Abstract
Lung adenocarcinoma (ADC) is the most common lung cancer subtype and presents a high mortality rate. Clinical recurrence is often associated with the emergence of metastasis and treatment resistance. The purpose of this study was to identify genes with high prometastatic activity which could potentially account for treatment resistance.
Global transcriptomic profiling was performed by robust microarray analysis in highly metastatic subpopulations. Extensive in vitro and in vivo functional studies were achieved by overexpression and by silencing gene expression.
We identified the small GTPase RHOB as a gene that promotes early and late stages of metastasis in ADC. Gene silencing of RHOB prevented metastatic activity in a systemic murine model of bone metastasis. These effects were highly dependent on tumor‐host interactions. Clinical analysis revealed a marked association between high RHOB levels and poor survival. Consistently, high RHOB levels promote metastasis progression, taxane‐chemoresistance, and contribute to the survival advantage to γ‐irradiation. We postulate that RHOB belongs to a novel class of “genes of recurrence” that have a dual role in metastasis and treatment resistance.
Keywords: GTPase, Non‐cell autonomous, Microenvironment, Tumor‐stroma, MMP, Chemotherapy, Radiotheraphy, Drug target
Highlights
RHOB is a novel prometastatic target in ADC.
RHOB promotes treatment resistance.
RHOB levels associate with clinical outcome.
Abbreviations
- ADC
Adenocarcinoma
- SQ
squamous cell carcinoma
- TP
time to progression
- i.c.
intracardiac
- i.t.
intratibial
- HMS
highly-metastatic subpopulations
- SCC
single-cell derived colony
- TRAP
tartrate-resistant acid phosphatase
- qPCR
real time quantitative RT-PCR
- MMP
metalloprotease
- CM
conditioned medium
1. Introduction
Adenocarcinoma (ADC) is the most frequent histological type and accounts for 30–40% of non‐small cell lung cancers (NSCLCs) (Jemal et al., 2010). Prognosis remains poor despite recent progress in multimodal management, which includes surgical resection, and combined chemo‐ and radiotherapy. Similarly to other lung cancer subtypes, ADC tumors metastasize to local or distant sites, including the brain, the adrenal medulla and the skeleton (Hess et al., 2006). This process may appear even during early stages of tumor progression because >30% of stage I tumors relapse after complete surgical resection (Feld et al., 1984). More often, following the course of treatment and a variable period of remission, recurrence involves refractory response to chemo‐ and radiotherapy and the appearance of metastasis or a secondary primary tumor (Martini et al., 1995).
Metastasis entails several consecutive steps in which tumor cells acquire novel functions such as invasiveness, resistance to shear‐stress in the circulation, and evasion of immune surveillance, which allows infiltration and colonization in target organs (Fidler, 2003). These functional advantages of cellular variants within the primary tumor are directed by an intrinsic genetic/epigenetic program (Gupta et al., 2007; Minn et al., 2005) that is highly modulated by microenvironmental cues (Joyce and Pollard, 2009). The complexity of this process has been previously established for some tumors, in which a subset of genes acts cooperatively and promotes different events in the metastatic process (Gupta et al., 2007; Yang et al., 2004). Their relevance is underscored by their significance as prognostic markers of the clinical course. Less frequently, a single gene that confers robust pleiotropic functions and predicts clinical outcome has been identified (Han et al., 2008; Yang et al., 2004). Recently, functional endothelial protein C receptor (EPCR) promotes metastasis and associates with survival of ADC patients (Antón et al., 2012).
In addition to cell intrinsic mechanisms, a variety of non‐tumor cells greatly influence tumor progression. These heterotypic cellular interactions as well as a variety of locally released cytokines and growth factors initiate critical signaling pathways that strongly influence the primary tumor, aggravating disease progression and promoting colonization at distant sites. In bone, a frequent target organ of ADC metastasis, paracrine loops between bone‐derived factors, such as transforming growth factor (TGF‐β), and tumor cells have been shown to exacerbate osseous colonization (Yin et al., 1999). Moreover, host‐related effects mediated by stromal platelet‐derived growth factor receptor signaling have been implicated in bone homing of lung cancer (Catena et al., 2011).
Similarly, host–tumor mechanisms and intrinsic tumor mutations can also greatly influence treatment resistance. A variety of mutations or acquired gene expression changes in molecular transporters, DNA repair pathways, or intracellular drug metabolism (Stewart, 2010) have been shown to confer cell resistance. Given this reciprocal influence of tumor microenvironment in both processes, we hypothesized that some genes that promote a metastatic phenotype may also endow cells with enhanced tolerance to therapeutic stress.
For the characterization of key determinants in both processes of lung ADC, we first identified genes with robust prometastatic activity. After extensive in vitro and in vivo functional analysis, we used data mining and clinical validation to specify further their potential contribution to treatment resistance. Using this approach, we identified RHOB as a critical component in lung ADC. This small GTPase acts as a signaling switch, that is implicated in the regulation of a variety of molecular pathways by cycling between an active GTP and inactive GDP‐bound state (Prendergast, 2001). Unlike other RHO GTPases, RHOB participates in endosome trafficking (Jaffe and Hall, 2002). Consistent with this, RHOB displays a unique pattern of cellular distribution that contributes to the cytoplasm to membrane targeting of a variety of receptors and signaling molecules (Jaffe and Hall, 2005).
RHOB effects are stage and/or cell‐type dependent, and in some cellular contexts, ectopic expression of RHOB promotes proliferation and transformation (Prendergast et al., 1995), whereas in others, high RHOB levels inhibit invasive and migratory cell behavior (Bousquet et al., 2009; Jiang et al., 2004). This context‐specificity has also been observed in vivo in some tumors (Du and Prendergast, 1999), including lung cancer in which RHOB levels decreased between preinvasive and invasive stages in the tumorigenic progression (Mazieres et al., 2004). Because of these findings in lung cancer and in other tumors (Couderc et al., 2008, 2001, 2001, 2011), RHOB has been considered to function as a tumor suppressor (Prendergast, 2001). Yet, recent evidence challenges this prevailing view by unveiling the dominant host‐derived effects in tumor vasculature elicited by RHOB (Kazerounian et al., 2013). In this study, we found that ablation of RHOB levels in tumor cells dramatically decreased bone metastasis in an experimental model. In contrast, RHOB overexpression was associated with enhanced dissemination from the primary location and increased resistance to chemotherapy and radiation.
2. Material and methods
2.1. Plasmids
Human RHOB cDNA was cloned in pBABE retroviral vector. Lentiviral vectors containing short hairpin for RHOB were obtained from shRNA Mission (Sigma).
2.2. Cell lines
A549 cell line was a kind gift from Dr. Gazdar (University of Texas Southwestern, Dallas, TX). The cell line is authenticated by sequencing of several described mutations every 6 months. Murine stromal ST‐2 cell line was authenticated by qPCR.
2.3. RHO activity assays
Pull down assays were performed using Rhotekin‐RBD (for RHO proteins) and PAK‐PBD beads (for Rac1 and cdc42) and samples were subjected to western blot analysis according to manufacturer specifications (Cell Biolabs).
2.4. In silico experiments
Gene expression and clinical data for 111 cases of primary lung carcinomas were obtained from Bild et al. Data were retrieved from the Gene expression Omnibus (GSE3141).
2.5. Chemoresistance and radioresistance clonogenic assay
Cells (1 × 104 per well) were seeded into 48‐well plate. After 24 h, cells were treated for 24 h with paclitaxel (Sigma), 2 h with cisplatin (Sigma), and 9 h with gemcitabine (Eli Lilly) for the indicated doses. After culturing the cells in drug‐free media for additional 48 h, clonogenic assay was performed as previously described (Hu et al., 2009).
For resistance to radiation, cells (1 × 105) alone or with ST‐2 (1 × 105) were exposed to γ‐rays (5.5 Gy) on 60 mm dishes using a Gammacell 3000 irradiator (Nordion, Ottawa, Canada). Cells were recovered for 48 h and harvested in 8 mL and a 100 μL aliquot was seeded onto 6‐well plates for 8 days. Cells were fixed, stained and the total area of resistant clones was evaluated with a computerized image analysis system.
2.6. In vitro assays
Global MMP activity was performed in coculture supernatants using an assay based on the cleavage of synthetic fluorogenic peptide for MMP‐3 from Bachem (M‐2110) and fluorogenic peptide substrate II (R&D systems), an excellent substrate for MMP‐3 and MMP‐10. Cell adhesion, invasion assays have been described elsewhere (22).
2.7. In vivo assays
Intracardiac inoculation (i.c.) and intratibial injection (i.t.) were performed as previously described (Vicent et al., 2008). Oropharyngeal aspiration was performed according to Lakatos et al. (2006).
2.8. Statistical analysis
Log‐rank test was used to calculate the statistical significance (p value) of differences observed among Kaplan–Meier curves. To study differences in proliferation rates, tumor growth, differences in metastatic area, SCC number and metalloproteolytic activity data were analyzed by different comparison test. For parametric analysis, ANOVA followed by Tukey Post hoc test was performed in cases with variance homogeneity and Brown‐Forsythe and T2 Tamhane when this did not happen. Data are presented as mean ±SD in these cases. For non‐parametric statistics, data were analyzed by Kruskall–Wallis test followed by Mann‐Whithney multiple comparison test with Bonferroni's adjustment. Statistical results were defined as statistically significant (*p < 0.05, **p < 0.01, ***p < 0.001).
Supplementary methods provide additional information.
3. Results
3.1. Transcriptomic identification of RHOB
Using the human ADC A549 cell line, we established a dual screening strategy by transcriptomic selection of genes with robust prometastatic activity (Kang et al., 2003), and subsequently tested their importance in human lung cancer. This approach identified a triple gene signature of HDAC4, PITX1 and ROBO1 which concomitantly confer strong prometastatic activity to bone even though the individual contribution of each gene proved to be irrelevant (Luis‐Ravelo et al., 2013).
This transcriptomic analysis also enabled to identify RHOB as another top overexpressed gene (Figure 1a). A complete list of the top 40 gene probe sets (FDR cutoff <0.052) can be found in Figure 1a. Expression of this gene was confirmed by using western blotting (Figure 1b) and real‐time quantitative PCR (qPCR) (Figure 1c). Because RHOB participates in a variety of cellular processes, we performed an in silico analysis of microarray data of RHOB expression levels derived from human lung tumor samples in a previously published database (Sup. methods). Bioinformatics analysis revealed no statistical differences when all histological subtypes were included (data not shown). However, when only ADC was considered, patients with high RHOB expression levels had a poor prognosis as compared to those with low levels of RHOB expression in lung tumors (Figure 1d).
Figure 1.
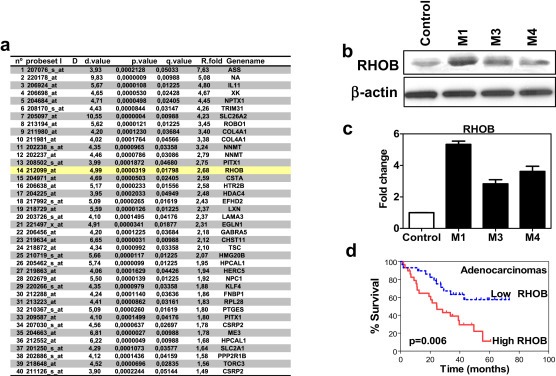
Transcriptomic selection of relevant candidate genes. a. Top 40 differentially overexpressed genes obtained after the filtering analysis derived from microarray expression assessed in highly‐metastatic subpopulations (HMS) versus control cells. Probeset number, statistical values and fold expression are shown in columns. b. Western blot analysis of RHOB protein levels in parental and HMS (M1, M3 and M4). c. Transcriptomic validation by real‐time PCR for RHOB. Graph represent the results obtained by relative standard curve method using parental cells as calibrator (control). GAPDH was used as endogenous control. d. Kaplan–Meier survival curve of patients with lung ADC (n = 58) segregated by the levels of RHOB using the microarray data set from Bild et al. (Bild et al., 2006).
Next, we performed an immunohistochemical analysis besides limitations inherent to the specificity of available antibodies (Sup. Figure 1, 2, 3). However, experiments on two independent cohorts, using an anti‐RHOB antibody (Sup. Figure 3), strongly suggest an association between RHOB expression levels and survival time in ADC patients treated with adjuvant therapy (Sup. Figure 3). More importantly, a survival analysis of a cohort of NSCLC tumors subjected to adjuvant taxane‐based chemotherapy and/or radiotherapy was also performed and patients with high RHOB levels were found to have a worse survival rates than those with low levels (Sup. Figure 4).
These data suggest that RHOB might confer cell advantageous functions associated with poor clinical outcome.
3.2. Identification of RHOB as a prometastatic gene in ADC
We assessed the contribution of RHOB to the prometastatic activity by downregulating its expression levels (Figure 2a). Proliferation was unaffected by gene knockdown in vitro (Figure 2a). To substantiate the role of RHOB, we performed i.c. inoculation using two different knockdown constructs. Animals inoculated with RHOB knockdown cells showed a longer latency in the appearance of metastasis with fewer metastatic lesions (Figure 2b) and a significant decrease in tumor burden (Figure 2c). Bioluminescence image analysis, histological analysis and μCT scans, revealed decreased colonization of the bone marrow compartment, with a lower degree of cortical bone destruction in the metaphyseal region, and colonization of extraosseous tissues (Figure 2d). These findings suggest that RHOB levels could confer prometastatic activity.
Figure 2.
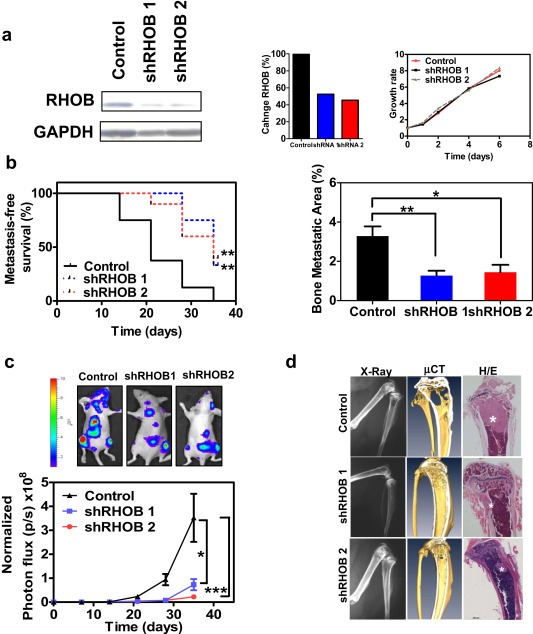
Identification of RHOB as a robust prometastatic contributor. a. Left Panel: Western blot analysis of RHOB levels in cell lysates derived from 2 different RHOB knock‐down transduced cell populations. Middle Panel: Quantification of RHOB inhibition levels. Right Panel: Cell growth kinetics in vitro of cells with silenced RHOB levels. b. Evaluation of RHOB knockdown effects after i.c. inoculation of cells carrying two different shRNA targeting RHOB in nude mice (n = 10 per group), by Kaplan–Meier curves of metastasis‐free survival (left) and microradiographic computerized analysis of hind limbs (right). c. Assessment of tumor burden by bioluminescence imaging. Representative images are shown at the bottom. d. Representative X‐ray images (left), three dimensional reconstruction of micro‐CT scans (middle), and hematoxylin and eosin staining (right). Asterisk indicates presence of tumor cells.
3.3. RHOB levels modulate different steps of the metastatic cascade
We retrovirally overexpressed RHOB in parental cells using two different vectors (Figure 3a). The overexpressors did not show differences in their growth kinetics (Sup. Figure 6). RHOB overexpression led to an increase in the active form of the protein, but the activity of other RHO GTPases such as RHOA, RHOC, RAC1 or CDC42 was not affected (Figure 3a and Sup. Figure 7).
Figure 3.
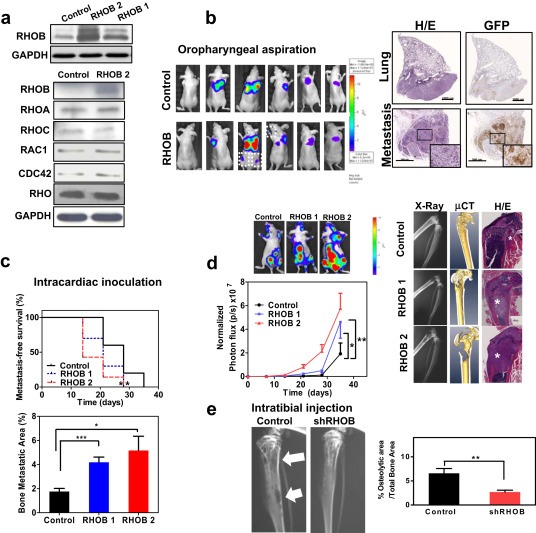
Role of RHOB in the multistep process of metastasis. a. Top panel: Western blot analysis of two independent RHOB‐overexpressing pools (RHOB1 and RHOB2). Bottom panel: Western blot analysis after RHO activation pull‐down assays in RHOB overexpressors. Blots were performed using specific RHOA, RHOB, RHOC, RAC1 and CDC42 antibodies. Total RHO was assessed with a Pan‐Rho antibody. b. Evaluation of RHOB effects in orthotopic model after oropharyngeal aspiration of RHOB overexpressors and control cells (n = 7/group). Representative bioluminescence images are shown (left panel). Metastatic cells were found in extrapulmonary sites in mice bearing RHOB cells. The experiment was carried out with both RHOB1 and RHOB2 overexpressing cells, obtaining similar results. Right Panel: Histological and immunohistochemical examination of tumor cells in the lungs (Upper panels, 5×) and extrapulmonary sites (Lower panels, 20×). Serial sections were stained with H&E (left panels) or incubated with an anti‐green‐fluorescent‐protein antibody (right panels) to confirm the presence of disseminated RHOB‐overexpressing cells. Scale bars: 1 mm (lungs) and 500 μm (metastasis). c. Top: Effects of RHOB overexpression after i.c. inoculation in nude mice, by Kaplan–Meier curves of metastasis‐free survival (n = 10 mice/group). Bottom: X‐ray computerized image analysis. d. Left: Bioluminescence imaging assessment. Right: Representative images. Asterisk denotes the presence of tumor cells. e. Bone colonization effects of RHOB assessed after intratibial injection in the osseous compartment. Representative X‐ray images (arrows indicate osteolytic lesions) and quantification of X‐ray image analysis at day 24 post‐inoculation.
We sought to explore whether high RHOB levels could also influence early metastatic events such as invasion and intravasation. We orthotopically inoculated RHOB‐overexpressing cells. Bioluminescence image analysis of the thoracic cavity showed no significant differences in tumor burden between groups at 7 months post‐inoculation. Although no metastases were observed in control animals during the experimental period, several metastatic foci were detected in some mice inoculated with RHOB‐overexpressing cells (Figure 3b). The presence of cells that colonized extrapulmonary organs in this group was detected. No other macroscopic lesions were observed after systematic necropsy. Taken together, these data suggest that cells with high RHOB levels in the lung microenvironment showed an increased ability to form distant metastases.
To investigate the effects of high RHOB levels in late stages of metastasis, we i.c. inoculated athymic nude mice with RHOB‐overexpressing cells. Mice showed decreased latency for initiation of bone metastatic lesions, an increase in osteolytic lesions (Figure 3c) and tumor burden (Figure 3d).
To distinguish the contribution to colonization, we injected shRHOB and control cells intratibially. Twenty‐five days after injection, control cells induced overt osteolytic lesions, whereas shRHOB cells induced smaller bone lesions (Figure 3e). These findings indicate that the decreased metastatic activity observed in shRHOB cells could be mediated, at least in part, by the impaired ability of shRHOB cells to colonize and thrive in the bone compartment.
Taken together, these data suggest that RHOB levels in lung cancer cells play an important role in early and late steps of metastasis by increasing the prometastatic activity.
3.4. RHOB modulates multiple metastasis‐related mechanisms
It has been well established that MMPs significantly contribute to bone matrix degradation in bone metastases (Lu et al., 2009). Although osseous collagens can be degraded by several MMPs, we have previously shown that MMP‐3/MMP‐10 activities are rapidly induced upon tumor–stromal interactions (Luis‐Ravelo et al., 2011). To establish the cellular RHOB levels that could account for the metastatic effects observed in vivo, we assessed MMP3/10 matrix metalloproteinase (MMP) activities. Conditioned medium (CM) derived from knockdown cells cultured with stromal ST‐2 cells resulted in a decrease in MMP activity (Figure 4a and Sup. Figure 8). Consistently, when RHOB‐overexpressing cells were cultured under the same conditions, global MMP activity increased with a fluorogenic substrate recognizing MMP‐3 and ‐10, an activity that was completely abrogated by the global MMP inhibitor GM6001 (Figure 4a and Sup. Figure 8). Thus, RHOB levels modulated global MMP activity. RHOB was upregulated in tumor cells cultured with murine stromal ST‐2 cells, whereas no effects were observed in A549 cells incubated with the CM derived from ST‐2 cells cultured alone or with A549 cells (Figure 4b). Consistent with the in vivo metastatic effects, RHOB knockdown cells displayed decreased ability to invade collagen type I; the most abundant bone matrix protein. In contrast, RHOB‐overexpressing cells displayed increased invasiveness under the same conditions (Figure 4c). Moreover, overexpression of RHOB in a panel of human ADC cell lines led to increased invasiveness in 5/7 cell lines as compared to mock‐transduced cells (Figure 4d). These data support the conclusion that high RHOB levels enhance invasiveness of ADC cancer cell lines. No differences in osteoclastogenic activities were found in an in vitro osteoclastogenic assay (data not shown).
Figure 4.
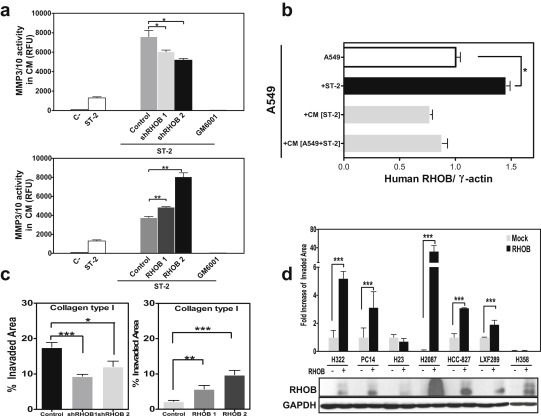
Functional relevance of RHOB in vitro. a. Global MMP activity in the conditioned medium (CM) of shRHOB (upper panel) and RHOB (lower panel) cells in co‐culture with ST‐2 cells for 3 days, assessed by digestion of fluorogenic substrate recognizing MMP‐3 and ‐10. (RFU: Relative Fluorescent Units). b. Assessment of RHOB expression levels in A549 cells cultured alone, in coculture with murine ST‐2 cells, or after incubation with CM derived either from ST‐2 cells alone or in coculture with A549, by using specific human primers in RT‐qPCR. Heterotypic interactions between A549 and ST‐2 cells (A549 + ST‐2) increased RHOB expression levels in tumor cells, whereas conditioned media from stromal cells (CM ST‐2) or from co‐culture (CM A549 + ST‐2) had no effect. c. Boyden chamber invasion assay of cells with knockdown levels of RHOB (left) or overexpressors (right) in collagen type I. d. Invasion assay of several human ADC cell lines retrovirally transduced with RHOB as compared to mock cells, which showed increased invasion in several cell lines. Western blot with an anti‐RHOB antibody in the panel of ADC human cell lines.
RHOB has been implicated in adhesion‐perturbing integrin receptor signaling therefore, we tested the ability of different RHOB clones to adhere to different substrates. These cells displayed greater adhesion to fibronectin, while knockdown cells showed impaired adhesion to this substrate. Differences were also found for hyaluronic acid (Sup. Figure 9).
3.5. RHOB mediates chemo‐ and radioresistance in lung ADC
To establish the possible contribution of RHOB expression levels in the clinical findings, we first studied the effect of RHOB levels in in vitro chemoresistance to three currently used drugs: cisplatin, paclitaxel, and gemcitabine. Incubation of RHOB knockdown cells with increasing doses of paclitaxel showed increased chemosensitivity as compared to control cells (Figure 5a), but not to other tested drugs (data not shown). In contrast, incubation of RHOB‐overexpressing cells with increasing doses of paclitaxel led to an increase in the number of surviving colonies in clonogenic assays, as compared to mock control cells, in a dose‐dependent manner (Figure 5a). These data suggest that RHOB levels modulate paclitaxel chemoresistance in A549 cells.
Figure 5.
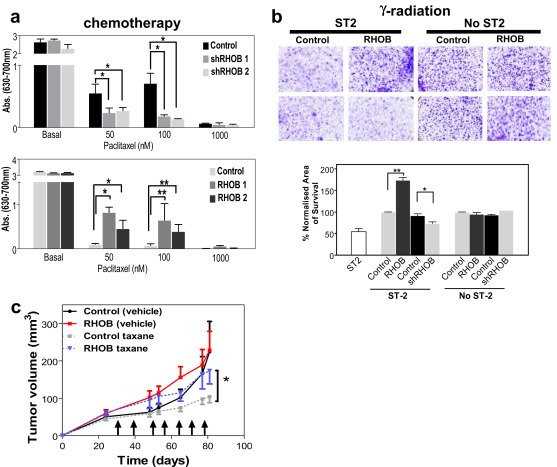
RHOB modulates chemo‐ and γ‐radioresistance. a. Upper panel: Quantification of resistance to increasing doses of paclitaxel in shRHOB cells in coculture conditions with ST‐2 cells. Lower panel: Similar quantification in RHOB‐overexpressing cells in conditions of coculture with ST‐2 cells. b. Upper panel: Representative images of radioresistance after 5 Gy γ‐irradiation of lung cancer cells that overexpressed RHOB, or with knockdown levels of RHOB, cultured with stromal ST‐2 cells. Lower panel: Quantification of radioresistant clones of RHOB‐overexpressing cells and shRHOB cells cocultured with ST‐2 cells. c. Measurement of tumor size after subcutaneous injection of RHOB‐overexpressing and mock‐transduced cells in athymic nude mice (n = 10 tumors per group), treated with vehicle or paclitaxel (15 mg/kg). Mean ± SEM (*p < 0.05, control taxane vs. RHOB taxane) at day 81. Arrows indicate the days of injection.
We also studied the influence of RHOB levels in radioresistance. RHOB levels had no influence on radioresistance when cells were cultured alone. However, stromal cells can contribute to radioresistance on tumor cells (Josson et al., 2010). Interestingly, RHOB‐overexpressing cells co‐cultured with stromal ST‐2 cells showed an increase in resistance to γ‐irradiation, whereas low levels of RHOB increased radiosensitivity (Figure 5b). Next, we assessed the contribution of RHOB in taxane‐resistance in vivo using a xenotransplant model in nude mice. RHOB‐overexpressing tumors showed increased resistance to paclitaxel treatment as compared to control‐treated tumors (Figure 5c). Thus, these data suggest that RHOB levels play a role in conferring taxane chemoresistance and radioresistance.
4. Discussion
In this report, we identified RHOB as a strong metastatic effector with robust functional activities that also contributes to treatment resistance in lung cancer. All these tumor cell functions are highly modulated by the host milieu where contextual signals mediated by RHOB could dominate over its tumor‐intrinsic effects.
The role of RHOB in resistance is in agreement with previous reports describing mechanisms of tolerance to radiation. In agreement with our findings, decreased levels of RHOB lead to high radiosensitization by enhanced tumor oxygenation (Ader et al., 2002, 2003), through a mechanism that involves HIF‐1α downregulation (Skuli et al., 2006) and altered DNA repair via integrins (Monferran et al., 2008). In contrast, in other models, genetic ablation of RHOB renders cells resistant to agents that target microtubules or DNA, as well as γ‐radiation (Liu et al., 2001a). This apparent discrepancy could be explained by non‐cell autonomous effects derived by host–cell contacts (Kazerounian et al., 2013) that markedly softened the long‐standing perception of RHOB as a tumor‐suppressor (Prendergast et al., 1995). In a complementary sense, mechanisms of taxane‐resistance in microtubule dynamics instability (Goncalves et al., 2001) are consistent with the well‐characterized actions of RHOB that are involved in cytoskeletal remodeling and microtubule polymerization. It is tempting to consider the possibility that class III β‐tubulin, which has been reported as a clinical outcome predictor and a mediator of taxane resistance in lung cancer, could be regulated by RHOB (Gan et al., 2007). This would explain why RHOB levels did not affect chemosensitivity to gemcitabine and cisplatin. Thus, RHOB could confer treatment resistance by cell‐autonomous effects related to its actions on microtubule regulation, or/and by increasing tumor‐host dependent MMPs secretion, which modulates radiosensitivity (Ganji et al., 2010). Furthermore, radio‐ and chemoresistance by RHOB could be mediated by tumor‐host interactions through changes in stromal angiogenesis (Kazerounian et al., 2013), and/or changes in host‐derived MMP secretion (Luis‐Ravelo et al., 2011).
Advantages acquired during treatment might also have influenced the cell functions required for single or multiple metastatic steps. At this point, we cannot establish how and when high RHOB‐expressing cells might appear in the primary tumor. Based on the clinical data, one could consider the intriguing possibility that high RHOB‐expressing cells might be selected during conventional treatment, and subsequently escape from the primary tumor. Complementary to this view, rare variants with high RHOB levels, which arise from the interaction of tumor cells with the rich stromal component of ADC tumors, might be more prone to initiate early metastatic spread to distant organs and overcome resistance. Indeed, in this context of RHOB alone was sufficient to confer dissemination in our orthotopic model; presumably by means of increased invasiveness and MMP proteolytic degradation. In agreement with these findings, RHOB regulates migration and is a key regulator of uPAR signaling in cell adhesion, migration and invasion (Alfano et al., 2012; Vega et al., 2012). These cell functions may also account for the overt osseous colonization and participate in resistance to ionizing radiation, since MMP secretion has been shown to modulate radiosensitivity (Ganji et al., 2010). In our model, tumor–stroma interactions induced an increase in RHOB expression levels under co‐culture conditions; an event that could be relevant in the bone compartment. It is worthy to note that other contextual signals such as TGF‐β, a host‐derived growth factor RHOB which is highly abundant in the bone matrix, could further exacerbate RHOB effects in bone (Engel et al., 1998). Thus, RHOB might confer prometastatic functions such as invasiveness, in a cell‐intrinsic manner, and increase MMP activity important during early and late stages of metastasis, which could be exacerbated by tumor‐host interactions that further modulate RHOB levels.
The complexity of the mechanisms triggered by RHOB in lung ADC might ultimately endow cells with enhanced tolerance to stress, by conferring dual properties in metastasis and treatment resistance. Similarly, a recent study has uncovered the role of metadherin, which is frequently amplified in breast cancer metastasis and chemoresistance, and it is also associated with poor prognosis (Hu et al., 2009). More recently, a CXCL1 network links resistance and metastasis (Acharyya et al., 2012). Thus, we postulate the emergence of a novel class of “genes of recurrence” that confer dual roles in resistance and metastasis.
Our results highlight the relevance of the subtype‐specific RHOB effects such as survival of patients with lung ADCs, whereas in other histological subsets, RHOB levels did not correlated with survival as previously reported (Mazieres et al., 2004; Sato et al., 2007). As formerly pointed out (Kazerounian et al., 2013), this context specificity could explain the apparent paradoxical observation that in early stages of lung tumorigenesis, loss of RhoB expression occurred between preinvasive and invasive stages (Mazieres et al., 2004; Sato et al., 2007). As well as in vitro findings, where ectopic expression of RhoB led to inhibition of invasive and migratory properties (Jiang et al., 2004), whereas opposite effects were observed in bronchiolar cells despite their normal growth kinetics (Bousquet et al., 2009).
In line with previous findings emphasizing the predominant role of RHOB driven by non‐cell autonomous mechanisms (Kazerounian et al., 2013), our study supports the requirement of host‐induced complementary activation of other stage‐ and subtype‐specific genes or cell signaling pathways. Indeed, RHOB can recruit different effector proteins involved in intracellular trafficking to endosomal membranes, such as Dia1 (Fernandez‐Borja et al., 2005) and PRK1 (Mellor et al., 1998), which could mediate the various responses elicited by RHOB in a stage‐ or subtype‐specific manner. This subtype specificity is consistent with the ADC‐restricted activation of the WNT/TCF signaling pathway that confers robust metastatic activity to bone and brain (Nguyen et al., 2009) only in this histological subset. The fact that WNT5 acts through different receptors and cooperates with RHOB to control motility suggests an intriguing link between WNT hyperactivation and RHOB overexpression that requires further validation in lung cancer (Witze et al., 2008).
It is noteworthy that our findings shed some doubt on early reports concerning the presumed role of RHOB in lung cancer (Mazieres et al., 2004; Sato et al., 2007). First, Mazieres et al. showed stunted inhibition in anchorage independent growth and cell growth kinetics upon RHOB transfection in A549 cells, findings that could be explained by the analysis of only two isolated transfected clones and the potential toxic effects derived from high doses of the transgene. These results contradicted the findings by Sato et al. and to our own findings, where no differences in cell growth kinetics were found after RHOB overexpression. Second, Mazières et al. used immunohistochemistry to compare different lesions including normal lung, preinvasive lesions, low‐grade tumor, and highly invasive tumors, and in an independent study, neoplastic and adjacent non‐neoplastic tissues observing a progressive decline in immunoreactivity associated with aggressive lesions. However, a rigorous study of different RHOB levels within the histological subset of ADC was not performed. In the Sato et al. study, the analysis of RHOB immunoreactivity did not reach statistical significance in all NSCLC samples, which is also in agreement with our findings. Thus, both reports remain far from conclusive on the role of RHOB levels in the ADC histological subtype.
In summary, we identified RHOB as a key “gene of recurrence” in lung ADC. Our data also suggest that RHOB could be an effective target to develop new therapeutic modalities for the treatment of lung ADC.
5. Conclusions
We identified the small GTPase RHOB which markedly enhanced the ability of cancer cells to promote early lung tumor dissemination and osseous colonization in a murine model of bone metastasis. RHOB promoted taxane‐resistance and survival advantage to radiation. Thus, RHOB likely contributes to the aggressive metastatic phenotype and resistance to treatment of ADC. It might be possible to use RHOB as a predictive factor in lung ADC relapse and as an appealing target to enhance chemosensitivity.
Supporting information
The following are the supplementary data related to this article.
Supplementary data
Supplementary data
Acknowledgments
We are grateful to R. Pío, S. Martínez, L. Martínez and P. Martín for their outstanding contribution. We are indebted to all people from the Bioinformatics and the Core Histology Units.
Supplementary data 1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2013.11.001.
Luis-Ravelo Diego, Antón Iker, Zandueta Carolina, Valencia Karmele, Pajares María-José, Agorreta Jackeline, Montuenga Luis, Vicent Silvestre, Wistuba Ignacio I., De Las Rivas Javier and Lecanda Fernando, (2014), RHOB influences lung adenocarcinoma metastasis and resistance in a host‐sensitive manner, Molecular Oncology, 8, doi: 10.1016/j.molonc.2013.11.001.
This work was supported by “UTE project FIMA” agreement The Cancer Research Thematic Network of the Health Institute Carlos III (RTICC RD06/0020/0066), Spanish Ministry of Science and Innovation & European Regional Development Fund (ERDF) “Una manera de hacer Europa”, PI042282, FIT‐090100‐2005‐46, SAF‐2009‐11280, SAF‐2012‐40056 grants 67/2005 and 09/2009 from the Government of Navarra, and “La Caixa Foundation” awards to F.L. PI10/00166 to L.M. D.L‐R was supported by the FIMA and FPU. I.A. was funded by the Basque Government. F.L. is an investigator from the I3 Program.
References
- Acharyya, S. , Oskarsson, T. , Vanharanta, S. , Malladi, S. , Kim, J. , Morris, P.G. , Manova-Todorova, K. , Leversha, M. , Hogg, N. , Seshan, V.E. , Norton, L. , Brogi, E. , Massague, J. , 2012. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 150, 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ader, I. , Toulas, C. , Dalenc, F. , Delmas, C. , Bonnet, J. , Cohen-Jonathan, E. , Favre, G. , 2002. RhoB controls the 24 kDa FGF-2-induced radioresistance in HeLa cells by preventing post-mitotic cell death. Oncogene. 21, 5998–6006. [DOI] [PubMed] [Google Scholar]
- Ader, I. , Delmas, C. , Bonnet, J. , Rochaix, P. , Favre, G. , Toulas, C. , Cohen-Jonathan-Moyal, E. , 2003. Inhibition of Rho pathways induces radiosensitization and oxygenation in human glioblastoma xenografts. Oncogene. 22, 8861–8869. [DOI] [PubMed] [Google Scholar]
- Alfano, D. , Ragno, P. , Stoppelli, M.P. , Ridley, A.J. , 2012. RhoB regulates uPAR signalling. J. Cell Sci.. 125, 2369–2380. [DOI] [PubMed] [Google Scholar]
- Antón, I. , Luis-Ravelo, E.M.D. , Zandueta, C. , Valencia, K. , Ormazabal, C. , Martínez-Canarias, S. , Perurena, N. , Pajares, M.J. , Agorreta, J. , Montuenga, L.M. , Segura, V. , Wistuba, I.I. , De Las Rivas, J. , Hermida, J. , Lecanda, F. , 2012. Receptor of activated protein C promotes metastasis and correlates with clinical outcome in lung adenocarcinoma. Am. J. Resp. & Crit. Care Med.. 186, 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bild, A.H. , Yao, G. , Chang, J.T. , Wang, Q. , Potti, A. , Chasse, D. , Joshi, M.B. , Harpole, D. , Lancaster, J.M. , Berchuck, A. , Olson, J.A. , Marks, J.R. , Dressman, H.K. , West, M. , Nevins, J.R. , 2006. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 439, 353–357. [DOI] [PubMed] [Google Scholar]
- Bousquet, E. , Mazieres, J. , Privat, M. , Rizzati, V. , Casanova, A. , Ledoux, A. , Mery, E. , Couderc, B. , Favre, G. , Pradines, A. , 2009. Loss of RhoB expression promotes migration and invasion of human bronchial cells via activation of AKT1. Cancer Res.. 69, (15) 6092–6099. [DOI] [PubMed] [Google Scholar]
- Catena, R. , Luis-Ravelo, D. , Anton, I. , Zandueta, C. , Salazar-Colocho, P. , Larzabal, L. , Calvo, A. , Lecanda, F. , 2011. PDGFR signaling blockade in marrow stroma impairs lung cancer bone metastasis. Cancer Res.. 71, 164–174. [DOI] [PubMed] [Google Scholar]
- Couderc, B. , Pradines, A. , Rafii, A. , Golzio, M. , Deviers, A. , Allal, C. , Berg, D. , Penary, M. , Teissie, J. , Favre, G. , 2008. In vivo restoration of RhoB expression leads to ovarian tumor regression. Cancer Gene Ther.. 15, 456–464. [DOI] [PubMed] [Google Scholar]
- Du, W. , Prendergast, G.C. , 1999. Geranylgeranylated RhoB mediates suppression of human tumor cell growth by farnesyltransferase inhibitors. Cancer Res.. 59, 5492–5496. [PubMed] [Google Scholar]
- Engel, M.E. , Datta, P.K. , Moses, H.L. , 1998. RhoB is stabilized by transforming growth factor beta and antagonizes transcriptional activation. J. Biol. Chem.. 273, 9921–9926. [DOI] [PubMed] [Google Scholar]
- Feld, R. , Rubinstein, L.V. , Weisenberger, T.H. , 1984. Sites of recurrence in resected stage I non-small-cell lung cancer: a guide for future studies. J. Clin. Oncol.. 2, 1352–1358. [DOI] [PubMed] [Google Scholar]
- Fernandez-Borja, M. , Janssen, L. , Verwoerd, D. , Hordijk, P. , Neefjes, J. , 2005. RhoB regulates endosome transport by promoting actin assembly on endosomal membranes through Dia1. J. Cell Sci.. 118, 2661–2670. [DOI] [PubMed] [Google Scholar]
- Fidler, I.J. , 2003. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer. 3, 453–458. [DOI] [PubMed] [Google Scholar]
- Gan, P.P. , Pasquier, E. , Kavallaris, M. , 2007. Class III beta-tubulin mediates sensitivity to chemotherapeutic drugs in non small cell lung cancer. Cancer Res.. 67, 9356–9363. [DOI] [PubMed] [Google Scholar]
- Ganji, P.N. , Nalla, A.K. , Gupta, R. , Mohanam, S. , Gujrati, M. , Dinh, D.H. , Rao, J.S. , 2010. siRNA-mediated downregulation of MMP-9 and uPAR in combination with radiation induces G2/M cell cycle arrest in medulloblastoma. Mol. Cancer Res.. 9, (1) 51–66. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Goncalves, A. , Braguer, D. , Kamath, K. , Martello, L. , Briand, C. , Horwitz, S. , Wilson, L. , Jordan, M.A. , 2001. Resistance to Taxol in lung cancer cells associated with increased microtubule dynamics. Proc. Natl. Acad. Sci. U S A. 98, 11737–11742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, G.P. , Nguyen, D.X. , Chiang, A.C. , Bos, P.D. , Kim, J.Y. , Nadal, C. , Gomis, R.R. , Manova-Todorova, K. , Massague, J. , 2007. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature. 446, 765–770. [DOI] [PubMed] [Google Scholar]
- Han, H.J. , Russo, J. , Kohwi, Y. , Kohwi-Shigematsu, T. , 2008. SATB1 reprogrammes gene expression to promote breast tumour growth and metastasis. Nature. 452, 187–193. [DOI] [PubMed] [Google Scholar]
- Hess, K.R. , Varadhachary, G.R. , Taylor, S.H. , Wei, W. , Raber, M.N. , Lenzi, R. , Abbruzzese, J.L. , 2006. Metastatic patterns in adenocarcinoma. Cancer. 106, 1624–1633. [DOI] [PubMed] [Google Scholar]
- Hu, G. , Chong, R.A. , Yang, Q. , Wei, Y. , Blanco, M.A. , Li, F. , Reiss, M. , Au, J.L. , Haffty, B.G. , Kang, Y. , 2009. MTDH activation by 8q22 genomic gain promotes chemoresistance and metastasis of poor-prognosis breast cancer. Cancer Cell. 15, 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe, A.B. , Hall, A. , 2002. Rho GTPases in transformation and metastasis. Adv. Cancer Res.. 84, 57–80. [DOI] [PubMed] [Google Scholar]
- Jaffe, A.B. , Hall, A. , 2005. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol.. 21, 247–269. [DOI] [PubMed] [Google Scholar]
- Jemal, A. , Siegel, R. , Xu, J. , Ward, E. , 2010. Cancer statistics, 2010. CA Cancer J. Clin.. 60, 277–300. [DOI] [PubMed] [Google Scholar]
- Jiang, K. , Sun, J. , Cheng, J. , Djeu, J.Y. , Wei, S. , Sebti, S. , 2004. Akt mediates Ras downregulation of RhoB, a suppressor of transformation, invasion, and metastasis. Mol. Cell Biol.. 24, 5565–5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josson, S. , Sharp, S. , Sung, S.Y. , Johnstone, P.A. , Aneja, R. , Wang, R. , Gururajan, M. , Turner, T. , Chung, L.W. , Yates, C. , 2010. Tumor-stromal interactions influence radiation sensitivity in epithelial-versus mesenchymal-like prostate cancer cells. J. Oncol.. 2010, 10 Article ID 232831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce, J.A. , Pollard, J.W. , 2009. Microenvironmental regulation of metastasis. Nat. Rev. Cancer. 9, 239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, Y. , Siegel, P.M. , Shu, W. , Drobnjak, M. , Kakonen, S.M. , Cordon-Cardo, C. , Guise, T.A. , Massague, J. , 2003. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 3, 537–549. [DOI] [PubMed] [Google Scholar]
- Kazerounian, S. , Gerald, D. , Huang, M. , Chin, Y.R. , Udayakumar, D. , Zheng, N. , O'Donnell, R.K. , Perruzzi, C. , Mangiante, L. , Pourat, J. , Phung, T.L. , Bravo-Nuevo, A. , Shechter, S. , McNamara, S. , Duhadaway, J.B. , Kocher, O.N. , Brown, L.F. , Toker, A. , Prendergast, G.C. , Benjamin, L.E. , 2013. RhoB differentially controls Akt function in tumor cells and stromal endothelial cells during breast tumorigenesis. Cancer Res.. 73, 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos, H.F. , Burgess, H.A. , Thatcher, T.H. , Redonnet, M.R. , Hernady, E. , Williams, J.P. , Sime, P.J. , 2006. Oropharyngeal aspiration of a silica suspension produces a superior model of silicosis in the mouse when compared to intratracheal instillation. Exp. Lung Res.. 32, 181–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, A. , Cerniglia, G.J. , Bernhard, E.J. , Prendergast, G.C. , 2001. RhoB is required to mediate apoptosis in neoplastically transformed cells after DNA damage. Proc. Natl. Acad. Sci. U S A. 98, 6192–6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, A.X. , Rane, N. , Liu, J.P. , Prendergast, G.C. , 2001. RhoB is dispensable for mouse development, but it modifies susceptibility to tumor formation as well as cell adhesion and growth factor signaling in transformed cells. Mol. Cell Biol.. 21, 6906–6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, X. , Wang, Q. , Hu, G. , Van Poznak, C. , Fleisher, M. , Reiss, M. , Massague, J. , Kang, Y. , 2009. ADAMTS1 and MMP1 proteolytically engage EGF-like ligands in an osteolytic signaling cascade for bone metastasis. Genes Dev.. 23, 1882–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis-Ravelo, D. , Anton, I. , Vicent, S. , Hernandez, I. , Valencia, K. , Zandueta, C. , Martinez-Canarias, S. , Gurpide, A. , Lecanda, F. , 2011. Tumor-stromal interactions of the bone microenvironment: in vitro findings and potential in vivo relevance in metastatic lung cancer models. Clin. Exp. Metastasis. 28, 779–791. [DOI] [PubMed] [Google Scholar]
- Luis-Ravelo, D. , Anton, I. , Zandueta, C. , Valencia, K. , Ormazabal, C. , Martinez-Canarias, S. , Guruceaga, E. , Perurena, N. , Vicent, S. , De Las Rivas, J. , Lecanda, F. , 2013. A gene signature of bone metastatic colonization sensitizes for tumor-induced osteolysis and predicts survival in lung cancer. Oncogene. 10.1038/onc.2013.440 [DOI] [PubMed] [Google Scholar]
- Martini, N. , Bains, M.S. , Burt, M.E. , Zakowski, M.F. , McCormack, P. , Rusch, V.W. , Ginsberg, R.J. , 1995. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J. Thorac. Cardiovasc. Surg.. 109, 120–129. [DOI] [PubMed] [Google Scholar]
- Mazieres, J. , Antonia, T. , Daste, G. , Muro-Cacho, C. , Berchery, D. , Tillement, V. , Pradines, A. , Sebti, S. , Favre, G. , 2004. Loss of RhoB expression in human lung cancer progression. Clin. Cancer Res.. 10, 2742–2750. [DOI] [PubMed] [Google Scholar]
- Mellor, H. , Flynn, P. , Nobes, C.D. , Hall, A. , Parker, P.J. , 1998. PRK1 is targeted to endosomes by the small GTPase, RhoB. J. Biol. Chem.. 273, 4811–4814. [DOI] [PubMed] [Google Scholar]
- Minn, A.J. , Gupta, G.P. , Siegel, P.M. , Bos, P.D. , Shu, W. , Giri, D.D. , Viale, A. , Olshen, A.B. , Gerald, W.L. , Massague, J. , Kang, Y. , Serganova, I. , Doubrovin, M. , Ponomarev, V. , Blasberg, R. , Drobnjak, M. , Kakonen, S.M. , Cordon-Cardo, C. , Guise, T.A. , Cardiff, R.D. , Muller, W.J. , 2005. Genes that mediate breast cancer metastasis to lung. Nature. 436, 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monferran, S. , Skuli, N. , Delmas, C. , Favre, G. , Bonnet, J. , Cohen-Jonathan-Moyal, E. , Toulas, C. , 2008. Alphavbeta3 and alphavbeta5 integrins control glioma cell response to ionising radiation through ILK and RhoB. Int. J. Cancer. 123, 357–364. [DOI] [PubMed] [Google Scholar]
- Nguyen, D.X. , Chiang, A.C. , Zhang, X.H. , Kim, J.Y. , Kris, M.G. , Ladanyi, M. , Gerald, W.L. , Massague, J. , 2009. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 138, 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast, G.C. , 2001. Actin’ up: RhoB in cancer and apoptosis. Nat. Rev. Cancer. 1, 162–168. [DOI] [PubMed] [Google Scholar]
- Prendergast, G.C. , Khosravi-Far, R. , Solski, P.A. , Kurzawa, H. , Lebowitz, P.F. , Der, C.J. , 1995. Critical role of Rho in cell transformation by oncogenic Ras. Oncogene. 10, 2289–2296. [PubMed] [Google Scholar]
- Sato, N. , Fukui, T. , Taniguchi, T. , Yokoyama, T. , Kondo, M. , Nagasaka, T. , Goto, Y. , Gao, W. , Ueda, Y. , Yokoi, K. , Minna, J.D. , Osada, H. , Kondo, Y. , Sekido, Y. , 2007. RhoB is frequently downregulated in non-small-cell lung cancer and resides in the 2p24 homozygous deletion region of a lung cancer cell line. Int. J. Cancer. 120, 543–551. [DOI] [PubMed] [Google Scholar]
- Skuli, N. , Monferran, S. , Delmas, C. , Lajoie-Mazenc, I. , Favre, G. , Toulas, C. , Cohen-Jonathan-Moyal, E. , 2006. Activation of RhoB by hypoxia controls hypoxia-inducible factor-1alpha stabilization through glycogen synthase kinase-3 in U87 glioblastoma cells. Cancer Res.. 66, 482–489. [DOI] [PubMed] [Google Scholar]
- Stewart, D.J. , 2010. Tumor and host factors that may limit efficacy of chemotherapy in non-small cell and small cell lung cancer. Crit. Rev. Oncol. Hematol.. 75, 173–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega, F.M. , Colomba, A. , Reymond, N. , Thomas, M. , Ridley, A.J. , 2012. RhoB regulates cell migration through altered focal adhesion dynamics. Open Biol.. 2, 120076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicent, S. , Luis-Ravelo, D. , Anton, I. , Garcia-Tunon, I. , Borras-Cuesta, F. , Dotor, J. , De Las Rivas, J. , Lecanda, F. , 2008. A novel lung cancer signature mediates metastatic bone colonization by a dual mechanism. Cancer Res.. 68, 2275–2285. [DOI] [PubMed] [Google Scholar]
- Witze, E.S. , Litman, E.S. , Argast, G.M. , Moon, R.T. , Ahn, N.G. , 2008. Wnt5a control of cell polarity and directional movement by polarized redistribution of adhesion receptors. Science. 320, 365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J. , Mani, S.A. , Donaher, J.L. , Ramaswamy, S. , Itzykson, R.A. , Come, C. , Savagner, P. , Gitelman, I. , Richardson, A. , Weinberg, R.A. , 2004. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 117, 927–939. [DOI] [PubMed] [Google Scholar]
- Yin, J.J. , Selander, K. , Chirgwin, J.M. , Dallas, M. , Grubbs, B.G. , Wieser, R. , Massague, J. , Mundy, G.R. , Guise, T.A. , 1999. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J. Clin. Invest.. 103, 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J. , Zhu, Y. , Zhang, G. , Liu, N. , Sun, L. , Liu, M. , Qiu, M. , Luo, D. , Tang, Q. , Liao, Z. , Zheng, Y. , Bi, F. , 2011. A distinct role of RhoB in gastric cancer suppression. Int. J. Cancer. 128, 1057–1068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following are the supplementary data related to this article.
Supplementary data
Supplementary data


