Abstract
The use of immunohistochemistry (IHC) in clinical cohorts is of paramount importance in determining the utility of a biomarker in clinical practice. A major bottleneck in translating a biomarker from bench‐to‐bedside is the lack of well characterized, specific antibodies suitable for IHC. Despite the widespread use of IHC as a biomarker validation tool, no universally accepted standardization guidelines have been developed to determine the applicability of particular antibodies for IHC prior to its use. In this review, we discuss the technical challenges faced by the use of immunohistochemical biomarkers and rigorously explore classical and emerging antibody validation technologies. Based on our review of these technologies, we provide strict criteria for the pragmatic validation of antibodies for use in immunohistochemical assays.
Keywords: Immunohistochemistry, Biomarker discovery, Antibody reliability, Antibody validation, Workflow
Highlights
Immunohistochemistry is an important tool for biomarker development.
Few potential immunohistochemistry biomarkers reach the clinical utility.
We describe the various factors influencing the IHC process.
We discuss classical and emerging antibody validation technologies.
We provide recommended work‐flows for proper antibody validation.
1. Introduction
The classical method of immunohistochemistry (IHC) allows for visualization of specific antigens in tissues or cells based on antibody‐antigen recognition, using brightfield or fluorescent microscopy. The history of IHC goes back to the early 1940s, when Coons and colleagues detected antigens in frozen tissue sections by developing an immunofluorescence technique (Coons et al., 1941). Introduction of a method based on peroxidase‐labelled antibodies opened the door to development of more advanced approaches (Mason et al., 1969; Nakane, 1968), enabling IHC to be used on routinely processed tissue sections, such as formalin‐fixed paraffin‐embedded (FFPE) tissues. However, it took until the early 1990s for the method to become generally accepted in diagnostic pathology (Leong, 1992; Taylor, 1994).
IHC is today a widely used method that can be rapidly performed in most laboratories. The procedure is short, simple and cost‐effective. Indeed, IHC has emerged as an important tool to detect cellular markers defining specific phenotypes relative to disease status and biology. Moreover, IHC is utilized for basic and clinical research, from small projects to high‐throughput strategies, to evaluate potential biomarkers in clinical patient cohorts. However, the lack of standardized guidelines for determining the specificity and functionality of antibodies renders the translation of promising biomarkers to the clinic difficult. Herein, we discuss the various limitations and technical challenges that need to be addressed when using IHC for biomarker development and clinical validation.
2. Review of clinically used IHC markers approved by FDA
A biomarker is defined as a molecule that is objectively measured and evaluated as an indicator of normal biological process, pathogenic process, or pharmacological responses to therapeutic intervention (Biomarkers‐Definitions‐Working‐Group, 2001). Although great efforts have been made in the last decade to discover novel cancer biomarkers for use in clinical practice, a striking number of these efforts fail to make it into the clinic (Fuzery et al., 2013). One of the causes of this failure of translation could be the limited knowledge that scientists working in biomarker discovery have in analytical, diagnostic and regulatory requirements for clinical assays (Fuzery et al., 2013). Over the last few decades a number of key FDA approved cancer biomarkers have been introduced into the clinic for differential diagnosis of specific tumours, leading to improvement of cancer detection and staging, identification of tumour subclasses, prediction of outcome after treatment, and selection of patients for different treatment options. However, of these approved biomarkers, only five are individual IHC‐based biomarkers (Fuzery et al., 2013) (Table 1). The earliest FDA approved biomarkers for IHC application were assays to detect the estrogen receptor (ER), progesterone receptor (PR) and HER‐2/neu (c‐erbB‐2). The presence of these biomarkers in breast cancer tissue serves as a diagnostic, prognostic and predictive method to assist pathologists in identifying breast cancer subtypes and determine whether patients are suitable candidates to receive certain targeted therapies such as Tamoxifen (ER positive patients) or Trastuzumab (Her‐2 positive patients). The IHC biomarker c‐kit (CD117), which is used in the clinic to detect gastrointestinal stromal tumours (GISTs) (Debiec‐Rychter et al., 2004), and p63, which is used to detect the presence of basal cells indicative of normal prostate glands (Shah et al., 2002; Weinstein et al., 2002), are the latest FDA approved single marker IHC‐based assays which were approved almost a decade ago in 2004 and 2005, respectively. Since then no other individual biomarker developed for detection in an IHC assay has been FDA approved. However, despite lack of FDA approval, there are many IHC markers utilized in some clinics to assist pathologists in diagnosis and decision making. Such examples include the use of E‐Cadherin and/or p120 staining to assist diagnosis of invasive lobular breast carcinoma (Rakha et al., 2010), various antibody panels for diagnosis and sub‐classification of malignant lymphomas, as well as the use of the proliferating nuclear marker, Ki67.
Table 1.
IHC biomarker assays for FFPE tissues.
| Biomarker | Cancer type | Year of approval or clearance | Clinical use |
|---|---|---|---|
| FDA approved single IHC biomarkers | |||
| p63 protein | Prostate | 2005 | Nuclear basal cell marker for differential diagnosis |
| c‐Kit (CD117) | Gastrointestinal stromal tumours | 2004 | Diagnosis |
| Estrogen receptor (ER) | Breast | 1999 | Prognosis, response to therapy |
| Progesterone receptor (PR) | Breast | 1999 | Prognosis, response to therapy |
| HER‐2/neu | Breast | 1998 | Prognosis, response to therapy |
| Biomarker assay | Cancer type | Company | Clinical use |
|---|---|---|---|
| Emerging panel‐based IHC biomarker assays | |||
| Mammostrat® (p53, HTF9C, CEACAM5, NDRG1 and SLC7A5 IHC combined with a defined mathematical algorithm) | Breast | Clarient InsightDx® | Recurrence risk index for hormone‐receptor positive, early stage breast cancer, independent of proliferation and grade |
| IHC4 (AQUA® Technology combined with ER/PR, HER2 and Ki‐67 IHC). | Breast | Genoptix® Medical Laboratory | Recurrence risk assessment |
An ideal biomarker demonstrating clinical sensitivity and specificity of 100% is almost never achieved in practice due the fact that increasing one of the parameters is only achieved at the expense of the other. As a result, panel biomarker assays are becoming more relevant. Two emerging IHC panel‐based assays are Mammostrat by Clarient InsightDx and IHC4 by Genoptix Medical Laboratory. Mammostrat is an IHC‐based panel assay that can estimate risk of recurrence in hormone receptor‐positive, early stage breast cancer patients which is independent of proliferation and grade. This assay quantifies p53, HTF9C, CEACAM5, NDRG1 and SLC7A5 by a defined mathematical algorithm resulting in a risk index (Bartlett et al., 2010, 2012). Similarly, IHC4 is another emerging assay which estimates recurrence risk for early stage breast cancer patients by quantifying IHC measurement of ER, PR, HER2 and Ki‐67 using Aqua® technology (Cuzick et al., 2011).
IHC‐based biomarker assays represent an attractive approach for biomarker detection in the clinic as the IHC technique is routinely carried out in clinical laboratories, there is a fast turn‐around time from assay to results and it is cost‐effective. However, the paucity of FDA‐approved biomarkers for IHC‐based assays emphasizes the importance and urgent requirement of standardized guidelines and workflows for IHC assay development which should be implemented at an early stage of biomarker discovery. This will ensure robust analytical and clinical performance and ultimately lead to a better chance of an IHC‐based biomarker assay achieving FDA approval.
3. Review of factors influencing the IHC process
The standard brightfield IHC technique is comprised of three components; slide preparation, IHC procedure and interpretation. Antibodies used in the clinic have undergone thorough testing and every step of the protocol has been well established, including both positive and negative controls. Factors which may affect the outcome of IHC include tissue handling, epitope retrieval, storage and handling of tissue sections, choice of antibody, detection method and interpretation procedure. To yield the expected staining pattern when establishing a new antibody, all factors which may influence the standardization and reproducibility of the process need to be carefully considered. These factors are summarized in Figure 1 and will be described more in detail below.
Figure 1.
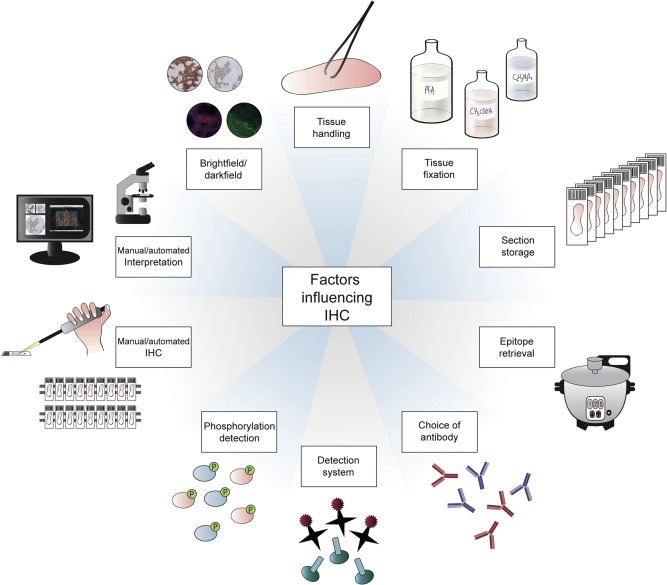
A schematic representation of various factors which may influence the standardization and reproducibility of the IHC process.
3.1. Tissue handling immediately after surgery, fixation and processing
‘Ischemia time’ refers to the time from when a tissue or organ is cut off from O2 supply through removal of a specimen from the body in surgery, to fixation of the specimen. Ischemia results in degradation of protein, RNA and DNA, as well as activation of tissue enzymes and autolysis (Kumar et al., 2005) and can therefore be a major factor influencing IHC results. Recently, Pekmezci et al. demonstrated that longer cold ischemia time affects the detection of ER and PR by IHC in breast cancer (Pekmezci et al., 2012). Although the American Society of Clinical Oncology and College of American Pathologists (ASCO/CAP) has developed guidelines for handling of tissues for ER, PR and HER‐2 detection in breast cancer patients, such guidelines are not available for other surgical specimens (Comanescu et al., 2012; Hammond et al., 2010). Fixation is another critical step in the IHC process to preserve tissue morphology and retain antigenicity of the target molecules. Two types of fixatives are commonly used in histopathology; (1) non‐coagulating fixatives (formaldehyde, glutaraldehyde, osmium teroxide, potassium dichromate and acetic acid) and (2) coagulating fixatives (alcohol, zinc salts, mercuric chloride, chromium trioxide and picric acid). The most common fixative used in histopathology is 10% neutral‐buffered formalin. This is composed of 4% paraformaldehyde solution which is buffered to a neutral pH. Formalin cross‐links peptides by formation of hydroxymethyl groups on reactive amino acid side chains, providing excellent preservation of tissue architecture; however, formalin fixation can mask epitopes and result in decreased antigenicity. Several factors influence the formalin fixation method, such as temperature, time, penetration rate, specimen dimension, volume ratio, pH of the buffer and osmolality, but unfortunately, there is a lack of available guidelines to establish a standard practise across pathology laboratories.
3.2. Appropriate storage and handling of tissue sections
Another factor that may influence the IHC outcome is storage of prepared tissue sections (Wester et al., 2000; Williams et al., 1997). It has been suggested that storing tissue sections longer than two months leads to loss of p53 antigen reactivity (Prioleau and Schnitt, 1995). The mechanisms underlying the loss of antigenicity in FFPE tissue is unclear. It has been hypothesised that oxidation may be the key contributor of antigenicity loss (Blind et al., 2008; Sauter and Mirlacher, 2002). Due to this and the fact that degradation of protein is temperature dependent, a large variety of storage conditions for cut sections have been advocated such as cold storage, paraffin coating or vacuum sealed desiccators. However, recently it has been suggested by Xie et al. (2011) that the presence of water both endogenously and exogenously plays a central role in loss of antigenicity. Therefore, slide storage conditions that are protected from oxidization by vacuum storage or paraffin coating are not completely protecting slides from loss of antigenicity if residual water from inadequate tissue processing is present on the tissue (Xie et al., 2011). Thus, the optimal storage of unstained sections is yet to be defined, making freshly cut sections or sections stored for less than two months most ideal. For long‐term storage, vacuum containers or storage in colder conditions (+4/−18°) is often recommended.
3.3. Appropriate and efficient epitope retrieval
Another major step that should be considered carefully when performing IHC is antigen retrieval (AR). The two methods of antigen retrieval are (1) heat‐induced epitope retrieval (HIER) (e.g. citrate pH 6.0, Tris–EDTA pH 9.0 and EDTA pH 8.0) and (2) proteolytic enzyme‐induced epitope retrieval (PIER) (e.g. proteinase K, trypsin, pepsin, pronase). Of the two methods, HIER is most commonly used. The technique was first described by Shi and colleagues (Shi et al., 1991) and has been improved by a number of investigators (Cattoretti et al., 1993, 1991, 1993) for its routine use in laboratories throughout the world. However, the mechanisms of AR are not fully understood. It is speculated that both HIER and PIER serve to break the methylene bridges created during fixation, exposing the antigenic sites in order to allow the antibodies to bind (D'Amico et al., 2009; Fowler et al., 2011; Kakimoto et al., 2008; Leong and Leong, 2007; Suurmeijer and Boon, 1993).
There are several different AR variables that can affect IHC staining results such as heating, the choice of AR solution, its pH and molarity, and the effect of metal ions (D'Amico et al., 2009; Emoto et al., 2005). An appropriately controlled AR method can restore antigenicity in formalin fixed paraffin embedded (FFPE) tissue to resemble the antigenicity of frozen tissue. Moreover, it can facilitate IHC standardization, despite variations in tissue fixation and subsequent handling (von Boguslawsky, 1994) (Shi et al., 2007; Taylor, 2006). However, the appropriate AR protocol is dependent on both the antibody and the target protein, and needs to be optimized for every antibody.
3.4. Appropriate choice of antibody (monoclonal vs polyclonal)
The three cardinal points that must be considered when buying commercial primary antibodies for IHC are as follows: (1) use reliable, recommended companies, (2) obtain complete information about the antibody to ensure it is applicable or recommended for IHC and, (3) characterize the specificity of the antibody. A significant number of commercial antibodies are not thoroughly analysed for off‐target binding, e.g. using protein arrays (Chang, 1983; Nilsson et al., 2005). In addition, several companies do not provide the sequence of the antigen the antibody was raised against (Saper, 2009) and, therefore, antibody validation is a mandatory step before proceeding with IHC.
The choice of using either monoclonal or polyclonal primary antibodies for IHC further complicates the issue of epitope specificity and determining which antibody would be more suitable for IHC (Bordeaux et al., 2003). Polyclonal antibodies are a collection of antibodies targeted against multiple epitopes of a particular antigen. Generally, when an animal is injected with a specific antigen, the immune system elicits a primary immune response by producing multiple B cell clones against the antigen. After subsequent immunization with the same antigen, these B cells differentiate into plasma cells producing and secreting antibodies found in the serum. The serum containing polyclonal antibodies can be affinity purified using the antigen as a ligand, which eliminates 99% of antibodies recognizing other targets than the antigen. This procedure results in antibodies with higher specificity than conventional polyclonal antibodies, still retaining the ability to recognize different epitopes on the same antigen (Lindskog et al., 2005). A monoclonal antibody is generated by selection of one single B cell from spleen or bone marrow of the immunized animal and fusing this cell with immortal myeloma cells to produce hybridoma cells (Kohler and Milstein, 1975). As such, the culture supernatant contains only one type of antibody specific for a single epitope of the immunizing peptide. The advantages and disadvantages of using polyclonal and monoclonal antibodies for IHC are summarized in Table 2.
Table 2.
Monoclonal versus polyclonal antibodies.
| Properties | Monoclonal antibody | Polyclonal antibody |
|---|---|---|
| Epitope selectivity | One antibody selective for a single epitope on an antigen | A mixture of antibodies recognizing multiple epitopes on an antigen |
| Source | Usually generated in mice or rabbit | Generated in a variety of species including rabbit, goat, sheep, and donkey |
| Reproducibility | Always identical (produced from the same hybridoma) | Prone to batch to batch variability (produced from animal sera) |
| Cross‐reactivity | Less likely to cross‐react with other proteins → lower background | May contain non‐specific antibodies→ background staining |
| Specificity/Sensitivity | More specific due to single epitope recognition but less sensitive because often unable to detect masked antigen. | More sensitive due to targeting multiple epitopes of an antigen but less specific than monoclonal antibodies |
A useful tool to search for appropriate antibodies suitable for IHC is the portal, Antibodypedia (http://www.antibodypedia.com). Here, antibodies are listed with reference to antibody companies and associated validation data (Bjorling and Uhlen, 2008).
3.5. Use of a sensitive and robust detection system
The outcome of an IHC assay depends on the use of sensitive protein detection system in order to visualize the antigen–antibody reaction. The most popular methods of detection are enzyme and fluorophore‐mediated detection systems. With chromogenic substrates, an enzyme label is reacted with the substrate to yield a strong colour product visualized by brightfield imaging. Alkaline phosphatase (AP) and horseradish peroxidase (HRP) are the two most extensively used enzymes, both with available chromogenic, fluorogenic and chemiluminescent substrates.
Detection systems in IHC can be divided into two broad categories, namely direct or indirect. In the direct detection method, the primary antibody is labelled with enzymes or fluorochromes, enabling direct detection of the antigen on the tissue section without the requirement of a secondary antibody. This method of detection is simpler and less time consuming; however, it has the disadvantage of lower sensitivity compared with indirect methods. The indirect detection method involves the use of unlabelled primary antibodies and labelled secondary/tertiary antibodies, which are specific for the bound primary antibody. Although this method is time consuming and complicated by multiple steps, indirect detection method is more sensitive in detecting tissue antigens. Some commonly used indirect detections mechanisms are as follows; the avidin‐biotin complex (ABC) method, the labelled streptavidin biotin (LSAB) method, the phosphatase‐anti‐phosphatase (PAP) and the polymer‐based detection system.
There are several other immunohistochemical detection methods such as tyramide amplification, cycled tyramide amplification, fluorescyl‐tyramide amplification and rolling circle amplification, but these are not heavily used to date in routine IHC.
3.6. Detection of phosphorylation using IHC
Post‐translational modifications are important biological events that control the behaviour of a protein. Phosphorylation is a post‐translational process regulating protein activity by the addition and removal of a phosphate group. Tissue phosphoproteomic studies show promise for the discovery of key phosphorylated proteins and signalling pathways in many diseases (Bodo and Hsi, 2011). The detection and quantification of phosphorylation has been well established using techniques such as Western blotting on cell lysates but it represents a new era in diagnostic pathology. Many phospho‐specific antibodies have been generated for immunohistochemical application; however, the detection step remains challenging due to the labile nature of phosphorylated proteins, reflecting dynamic processes. In addition, tissues become oxygen deficient shortly after being isolated from the blood supply and subsequently undergo rapid protein dephosphorylation (Blow, 2007). Therefore, if the tissues are not fixed within 60 min post‐surgical removal from the living body, the majority of phospho‐epitopes are lost (Baker et al., 2005; Jones et al., 2008). Due to this, most phosphorylation studies have not been reproduced. Other variations between studies leading to these discrepant results can include sample procurement, processing, scoring/quantification and subjectively selected cut‐offs (Bodo and Hsi, 2011). Therefore, rigorous standardization of laboratory procedures for tissue preservation and for the overall IHC technique as well as quantification is required for success in quantifying phosphorylation by IHC in tissue. Post‐translational modifications such as phosphorylation can also be studied with proximity ligation assay (PLA), described in Section 4.3. However, many of the same issues discussed here will also apply to PLA.
3.7. Use of manual immunohistochemistry versus automated immunohistochemistry platforms
A major milestone in the standardization, reliability and reproducibility of IHC is the invention of automated IHC platforms. Many critical steps in the manual IHC method are operator‐dependent and essential to the quality of the final IHC result and its reproducibility (Shi and Taylor, 2011). These include the critical antigen retrieval step, reagent preparation, application of reagents, appropriate washing steps and multiple incubation times. The use of automated IHC not only allows for larger volumes of slides to be stained simultaneously under standardized conditions, but also provides assistance to operators through additional processing monitoring errors such as alarms for inappropriate temperatures, insufficient volumes of reagent, expired reagents and even the selection of an incorrect reagent via the use of barcode scanning (Fetsch and Abati, 1999; Moreau et al., 1998; Prichard et al., 2011).
Many automated IHC machines, particularly those used in a clinical setting, are what is termed as “closed systems” which means the instrument is closed to introducing variations. Although this is an important advantage for standardization of IHC staining, it can be a drawback for research as the flexibility of choosing reagents, retrieval methods and introduction of subtle variation to the technique is lost. This has led to the development of “open” automated systems, offering similar flexibility as manual staining (Prichard et al., 2011). However, as HIER is not performed on an “open” platform, some of the same limitations of manual IHC discussed previously apply to this type of automated IHC. Clearly, there are advantages and disadvantages to the manual staining method and the “open” and “closed” automated systems so the choice of method should be influenced by the laboratory's purpose (Prichard et al., 2011). However, for large‐scale IHC efforts where planning and standardized IHC protocols are necessary (Uhlen et al., 2005; Warford et al., 2004) it can be anticipated that automated IHC may lead to reduction in error rate as each step of the staining procedure is recorded (Howat et al., 2014). Together with tissue microarray (TMA) technology (Battifora, 1986; Kononen et al., 1998), where a large number of tissues from different organs or individuals are assembled on a single slide, high‐throughput IHC minimizes reproducibility issues.
3.8. Interpretation via manual and automated approaches
Manual assessment of IHC staining remains the traditional method for most diagnostic and predictive decisions in pathology. However, manual interpretation of IHC data can be time intensive, laborious and an inherently subjective and semi‐quantitative process (Fiore et al., 2012). Observer variability can exist in three forms; intra‐observer variability, inter‐observer variability and inter‐laboratory variability (Conway et al., 2008). The latter is usually attributed to issues regarding tissue fixation and processing, antibodies used and detection systems. Intra‐observer variability, referring to the lack of consistent assessment by the observer, occurs less frequently than inter‐observer variability due to the fact that pathologists adhere to their own internal standards (Kay et al., 1994). Inter‐observer variability is the greatest problem associated with human‐based assessment of IHC staining, influenced by factors such as misplaced orientation on a TMA slide, eye fatigue, complexity of data management following differential categorical scoring, quality of microscope, illumination of microscope and individual human vision limitations (Conway et al., 2008).
Utilizing image analysis systems on virtual microscopy slides or whole slide images has been proposed as solving the problem of standardized quantification of IHC data, due to its capability of producing continuous datasets eliminating categorical and biased assessment. High‐throughput image analysis methods can also reduce workloads and outperform human manual scoring in terms of reproducibility and precision, as they are not affected by fatigue or subjectivity. Enormous advances in image analysis systems on tissue sections have been achieved over the years (Taylor and Levenson, 2006). However, despite these advances, image analysis is far from ready to replace the expert pathologist, as it is still very much a semi‐automated approach as most algorithms require specific input and training by a pathologist in order to produce accurate output. In addition, image analysis approaches are highly influenced by a number of factors that can affect the quality of their performance. For example, the quality of sections/TMAs hugely affects the resulting data obtained from image analysis. This is due to the inability of most of the current automated image analysis systems to identify irregularities on a section that the human eye can ignore, such as artefacts, edge effect staining, folding of tissue and thickness of tissue section, which may produce a false score. Moreover, image analysis often fails to distinguish tumour from benign tissue. Nevertheless, it is widely accepted that the continuous development of computer‐aided image analysis technologies will lead to quantitative systems that will compliment and support the pathologist/human expert to produce a less subjective and accurate IHC assessment.
3.9. Multiplexing: brightfield vs. darkfield
When measuring protein expression levels in tissue, a decision must be made as to whether assessment should be performed by IHC using brightfield imaging or immunofluorescence (IF) using fluorescent imaging, where both techniques offer advantages over the other. Brightfield imaging utilizes visible white light to illuminate the tissue, and protein expression is classically observed and graded based on the intensity of 3,3′‐diaminobenzidine (DAB), generating a brown staining (Gustashaw et al., 2010). Counterstaining with haematoxylin keeps morphological detail of the surrounding tissue intact and allows visualisation and analysis of localized protein. The IF technique visualizes protein expression in tissue against a dark background, using an antibody with a chemically attached fluorochrome, such as fluorescein isothiocyanate (FITC) or tetramethyl rhodamine isothiocyanate (TRITC) (Jordan et al., 2002). The antigen–antibody complex can be visualized using a fluorescent imaging instrument such as a microscope or scanner.
IHC using brightfield imaging is one of the pillars of modern pathology and a fundamental research tool in both pathology and translational research (Robertson and Savage, 2008), due to the many advantages associated with the technique. It can be performed routinely on FFPE tissue, which permits a pathologist or researcher to work with a familiar, conventional microscope (Jordan et al., 2002). In addition, it can detect antigens expressed at relatively low levels due to chromogenic enhancement steps, the equipment cost is low, and only minimal laboratory space is required. Most importantly in a clinical setting, the chromogens are very stable and long‐term slide storage is possible for many years. However, as a research tool, there are some major limitations associated with the technique. Firstly, the resolution of antigen localization is limited due to the chromogenic substrate precipitate, as well as the thickness of the sections imaged in the light microscope. Secondly, saturation of chromogenic systems occurs easily, which restricts quantitative analyses (Robertson and Savage, 2008). Above all, IHC using brightfield microscopy has a narrow dynamic range limiting its capability of multiplexing, and as cross reactivity is common, three antibodies/chromogens at a time is a maximum. Therefore, sequential or multi‐step staining is crucial to ensure cross reactivity does not occur with enzymes used or with primary/secondary antibodies raised in the same species. In addition, choosing colour combinations that are distinguishable by eye from each other and from the counterstain can be challenging, particularly when looking at co‐localized proteins. The concentration of precipitate may also inhibit further reaction, making it difficult to visualize rare targets and highly abundant targets on the same slide (Christensen and Winthers, 2009). Moreover, quantitation of multi‐staining using brightfield microscopy is even more limited, as most brightfield image analysis tools are primarily designed to quantify single chromogens. However, the use of spectral imaging technologies allows unmixing of stains and individual quantification of each chromogen.
In contrast to brightfield IHC, IF has a better capability of multiple labelling, as IF is of higher resolution due to the fluorophores being directly conjugated to the antibody (Robertson and Savage, 2008). Although choosing dyes with distinguishable spectral properties is still an issue, fluorescent imaging has a much broader dynamic range compared with brightfield imaging (Christensen and Winthers, 2009). On the other hand, IF‐based detection presents certain difficulties in respect to interpretation of tissue morphology, as well as the cost of reagents and equipment. Moreover, a fluorescent signal can be quenched when the fluorophores are in close proximity, and as fluorophores are not as stable as chromogens, photobleaching of stored slides is an issue. The most restraining aspect of IF is inherent autofluorescence of FFPE material, making high quality immunofluorescence imaging capricious (Robertson and Savage, 2008) and limiting the use of clinical material. Examples of consecutive sections stained with both brightfield and darkfield are displayed in Figure 2, illustrating some of the advantages and disadvantages with both methods.
Figure 2.
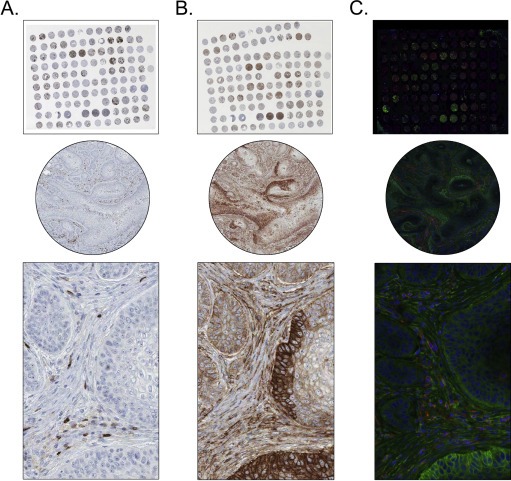
A lung cancer TMA stained with antibodies towards PTPRC (DakoCytomation) and CD99 (DakoCytomation), utilizing both brightfield IHC (A and B) and darkfield IF (C) on consecutive sections. (A) and (B), IHC staining of PTPRC and CD99, respectively. PTPRC shows distinct cytoplasmic positivity in lymphoid cells, while CD99 is strongly expressed in both tumour cells and surrounding tumour stroma. The IHC stained images show clear tissue morphology and manual interpretation of staining intensity can be easily determined. (C), IF staining of PTPRC (red) and CD99 (green). The IF stained images show autofluorescence and not as clear morphology; however, the different dyes and antibodies can be easily distinguishable from each other.
The use of multispectral imaging has overcome many of the issues regarding autofluorescence on FFPE tissue (Mansfield et al., 2005; Robertson and Savage, 2008). However, many reports using IF labelling of FFPE sections (Bataille et al., 2006; Bossard et al., 2006; Ferri et al., 1997; Hoover et al., 1998; Mason et al., 2000; Niki et al., 2004; Nurnberger et al., 2006; Papaxoinis et al., 2007; Scott et al., 2004; Suetterlin et al., 2004) have not been widely acknowledged by the scientific community (Robertson and Savage, 2008) rendering IHC by brightfield microscopy a more accepted assay for clinical use in quantifying protein expression. However, continuous research and development of new methods in the area of IF and image analysis, such as the new technique MxIF (Gerdes et al., 2013), will bridge the gap between classical IHC of FFPE material and the acceptance of IF analysis of human FFPE tissues.
One potentially might also consider application of both brightfield and fluorescent imaging, e.g. use of H&E staining/brightfield imaging for localisation of tumour regions and use of fluorescence‐based imaging for quantitation of consecutive tissue sections.
4. Review of currently used validation methods for antibodies for IHC
Commercial production of antibodies is well established; however, there are no universally accepted guidelines or standardized methods for determining the validity of these reagents (Bordeaux et al., 2003). The production and validation of specific antibodies is a challenging, costly and time consuming process. Perhaps as a result, the quality control by the antibody vendors is not always what it should be (Couchman, 2009). Moreover, the information supplied in academic publications where the antibodies are used is often insufficient. Therefore, it is imperative that investigators take requisite steps to assure themselves that the specificity of each antibody is as advertised. Here we explore both classical and emerging technologies for antibody validation.
4.1. Which staining pattern is expected?
The signal intensity is generally related to the antibody concentration (Dabbs, 2006). In order to get an optimal dilution of an antibody, rendering the greatest contrast between desired (specific) positivity and unwanted (non‐specific) background, it is necessary to know which staining pattern to expect. Hence, the first crucial step in antibody validation is to understand the nature of the target protein. For well‐known or partly characterized proteins, information regarding the expected staining pattern can be obtained from available databases such as Uniprot (www.uniprot.org), the Human Protein Atlas (www.proteinatlas.org), or by searches in published literature. Bioinformatic prediction algorithms for expected subcellular localisation, including presence of signal peptides or transmembrane regions, is gathered in online sources such as MDM (Fagerberg et al., 2010), SPOCTOPUS (Viklund et al., 2008) and Phobius (Kall et al., 2004). Furthermore, information on post‐translational modifications or splice variants is important in order to predict detection of multiple bands in Western blotting. Such information can be retrieved from e.g. OMIM (www.ncbi.nlm.nih.gov/omim) or Genecards (www.genecards.org). A large fraction of the human proteins are essentially uncharacterized and experimental data is needed for validation of the generated staining pattern in IHC.
4.2. Western blotting
The standard antibody validation method is Western blotting, whereby antibody specificity is confirmed by the presence of a single band corresponding to the predicted molecular weight of the target protein. However, as many proteins have a similar molecular weight, a band of the correct size is not full evidence for targeting the intended protein. Moreover, the kinetics of antibody‐antigen binding is context dependent and validation needs to be performed in an application‐specific manner. Therefore, even if an antibody yields a band of correct predicted size in Western blotting, it does not necessarily imply that the antibody is functional in IHC assays on FFPE tissue. This is mainly due to the fact that immunogenic epitopes are exposed differently in SDS‐PAGE compared to formalin fixation. Proteins are denatured during the Western blotting process so post‐translational modifications on the native protein may not be represented, while epitope masking (Hawkes et al., 1982) can occur with formalin fixation. Furthermore, as Western blot is dependent on the relative concentration of both the target and other proteins in the sample, even antibodies validated as highly specific may generate cross‐reactivity to off‐target proteins in the sample. This may be overcome by using cell lysates overexpressing the full‐length target protein, as the probability of correct protein detection is higher when a protein is present at sufficiently high level (Algenas et al., 2014).
4.3. Paired antibodies and proximity ligation assay
Paired antibodies are defined as antibodies raised against different, non‐overlapping epitopes on the same target protein. A similar IHC staining pattern yielded by two separate antibodies towards the same target protein on consecutive sections suggests a higher level of reliability, especially of importance for proteins lacking previous characterization (Uhlen et al., 2010). A dissimilar staining pattern does not however necessarily imply that both antibodies are unspecific, as one of them still could show the correct pattern. In addition, dissimilar antibodies could potentially mean that the antibodies are directed towards different isoforms of the same target protein, and other methods are necessary to decide if the antibody is specific. Even a similar staining patterns obtained by a set of paired antibodies can be difficult to interpret, and do not conclude if the two antibodies display the same unspecific background. The latter can be further elucidated using in situ proximity ligation assay (PLA).
The PLA technique is highly sensitive method determining protein interactions and analysing post‐translational modifications (Blokzijl et al., 2010; Lizardi et al., 1998; Soderberg et al., 2006). It is based on the principle that two or more oligonucleotide‐conjugated antibodies need to bind in close proximity in order to detect a signal, and can be utilized directly in frozen or FFPE tissue sections (Soderberg et al., 2008; Zieba et al., 2010). The binding is visualized by labelling the oligonucleotides with fluorophores or HRP. As two separate binding events are required to produce a signal, PLA also serves as a useful and reliable tool for antibody validation, using antibodies directed towards different epitopes on the same target protein. The signal generated by PLA can be quantified, and as each event produces a single “dot”, the outcome can be measured more easily compared to IHC staining intensity, facilitating automated image analysis.
4.4. Comparison with RNA sequencing data
The central dogma suggests a direct relationship between mRNA expression and protein levels in a population of cells at steady state. Lately, development of RNA sequencing (RNA‐Seq) has provided sensitive and reproducible expression analyses which can be easily applied for large scale exploration (Brawand et al., 2011; Wang et al., 2009). Comparison with transcription data may be a valuable antibody validation tool, whereby the quantitative measurement of the transcript abundance can be used to support the validation of protein expression. Several comprehensive RNA expression datasets are available online, e.g. at the Human Protein Atlas (www.proteinatlas.org) (Fagerberg et al., 2013), the RNA‐Seq atlas (www.medicalgenomics.org) (Krupp et al., 2012) and the BioGPS portal (www.biogps.org) (Wu et al., 2009). However, expression and abundance data is more noisy and complex than the underlying genomic sequence information, and protein levels are influenced by translational and post‐translational mechanisms. Some proteins are secreted or transported to other sites, and may not be observed in the organ where mRNA is expressed. This is the case for e.g. liver, where a large set of genes displaying high liver‐specific mRNA expression are negative for the corresponding proteins in liver, while positive in plasma (Kampf et al., 2014). Hence, some proteins may be present at levels not readily predicted by mRNA levels (Ghaemmaghami et al., 2003; Schwanhausser et al., 2011). On the contrary, a high correlation between mRNA and protein levels has still been shown in a number of studies (Greenbaum et al., 2002; Lu et al., 2007). The molecular pathways determining the expression patterns need to be further elucidated, in order to answer the fundamental question to what extent mRNA and protein expression correlate.
4.5. In situ hybridization
The RNA‐Seq technique may provide quantitative measurements of transcript levels; however, the comparison to IHC data is quite crude. The sequence mRNA pool from a tissue sample reflects all the different cell types present in the sample, and the RNA‐Seq lacks the precise localization and high cellular resolution provided by IHC. For morphological information on spatial distribution, in situ hybridization (ISH) uses RNA probes labelled with e.g. biotin that can be visualized in FFPE tissues (Carson et al., 2002; Gall and Pardue, 1969; Jin and Lloyd, 1997). One example of a large‐scale initiative using ISH spatial data is the Allen Brain Atlas (Lein et al., 2007), extensively used in the field of neuroscience. ISH renders a staining that can be compared with that of IHC and may thus serve as an antibody validation technique, e.g. identifying false positive results (Kiflemariam et al., 2012). However, as for several other methods, blocking of endogenous peroxidase and biotin could be a limiting factor (Qian and Lloyd, 2003), and in addition, ISH lacks the sensitivity to distinguish between sequences of high homology.
4.6. Mass spectrometry
Mass spectrometry provides the standard for detecting and quantifying a targeted set of proteins in a sample. The method uses the principle of ionizing peptides derived by proteolysis, and measuring the signal intensity of fragment ions over time, which indicates the abundance of the peptide in the sample (Anderson and Hunter, 2006; Towbin et al., 1979). As mass spectrometry yields a quantitative measurement of the target protein, it may be an important complement in validating the expression pattern rendered by an antibody, i.e. in analysing unexpected bands yielded by Western blotting. However, mass spectrometry lacks the spatial resolution that can be provided by IHC, and has sensitivity problems. It has been shown that the signal response of different peptides from the same protein can vary as much as 100‐fold in intensity (Picotti et al., 2007). Mass spectrometry also has a bias towards highly expressed proteins, as a low detection limit results in a reduced signal‐to‐noise‐ratio (Hack, 2004; Lange et al., 2008).
4.7. Appropriate positive and negative cell/tissue controls
Another approach to ensure antibody specificity is to perform IHC on positive and negative FFPE control cell lines known to express or not express the target protein, and to perform Western blotting on their subsequent lysates. This is also a useful tool to ensure your antibody is applicable to use on FFPE material prior to its use on valuable FFPE tissue. However, cell lines in which targets have appropriate levels of expression or lack of expression can be limited. In these instances, alternative approaches of cell manipulation can be performed to create positive and negative control cells. Overexpression models can be created and used as positive controls by introducing viral constructs that contain the gene/protein of interest into a cell line via lentiviral or retroviral transduction or plasmid‐based transfection (Seth, 2005). Similarly, negative control cell lines can be derived by RNA interference (RNAi), whereby expression of a target gene can be knocked down with high specificity (Rao et al., 2009). Alternatively, the use of the recently developed approach of clustered regularly interspaced short palindromic repeats (CRISPR) (Cho and Kim, 2013; Mali et al., 2013) could be used to generate a negative control. Unlike RNAi knockdown where transfection efficiency rarely reaches 100%, the CRISPR approach allows for complete knockdown which is ideal for insurance of antibody specificity. In addition, the use of tissue where a knockout of the gene has been engineered can be used to argue specificity of the primary antibody. It must also be noted that there is an increasing provision of commercial recombinant cell lines on the market with either ectopic overexpression of specific proteins (e.g. from Origene Technologies Inc.) or knockouts in cell lines (e.g. Horizon Discovery Ltd.).
4.8. Other commercially available controls
Many other techniques available through antibody suppliers can be carried out on tissue to test for antibody specificity. Isotope controls can be used to control for cross‐reactivity. This method ensures that the staining observed is not a result of immunoglobulins binding non‐specifically to Fc receptors present on the cell surface. However, the method does not prove that the antibody is binding to the target antigen.
Synthetic peptides towards which the commercial antibodies were generated can be used in competitive assays, where antiserum is incubated with the synthetic peptide prior to staining. If the staining component of the antiserum is raised against that antigen, the antibodies should adsorb to the peptide and little or no staining should be observed (Saper, 2005). However, although this is an acceptable assay for validation of polyclonal antibodies, the technique cannot be used for monoclonal antibodies as they will always be adsorbed by their antigen, even if they are staining something entirely different in the tissue (Saper, 2005). Furthermore, even as a polyclonal antibody validation tool, it does not rule out that other tissue proteins cross‐react with the synthetic peptide.
5. Ideal work‐flow
There does not exist an unflawed antibody validation method for IHC, and each method has its own advantages and disadvantages. In this section, we describe and discuss two alternative recommended work‐flows to follow in order to ensure an antibody is of highest quality prior to use in IHC. One is intended for IHC in high‐throughput strategies, such as the Human Protein Atlas project (Figure 3), and one is suitable for IHC in mainstream biomarker development applications, particularly those intending clinical application (Figure 4).
Figure 3.
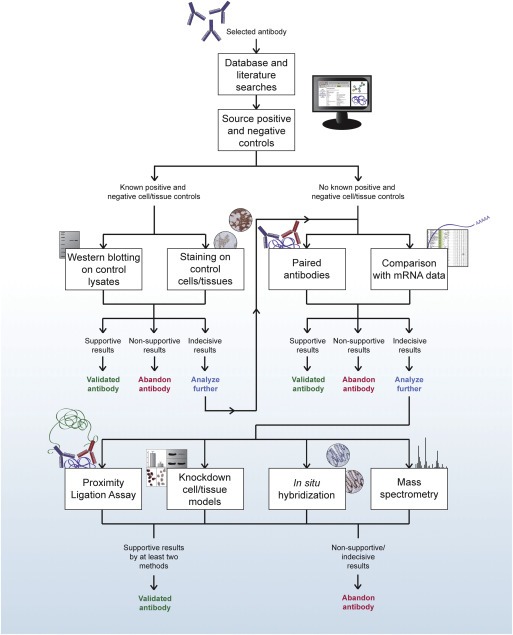
A schematic representation of recommended techniques to use for antibody validation in high‐throughput systematic investigations, such as the Human Protein Atlas project.
Figure 4.
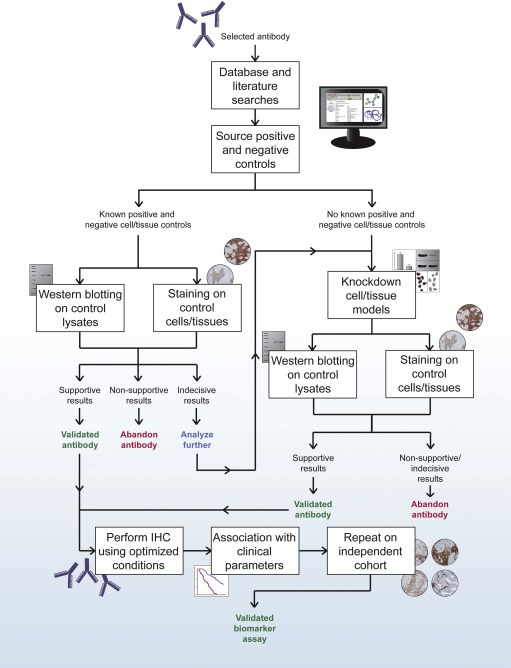
A schematic representation of recommended techniques to use for antibody validation in mainstream biomarker development projects, oriented towards clinical application.
Both approaches firstly involve the identification and selection of an appropriate antibody, and searches of literature and databases in order to fully understand the target protein and identify positive and negative controls. In the case of well characterized differentially expressed genes, IHC staining on cell lines or tissues known to express or not express the target protein is a relatively inexpensive, fast and an easily assessed method. Ideally, the validation should be complemented by Western blotting of the corresponding cell or tissue lysates. Previous experiments suggest, however, that a large fraction of all proteins are expressed in a house‐keeping manner (Fagerberg et al., 2013; Ponten et al., 2009). For such ubiquitously expressed proteins, this validation strategy has limitations as to lack of negative controls, as almost any antibody could render a ubiquitous staining pattern in IHC depending on the antibody concentration used. In addition, many proteins are largely uncharacterized and a more thorough investigation needs to be performed in order to ensure the antibody binds to its intended target.
The recommended antibody validation techniques to consider next largely rely on the cost and time that can be spent for thorough validation, and the laboratory's access to tissues and certain equipment. Moreover, it needs to be taken into consideration that the desired level of accuracy and specificity versus sensitivity may differ depending on the aim of the study. A biomarker intended to be used for labelling of beta cells in pancreas may only require absent staining in other cells of islet of Langerhans and abdominal organs adjacent to pancreas, while unspecific antibody binding in other tissues does not interfere with the result (Lindskog et al., 2012). In contrast, a potential diagnostic marker with the aim to accurately determine the origin of a metastasis tumour needs a higher level of specificity, in order to set the correct diagnosis (Gremel et al., 2014). The strategies also differ between validation of antibodies in high‐throughput projects and antibodies intended to be used in biomarker assays.
One example of a high‐throughput IHC initiative is the Human Protein Atlas project, which systematically explores the human proteome using in‐house generated affinity purified polyclonal antibodies on TMAs (Kampf et al., 2012; Uhlen et al., 2005). The TMAs contain samples from 44 different normal tissues, the 20 most common cancer types, 46 cell lines and six samples of primary cells. The current publically available version of the online atlas (www.proteinatlas.org) covers 16,621 human genes, represented by data from 21,984 antibodies, and thus serves as a valuable resource in biomarker discovery (Asplund and Edqvist, 2012; Ponten et al., 2011). The Human Protein Atlas utilizes paired antibodies and comparison with mRNA data, which in conjunction with IHC staining on test TMAs and Western blotting suggest a high level of antibody reliability. In challenging cases where the obtained results are contradictive or indecisive, thorough investigation with other methods such as PLA, knockdown models, in situ hybridization or mass spectrometry could add potential value in determining an antibody's specificity. A flow‐chart recommended for such high‐throughput projects is displayed in Figure 3.
In oncology drug research and development, where researchers seek to introduce drugs targeted to molecular pathways and reduce development timelines, there is an increasing demand for specific and sensitive cancer tissue‐based IHC biomarkers (Smith and Womack, 2014). The two most critical elements of a successful IHC assay are reliable antibodies and tissue sample integrity, and a failure to validate these elements sufficiently will lead to conflicting, irreproducible results (Smith and Womack, 2014). Therefore, we propose a strict but appropriate IHC workflow that should be adhered to for research and development of potential biomarkers (Figure 4). In this workflow, inclusion of definite positive and negative FFPE controls is imperative in every IHC run where antibody specificity can be verified as well as controlling for additional run variations. These controls may be in the form of either cell or tissue controls. Moreover, the use of automated systems is recommended to limit errors due to technical and laboratory variability.
6. Discussion and conclusion
IHC is an invaluable validation tool in biomarker discovery. However, considering the excessive number of existing studies proposing novel IHC biomarkers, markers validated in several clinical cohorts are extremely few, stressing the need to raise quality standards for clinical biomarker studies. Even if results can be reproduced, the transition towards a routinely used marker is complex. For a new factor to become of potential value in the clinic, it has to add an important value compared with other already used factors. Moreover, it also has to be taken into account in which patient material the factor was analysed and if it fits with the population where it potentially will be used. To be able to perform and reproduce a multitude of studies for the same marker, a specific antibody and standardized antibody validation workflow is crucial. We agree with the proposal recently made by (Howat et al., 2014), suggesting that the antibody conditions should be published on an open access site following publication in order to keep the knowledge already gained by research groups. This would aid in protocol optimization, minimize waste of valuable patient material and improve the quality of publications.
In this review, we described and discussed methods available for the validation of antibodies prior to usage in IHC, as well as numerous factors in the IHC procedure that can potentially influence the end result. In addition, we provide strict criteria that should be adhered for the pragmatic validation of antibodies for use in both high‐throughput, systematic investigations and mainstream biomarker discovery‐oriented immunohistochemical assays.
Acknowledgements
The work was financially supported by the Marie Curie Industry‐Academia Partnerships and Pathways program, FAST‐PATH (No. 285910) (www.fastpathproject.com), as well as the FP7 Collaborative Projects, APO‐DECIDE (www.apodecide.eu) and RATHER (www.ratherproject.com). Support is also acknowledged from the Irish Cancer Society Collaborative Cancer Research Centre, BREAST‐PREDICT (www.breastpredict.com), as well as the Wallenberg Research Foundation (KAW).
O'Hurley Gillian, Sjöstedt Evelina, Rahman Arman, Li Bo, Kampf Caroline, Pontén Fredrik, Gallagher William M., Lindskog Cecilia, (2014), Garbage in, garbage out: A critical evaluation of strategies used for validation of immunohistochemical biomarkers, Molecular Oncology, 8, doi: 10.1016/j.molonc.2014.03.008.
Contributor Information
Fredrik Pontén, Email: Fredrik.Ponten@igp.uu.se.
William M. Gallagher, Email: William.Gallagher@ucd.ie
References
- Algenas, C. , Agaton, C. , Fagerberg, L. , Asplund, A. , Bjorling, L. , Bjorling, E. , Kampf, C. , Lundberg, E. , Nilsson, P. , Persson, A. , Wester, K. , Ponten, F. , Wernerus, H. , Uhlen, M. , Ottosson Takanen, J. , Hober, S. , 2014. Antibody performance in western blot applications is context-dependent. Biotechnol. J.. 9, (3) 435–445. [DOI] [PubMed] [Google Scholar]
- Anderson, L. , Hunter, C.L. , 2006. Quantitative mass spectrometric multiple reaction monitoring assays for major plasma proteins. Mol. Cell Proteomics. 5, (4) 573–588. [DOI] [PubMed] [Google Scholar]
- Asplund, A. , Edqvist, P.H. , Schwenk, J.M. , Ponten, F. , 2012. Antibodies for profiling the human proteome – the human protein atlas as a resource for cancer research. Proteomics. 12, (13) 2067–2077. [DOI] [PubMed] [Google Scholar]
- Baker, A.F. , Dragovich, T. , Ihle, N.T. , Williams, R. , Fenoglio-Preiser, C. , Powis, G. , 2005. Stability of phosphoprotein as a biological marker of tumor signaling. Clin. Cancer Res.. 11, (12) 4338–4340. [DOI] [PubMed] [Google Scholar]
- Bartlett, J.M. , Thomas, J. , Ross, D.T. , Seitz, R.S. , Ring, B.Z. , Beck, R.A. , Pedersen, H.C. , Munro, A. , Kunkler, I.H. , Campbell, F.M. , Jack, W. , Kerr, G.R. , Johnstone, L. , Cameron, D.A. , Chetty, U. , 2010. Mammostrat as a tool to stratify breast cancer patients at risk of recurrence during endocrine therapy. Breast Cancer Res.: BCR. 12, (4) R47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett, J.M. , Bloom, K.J. , Piper, T. , Lawton, T.J. , van de Velde, C.J. , Ross, D.T. , Ring, B.Z. , Seitz, R.S. , Beck, R.A. , Hasenburg, A. , Kieback, D. , Putter, H. , Markopoulos, C. , Dirix, L. , Seynaeve, C. , Rea, D. , 2012. Mammostrat as an immunohistochemical multigene assay for prediction of early relapse risk in the tamoxifen versus exemestane adjuvant multicenter trial pathology study. J. Clin. Oncol. : Off. J. Am. Soc. Clin. Oncol.. 30, (36) 4477–4484. [DOI] [PubMed] [Google Scholar]
- Bataille, F. , Troppmann, S. , Klebl, F. , Rogler, G. , Stoelcker, B. , Hofstadter, F. , Bosserhoff, A.K. , Rummele, P. , 2006. Multiparameter immunofluorescence on paraffin-embedded tissue sections. Appl. Immunohistochem. Mol. Morphol.: AIMM/Off. Publ. Soc. Appl. Immunohistochem.. 14, (2) 225–228. [DOI] [PubMed] [Google Scholar]
- Battifora, H. , 1986. The multitumor (sausage) tissue block: novel method for immunohistochemical antibody testing. Lab Invest.. 55, (2) 244–248. [PubMed] [Google Scholar]
- Biomarkers-Definitions-Working-Group 2001. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther.. 69, (3) 89–95. [DOI] [PubMed] [Google Scholar]
- Bjorling, E. , Uhlen, M. , 2008. Antibodypedia, a portal for sharing antibody and antigen validation data. Mol Cell Proteom.. 7, (10) 2028–2037. [DOI] [PubMed] [Google Scholar]
- Blind, C. , Koepenik, A. , Pacyna-Gengelbach, M. , Fernahl, G. , Deutschmann, N. , Dietel, M. , Krenn, V. , Petersen, I. , 2008. Antigenicity testing by immunohistochemistry after tissue oxidation. J. Clin. Pathol.. 61, (1) 79–83. [DOI] [PubMed] [Google Scholar]
- Blokzijl, A. , Friedman, M. , Ponten, F. , Landegren, U. , 2010. Profiling protein expression and interactions: proximity ligation as a tool for personalized medicine. J. Intern. Med.. 268, (3) 232–245. [DOI] [PubMed] [Google Scholar]
- Blow, N. , 2007. Tissue preparation: tissue issues. Nature. 448, (7156) 959–963. [DOI] [PubMed] [Google Scholar]
- Bodo, J. , Hsi, E.D. , 2011. Phosphoproteins and the dawn of functional phenotyping. Pathobiol.: J. Immunopathol. Mol. Cell. Biol.. 78, (2) 115–121. [DOI] [PubMed] [Google Scholar]
- Bordeaux, J. , Welsh, A. , Agarwal, S. , Killiam, E. , Baquero, M. , Hanna, J. , Anagnostou, V. , Rimm, D. , 2003. Antibody validation. Biotechniques. 48, (3) 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossard, C. , Jarry, A. , Colombeix, C. , Bach-Ngohou, K. , Moreau, A. , Loussouarn, D. , Mosnier, J.F. , Laboisse, C.L. , 2006. Phosphohistone H3 labelling for histoprognostic grading of breast adenocarcinomas and computer-assisted determination of mitotic index. J. Clin. Pathol.. 59, (7) 706–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawand, D. , Soumillon, M. , Necsulea, A. , Julien, P. , Csardi, G. , Harrigan, P. , Weier, M. , Liechti, A. , Aximu-Petri, A. , Kircher, M. , Albert, F.W. , Zeller, U. , Khaitovich, P. , Grutzner, F. , Bergmann, S. , Nielsen, R. , Paabo, S. , Kaessmann, H. , 2011. The evolution of gene expression levels in mammalian organs. Nature. 478, (7369) 343–348. [DOI] [PubMed] [Google Scholar]
- Carson, J.P. , Thaller, C. , Eichele, G. , 2002. A transcriptome atlas of the mouse brain at cellular resolution. Curr. Opin. Neurobiol.. 12, (5) 562–565. [DOI] [PubMed] [Google Scholar]
- Cattoretti, G. , Pileri, S. , Parravicini, C. , Becker, M.H. , Poggi, S. , Bifulco, C. , Key, G. , D'Amato, L. , Sabattini, E. , Feudale, E. , 1993. Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J. Pathol.. 171, (2) 83–98. [DOI] [PubMed] [Google Scholar]
- Chang, T.W. , 1983. Binding of cells to matrixes of distinct antibodies coated on solid surface. J. Immunol. Methods. 65, (1–2) 217–223. [DOI] [PubMed] [Google Scholar]
- Cho, S.W. , Kim, S. , Kim, J.M. , Kim, J.S. , 2013. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat. Biotechnol.. 31, (3) 230–232. [DOI] [PubMed] [Google Scholar]
- Christensen, N.K. , Winthers, L. , 2009. Multi-staining immunohistochemistry. IHC Stain. Meth.. 103–108. [Google Scholar]
- Comanescu, M. , Arsene, D. , Ardeleanu, C. , Bussolati, G. , 2012. The mandate for a proper preservation in histopathological tissues. Rom. J. Morphol. Embryol.=Revue roumaine de morphologie embryologie. 53, (2) 233–242. [PubMed] [Google Scholar]
- Conway, C. , Dobson, L. , O'Grady, A. , Kay, E. , Costello, S. , O'Shea, D. , 2008. Virtual microscopy as an enabler of automated/quantitative assessment of protein expression in TMAs. Histochem. Cell Biol.. 130, (3) 447–463. [DOI] [PubMed] [Google Scholar]
- Coons, A.H. , Creech, H.J. , Jones, R.N. , 1941. Immunological properties of an antibody containing a fluorescent group. Proc. Soc. Exp. Biol. Med.. 47, 200–202. [Google Scholar]
- Couchman, J.R. , 2009. Commercial antibodies: the good, bad, and really ugly. J. Histochem. Cytochem.: Off. J. Histochem. Soc.. 57, (1) 7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzick, J. , Dowsett, M. , Pineda, S. , Wale, C. , Salter, J. , Quinn, E. , Zabaglo, L. , Mallon, E. , Green, A.R. , Ellis, I.O. , Howell, A. , Buzdar, A.U. , Forbes, J.F. , 2011. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J. Clin. Oncol. : Off. J. Am. Soc. Clin. Oncol.. 29, (32) 4273–4278. [DOI] [PubMed] [Google Scholar]
- D'Amico, F. , Skarmoutsou, E. , Stivala, F. , 2009. State of the art in antigen retrieval for immunohistochemistry. J. Immunol. Methods. 341, (1–2) 1–18. [DOI] [PubMed] [Google Scholar]
- Dabbs, D.J. , 2006. Diagnostic Immunohistochemistry Elsevier; Pittsburgh: [Google Scholar]
- Debiec-Rychter, M. , Wasag, B. , Stul, M. , De Wever, I. , Van Oosterom, A. , Hagemeijer, A. , Sciot, R. , 2004. Gastrointestinal stromal tumours (GISTs) negative for KIT (CD117 antigen) immunoreactivity. J. Pathol.. 202, (4) 430–438. [DOI] [PubMed] [Google Scholar]
- Emoto, K. , Yamashita, S. , Okada, Y. , 2005. Mechanisms of heat-induced antigen retrieval: does pH or ionic strength of the solution play a role for refolding antigens?. J. Histochem. Cytochem.: Off. J. Histochem. Soc.. 53, (11) 1311–1321. [DOI] [PubMed] [Google Scholar]
- Fagerberg, L. , Jonasson, K. , von Heijne, G. , Uhlen, M. , Berglund, L. , 2010. Prediction of the human membrane proteome. Proteomics. 10, (6) 1141–1149. [DOI] [PubMed] [Google Scholar]
- Fagerberg, L. , Hallstrom, B.M. , Oksvold, P. , Kampf, C. , Djureinovic, D. , Odeberg, J. , Habuka, M. , Tahmasebpoor, S. , Danielsson, A. , Edlund, K. , Asplund, A. , Sjostedt, E. , Lundberg, E. , Al-Khalili Szigyarto, C. , Skogs, M. , Ottosson Takanen, J. , Berling, H. , Tegel, H. , Mulder, J. , Nilsson, P. , Schwenk, J.M. , Lindskog, C. , Danielsson, F. , Mardinoglu, A. , Sivertsson, A. , von Felitzen, K. , Forsberg, M. , Zwahlen, M. , Olsson, I. , Navani, S. , Huss, M. , Nielsen, J. , Ponten, F. , Uhlen, M. , 2013. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteom.. 13, (2) 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri, G.L. , Gaudio, R.M. , Castello, I.F. , Berger, P. , Giro, G. , 1997. Quadruple immunofluorescence: a direct visualization method. J. Histochem. Cytochem.: Off. J. Histochem. Soc.. 45, (2) 155–158. [DOI] [PubMed] [Google Scholar]
- Fetsch, P.A. , Abati, A. , 1999. Overview of the clinical immunohistochemistry laboratory: regulations and troubleshooting guidelines. Methods Mol. Biol.. 115, 405–414. [DOI] [PubMed] [Google Scholar]
- Fiore, C. , Bailey, D. , Conlon, N. , Wu, X. , Martin, N. , Fiorentino, M. , Finn, S. , Fall, K. , Andersson, S.O. , Andren, O. , Loda, M. , Flavin, R. , 2012. Utility of multispectral imaging in automated quantitative scoring of immunohistochemistry. J. Clin. Pathol.. 65, (6) 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler, C.B. , Evers, D.L. , O'Leary, T.J. , Mason, J.T. , 2011. Antigen retrieval causes protein unfolding: evidence for a linear epitope model of recovered immunoreactivity. J. Histochem. Cytochem.: Off. J. Histochem. Soc.. 59, (4) 366–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuzery, A.K. , Levin, J. , Chan, M.M. , Chan, D.W. , 2013. Translation of proteomic biomarkers into FDA approved cancer diagnostics: issues and challenges. Clin. Proteomics. 10, (1) 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall, J.G. , Pardue, M.L. , 1969. Formation and detection of RNA-DNA hybrid molecules in cytological preparations. Proc. Natl. Acad. Sci. U S A. 63, (2) 378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes, M.J. , Sevinsky, C.J. , Sood, A. , Adak, S. , Bello, M.O. , Bordwell, A. , Can, A. , Corwin, A. , Dinn, S. , Filkins, R.J. , Hollman, D. , Kamath, V. , Kaanumalle, S. , Kenny, K. , Larsen, M. , Lazare, M. , Li, Q. , Lowes, C. , McCulloch, C.C. , McDonough, E. , Montalto, M.C. , Pang, Z. , Rittscher, J. , Santamaria-Pang, A. , Sarachan, B.D. , Seel, M.L. , Seppo, A. , Shaikh, K. , Sui, Y. , Zhang, J. , Ginty, F. , 2013. Highly multiplexed single-cell analysis of formalin-fixed, paraffin-embedded cancer tissue. Proc. Natl. Acad. Sci. U S A. 110, (29) 11982–11987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaemmaghami, S. , Huh, W.K. , Bower, K. , Howson, R.W. , Belle, A. , Dephoure, N. , O'Shea, E.K. , Weissman, J.S. , 2003. Global analysis of protein expression in yeast. Nature. 425, (6959) 737–741. [DOI] [PubMed] [Google Scholar]
- Greenbaum, D. , Jansen, R. , Gerstein, M. , 2002. Analysis of mRNA expression and protein abundance data: an approach for the comparison of the enrichment of features in the cellular population of proteins and transcripts. Bioinformatics. 18, (4) 585–596. [DOI] [PubMed] [Google Scholar]
- Greenwell, A. , Foley, J.F. , Maronpot, R.R. , 1991. An enhancement method for immunohistochemical staining of proliferating cell nuclear antigen in archival rodent tissues. Cancer Lett.. 59, (3) 251–256. [DOI] [PubMed] [Google Scholar]
- Greenwell, A. , Foley, J.F. , Maronpot, R.R. , 1993. Detecting proliferating cell nuclear antigen in archival rodent tissues. Environ. Health Perspect.. 101, (Suppl. 5) 207–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel, G. , Bergman, J. , Djureinovic, D. , Edqvist, P.H. , Maindad, V. , Bharambe, B.M. , Khan, W.A. , Navani, S. , Elebro, J. , Jirstrom, K. , Hellberg, D. , Uhlen, M. , Micke, P. , Ponten, F. , 2014. A systematic analysis of commonly used antibodies in cancer diagnostics. Histopathology. 64, (2) 293–305. [DOI] [PubMed] [Google Scholar]
- Gustashaw, K.M. , Najmabadi, P. , Potts, S. , 2010. Measuring protein expression in Tissue: the complemenatary roles of brightfield and fluoescence in whole slide scanning. LABMEDICINE. 41, (3) 135–142. [Google Scholar]
- Hack, C.J. , 2004. Integrated transcriptome and proteome data: the challenges ahead. Brief Funct. Genomic Proteomic. 3, (3) 212–219. [DOI] [PubMed] [Google Scholar]
- Hammond, M.E. , Hayes, D.F. , Dowsett, M. , Allred, D.C. , Hagerty, K.L. , Badve, S. , Fitzgibbons, P.L. , Francis, G. , Goldstein, N.S. , Hayes, M. , Hicks, D.G. , Lester, S. , Love, R. , Mangu, P.B. , McShane, L. , Miller, K. , Osborne, C.K. , Paik, S. , Perlmutter, J. , Rhodes, A. , Sasano, H. , Schwartz, J.N. , Sweep, F.C. , Taube, S. , Torlakovic, E.E. , Valenstein, P. , Viale, G. , Visscher, D. , Wheeler, T. , Williams, R.B. , Wittliff, J.L. , Wolff, A.C. , 2010. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol.. 28, (16) 2784–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes, R. , Niday, E. , Gordon, J. , 1982. A dot-immunobinding assay for monoclonal and other antibodies. Anal. Biochem.. 119, (1) 142–147. [DOI] [PubMed] [Google Scholar]
- Hoover, K.B. , Liao, S.Y. , Bryant, P.J. , 1998. Loss of the tight junction MAGUK ZO-1 in breast cancer: relationship to glandular differentiation and loss of heterozygosity. Am. J. Pathol.. 153, (6) 1767–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howat, W.J. , Lewis, A. , Jones, P. , Kampf, C. , Ponten, F. , van der Loos, C.M. , Gray, N. , Womack, C. , Warford, A. , 2014 Feb 11. Antibody validation of immunohistochemistry for biomarker discovery: recommendations of a consortium of academic and pharmaceutical based histopathology researchers. Methods. 10.1016/j.ymeth.2014.01.018 pii: S1046-2023(14)00028-0 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, L. , Lloyd, R.V. , 1997. In situ hybridization: methods and applications. J. Clin. Lab. Anal.. 11, (1) 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, R.J. , Boyce, T. , Fennell, M. , Jacobs, V. , Pinto, F. , Duffield, E. , Clack, G. , Green, T. , Kelly, J. , Robertson, J. , 2008. The impact of delay in cryo-fixation on biomarkers of Src tyrosine kinase activity in human breast and bladder cancers. Cancer Chemother. Pharmacol.. 61, (1) 23–32. [DOI] [PubMed] [Google Scholar]
- Jordan, R.C. , Daniels, T.E. , Greenspan, J.S. , Regezi, J.A. , 2002. Advanced diagnostic methods in oral and maxillofacial pathology. Part II: immunohistochemical and immunofluorescent methods. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod.. 93, (1) 56–74. [DOI] [PubMed] [Google Scholar]
- Kakimoto, K. , Takekoshi, S. , Miyajima, K. , Osamura, R.Y. , 2008. Hypothesis for the mechanism for heat-induced antigen retrieval occurring on fresh frozen sections without formalin-fixation in immunohistochemistry. J. Mol. Histol.. 39, (4) 389–399. [DOI] [PubMed] [Google Scholar]
- Kall, L. , Krogh, A. , Sonnhammer, E.L. , 2004. A combined transmembrane topology and signal peptide prediction method. J. Mol. Biol.. 338, (5) 1027–1036. [DOI] [PubMed] [Google Scholar]
- Kampf, C. , Mardinoglu, A. , Fagerberg, L. , Hallström, B.M. , Edlund, K. , Lundberg, E. , Pontén, F. , Nielsen, J. , Uhlen, M. , 2014 Mar 19. The human liver-specific proteome defined by transcriptomics and antibody-based profiling. FASEB J.. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Kampf, C. , Olsson, I. , Ryberg, U. , Sjostedt, E. , Ponten, F. , 2012. Production of tissue microarrays, immunohistochemistry staining and digitalization within the human protein atlas. J. Vis. Exp.. 63, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay, E.W. , Walsh, C.J. , Cassidy, M. , Curran, B. , Leader, M. , 1994. C-erbB-2 immunostaining: problems with interpretation. J. Clin. Pathol.. 47, (9) 816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiflemariam, S. , Andersson, S. , Asplund, A. , Ponten, F. , Sjoblom, T. , 2012. Scalable in situ hybridization on tissue arrays for validation of novel cancer and tissue-specific biomarkers. PLoS One. 7, (3) e32927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler, G. , Milstein, C. , 1975. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 256, (5517) 495–497. [DOI] [PubMed] [Google Scholar]
- Kononen, J. , Bubendorf, L. , Kallioniemi, A. , Barlund, M. , Schraml, P. , Leighton, S. , Torhorst, J. , Mihatsch, M.J. , Sauter, G. , Kallioniemi, O.P. , 1998. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat. Med.. 4, (7) 844–847. [DOI] [PubMed] [Google Scholar]
- Krupp, M. , Marquardt, J.U. , Sahin, U. , Galle, P.R. , Castle, J. , Teufel, A. , 2012. RNA-Seq Atlas – a reference database for gene expression profiling in normal tissue by next-generation sequencing. Bioinformatics. 28, (8) 1184–1185. [DOI] [PubMed] [Google Scholar]
- Kumar, V.A. , Fausto, A.K. , 2005. Robbins and Cotran Pathologic Basis of Disease Elsevier Inc. [Google Scholar]
- Lange, V. , Picotti, P. , Domon, B. , Aebersold, R. , 2008. Selected reaction monitoring for quantitative proteomics: a tutorial. Mol. Syst. Biol.. 4, 222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein, E.S. , Hawrylycz, M.J. , Ao, N. , Ayres, M. , Bensinger, A. , Bernard, A. , Boe, A.F. , Boguski, M.S. , Brockway, K.S. , Byrnes, E.J. , Chen, L. , Chen, L. , Chen, T.M. , Chin, M.C. , Chong, J. , Crook, B.E. , Czaplinska, A. , Dang, C.N. , Datta, S. , Dee, N.R. , Desaki, A.L. , Desta, T. , Diep, E. , Dolbeare, T.A. , Donelan, M.J. , Dong, H.W. , Dougherty, J.G. , Duncan, B.J. , Ebbert, A.J. , Eichele, G. , Estin, L.K. , Faber, C. , Facer, B.A. , Fields, R. , Fischer, S.R. , Fliss, T.P. , Frensley, C. , Gates, S.N. , Glattfelder, K.J. , Halverson, K.R. , Hart, M.R. , Hohmann, J.G. , Howell, M.P. , Jeung, D.P. , Johnson, R.A. , Karr, P.T. , Kawal, R. , Kidney, J.M. , Knapik, R.H. , Kuan, C.L. , Lake, J.H. , Laramee, A.R. , Larsen, K.D. , Lau, C. , Lemon, T.A. , Liang, A.J. , Liu, Y. , Luong, L.T. , Michaels, J. , Morgan, J.J. , Morgan, R.J. , Mortrud, M.T. , Mosqueda, N.F. , Ng, L.L. , Ng, R. , Orta, G.J. , Overly, C.C. , Pak, T.H. , Parry, S.E. , Pathak, S.D. , Pearson, O.C. , Puchalski, R.B. , Riley, Z.L. , Rockett, H.R. , Rowland, S.A. , Royall, J.J. , Ruiz, M.J. , Sarno, N.R. , Schaffnit, K. , Shapovalova, N.V. , Sivisay, T. , Slaughterbeck, C.R. , Smith, S.C. , Smith, K.A. , Smith, B.I. , Sodt, A.J. , Stewart, N.N. , Stumpf, K.R. , Sunkin, S.M. , Sutram, M. , Tam, A. , Teemer, C.D. , Thaller, C. , Thompson, C.L. , Varnam, L.R. , Visel, A. , Whitlock, R.M. , Wohnoutka, P.E. , Wolkey, C.K. , Wong, V.Y. , Wood, M. , Yaylaoglu, M.B. , Young, R.C. , Youngstrom, B.L. , Yuan, X.F. , Zhang, B. , A. Zwingman, T. , Jones, A.R. , 2007. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 445, (7124) 168–176. [DOI] [PubMed] [Google Scholar]
- Leong, A.S. , 1992. Diagnostic immunohistochemistry – problems and solutions. Pathology. 24, (1) 1–4. [DOI] [PubMed] [Google Scholar]
- Leong, T.Y. , Leong, A.S. , 2007. How does antigen retrieval work?. Adv. Anat. Pathol.. 14, (2) 129–131. [DOI] [PubMed] [Google Scholar]
- Lindskog, M. , Rockberg, J. , Uhlen, M. , Sterky, F. , 2005. Selection of protein epitopes for antibody production. Biotechniques. 38, (5) 723–727. [DOI] [PubMed] [Google Scholar]
- Lindskog, C. , Korsgren, O. , Ponten, F. , Eriksson, J.W. , Johansson, L. , Danielsson, A. , 2012. Novel pancreatic beta cell-specific proteins: antibody-based proteomics for identification of new biomarker candidates. J. Proteomics. 75, (9) 2611–2620. [DOI] [PubMed] [Google Scholar]
- Lizardi, P.M. , Huang, X. , Zhu, Z. , Bray-Ward, P. , Thomas, D.C. , Ward, D.C. , 1998. Mutation detection and single-molecule counting using isothermal rolling-circle amplification. Nat. Genet.. 19, (3) 225–232. [DOI] [PubMed] [Google Scholar]
- Lu, P. , Vogel, C. , Wang, R. , Yao, X. , Marcotte, E.M. , 2007. Absolute protein expression profiling estimates the relative contributions of transcriptional and translational regulation. Nat. Biotechnol.. 25, (1) 117–124. [DOI] [PubMed] [Google Scholar]
- Mali, P. , Yang, L. , Esvelt, K.M. , Aach, J. , Guell, M. , DiCarlo, J.E. , Norville, J.E. , Church, G.M. , 2013. RNA-guided human genome engineering via Cas9. Science. 339, (6121) 823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield, J.R. , Gossage, K.W. , Hoyt, C.C. , Levenson, R.M. , 2005. Autofluorescence removal, multiplexing, and automated analysis methods for in-vivo fluorescence imaging. J. Biomed. Opt.. 10, (4) 41207 [DOI] [PubMed] [Google Scholar]
- Mason, T.E. , Phifer, R.F. , Spicer, S.S. , Swallow, R.A. , Dreskin, R.B. , 1969. An immunoglobulin-enzyme bridge method for localizing tissue antigens. J. Histochem. Cytochem.. 17, (9) 563–569. [DOI] [PubMed] [Google Scholar]
- Mason, D.Y. , Micklem, K. , Jones, M. , 2000. Double immunofluorescence labelling of routinely processed paraffin sections. J. Pathol.. 191, (4) 452–461. [DOI] [PubMed] [Google Scholar]
- Moreau, A. , Le Neel, T. , Joubert, M. , Truchaud, A. , Laboisse, C. , 1998. Approach to automation in immunohistochemistry. Clin. Chim. Acta. 278, (2) 177–184. [DOI] [PubMed] [Google Scholar]
- Nakane, P.K. , 1968. Simultaneous localization of multiple tissue antigens using the peroxidase-labeled antibody method: a study on pituitary glands of the rat. J. Histochem. Cytochem.. 16, (9) 557–560. [DOI] [PubMed] [Google Scholar]
- Niki, H. , Hosokawa, S. , Nagaike, K. , Tagawa, T. , 2004. A new immunofluorostaining method using red fluorescence of PerCP on formalin-fixed paraffin-embedded tissues. J. Immunol. Methods. 293, (1–2) 143–151. [DOI] [PubMed] [Google Scholar]
- Nilsson, P. , Paavilainen, L. , Larsson, K. , Odling, J. , Sundberg, M. , Andersson, A.C. , Kampf, C. , Persson, A. , Al-Khalili Szigyarto, C. , Ottosson, J. , Bjorling, E. , Hober, S. , Wernerus, H. , Wester, K. , Ponten, F. , Uhlen, M. , 2005. Towards a human proteome atlas: high-throughput generation of mono-specific antibodies for tissue profiling. Proteomics. 5, (17) 4327–4337. [DOI] [PubMed] [Google Scholar]
- Nurnberger, J. , Kavapurackal, R. , Zhang, S.J. , Opazo Saez, A. , Heusch, G. , Philipp, T. , Pietruck, F. , Kribben, A. , 2006. Differential tissue distribution of the Invs gene product inversin. Cell Tissue Res.. 323, (1) 147–155. [DOI] [PubMed] [Google Scholar]
- Papaxoinis, K. , Patsouris, E. , Kittas, C. , Nicolopoulou-Stamati, P. , 2007. Insulinlike growth factor I receptor and estrogen receptor beta expressions are inversely correlated in colorectal neoplasms and affected by the insulin resistance syndrome. Hum. Pathol.. 38, (7) 1037–1046. [DOI] [PubMed] [Google Scholar]
- Pekmezci, M. , Szpaderska, A. , Osipo, C. , Ersahin, C. , 2012. The effect of cold ischemia time and/or formalin fixation on estrogen receptor, progesterone receptor, and human epidermal growth factor Receptor-2 results in breast carcinoma. Pathol, Res. Int.. 2012, 947041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picotti, P. , Aebersold, R. , Domon, B. , 2007. The implications of proteolytic background for shotgun proteomics. Mol. Cell Proteom.. 6, (9) 1589–1598. [DOI] [PubMed] [Google Scholar]
- Ponten, F. , Gry, M. , Fagerberg, L. , Lundberg, E. , Asplund, A. , Berglund, L. , Oksvold, P. , Bjorling, E. , Hober, S. , Kampf, C. , Navani, S. , Nilsson, P. , Ottosson, J. , Persson, A. , Wernerus, H. , Wester, K. , Uhlen, M. , 2009. A global view of protein expression in human cells, tissues, and organs. Mol. Syst. Biol.. 5, 337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponten, F. , Schwenk, J.M. , Asplund, A. , Edqvist, P.H. , 2011. The Human Protein Atlas as a proteomic resource for biomarker discovery. J. Intern. Med.. 270, (5) 428–446. [DOI] [PubMed] [Google Scholar]
- Prichard, J. , Bitting, A. , Meyers, J. , 2011. Handbook of Practical Immunohistochemistry Springer; New York: [Google Scholar]
- Prioleau, J. , Schnitt, S.J. , 1995. p53 antigen loss in stored paraffin slides. N. Engl. J. Med.. 332, (22) 1521–1522. [DOI] [PubMed] [Google Scholar]
- Qian, X. , Lloyd, R.V. , 2003. Recent developments in signal amplification methods for in situ hybridization. Diagn. Mol. Pathol.. 12, (1) 1–13. [DOI] [PubMed] [Google Scholar]
- Rakha, E.A. , Patel, A. , Powe, D.G. , Benhasouna, A. , Green, A.R. , Lambros, M.B. , Reis-Filho, J.S. , Ellis, I.O. , 2010. Clinical and biological significance of E-cadherin protein expression in invasive lobular carcinoma of the breast. Am. J. Surg. Pathol.. 34, (10) 1472–1479. [DOI] [PubMed] [Google Scholar]
- Rao, D.D. , Vorhies, J.S. , Senzer, N. , Nemunaitis, J. , 2009. siRNA vs. shRNA: similarities and differences. Adv. Drug. Deliv. Rev.. 61, (9) 746–759. [DOI] [PubMed] [Google Scholar]
- Robertson, D. , Savage, K. , Reis-Filho, J.S. , Isacke, C.M. , 2008. Multiple immunofluorescence labelling of formalin-fixed paraffin-embedded (FFPE) tissue. BMC Cell Biol.. 9, 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper, C.B. , 2005. An open letter to our readers on the use of antibodies. J. Comp. Neurol.. 493, (4) 477–478. [DOI] [PubMed] [Google Scholar]
- Saper, C.B. , 2009. A guide to the perplexed on the specificity of antibodies. J. Histochem. Cytochem.. 57, (1) 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter, G. , Mirlacher, M. , 2002. Tissue microarrays for predictive molecular pathology. J. Clin. Pathol.. 55, (8) 575–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanhausser, B. , Busse, D. , Li, N. , Dittmar, G. , Schuchhardt, J. , Wolf, J. , Chen, W. , Selbach, M. , 2011. Global quantification of mammalian gene expression control. Nature. 473, (7347) 337–342. [DOI] [PubMed] [Google Scholar]
- Scott, I.S. , Heath, T.M. , Morris, L.S. , Rushbrook, S.M. , Bird, K. , Vowler, S.L. , J. Arends, M. , Coleman, N. , 2004. A novel immunohistochemical method for estimating cell cycle phase distribution in ovarian serous neoplasms: implications for the histopathological assessment of paraffin-embedded specimens. Br. J. Cancer. 90, (8) 1583–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth, P. , 2005. Vector-mediated cancer gene therapy: an overview. Cancer Biol. Ther.. 4, (5) 512–517. [DOI] [PubMed] [Google Scholar]
- Shah, R.B. , Zhou, M. , LeBlanc, M. , Snyder, M. , Rubin, M.A. , 2002. Comparison of the basal cell-specific markers, 34betaE12 and p63, in the diagnosis of prostate cancer. Am. J. Surg. Pathol.. 26, (9) 1161–1168. [DOI] [PubMed] [Google Scholar]
- Shi, S.R. , Taylor, C.R. , 2011. Antigen Retrieval Immunohistochemistry Based Research and Diagnostics John Wiley & Sons; [Google Scholar]
- Shi, S.R. , Key, M.E. , Kalra, K.L. , 1991. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J. Histochem. Cytochem.: Off. J. Histochem. Soc.. 39, (6) 741–748. [DOI] [PubMed] [Google Scholar]
- Shi, S.R. , Liu, C. , Taylor, C.R. , 2007. Standardization of immunohistochemistry for formalin-fixed, paraffin-embedded tissue sections based on the antigen-retrieval technique: from experiments to hypothesis. J. Histochem. Cytochem.: Off. J. Histochem. Soc.. 55, (2) 105–109. [DOI] [PubMed] [Google Scholar]
- Smith, N.R. , Womack, C. , 2014. A matrix approach to guide IHC-based tissue biomarker development in oncology drug discovery. J. Pathol.. 232, (2) 190–198. [DOI] [PubMed] [Google Scholar]
- Soderberg, O. , Gullberg, M. , Jarvius, M. , Ridderstrale, K. , Leuchowius, K.J. , Jarvius, J. , Wester, K. , Hydbring, P. , Bahram, F. , Larsson, L.G. , Landegren, U. , 2006. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods. 3, (12) 995–1000. [DOI] [PubMed] [Google Scholar]
- Soderberg, O. , Leuchowius, K.J. , Gullberg, M. , Jarvius, M. , Weibrecht, I. , Larsson, L.G. , Landegren, U. , 2008. Characterizing proteins and their interactions in cells and tissues using the in situ proximity ligation assay. Methods. 45, (3) 227–232. [DOI] [PubMed] [Google Scholar]
- Suetterlin, R. , Baschong, W. , Laeng, R.H. , 2004. Immunofluorescence and confocal laser scanning microscopy of chronic myeloproliferative disorders on archival formaldehyde-fixed bone marrow. J. Histochem. Cytochem.: Off. J. Histochem. Soc.. 52, (3) 347–354. [DOI] [PubMed] [Google Scholar]
- Suurmeijer, A.J. , Boon, M.E. , 1993. Notes on the application of microwaves for antigen retrieval in paraffin and plastic tissue sections. Euro. J. Morphol.. 31, (1–2) 144–150. [PubMed] [Google Scholar]
- Taylor, C.R. , 1994. An exaltation of experts: concerted efforts in the standardization of immunohistochemistry. Hum Pathol.. 25, (1) 2–11. [DOI] [PubMed] [Google Scholar]
- Taylor, C.R. , 2006. Standardization in immunohistochemistry: the role of antigen retrieval in molecular morphology. Biotech. Histochem.: Off. Publ. Biol. Stain Comm.. 81, (1) 3–12. [DOI] [PubMed] [Google Scholar]
- Taylor, C.R. , Levenson, R.M. , 2006. Quantification of immunohistochemistry–issues concerning methods, utility and semiquantitative assessment II. Histopathology. 49, (4) 411–424. [DOI] [PubMed] [Google Scholar]
- Towbin, H. , Staehelin, T. , Gordon, J. , 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad Sci. U S A. 76, (9) 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlen, M. , Bjorling, E. , Agaton, C. , Szigyarto, C.A. , Amini, B. , Andersen, E. , Andersson, A.C. , Angelidou, P. , Asplund, A. , Asplund, C. , Berglund, L. , Bergstrom, K. , Brumer, H. , Cerjan, D. , Ekstrom, M. , Elobeid, A. , Eriksson, C. , Fagerberg, L. , Falk, R. , Fall, J. , Forsberg, M. , Bjorklund, M.G. , Gumbel, K. , Halimi, A. , Hallin, I. , Hamsten, C. , Hansson, M. , Hedhammar, M. , Hercules, G. , Kampf, C. , Larsson, K. , Lindskog, M. , Lodewyckx, W. , Lund, J. , Lundeberg, J. , Magnusson, K. , Malm, E. , Nilsson, P. , Odling, J. , Oksvold, P. , Olsson, I. , Oster, E. , Ottosson, J. , Paavilainen, L. , Persson, A. , Rimini, R. , Rockberg, J. , Runeson, M. , Sivertsson, A. , Skollermo, A. , Steen, J. , Stenvall, M. , Sterky, F. , Stromberg, S. , Sundberg, M. , Tegel, H. , Tourle, S. , Wahlund, E. , Walden, A. , Wan, J. , Wernerus, H. , Westberg, J. , Wester, K. , Wrethagen, U. , Xu, L.L. , Hober, S. , Ponten, F. , 2005. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol Cell Proteomics. 4, (12) 1920–1932. [DOI] [PubMed] [Google Scholar]
- Uhlen, M. , Oksvold, P. , Fagerberg, L. , Lundberg, E. , Jonasson, K. , Forsberg, M. , Zwahlen, M. , Kampf, C. , Wester, K. , Hober, S. , Wernerus, H. , Bjorling, L. , Ponten, F. , 2010. Towards a knowledge-based human protein atlas. Nat. Biotechnol.. 28, (12) 1248–1250. [DOI] [PubMed] [Google Scholar]
- Viklund, H. , Bernsel, A. , Skwark, M. , Elofsson, A. , 2008. SPOCTOPUS: a combined predictor of signal peptides and membrane protein topology. Bioinformatics. 24, (24) 2928–2929. [DOI] [PubMed] [Google Scholar]
- von Boguslawsky, K. , 1994. Immunohistochemical detection of progesterone receptors in paraffin sections. A novel method using microwave oven pretreatment. APMIS. 102, (9) 641–646. [DOI] [PubMed] [Google Scholar]
- Wang, Z. , Gerstein, M. , Snyder, M. , 2009. RNA-Seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet.. 10, (1) 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warford, A. , Howat, W. , McCafferty, J. , 2004. Expression profiling by high-throughput immunohistochemistry. J Immunol. Methods. 290, (1–2) 81–92. [DOI] [PubMed] [Google Scholar]
- Weinstein, M.H. , Signoretti, S. , Loda, M. , 2002. Diagnostic utility of immunohistochemical staining for p63, a sensitive marker of prostatic basal cells. Mod Pathol.. 15, (12) 1302–1308. [DOI] [PubMed] [Google Scholar]
- Wester, K. , Wahlund, E. , Sundstrom, C. , Ranefall, P. , Bengtsson, E. , Russell, P.J. , Ow, K.T. , Malmstrom, P.U. , Busch, C. , 2000. Paraffin section storage and immunohistochemistry. Effects of time, temperature, fixation, and retrieval protocol with emphasis on p53 protein and MIB1 antigen. Appl. Immunohistochem. Mol. Morphol.. 8, (1) 61–70. [PubMed] [Google Scholar]
- Williams, J.H. , Mepham, B.L. , Wright, D.H. , 1997. Tissue preparation for immunocytochemistry. J. Clin. Pathol.. 50, (5) 422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, C. , Orozco, C. , Boyer, J. , Leglise, M. , Goodale, J. , Batalov, S. , Hodge, C.L. , Haase, J. , Janes, J. , Huss, J.W. , Su, A.I. , 2009. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol.. 10, (11) R130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, R. , Chung, J.Y. , Ylaya, K. , Williams, R.L. , Guerrero, N. , Nakatsuka, N. , Badie, C. , Hewitt, S.M. , 2011. Factors influencing the degradation of archival formalin-fixed paraffin-embedded tissue sections. J. Histochem. Cytochem. : Off. J. Histochem. Soc.. 59, (4) 356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieba, A. , Wahlby, C. , Hjelm, F. , Jordan, L. , Berg, J. , Landegren, U. , Pardali, K. , 2010. Bright-field microscopy visualization of proteins and protein complexes by in situ proximity ligation with peroxidase detection. Clin. Chem.. 56, (1) 99–110. [DOI] [PubMed] [Google Scholar]


