L. , Xia, C. , Simantov, R. , Schwartz, B. , Poulin-Costello, M. , O'Dwyer, P.J. , Ratain, M.J. , 2006. Sorafenib in advanced melanoma: a phase II randomised discontinuation trial analysis. Br. J. Cancer. 95, 581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erber, R. , Thurnher, A. , Katsen, A.D. , Groth, G. , Kerger, H. , Hammes, H.P. , Menger, M.D. , Ullrich, A. , Vajkoczy, P. , 2004. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J.. 18, 338–340. [DOI] [PubMed] [Google Scholar]
- Erhard, H. , Rietveld, F.J. , van Altena, M.C. , Brocker, E.B. , Ruiter, D.J. , de Waal, R.M. , 1997. Transition of horizontal to vertical growth phase melanoma is accompanied by induction of vascular endothelial growth factor expression and angiogenesis. Melanoma Res.. 7, (Suppl. 2) S19–S26. [PubMed] [Google Scholar]
- Escudier, B. , Eisen, T. , Stadler, W.M. , Szczylik, C. , Oudard, S. , Siebels, M. , Negrier, S. , Chevreau, C. , Solska, E. , Desai, A.A. , 2007. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med.. 356, 125–134. [DOI] [PubMed] [Google Scholar]
- Eskens, F.A. , Dumez, H. , Hoekstra, R. , 2003. Phase I and pharmacokinetic study of continuous twice weekly intravenous administration of Cilengitide (EMD 121974), a novel inhibitor of the integrins alphavbeta3 and alphavbeta5 in patients with advanced solid tumours. Eur. J. Cancer. 39, (7) 917–926. [DOI] [PubMed] [Google Scholar]
- Fallowfield, M.E. , Cook, M.G. , 1991. The vascularity of primary cutaneous melanoma. J. Pathol.. 164, 241–244. [DOI] [PubMed] [Google Scholar]
- Farhadi, M.R. , Capelle, H.H. , Erber, R. , Ullrich, A. , Vajkoczy, P. , 2005. Combined inhibition of vascular endothelial growth factor and platelet-derived growth factor signaling: effects on the angiogenesis, microcirculation, and growth of orthotopic malignant gliomas. J. Neurosurg.. 102, 363–370. [DOI] [PubMed] [Google Scholar]
- Feron, O. , 2004. Targeting the tumor vascular compartment to improve conventional cancer therapy. Trends Pharmacol. Sci.. 25, 536–542. [DOI] [PubMed] [Google Scholar]
- Ferrara, N. , Hillan, K.J. , Gerber, H.P. , Novotny, W. , 2004. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov.. 3, 391–400. [DOI] [PubMed] [Google Scholar]
- Fiedler, U. , Augustin, H.G. , 2006. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol.. 27, 552–558. [DOI] [PubMed] [Google Scholar]
- Fiedler, U. , Scharpfenecker, M. , Koidl, S. , Hegen, A. , Grunow, V. , Schmidt, J.M. , Kriz, W. , Thurston, G. , Augustin, H.G. , 2004. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 103, 4150–4156. [DOI] [PubMed] [Google Scholar]
- Fiedler, U. , Reiss, Y. , Scharpfenecker, M. , Grunow, V. , Koidl, S. , Thurston, G. , Gale, N.W. , Witzenrath, M. , Rosseau, S. , Suttorp, N. , 2006. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat. Med.. 12, 235–239. [DOI] [PubMed] [Google Scholar]
- Findley, C.M. , Cudmore, M.J. , Ahmed, A. , Kontos, C.D. , 2007. VEGF induces Tie2 shedding via a phosphoinositide 3-kinase/Akt dependent pathway to modulate Tie2 signaling. Arterioscler. Thromb. Vasc. Biol.. 27, 2619–2626. [DOI] [PubMed] [Google Scholar]
- Flaherty, K.T. , Puzanov, I. , Kim, K.B. , Ribas, A. , McArthur, G.A. , Sosman, J.A. , O'Dwyer, P.J. , Lee, R.J. , Grippo, J.F. , Nolop, K. , Chapman, P.B. , 2010. Inhibition of mutated, activated BRAF in metastatic melanoma. N. Engl. J. Med.. 363, 809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty, K.T. , Lee, S.J. , Schuchter, L.M. , Flaherty, L.E. , Wright, J.J. , Leming, P.D. , Kirkwood, J.M. , 2010. Final results of E2603: a double-blind, randomized phase III trial comparing carboplatin ©/paclitaxel (P) with or without sorafenib (S) in metastatic melanoma. J. Clin. Oncol.. 28, (suppl. 15s) abstr 8511 [Google Scholar]
- Folkman, J. , Hanahan, D. , 1991. Switch to the angiogenic phenotype during tumorigenesis. Princess Takamatsu Symp.. 22, 339–347. [PubMed] [Google Scholar]
- Folkman, J. , Watson, K. , Ingber, D. , Hanahan, D. , 1989. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 339, 58–61. [DOI] [PubMed] [Google Scholar]
- Folkman, J. , 1971. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med.. 285, 1182–1186. [DOI] [PubMed] [Google Scholar]
- Folkman, J. , 2006. Angiogenesis. Annu. Rev. Med.. 57, 1–18. [DOI] [PubMed] [Google Scholar]
- Fong, G.H. , Takeda, K. , 2008. Role and regulation of prolyl hydroxylase domain proteins. Cell Death Differ.. 15, 635–641. [DOI] [PubMed] [Google Scholar]
- Fruehauf, J. , Lutzky, J. , McDermott, D. , Brown, C.K. , Pithavala, Y.K. , Bycott, P.W. , Shalinsky, D. , Liau, K.F. , Niethammer, A. , Rixe, O. , 2008. Axitinib (AG-013736) in patients with metastatic melanoma: a phase II study. J. Clin. Oncol.. 26, (Abstract 9006) [Google Scholar]
- Gaengel, K. , Genove, G. , Armulik, A. , Betsholtz, C. , 2009. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler. Thromb. Vasc. Biol.. 29, 630–638. [DOI] [PubMed] [Google Scholar]
- Gerhardt, H. , Betsholtz, C. , 2003. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res.. 314, 15–23. [DOI] [PubMed] [Google Scholar]
- Gilead, A. , Neeman, M. , 1999. Dynamic remodeling of the vascular bed precedes tumor growth: MLS ovarian carcinoma spheroids implanted in nude mice. Neoplasia. 1, 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, C.H. , Rivers, J. , Kerbel, R.S. , Stankiewicz, K.S. , White, W.L. , 1994. Extent of vascularization as a prognostic indicator in thin (< 0.76 mm) malignant melanomas. Am. J. Pathol.. 145, 510–514. [PMC free article] [PubMed] [Google Scholar]
- Greenberg, J.I. , Shields, D.J. , Barillas, S.G. , Acevedo, L.M. , Murphy, E. , Huang, J. , Scheppke, L. , Stockmann, C. , Johnson, R.S. , Angle, N. , 2008. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 456, 809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainsworth, J.D. , Sosman, J.A. , Spigel, D.R. , Edwards, D.L. , Baughman, C. , Greco, A. , 2005. Treatment of metastatic renal cell carcinoma with a combination of bevacizumab and erlotinib. J. Clin. Oncol.. 23, 7889–7896. [DOI] [PubMed] [Google Scholar]
- Hanahan, D. , Weinberg, R.A. , 2000. The hallmarks of cancer. Cell. 100, 57–70. [DOI] [PubMed] [Google Scholar]
- Hashizume, H. , Baluk, P. , Morikawa, S. , McLean, J.W. , Thurston, G. , Roberge, S. , Jain, R.K. , McDonald, D.M. , 2000. Openings between defective endothelial cells explain tumor vessel leakiness. Am. J. Pathol.. 156, 1363–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschild, A. , Agarwala, S.S. , Trefzer, U. , Hogg, D. , Robert, C. , Hersey, P. , Eggermont, A. , Grabbe, S. , Gonzalez, R. , Gille, J. , Peschel, C. , Schadendorf, D. , Garbe, C. , O'Day, S. , Daud, A. , White, J.M. , Xia, C. , Patel, K. , Kirkwood, J.M. , Keilholz, U. , 2009. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J. Clin. Oncol.. 27, 2823–2830. [DOI] [PubMed] [Google Scholar]
- Helfrich, I. , Edler, L. , Sucker, A. , Thomas, M. , Christian, S. , Schadendorf, D. , Augustin, H.G. , 2009. Angiopoietin-2 levels are associated with disease progression in metastatic malignant melanoma. Clin. Cancer Res.. 15, 1384–1392. [DOI] [PubMed] [Google Scholar]
- Helfrich, I. , Scheffrahn, I. , Bartling, S. , Weis, J. , von, F.V. , Middleton, M. , Kato, M. , Ergun, S. , Schadendorf, D. , 2010. Resistance to antiangiogenic therapy is directed by vascular phenotype, vessel stabilization, and maturation in malignant melanoma. J. Exp. Med.. 207, 491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom, M. , Kalen, M. , Lindahl, P. , Abramsson, A. , Betsholtz, C. , 1999. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 126, 3047–3055. [DOI] [PubMed] [Google Scholar]
- Hellstrom, M. , Gerhardt, H. , Kalen, M. , Li, X. , Eriksson, U. , Wolburg, H. , Betsholtz, C. , 2001. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J. Cell Biol.. 153, 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix, M.J. , Seftor, E.A. , Meltzer, P.S. , Gardner, L.M. , Hess, A.R. , Kirschmann, D.A. , Schatteman, G.C. , Seftor, R.E. , 2001. Expression and functional significance of VE-cadherin in aggressive human melanoma cells: role in vasculogenic mimicry. Proc. Natl. Acad. Sci. U. S. A.. 98, 8018–8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess-Stumpp, H. , Haberey, M. , Thierauch, K.H. , 2005. PTK 787/ZK 222584, a tyrosine kinase inhibitor of all known VEGF receptors, represses tumor growth with high efficacy. Chembiochem. 6, 550–557. [DOI] [PubMed] [Google Scholar]
- Hillen, F. , van de, W.A. , Creytens, D. , Vermeulen, A.H. , Griffioen, A.W. , 2006. Proliferating endothelial cells, but not microvessel density, are a prognostic parameter in human cutaneous melanoma. Melanoma Res.. 16, 453–457. [DOI] [PubMed] [Google Scholar]
- Hirschi, K.K. , D'Amore, P.A. , 1996. Pericytes in the microvasculature. Cardiovasc. Res.. 32, 687–698. [PubMed] [Google Scholar]
- Hirschi, K.K. , Rohovsky, S.A. , D'Amore, P.A. , 1998. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J. Cell Biol.. 141, 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs, S.K. , Monsky, W.L. , Yuan, F. , Roberts, W.G. , Griffith, L. , Torchilin, V.P. , Jain, R.K. , 1998. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc. Natl. Acad. Sci. U. S. A.. 95, 4607–4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holash, J. , Maisonpierre, P.C. , Compton, D. , Boland, P. , Alexander, C.R. , Zagzag, D. , Yancopoulos, G.D. , Wiegand, S.J. , 1999. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 284, 1994–1998. [DOI] [PubMed] [Google Scholar]
- Holash, J. , Davis, S. , Papadopoulos, N. , Croll, S.D. , Ho, L. , Russell, M. , Boland, P. , Leidich, R. , Hylton, D. , Burova, E. , Ioffe, E. , 2002. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc. Nat. Acad. Sci.. 99, 11393–11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iivanainen, E. , Nelimarkka, L. , Elenius, V. , Heikkinen, S.M. , Junttila, T.T. , Sihombing, L. , Sundvall, M. , Maatta, J.A. , Laine, V.J. , Yla-Herttuala, S. , 2003. Angiopoietin-regulated recruitment of vascular smooth muscle cells by endothelial-derived heparin binding EGF-like growth factor. FASEB J.. 17, 1609–1621. [DOI] [PubMed] [Google Scholar]
- Ilmonen, S. , Kariniemi, A.L. , Vlaykova, T. , Muhonen, T. , Pyrhonen, S. , sko-Seljavaara, S. , 1999. Prognostic value of tumour vascularity in primary melanoma. Melanoma Res.. 9, 273–278. [DOI] [PubMed] [Google Scholar]
- Imhof, B.A. , urrand-Lions, M. , 2006. Angiogenesis and inflammation face off. Nat. Med.. 12, 171–172. [DOI] [PubMed] [Google Scholar]
- Jain, R.K. , 1998. The next frontier of molecular medicine: delivery of therapeutics. Nat. Med.. 4, 655–657. [DOI] [PubMed] [Google Scholar]
- Jayson, G.C. , Parker, G.J. , Mullamitha, S. , Valle, J.W. , Saunders, M. , Broughton, L. , Lawrance, J. , Carrington, B. , Roberts, C. , Issa, B. , 2005. Blockade of platelet-derived growth factor receptor-beta by CDP860, a humanized, PEGylated di-Fab’, leads to fluid accumulation and is associated with increased tumor vascularized volume. J. Clin. Oncol.. 23, 973–981. [DOI] [PubMed] [Google Scholar]
- Kerbel, R.S. , 2008. Tumor angiogenesis. N. Engl. J. Med.. 358, 2039–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y. , Imdad, R.Y. , Stephenson, A.H. , Sprague, R.S. , Lonigro, A.J. , 1998. Vascular endothelial growth factor mRNA in pericytes is upregulated by phorbol myristate acetate. Hypertension. 31, 511–515. [DOI] [PubMed] [Google Scholar]
- Kiss, J. , Timar, J. , Somlai, B. , Gilde, K. , Fejos, Z. , Gaudi, I. , Ladanyi, A. , 2007. Association of microvessel density with infiltrating cells in human cutaneous malignant melanoma. Pathol. Oncol. Res.. 13, 21–31. [DOI] [PubMed] [Google Scholar]
- Kuboki, S. , Shimizu, H. , Mitsuhashi, N. , Kusashio, K. , Kimura, F. , Yoshidome, H. , Ohtsuka, M. , Kato, A. , Yoshitomi, H. , Miyazaki, M. , 2008. Angiopoietin-2 levels in the hepatic vein as a useful predictor of tumor invasiveness and prognosis in human hepatocellular carcinoma. J. Gastroenterol. Hepatol.. 23, e157–e164. [DOI] [PubMed] [Google Scholar]
- Kunkel, P. , Muller, S. , Schirmacher, P. , Stavrou, D. , Fillbrandt, R. , Westphal, M. , Lamszus, K. , 2001. Expression and localization of scatter factor/hepatocyte growth factor in human astrocytomas. Neuro. Oncol.. 3, 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurschat, P. , Eming, S. , Nashan, D. , Krieg, T. , Mauch, C. , 2007. Early increase in serum levels of the angiogenesis-inhibitor endostatin and of basic fibroblast growth factor in melanoma patients during disease progression. Br. J. Dermatol.. 156, 653–658. [DOI] [PubMed] [Google Scholar]
- Kusters, B. , Leenders, W.P. , Wesseling, P. , Smits, D. , Verrijp, K. , Ruiter, D.J. , Peters, J.P. , van Der Kogel, A.J. , de Waal, R.M. , 2002. Vascular endothelial growth factor-A(165) induces progression of melanoma brain metastases without induction of sprouting angiogenesis. Cancer Res.. 62, 341–345. [PubMed] [Google Scholar]
- Lacal, P.M. , Failla, C.M. , Pagani, E. , Odorisio, T. , Schietroma, C. , Falcinelli, S. , Zambruno, G. , D'Atri, S. , 2000. Human melanoma cells secrete and respond to placenta growth factor and vascular endothelial growth factor. J. Invest. Dermatol.. 115, 1000–1007. [DOI] [PubMed] [Google Scholar]
- Lee, S. , Chen, T.T. , Barber, C.L. , Jordan, M.C. , Murdock, J. , Desai, S. , Ferrara, N. , Nagy, A. , Roos, K.P. , Iruela-Arispe, M.L. , 2007. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 130, 691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenders, W. , Lubsen, N. , van, A.M. , Clauss, M. , Deckers, M. , Lowik, C. , Breier, G. , Ruiter, D. , de, W.R. , 2002. Design of a variant of vascular endothelial growth factor-A (VEGF-A) antagonizing KDR/Flk-1 and Flt-1. Lab. Invest.. 82, 473–481. [DOI] [PubMed] [Google Scholar]
- Leenders, W.P. , Kusters, B. , de Waal, R.M. , 2002. Vessel co-option: how tumors obtain blood supply in the absence of sprouting angiogenesis. Endothelium. 9, 83–87. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Wang, M.N. , Li, H. , King, K.D. , Bassi, R. , Sun, H. , Santiago, A. , Hooper, A.T. , Bohlen, P. , Hicklin, D.J. , 2002. Active immunization against the vascular endothelial growth factor receptor flk1 inhibits tumor angiogenesis and metastasis. J. Exp. Med.. 195, 1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, P. , Polverini, P. , Dewhirst, M. , Shan, S. , Rao, P.S. , Peters, K. , 1997. Inhibition of tumor angiogenesis using a soluble receptor establishes a role for Tie2 in pathologic vascular growth. J. Clin. Invest.. 100, 2072–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl, P. , Betsholtz, C. , 1998. Not all myofibroblasts are alike: revisiting the role of PDGF-A and PDGF-B using PDGF-targeted mice. Curr. Opin. Nephrol. Hypertens.. 7, 21–26. [DOI] [PubMed] [Google Scholar]
- Lindblom, P. , Gerhardt, H. , Liebner, S. , Abramsson, A. , Enge, M. , Hellstrom, M. , Backstrom, G. , Fredriksson, S. , Landegren, U. , Nystrom, H.C. , 2003. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev.. 17, 1835–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugassy, C. , Kleinman, H.K. , Engbring, J.A. , Welch, D.R. , Harms, J.F. , Rufner, R. , Ghanem, G. , Patierno, S.R. , Barnhill, R.L. , 2004. Pericyte-like location of GFP-tagged melanoma cells: ex vivo and in vivo studies of extravascular migratory metastasis. Am. J. Pathol.. 164, 1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttun, A. , Autiero, M. , Tjwa, M. , Carmeliet, P. , 2004. Genetic dissection of tumor angiogenesis: are PlGF and VEGFR-1 novel anti-cancer targets?. Biochim. Biophys. Acta. 1654, 79–94. [DOI] [PubMed] [Google Scholar]
- Mahabeleshwar, G.H. , Byzova, T.V. , 2007. Angiogenesis in melanoma. Semin. Oncol.. 34, 555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler, D.A. , Huang, S. , Tabrizi, M. , Bell, G.M. , 2004. Efficacy and safety of a monoclonal antibody recognizing interleukin-8 in COPD: a pilot study. Chest. 126, 926–934. [DOI] [PubMed] [Google Scholar]
- Maisonpierre, P.C. , Suri, C. , Jones, P.F. , Bartunkova, S. , Wiegand, S.J. , Radziejewski, C. , Compton, D. , McClain, J. , Aldrich, T.H. , Papadopoulos, N. , 1997. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 277, 55–60. [DOI] [PubMed] [Google Scholar]
- Maniotis, A.J. , Folberg, R. , Hess, A. , Seftor, E.A. , Gardner, L.M. , Pe'er, J. , Trent, J.M. , Meltzer, P.S. , Hendrix, M.J. , 1999. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am. J. Pathol.. 155, 739–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcoval, J. , Moreno, A. , Graells, J. , Vidal, A. , Escriba, J.M. , Garcia-Ramirez, M. , Fabra, A. , 1997. Angiogenesis and malignant melanoma. Angiogenesis is related to the development of vertical (tumorigenic) growth phase. J. Cutan. Pathol.. 24, 212–218. [DOI] [PubMed] [Google Scholar]
- Mazure, N.M. , Chen, E.Y. , Yeh, P. , Laderoute, K.R. , Giaccia, A.J. , 1996. Oncogenic transformation and hypoxia synergistically act to modulate vascular endothelial growth factor expression. Cancer Res.. 56, 3436–3440. [PubMed] [Google Scholar]
- Melnikova, V.O. , Bar-Eli, M. , 2006. Bioimmunotherapy for melanoma using fully human antibodies targeting MCAM/MUC18 and IL-8. Pigment Cell Res.. 19, 395–405. [DOI] [PubMed] [Google Scholar]
- Morikawa, S. , Baluk, P. , Kaidoh, T. , Haskell, A. , Jain, R.K. , McDonald, D.M. , 2002. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am. J. Pathol.. 160, 985–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld, G. , Cohen, T. , Gengrinovitch, S. , Poltorak, Z. , 1999. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J.. 13, 9–22. [PubMed] [Google Scholar]
- Nisancioglu, M.H. , Betsholtz, C. , Genove, G. , 2010. The absence of pericytes does not increase the sensitivity of tumor vasculature to vascular endothelial growth factor-A blockade. Cancer Res.. 70, 5109–5115. [DOI] [PubMed] [Google Scholar]
- O'Day, S.J. , Kim, K.B. , Sosman, J.A. , 2009. BEAM: a randomized phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untrated advanced melanoma. Eur. J. Cancer. (Suppl. 7) (Abstract 23LBA) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorisio, T. , Cianfarani, F. , Failla, C.M. , Zambruno, G. , 2006. The placenta growth factor in skin angiogenesis. J. Dermatol. Sci.. 41, 11–19. [DOI] [PubMed] [Google Scholar]
- Oku, T. , Tjuvajev, J.G. , Miyagawa, T. , Sasajima, T. , Joshi, A. , Joshi, R. , Finn, R. , Claffey, K.P. , Blasberg, R.G. , 1998. Tumor growth modulation by sense and antisense vascular endothelial growth factor gene expression: effects on angiogenesis, vascular permeability, blood volume, blood flow, fluorodeoxyglucose uptake, and proliferation of human melanoma intracerebral xenografts. Cancer Res.. 58, 4185–4192. [PubMed] [Google Scholar]
- Oliner, J. , Min, H. , Leal, J. , Yu, D. , Rao, S. , You, E. , Tang, X. , Kim, H. , Meyer, S. , Han, S.J. , 2004. Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell. 6, 507–516. [DOI] [PubMed] [Google Scholar]
- Orfanos, S.E. , Kotanidou, A. , Glynos, C. , Athanasiou, C. , Tsigkos, S. , Dimopoulou, I. , Sotiropoulou, C. , Zakynthinos, S. , Armaganidis, A. , Papapetropoulos, A. , 2007. Angiopoietin-2 is increased in severe sepsis: correlation with inflammatory mediators. Crit. Care Med.. 35, 199–206. [DOI] [PubMed] [Google Scholar]
- Padera, T.P. , Stoll, B.R. , Tooredman, J.B. , Capen, D. , di, T.E. , Jain, R.K. , 2004. Pathology: cancer cells compress intratumour vessels. Nature. 427, 695 [DOI] [PubMed] [Google Scholar]
- Paku, S. , 1998. Current concepts of tumor-induced angiogenesis. Pathol. Oncol. Res.. 4, 62–75. [DOI] [PubMed] [Google Scholar]
- Park, J.H. , Park, K.J. , Kim, Y.S. , Sheen, S.S. , Lee, K.S. , Lee, H.N. , Oh, Y.J. , Hwang, S.C. , 2007. Serum angiopoietin-2 as a clinical marker for lung cancer. Chest. 132, 200–206. [DOI] [PubMed] [Google Scholar]
- Perez, D.G. , Suman, V.J. , Fitch, T.R. , Amatruda, T. , Morton, R.F. , Jilani, S.Z. , Constantinou, C.L. , Egner, J.R. , Kottschade, L.A. , Markovic, S.N. , 2009. Phase 2 trial of carboplatin, weekly paclitaxel, and biweekly bevacizumab in patients with unresectable stage IV melanoma: a North Central Cancer Treatment group study, N047A. Cancer. 115, 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, B.A. , Diaz, L.A. , Polyak, K. , Meszler, L. , Romans, K. , Guinan, E.C. , Antin, J.H. , Myerson, D. , Hamilton, S.R. , Vogelstein, B. , 2005. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat. Med.. 11, 261–262. [DOI] [PubMed] [Google Scholar]
- Pezzella, F. , Pastorino, U. , Tagliabue, E. , Andreola, S. , Sozzi, G. , Gasparini, G. , Menard, S. , Gatter, K.C. , Harris, A.L. , Fox, S. , 1997. Non-small-cell lung carcinoma tumor growth without morphological evidence of neo-angiogenesis. Am. J. Pathol.. 151, 1417–1423. [PMC free article] [PubMed] [Google Scholar]
- Pietras, K. , Hanahan, D. , 2005. A multitargeted, metronomic, and maximum-tolerated dose "chemo-switch" regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J. Clin. Oncol.. 23, 939–952. [DOI] [PubMed] [Google Scholar]
- Pietras, K. , Rubin, K. , Sjoblom, T. , Buchdunger, E. , Sjoquist, M. , Heldin, C.H. , Ostman, A. , 2002. Inhibition of PDGF receptor signaling in tumor stroma enhances antitumor effect of chemotherapy. Cancer Res.. 62, 5476–5484. [PubMed] [Google Scholar]
- Qing, G. , Simon, M.C. , 2009. Hypoxia inducible factor-2alpha: a critical mediator of aggressive tumor phenotypes. Curr. Opin. Genet. Dev.. 19, 60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, J.A. , McNutt, N.S. , Albino, A.P. , 1994. Differential expression of basic fibroblast growth factor (bFGF) in melanocytic lesions demonstrated by in situ hybridization. Implications for tumor progression. Am. J. Pathol.. 144, 329–336. [PMC free article] [PubMed] [Google Scholar]
- Reinmuth, N. , Liu, W. , Jung, Y.D. , Ahmad, S.A. , Shaheen, R.M. , Fan, F. , Bucana, C.D. , McMahon, G. , Gallick, G.E. , Ellis, L.M. , 2001. Induction of VEGF in perivascular cells defines a potential paracrine mechanism for endothelial cell survival. FASEB J.. 15, 1239–1241. [DOI] [PubMed] [Google Scholar]
- Reynolds, L.P. , Grazul-Bilska, A.T. , Redmer, D.A. , 2000. Angiogenesis in the corpus luteum. Endocrine. 12, 1–9. [DOI] [PubMed] [Google Scholar]
- Ribatti, D. , Vacca, A. , Ria, R. , Marzullo, A. , Nico, B. , Filotico, R. , Roncali, L. , Dammacco, F. , 2003. Neovascularisation, expression of fibroblast growth factor-2, and mast cells with tryptase activity increase simultaneously with pathological progression in human malignant melanoma. Eur. J. Cancer. 39, 666–674. [DOI] [PubMed] [Google Scholar]
- Ribatti, D. , Nico, B. , Floris, C. , Mangieri, D. , Piras, F. , Ennas, M.G. , Vacca, A. , Sirigu, P. , 2005. Microvascular density, vascular endothelial growth factor immunoreactivity in tumor cells, vessel diameter and intussusceptive microvascular growth in primary melanoma. Oncol. Rep.. 14, 81–84. [PubMed] [Google Scholar]
- Rixe, O. , Bukowski, R.M. , Michaelson, M.D. , Wilding, G. , Hudes, G.R. , Bolte, O. , Motzer, R.J. , Bycott, P. , Liau, K.F. , Freddo, J. , Trask, P.C. , Kim, S. , Rini, B.I. , 2007. Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: a phase II study. Lancet Oncol.. 8, 975–984. [DOI] [PubMed] [Google Scholar]
- Salven, P. , Heikkila, P. , Joensuu, H. , 1997. Enhanced expression of vascular endothelial growth factor in metastatic melanoma. Br. J. Cancer. 76, 930–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadendorf, D. , Algarra, S.M. , Bastholt, L. , Cinat, G. , Dreno, B. , Eggermont, A.M. , Espinosa, E. , Guo, J. , Hauschild, A. , Petrella, T. , Schachter, J. , Hersey, P. , 2009. Immunotherapy of distant metastatic disease. Ann. Oncol.. (Suppl. 6) vi41–50. (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlingemann, R.O. , Rietveld, F.J. , Kwaspen, F. , van de Kerkhof, P.C. , de Waal, R.M. , Ruiter, D.J. , 1991. Differential expression of markers for endothelial cells, pericytes, and basal lamina in the microvasculature of tumors and granulation tissue. Am. J. Pathol.. 138, 1335–1347. [PMC free article] [PubMed] [Google Scholar]
- Seftor, R.E. , 1998. Role of the beta3 integrin subunit in human primary melanoma progression: multifunctional activities associated with alpha(v)beta3 integrin expression. Am. J. Pathol.. 153, (5) 1347–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen, R.M. , Ahmad, S.A. , Liu, W. , Reinmuth, N. , Jung, Y.D. , Tseng, W.W. , Drazan, K.E. , Bucana, C.D. , Hicklin, D.J. , Ellis, L.M. , 2001. Inhibited growth of colon cancer carcinomatosis by antibodies to vascular endothelial and epidermal growth factor receptors. Br. J. Cancer. 85, 584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen, R.M. , Tseng, W.W. , Davis, D.W. , Liu, W. , Reinmuth, N. , Vellagas, R. , Wieczorek, A.A. , Ogura, Y. , McConkey, D.J. , Drazan, K.E. , 2001. Tyrosine kinase inhibition of multiple angiogenic growth factor receptors improves survival in mice bearing colon cancer liver metastases by inhibition of endothelial cell survival mechanisms. Cancer Res.. 61, 1464–1468. [PubMed] [Google Scholar]
- Shepro, D. , Morel, N.M. , 1993. Pericyte physiology. FASEB J.. 7, 1031–1038. [DOI] [PubMed] [Google Scholar]
- Siemeister, G. , Schirner, M. , Reusch, P. , Barleon, B. , Marme, D. , Martiny-Baron, G. , 1998. An antagonistic vascular endothelial growth factor (VEGF) variant inhibits VEGF-stimulated receptor autophosphorylation and proliferation of human endothelial cells. Proc. Natl. Acad. Sci. U. S. A.. 95, 4625–4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims, D.E. , 2000. Diversity within pericytes. Clin. Exp. Pharmacol. Physiol.. 27, 842–846. [DOI] [PubMed] [Google Scholar]
- Song, N. , Huang, Y. , Shi, H. , Yuan, S. , Ding, Y. , Song, X. , Fu, Y. , Luo, Y. , 2009. Overexpression of platelet-derived growth factor-BB increases tumor pericyte content via stromal-derived factor-1alpha/CXCR4 axis. Cancer Res.. 69, 6057–6064. [DOI] [PubMed] [Google Scholar]
- Srivastava, A. , Laidler, P. , Davies, R.P. , Horgan, K. , Hughes, L.E. , 1988. The prognostic significance of tumor vascularity in intermediate-thickness (0.76-4.0 mm thick) skin melanoma. A quantitative histologic study. Am. J. Pathol.. 133, 419–423. [PMC free article] [PubMed] [Google Scholar]
- St, C.B. , Rago, C. , Velculescu, V. , Traverso, G. , Romans, K.E. , Montgomery, E. , Lal, A. , Riggins, G.J. , Lengauer, C. , Vogelstein, B. , 2000. Genes expressed in human tumor endothelium. Science. 289, 1197–1202. [DOI] [PubMed] [Google Scholar]
- Straume, O. , Salvesen, H.B. , Akslen, L.A. , 1999. Angiogenesis is prognostically important in vertical growth phase melanomas. Int. J. Oncol.. 15, 595–599. [DOI] [PubMed] [Google Scholar]
- Stupack, D.G. , Cheresh, D.A. , 2004. Integrins and angiogenesis. Curr. Top. Dev. Biol.. 64, 207–238. [DOI] [PubMed] [Google Scholar]
- Sundberg, C. , Kowanetz, M. , Brown, L.F. , Detmar, M. , Dvorak, H.F. , 2002. Stable expression of angiopoietin-1 and other markers by cultured pericytes: phenotypic similarities to a subpopulation of cells in maturing vessels during later stages of angiogenesis in vivo. Lab. Invest.. 82, 387–401. [DOI] [PubMed] [Google Scholar]
- Suri, C. , Jones, P.F. , Patan, S. , Bartunkova, S. , Maisonpierre, P.C. , Davis, S. , Sato, T.N. , Yancopoulos, G.D. , 1996. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 87, 1171–1180. [DOI] [PubMed] [Google Scholar]
- Sweeney, P. , Karashima, T. , Kim, S.J. , Kedar, D. , Mian, B. , Huang, S. , Baker, C. , Fan, Z. , Hicklin, D.J. , Pettaway, C.A. , 2002. Anti-vascular endothelial growth factor receptor 2 antibody reduces tumorigenicity and metastasis in orthotopic prostate cancer xenografts via induction of endothelial cell apoptosis and reduction of endothelial cell matrix metalloproteinase type 9 production. Clin. Cancer Res.. 8, 2714–2724. [PubMed] [Google Scholar]
- Teichert-Kuliszewska, K. , Maisonpierre, P.C. , Jones, N. , Campbell, A.I. , Master, Z. , Bendeck, M.P. , Alitalo, K. , Dumont, D.J. , Yancopoulos, G.D. , Stewart, D.J. , 2001. Biological action of angiopoietin-2 in a fibrin matrix model of angiogenesis is associated with activation of Tie2. Cardiovasc. Res.. 49, 659–670. [DOI] [PubMed] [Google Scholar]
- Thomas, A.L. , Trarbach, T. , Bartel, C. , Laurent, D. , Henry, A. , Poethig, M. , Wang, J. , Masson, E. , Steward, W. , Vanhoefer, U. , 2007. A phase IB, open-label dose-escalating study of the oral angiogenesis inhibitor PTK787/ZK 222584 (PTK/ZK), in combination with FOLFOX4 chemotherapy in patients with advanced colorectal cancer. Ann. Oncol.. 18, 782–788. [DOI] [PubMed] [Google Scholar]
- Ugurel, S. , Rappl, G. , Tilgen, W. , Reinhold, U. , 2001. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J. Clin. Oncol.. 19, 577–583. [DOI] [PubMed] [Google Scholar]
- Ugurel, S. , Hildenbrand, R. , Zimpfer, A. , La, R.P. , Paschka, P. , Sucker, A. , Keikavoussi, P. , Becker, J.C. , Rittgen, W. , Hochhaus, A. , 2005. Lack of clinical efficacy of imatinib in metastatic melanoma. Br. J. Cancer. 92, 1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varker, K.A. , Biber, J.E. , Kefauver, C. , Jensen, R. , Lehman, A. , Young, D. , Wu, H. , Lesinski, G.B. , Kendra, K. , Chen, H.X. , Walker, M.J. , Carson, W.E. , 2007. A randomized phase 2 trial of bevacizumab with or without daily low-dose interferon alfa-2b in metastatic malignant melanoma. Ann. Surg. Oncol.. 14, 2367–2376. [DOI] [PubMed] [Google Scholar]
- Wakui, S. , Yokoo, K. , Muto, T. , Suzuki, Y. , Takahashi, H. , Furusato, M. , Hano, H. , Endou, H. , Kanai, Y. , 2006. Localization of Ang-1, -2, Tie-2, and VEGF expression at endothelial-pericyte interdigitation in rat angiogenesis. Lab. Invest.. 86, 1172–1184. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Tasevski, V. , Wallace, E.M. , Gallery, E.D. , Morris, J.M. , 2007. Reduced maternal serum concentrations of angiopoietin-2 in the first trimester precede intrauterine growth restriction associated with placental insufficiency. BJOG. 114, 1427–1431. [DOI] [PubMed] [Google Scholar]
- Westphal, J.R. , Van't, H.R. , Peek, R. , Willems, R.W. , Crickard, K. , Crickard, U. , Askaa, J. , Clemmensen, I. , Ruiter, D.J. , de Waal, R.M. , 2000. Angiogenic balance in human melanoma: expression of VEGF, bFGF, IL-8, PDGF and angiostatin in relation to vascular density of xenografts in vivo. Int. J. Cancer. 86, 768–776. [DOI] [PubMed] [Google Scholar]
- Whitaker, G.B. , Limberg, B.J. , Rosenbaum, J.S. , 2001. Vascular endothelial growth factor receptor-2 and neuropilin-1 form a receptor complex that is responsible for the differential signaling potency of VEGF(165) and VEGF(121). J. Biol. Chem.. 276, 25520–25531. [DOI] [PubMed] [Google Scholar]
- Yana, I. , Sagara, H. , Takaki, S. , Takatsu, K. , Nakamura, K. , Nakao, K. , Katsuki, M. , Taniguchi, S. , Aoki, T. , Sato, H. , 2007. Crosstalk between neovessels and mural cells directs the site-specific expression of MT1-MMP to endothelial tip cells. J. Cell Sci.. 120, 1607–1614. [DOI] [PubMed] [Google Scholar]
- Zhou, Y.Z. , Fang, X.Q. , Li, H. , Diao, Y.T. , Yang, Y.F. , Zhao, D.L. , Wu, K. , Li, H.Q. , 2007. Role of serum angiopoietin-2 level in screening for esophageal squamous cell cancer and its precursors. Chin. Med. J. (Engl.). 120, 1216–1219. [PubMed] [Google Scholar]


