Abstract
Poly‐ADP‐ribose‐polymerase inhibitors (PARPi) are considered to be optimal tools for specifically enhancing radiosensitivity. This effect has been shown to be replication‐dependent and more profound in HR‐deficient tumors. Here, we present a new mode of PARPi‐mediated radiosensitization which was observed in four out of six HR‐proficient tumor cell lines (responders) investigated, but not in normal cells. This effect is replication‐independent, as the radiosensitization remained unaffected following the inhibition of replication using aphidicolin. We showed that responders are radiosensitized by Olaparib because their DSB‐repair is switched to PARP1‐dependent end‐joining (PARP1‐EJ), as evident by (i) the significant increase in the number of residual γH2AX foci following irradiation with 3Gy and treatment with Olaparib, (ii) the enhanced enrichment of PARP1 at the chromatin after 3Gy and (iii) the inhibition of end‐joining activity measured by a specific reporter substrate upon Olaparib treatment. This is the first study which directly demonstrates the switch to PARP1‐EJ in tumor cells and its contribution to the response to Olaparib as a radiosensitizer, findings which could widen the scope of application of PARPi in tumor therapy.
Keywords: DSB repair, Non‐homologous end-joining, Olaparib, Radiosensitivity, PARP1‐dependent end‐joining
Highlights
Clinical use of PARPi as a potent radiosensitizer is limited to tumors with high proliferation rate and/or HR deficiency.
We suggest a new mode for the tumor radiosensitization by PARPi which depends on the inhibition of the PARP1‐EJ repair.
Switch to PARP1‐EJ was seen in tumor but not in normal cells indicating a tumor specificity for radiosensitization by PARPi.
This could expand the application of PARPi as radiosensitizers to include tumors whose repair is switched to PARP1‐EJ.
1. Introduction
The primary goal of radiotherapy (RT) in the treatment of cancer is achieving a defined killing of tumor cells with minimal adverse effects for its patients. Although already very efficient, RT alone is often unable to induce complete remission, primarily because the maximal dose is limited by the surrounding normal tissue. Therefore, a principle challenge for researchers is to enhance response to radiotherapy by developing efficient radio‐sensitizers which act solely in tumors. RT achieves its cell killing effect mostly through the induction of various forms of DNA damage, among which double‐strand breaks (DSBs) are considered to be the most toxic. DSBs are repaired via two main pathways: non‐homologous end‐joining (NHEJ) and homologous recombination (HR). NHEJ is a re‐ligation of the two ends of a DSB without the use of significant homology, whereas HR uses homologous DNA sequences i.e. sister chromatids as a template for repair (Helleday et al., 2007). While NHEJ is active throughout all cell cycle phases and is more error‐prone compared with HR, HR predominates in S‐phase cells, when a sister chromatid is available, and is a high‐fidelity process (Helleday et al., 2007).
A strong radiosensitizing effect can be achieved by interfering with one of these two DSB repair pathways (Huhn et al., 2013). However, the cytotoxic effect on normal cells is a significant obstacle in targeting these pathways. In this context, the inhibition of the Poly [ADP‐ribose] polymerase 1 (PARP‐1) is considered to be of great potential. PARP1 is involved in the repair of single‐strand breaks (SSBs) via base excision repair (BER). PARP1 inhibition leads to the accumulation of unrepaired spontaneously occurring SSBs which collide with replication forks, generating one‐ended DSBs (Tutt et al., 2005). Such DSBs are specifically repaired by HR and accordingly, PARP inhibition is lethal in HR‐deficient cells. This concept (also referred to as synthetic lethality) has been successfully applied in breast and/or ovarian cancer patients defective in HR due to mutations in BRCA1/2.
When combined with irradiation (IR), PARP inhibition has also been found to enhance cellular radiosensitivity (Powell et al., 2010; Senra et al., 2011). Given the fact that ionizing radiation (IR) induces mostly SSBs, the radiosensitizing effect mediated by PARP inhibition is considered to occur through a block in the BER pathway, thereby increasing the risk of collapsed replication forks, and thus generating persistent DSBs. In line with this, the data available so far demonstrate that radiosensitization has been found to depend primarily on the proportion of cells undergoing DNA replication (Dungey et al., 2008), and is especially pronounced in cells deficient in HR (e.g. BRCA breast cancer). Therefore, exploiting these characteristics can provide a mechanistic basis for the use of PARP inhibitors (PARPi)as radiosensitizers in rapidly dividing tumors such as squamous cell carcinomas of the head and neck or HR‐deficient tumors such as BRCA‐deficient tumors.
Emerging evidence indicates that in addition to HR and NHEJ, DSBs may also be repaired by an alternative end‐joining pathway (Alt‐EJ). This pathway is activated when NHEJ is defective (Audebert et al., 2004, 2013, 2008, 2010, 2007). Previously, we and others have found that this Alt‐EJ – also termed B‐NHEJ (Iliakis, 2009, 2003, 2006)‐ requires PARP1 (PARP1‐dependent end‐joining, PARP1‐EJ) and, in contrast to NHEJ, is highly inaccurate (Audebert et al., 2004, 2013, 2010, 2013). Consequently, the inhibition of PARP1 in cells whose repair has switched to Alt‐EJ has been shown to enhance radiosensitivity (Loser et al., 2010; Mansour et al., 2010). Interestingly, previous data have demonstrated that many human tumors including glioblastomas as well as cancers of the bladder, breast and head and neck employ an inaccurate mode of DSB repair (Baldeyron et al., 2002; Bentley et al., 2004; Shin et al., 2006); whether this is in fact a case of PARP1‐EJ, however, is not yet clear. In the current study, we show for the first time that PARP inhibition by Olaparib may result in an efficient radiosensitization in tumor cells which is replication‐independent and does not require defective HR, instead resulting from the switch in DSB‐repair pathway to PARP1‐EJ. This finding suggests that the inhibition of Alt‐EJ by Olaparib may be used to improve RT, thus widening the scope of application for the therapeutic inhibition of PARP1.
2. Materials and methods
2.1. Cell lines and treatment
The tumor cell lines used in this study were grown in DMEM (Gibco–Invitrogen) supplemented with 10% fetal calf serum, 100 U/ml penicillin and 100 μg/ml streptomycin at 37 °C with 10% CO2 PARP was chemically inhibited using 1 μM Olaparib (Selleck).
2.2. Colony formation assay
The effect of PARP inhibition on survival after exposure to irradiation was assessed as previously described (Mansour et al., 2010). Briefly, cells were plated at 400 cells per T25 flask in the presence of PARPi in triplicate and then incubated for 6 h. Cells were then X‐irradiated (RS225 research system, GLUMAY MEDICAL, UK at 200 kV, 15 mA) and maintained for 2–3 weeks before staining with 1% crystal violet. Colonies containing more than 50 cells were counted as survivors (automated Colcount Mammalian Cell Colony Counter Oxford Optronix Ltd., UK). DMSO was used as a control instead of the inhibitor at the same concentration. The radiosensitization enhancement ratio for Olaparib at 50% survival, ER50, was calculated as follows:
2.3. DSB‐repair reporter assay
The cells containing single stably integrated copies of either repair substrate (pEJ or pGC) were electroporated with 50 μg of the I‐Sce‐I expression vector (pCMV3xnls‐I‐SceI, a kind gift of M. Jasin) as described to induce DSBs or with pCMV‐Neo as a control. 24 and 48 h after transfection, the cells were assessed for green fluorescence by flow cytometry (FACScan, BD Bioscience). All subsequent repair results were corrected according to transfection efficiency of each cell line.
For I‐PpoI experiments, GALV‐pseudotyped retroviral particles were produced by transient transfection of the pBABE‐ER‐IPpoI plasmid (kind gift from M. Kastan) in HEK293T cells (Lord and Ashworth, 2013). Supernatant was used to stably transduce PC3 and Du145 cells as previously described (Berkovich et al., 2007; Li et al., 2007). For DSB induction, 1 μM 4‐hydroxy tamoxifen (4‐OHT) was added to the cultures for 6 h to induce I‐PpoI nuclear translocation. Subsequently, cells were fixed at different time points to detect γH2AX foci using IF analysis (see below).
2.4. Cell cycle analysis
Cells were harvested and fixed with 80% cold ethanol (−20 °C). After washing, the DNA was stained with PI solution containing RNase A. Cell cycle distribution was monitored by flow cytometry (FACScan, BD Bioscience) and analyzed by the Mod‐Fit software (Verity Software House).
2.5. Immunofluorescence
Cells grown on cover slips were washed once with cold PBS and fixed with 2% formaldehyde/PBS for 10 min. Fixed cells were permeabilized with 0.2% Triton X‐100/PBS on ice for 5 min. The cells were incubated for 2 h with primary antibody, mouse monoclonal anti‐phospho‐S139‐H2AX antibody (Millipore) at a dilution of 1:300, and rabbit monoclonal anti‐53BP1pAb (Novus Biologicals) at a dilution of 1:600. After being washed three times with cold PBS, the cells were incubated for 1 h with secondary anti‐mouse Alexafluor594 (Invitrogen) or anti‐rabbit fluorescein (Amersham) at a dilution of 1:1000. The nuclei were counterstained with 4′‐6‐diamidino‐2‐phenylindole (DAPI, 10 ng/ml). Immuno‐fluorescence was observed with the Zeiss AxioObserver.Z1 microscope (objectives: EC PlnN 40x/0.75 DICII, resolution 0.44 μm; Pln Apo 63x/1.4 Oil DICII, resolution 0.24 μm; EC PlnN 100x/1.3 Oil DICII, resolution 0.26 μm and filters: Zeiss 43, Zeiss 38, Zeiss 49). Semi‐confocal images were obtained using the Zeiss Apotome, Zeiss AxioCamMRm and Zeiss AxioVision Software.
2.6. Caspase activity
Detection of caspase activity was performed utilizing the FAMFLICA™ Poly Caspases Assay Kit (Immunochemistry Technologies) according to the manufacturer's instructions. Flow‐cytometric analysis was performed on a FACS Canto with FACS Diva Software (Becton Dickinson).
2.7. Protein extraction and western blot
Whole cell lysates were isolated using Laemmli buffer (Laemmli, 1970) and different protein fractions were isolated using a subcellular protein fractionation kit (Thermo Scientific, Rockford, IL) following the manufacturer's instructions. Equal amounts of total protein (30 μg) were electrophoresed using SDS/PAGE. Immunoblot analysis was performed with the following antibodies: rabbit anti‐Ku80, mouse mAb (Abcam), mouse anti‐LigIV mAb (Santa Cruz Biotechnology), mouse anti‐PARP1 (DB Pharmigen), rabbit anti‐H2B pAb (Imgenex), mouse anti‐SP1 pAb (H‐225, Santa Cruz Biotechnology), as well as mouse anti‐beta‐actin (Sigma).
3. Results
3.1. Differences in the radio‐response of tumor cells to inhibition of PARP1 by Olaparib
Figure 1 shows the effects of PARP inhibition on the radiosensitivity of different human tumor cell lines (PC3, Du145, LNCaP, HeLa, H1299 and A549).PARP1 was inhibited by treatment with 1 μM Olaparib for 1 h prior to irradiation, which was found to efficiently block PARP activity in all six cell lines (Supplementary Figure S1). Four (H1299, LNCaP, PC3, HeLa) out of the six tumor cell lines tested showed a clear increase in the radiosensitivity, with an enhancement ratio (within a surviving fraction of 50%, ER50) varying between 1.3 and 2.2. In contrast, no radiosensitization was observed for A549 (ER50 = 1.04) or Du145 (ER50 = 1.05). Notably, no radiosensitization was found upon PARP inhibition for two normal human fibroblast cell lines (ER50 = 1.06 and 1.06 for NF184 and NF180 respectively) (Figure 1). For clarity, we will refer to tumors which respond to Olaparib as “responders” and those which did not as “non‐responders”.
Figure 1.
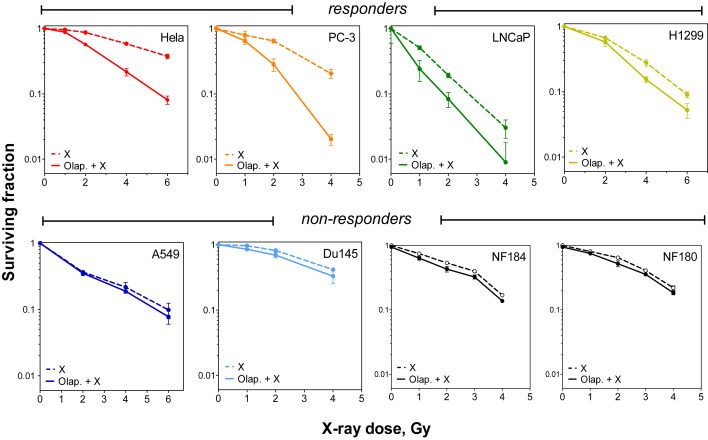
Olaparib treatment sensitizes some but not all tumor cells to IR. Cells were treated with either DMSO (dashed line) or 1 μM of Olaparib (solid line) prior to exposure to the indicated doses of ionizing radiation. The surviving fractions were then calculated by colony forming assay (see Materials and methods). Shown are the means ± SEM from three experiments.
3.2. Olaparib‐mediated radiosensitization in responders is mostly replication‐independent
Previously, it has been shown that PARP inhibition promotes the replication‐dependent conversion of unrepaired SSBs to potentially toxic DSBs (Helleday et al., 2007), a process which requires HR. To investigate whether the radiosensitizing effect of Olaparib in responders is mediated by this mechanism, we used the DNA polymerase inhibitor aphidicolin (APH) to inhibit DNA replication during the period of PARP inhibition (Supplementary Figure S2A). Cells were treated with both Olaparib and APH (2.5 μM) for 4 h (1 h pre‐ and 3 h‐post IR) and clonogenic survival assays were conducted in responders (HeLa, PC3, LNCaP, and H1299 cells) in the presence or absence of Olaparib. As shown in Figure 3A, the radiosensitizing effects of Olaparib were only mildly reduced after APH treatment, indicating that this effect is primarily replication‐independent in responders. In order to further reinforce this data, two responding cell lines (HeLa and H1299) were collected prior to replication by overnight incubation with 2.5 μM APH (Supplementary Figure S2A). Two hours before IR, synchronized cells were treated with Olaparib for 2 h before release from APH treatment. Confirming the results above, both synchronized HeLa and H1299 cells were radiosensitized by Olaparib (Supplementary Figure S2B).
Figure 3.
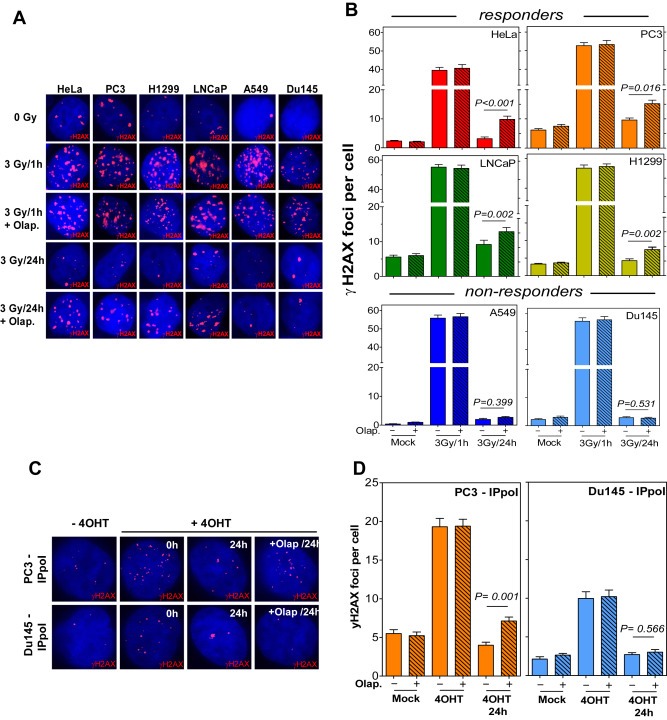
PARP1 is involved in the repair of IR‐induced DSB in responders. (A) Representative IF photos for yH2AX foci induction and clearance after 3Gy in the indicated cell lines. (B) The indicated cells were treated with either 1 μM Olaparib (+) or DMSO (−) for 2 h prior to irradiation with 3Gy. γH2AX foci were then evaluated at 1 h and 24 h time points. Shown are the means ± SEM from at least three different experiments. P‐values were derived using the Mann–Whitney test. (C) Representative IF photos for γH2AX foci after the induction of DSBs using I‐PpoI. Cells were transfected with an I‐PpoI‐expressing vector (PBABE‐ER‐I‐PpoI) and treated with 4‐OHT for 6 h to induce the nuclear translocation of I‐PpoI and hence the induction of DSBs. The number of γH2AX foci was evaluated at the indicated time points in the presence or absence of Olaparib. (D) Quantitative analysis of the number of γH2AX foci after 4‐OHT treatment in either PC3 or Du145 cells in the presence or absence of Olaparib. Shown are the mean values ± SEM from at least three experiments. P‐values were derived using the Mann–Whitney test.
It has previously been shown that radiosensitization by PARP inhibition strongly depends on replication in cells defective in HR (Dungey et al., 2008; Loser et al., 2010). Accordingly, the survival of HR‐deficient irs1SF Hamster cells (Xrcc3‐deficient) was greatly reduced by Olaparib (Figure 2B). As anticipated, this radiosensitizing effect resulting from PARP inhibition was abolished when replication was blocked by APH treatment (Figure 2B). Similar effects were observed whenirs1SF cells were synchronized pre‐replication by 18 h‐treatment with APH (Supplementary Figure S2B).
Figure 2.
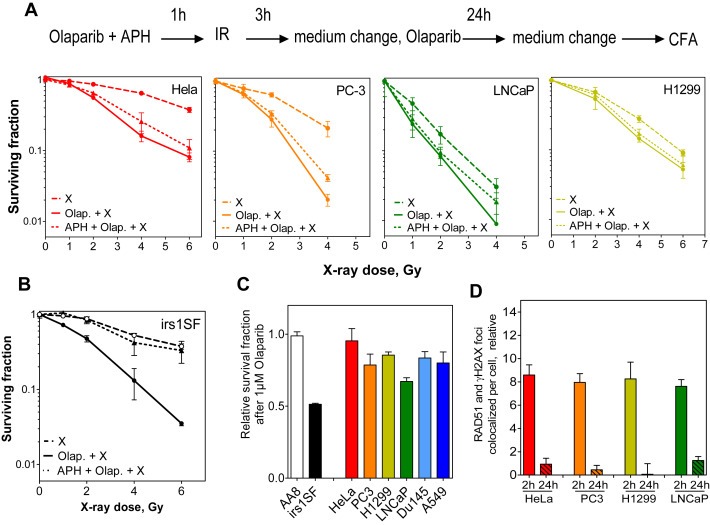
Olaparib‐mediated radiosensitization in responders is replication‐independent. (A) Replication was inhibited by incubating cells with 2.5 M aphidicolin (APH) for 1 h pre‐ and 3 h post‐ irradiation with the indicated doses. The surviving fractions were then calculated as in Figure 1. Shown are the means ± SEM from three experiments. (B) Replication was inhibited in HR‐deficient irs1‐SF hamster cells as in A, the relative surviving fractions after 1 μM Olaparib were calculated. Shown are the means ± SEM from three experiments. (C) Cells were treated with the indicated concentrations of Olaparib for 24 h and the surviving fractions were then measured after 3 weeks. Indicated are the means ± SEM from three experiments. (D) Quantification of the relative number of colocalized RAD51 and γH2AX foci at the indicated time points after 2Gy. At least 100 cells were counted for each time point.
Moreover, responders did not show a HR‐deficient phenotype, as evidenced by the finding that they (1) showed no particular sensitivity to Olaparib alone as described for HR‐deficient cells (Figure 2B & C and Supplementary Figure S3A&B) (Dungey et al., 2008; Loser et al., 2010) and (2) exhibited normal RAD51 loading and resolution after IR (Figure 2D and Supplementary Figure S3C). Collectively, these data clearly illustrate that the replication‐dependent conversion of a SSB to a DSB is responsible for the radiosensitization by PARPi when HR is impaired. However, some tumor cells (responders) which showed no evidence of HR‐deficiency were radiosensitized by PARPi in a replication‐independent manner.
3.3. PARP inhibition only impairs DNA double‐strand break repair in responders
The mechanism behind Olaparib‐mediated cellular radiosensitivity could potentially be explained by induced cell cycle arrest, enhanced apoptosis, or impaired DBS repair efficiency (Kasten‐Pisula et al., 2009). In order to test these possibilities, we first examined the effect of Olaparib treatment on the cell cycle with or without IR in both responders and non‐responders. Olaparib alone showed no effect on the cell cycle (data not shown). However, when combined with IR, Olaparib led to a transient (4–12 h), radiation‐induced accumulation of both responders (Supplementary Figure S5A) and non‐responders (Supplementary Figure S5B) in the S‐phase. This finding indicates that the radiosensitizing effect of Olaparib in responders is not related to the cell cycle. The effect of Olaparib on apoptosis was measured using caspase activity. The data obtained revealed no increase in caspase activity upon inhibition of PARP activity in either responders or non‐responders after irradiation (Supplementary Figure S5C).
Next we sought to examine the potential role of PARP1 in the repair of IR‐induced DSBs. To address this, we measured the effect of Olaparib on the induction and resolution of γH2AX foci after exposure to 3Gy (Figure 3A). In all cell lines, the inhibition of PARP activity did not affect the number of γH2AX foci present at 1 h following irradiation with 3Gy. However, PARP inhibition significantly increased the number of persistent γH2AX foci (at 24 h time point) solely in responders (HeLa, P < 0.001; PC3, P = 0.016; LNCaP, P = 0.002; and H1299, P = 0.002), but not in non‐responders (A549, P = 0.399; and Du145, P = 0.531) (Figure 3B), indicating a defect in DSB repair mediated by PARP inhibition that was only observed in responders. Noteworthy, Olaparib alone affected the number of γH2AX foci at 24 h time point in 4 cell lines (Supplementary Figure S4) including 2 responders (HeLa and PC3) as well as 2 non‐responders (A549 and Du145). Even in case of PC3 cells, Olaparib deceased the number of γH2AX foci at 24 h time point (from 6.18 to 5.7). Hence, this nonspecific effect cannot account for the specific inhibitory effect of Olaparib on responders after 3Gy. In order to reinforce this finding, we made use of the eukaryotic homing endonuclease I‐PpoI to generate frank DSBs at defined positions intra‐chromosomally (Berkovich et al., 2007). Using this assay allows follow‐up of DSB repair without being affected by other forms of DNA damage. Viral supernatant of the I‐PpoI (pBABE‐HA‐ER‐IPpoI) vector was used to transduce either PC3 (responder) or Du145 (non‐responder) as previously described (Berkovich et al., 2007). The transfected cells were incubated with 1 μM of 4‐hydroxy‐tamoxifen (4‐OHT) for 6 h to allow for the nuclear trans‐localization of I‐PpoI, and consequently γH2AX foci induction and clearance were monitored using immunofluorescence (Figure 3C). In both PC3 and Du145 cells, Olaparib had no effect on the number of initial γH2AX foci (P = 0.290 and P = 0.412, respectively). Importantly, Olaparib treatment led to a significant increase in the number of residual γH2AX foci measured at the 24 h time point in PC3 (P = 0.0001), but not in Du145 (P = 0.566) (Figure 3D). Overall, these data strongly indicate that the radiosensitization achieved by PARP1 inhibition in responders does not result from enhanced cell cycle arrest or elevated apoptosis, but rather from an impaired DSB repair.
3.4. DSB repair is switched to PARP1‐dependent end‐joining in responders
Previously, we and others have described an alternative DSB repair pathway which is PARP1‐dependent (PARP1‐EJ) (Audebert et al., 2004, 2010, 2013, 2003). Based on these data, we hypothesized that DSB repair in responders might switch partly to PARP1‐EJ, which would also explain the impaired repair reported in these cells and the radiosensitization upon PARP1 inhibition. In order to directly address these questions, both responders and non‐responders were transiently transfected with I‐SceI‐linearized pEJ plasmid (Figure 4A, upper panel) (Mansour et al., 2010) in the presence or absence of Olaparib. The frequency of GFP+ cells, which result from efficient end‐joining, was then analyzedafter48 h using flow cytometry. Interestingly, treatment with Olaparib only led to a significant decrease in the percentage of GFP+ cells in responders, but not in non‐responders (Figure 4A, lower panel and Supplementary Figure S6). Here we considered the extent of Olaparib‐mediated end‐joining inhibition as the efficiency of PARP1‐EJ in each cell line. As shown in Figure 4B, PARP1‐EJ efficiently shares NHEJ in repairing the induced DSB in responders (HeLa, PC3, LNCaP and H1299) while non‐responders (A549 and Du145) mainly restrict to NHEJ.
Figure 4.
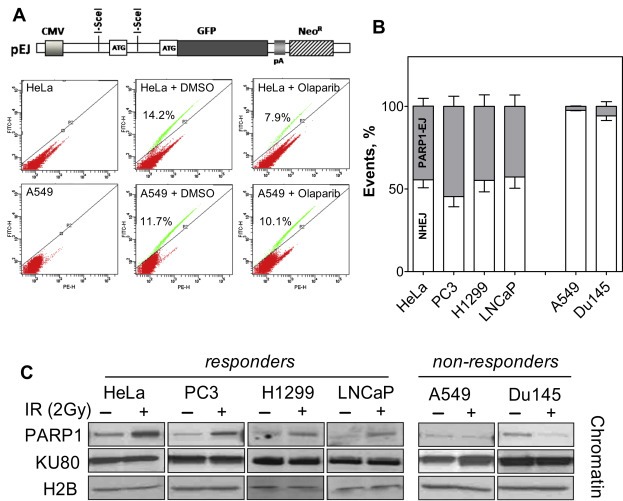
PARP1‐dependent end‐joining contributes to DSB repair in responders. (A) Upper panel: Schematic representation of the end‐joining substrate pEJ. Lower panel: Representative flow cytometry blots for the percentage of GFP+ cells 48 h after transfection with I‐SceI‐linearized pEJ in the presence or absence of 1 μM Olaparib. (B) The inhibition of end‐joining activity by Olaparib was expressed as the percentage of PARP1‐EJ (% PARP1‐EJ). Shown are the means ± SEM from at least four different experiments. (C) Cells were either mock irradiated (−) or irradiated with 3Gy (+) 2 h before the indicated proteins were assessed in the chromatin‐bound fractions using the Subcellular Protein Fractionation Kit (Thermo Scientific). H2B and SP1 were used as markers of the chromatin and nuclear fractions.
Recently, we have also demonstrated that the switch to PARP1‐EJ is marked by a specific signature of proteins in the chromatin‐bound fraction after IR (Mansour et al., 2013). This signature is characterized by an enriched fraction of PARP1‐EJ proteins i.e. PARP1.Here, we examined the chromatin of both responders and non‐responders 2 h after exposure to 2Gy. Strikingly, we revealed that PARP1 was found in a greater quantity at the damaged chromatin of responders (Figure 4D, upper panel) than in non‐responders (Figure 4D, lower panel).
Notably, using our previously described HR pGC substrate (Mansour et al., 2008), we found that HR efficiency was not affected by PARP inhibition (Supplementary Figure S3D)
Collectively, these data indicate that DSB repair is partly switched to the PARP1‐EJ, and that consequently the inhibition of PARP1 led to impaired DSB repair and enhanced radiosensitization, but only in responders.
4. Discussion
Much effort has been put towards identifying radiosensitizers which are specifically active in tumors. In this context, DNA repair is considered to be a promising target, as some of the core components of repair pathways are known to be frequently altered in tumor cells. The goal of these strategies would be to take advantage of these cancer cell defects in repairing DNA damage and exploit them to enhance radiotherapy (RT) response, with limited normal tissue toxicity. Among the newly studied compounds, PARP inhibitors (PARPi) are of special interest because of their specific lethal effect in tumors defective in DSB repair via HR (Bryant and Helleday, 2004; Farmer et al., 2005). This approach is based on the concept of synthetic lethality, in which two separate molecular pathways, which themselves are nonlethal when disrupted individually, are lethal when inhibited simultaneously (Hartwell et al., 1997; Kaelin, 2005; Kelland et al., 1988; Rehman et al., 2010). In the case of PARPi, these two molecular pathways are HR and base excision repair (BER), responsible for the repair of single‐strand DNA breaks (SSBs). In particular, the inhibition of PARP activity strongly delays the repair of SSBs, giving rise to DSBs upon their collision with an on‐going replication fork. These DSBs are normally repaired efficiently by HR, but become lethal in tumors defective in HR due to mutations in BRCA1 or BRCA2. Consequently, PARPi‐mediated radiosensitization is believed to rely primarily on the efficiency of the HR repair pathway and the proportion of cells undergoing DNA replication (Dungey et al., 2008; Loser et al., 2010).
Previously, we and others have shown that PARP inhibition enhances cellular radiosensitivity in a manner which is independent of replication and thus occurs in cells which show no evidence of HR impairment, but are instead deficient in NHEJ (Loser et al., 2010, 2010, 2013). Furthermore, we have demonstrated that the repair in these cells has shifted from classical NHEJ to an inaccurate alternative end‐joining (Alt‐EJ) pathway, which strongly depends on PARP1. Interestingly, several reports have indicated that DSB repair in tumor cells and tissues, but not in normal ones, is often performed by an inaccurate repair mechanism (Baldeyron et al., 2002; Bentley et al., 2004; Shin et al., 2006), suggesting a potential switch to Alt‐EJ; the dependence on PARP1, however, is still unclear. In the current study, we show that in four out of six tumor cell lines studied, DSB repair indeed switched to Alt‐EJ, which strongly depends on PARP1 (PARP1‐dependent end joining, PARP1‐EJ) (Figure 4). In these cell lines, the repair of DSBs was therefore impaired by Olaparib, as evidenced by the increase in the number of γH2AX foci at 24 h after IR and I‐PpoI treatment (Figure 3). Accordingly, tumor cell lines with a shift to PARP1‐EJ are specifically radiosensitized by Olaparib (Figure 1, upper panel; responders). This radiosensitizing effect could not be achieved in cell lines whose repair had not undergone the shift to PARP1‐EJ (Figure 1, lower panel; non‐responders). Importantly, this Olaparib‐mediated radiosensitization is tumor‐specific, as normal cells were shown to be non‐responders (Figure 1, lower panel). Moreover, we demonstrated that the responders are proficient in HR (Figure 2B and Supplementary Figure S2)and that the radiosensitization caused by Olaparib is primarily replication‐independent, as the inhibition of replication using APH showed an only minor effect on the Olaparib‐mediated radiosensitization (Figure 2A and Supplementary Figure S2B).
Collectively, our results here demonstrate a new manner in which tumors respond to Olaparib as a radiosensitizer (Figure 5). The repair is switched to the PARP1‐EJ in responders and therefore, the PARP1‐EJ shares NHEJ in repairing the induced DSB after IR. Consequently, the repair of DSBs is impaired upon inhibition of PARP activity by Olaparib, leading to an increase in radiosensitization. In contrast, the DSBs induced in non‐responders are repaired exclusively by NHEJ and hence, the inhibition of PARP1‐EJ does not impair DSB repair and the radiosensitivity is not affected.
Figure 5.
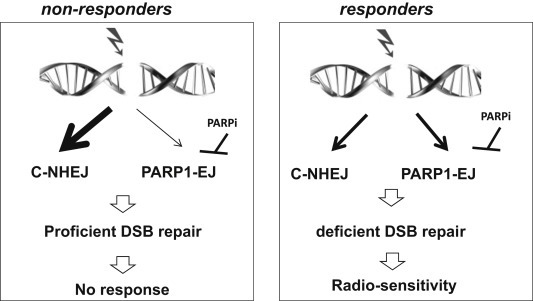
Model of the response to Olaparib as a radiosensitization agent. In non‐responders, the induced DSBs are repaired exclusively by NHEJ and hence, the inhibition of PARP1‐EJ fails to impair DSB repair and radiosensitivity is not affected. However, in responders, PARP1‐EJ shares NHEJ in repairing the DSBs induced after IR. Therefore, upon inhibition of PARP activity by Olaparib, the repair of DSBs is impaired, leading to the enhancement of radiosensitization.
The results of this study have several implications for the potential use of Olaparib in enhancing the radiosensitivity of tumors. First, our data reveal that the spectrum of tumors that can be radiosensitized by PARPi can clearly be expanded to include those whose repair has switched to PARP1‐EJ. Second, a major impediment to the clinical use of any radiosensitizing agent is the simultaneous sensitization of normal cells. Here, we report that the radiosensitizing effect is tumor‐specific, as normal cells showed no radiosensitization upon PARP inhibition.
According to our recent study (Mansour et al., 2013), DSB repair is switched to the PARP1‐EJ only in the absence of KU, but not in the absence of either DNA‐PK or XRCC4 (Mansour et al., 2013), indicating that the binding step between KU and the DNA is crucial in determining whether the repair will be performed via classical NHEJ or PARP1‐EJ. Although KU is expressed homogenously in tumors, changes in the DNA binding activity of KU have been previously described, even when KU protein levels remained unchanged (Muller et al., 2001). Interestingly, the KU–DNA binding activity was shown to be more frequently impaired in tumors compared to corresponding normal tissues (Pucci et al., 2001). For example, the BCL2 oncogene, which is often over‐expressed in tumors (Catz and Johnson, 2003), has been demonstrated to impede the binding of KU to the DNA by sequestrating KU70and preventing its assembly at DSB ends (Kumar et al., 2010; Wang et al., 2008). Alternatively, tumor cells may also switch to PARP1‐EJ when PARP1 is over‐expressed and, as a consequence, the dominance of KU over PARP1 and with that the inhibition of PARP‐EJ may be abolished (Walker et al., 2001; Wang et al., 2006). This aspect might be especially important for triple‐negative breast cancer (TNBC) patients over‐expressing PARP1 (Ossovskaya et al., 2010). For TNBC, phase II trials for combined treatment with carboplatin and the PARP inhibitor Iniparib had proved very promising (O'Shaughnessy et al., 2011); however, a phase III trial had to be stopped due to lack of response in 16 TNBC patients (Garber, 2013). The data presented here suggest that it might be reasonable to identify TNBC patients clearly over‐expressing PARP1 and whose repair is probably switched to PARP1‐EJ. These patients could respond favorably to a combined treatment with irradiation and PARPi.
As our understanding of the mechanisms and biochemical details involved in the regulation of the PARP1‐EJ repair mode increases, so does our potential to identify tumors which rely on this pathway, potentially having an enormous impact on future clinical trials. Therapeutic success will ultimately rely on the identification of patients that should be treated with Olaparib as a radiosensitizer.
In summary, it was shown here that DSB repair shifts to PARP1‐EJin tumor but not normal cells. This switch can then be exploited as an Achilles' heel to enhance radiosensitivity via PARP inhibition. This concept may greatly widen the application of PARPi in cancer treatment.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
The following is the supplementary data related to this article:
Supplementary data
Acknowledgments
The authors thank Jennifer Volquardsen for her valuable technical support.
Supplementary data 1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2014.06.008.
Kötter Annika, Cornils Kerstin, Borgmann Kerstin, Dahm-Daphi Jochen, Petersen Cordula, Dikomey Ekkehard and Mansour Wael Y., (2014), Inhibition of PARP1‐dependent end‐joining contributes to Olaparib‐mediated radiosensitization in tumor cells, Molecular Oncology, 8, doi: 10.1016/j.molonc.2014.06.008.
References
- Audebert, M. , Salles, B. , Calsou, P. , 2004. Involvement of poly(ADP-ribose) polymerase-1 and XRCC1/DNA ligase III in an alternative route for DNA double-strand breaks rejoining. J. Biol. Chem.. 279, 55117–55126. [DOI] [PubMed] [Google Scholar]
- Baldeyron, C. , Jacquemin, E. , Smith, J. , Jacquemont, C. , De Oliveira, I. , Gad, S. , Feunteun, J. , Stoppa-Lyonnet, D. , Papadopoulo, D. , 2002. A single mutated BRCA1 allele leads to impaired fidelity of double strand break end-joining. Oncogene. 21, 1401–1410. [DOI] [PubMed] [Google Scholar]
- Bentley, J. , Diggle, C.P. , Harnden, P. , Knowles, M.A. , Kiltie, A.E. , 2004. DNA double strand break repair in human bladder cancer is error prone and involves microhomology-associated end-joining. Nucleic Acids Res.. 32, 5249–5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkovich, E. , Monnat, R.J. , Kastan, M.B. , 2007. Roles of ATM and NBS1 in chromatin structure modulation and DNA double-strand break repair. Nat. Cell Biol.. 9, 683–690. [DOI] [PubMed] [Google Scholar]
- Bryant, H.E. , Helleday, T. , 2004. Poly(ADP-ribose) polymerase inhibitors as potential chemotherapeutic agents. Biochem. Soc. Trans.. 32, 959–961. [DOI] [PubMed] [Google Scholar]
- Catz, S.D. , Johnson, J.L. , 2003. BCL-2 in prostate cancer: a minireview. Apoptosis. 8, 29–37. [DOI] [PubMed] [Google Scholar]
- Dungey, F.A. , Loser, D.A. , Chalmers, A.J. , 2008. Replication-dependent radiosensitization of human glioma cells by inhibition of poly(ADP-ribose) polymerase: mechanisms and therapeutic potential. Int. J. Radiat. Oncol. Biol. Phys.. 72, 1188–1197. [DOI] [PubMed] [Google Scholar]
- Farmer, H. , McCabe, N. , Lord, C.J. , Tutt, A.N. , Johnson, D.A. , Richardson, T.B. , Santarosa, M. , Dillon, K.J. , Hickson, I. , Knights, C. , Martin, N.M. , Jackson, S.P. , Smith, G.C. , Ashworth, A. , 2005. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 434, 917–921. [DOI] [PubMed] [Google Scholar]
- Garber, K. , 2013. PARP inhibitors bounce back. Nat. Rev. Drug Discov.. 12, 725–727. [DOI] [PubMed] [Google Scholar]
- Hartwell, L.H. , Szankasi, P. , Roberts, C.J. , Murray, A.W. , Friend, S.H. , 1997. Integrating genetic approaches into the discovery of anticancer drugs. Science. 278, 1064–1068. [DOI] [PubMed] [Google Scholar]
- Helleday, T. , Lo, J. , van Gent, D.C. , Engelward, B.P. , 2007. DNA double-strand break repair: from mechanistic understanding to cancer treatment. DNA Repair (Amst). 6, 923–935. [DOI] [PubMed] [Google Scholar]
- Huhn, D. , Bolck, H.A. , Sartori, A.A. , 2013. Targeting DNA double-strand break signalling and repair: recent advances in cancer therapy. Swiss Med. Wkly.. 143, w13837 [DOI] [PubMed] [Google Scholar]
- Iliakis, G. , 2009. Backup pathways of NHEJ in cells of higher eukaryotes: cell cycle dependence. Radiother. Oncol.. 92, 310–315. [DOI] [PubMed] [Google Scholar]
- Jia, Q. , Dulk-Ras, A.D. , Shen, H. , Hooykaas, P.J. , de Pater, S. , 2013. Poly(ADP-ribose)polymerases are involved in microhomology mediated back-up non-homologous end joining in Arabidopsis thaliana . Plant Mol. Biol.. 82, 339–351. [DOI] [PubMed] [Google Scholar]
- Kaelin, W.G. , 2005. The concept of synthetic lethality in the context of anticancer therapy. Nat. Rev. Cancer. 5, 689–698. [DOI] [PubMed] [Google Scholar]
- Kasten-Pisula, U. , Menegakis, A. , Brammer, I. , Borgmann, K. , Mansour, W.Y. , Degenhardt, S. , Krause, M. , Schreiber, A. , Dahm-Daphi, J. , Petersen, C. , Dikomey, E. , Baumann, M. , 2009. The extreme radiosensitivity of the squamous cell carcinoma SKX is due to a defect in double-strand break repair. Radiother. Oncol.. 90, 257–264. [DOI] [PubMed] [Google Scholar]
- Kelland, L.R. , Burgess, L. , Steel, G.G. , 1988. Differential radiosensitization by the poly(ADP-ribose) transferase inhibitor 3-aminobenzamide in human tumor cells of varying radiosensitivity. Int. J. Radiat. Oncol. Biol. Phys.. 14, 1239–1246. [DOI] [PubMed] [Google Scholar]
- Kumar, T.S. , Kari, V. , Choudhary, B. , Nambiar, M. , Akila, T.S. , Raghavan, S.C. , 2010. Anti-apoptotic protein BCL2 down-regulates DNA end joining in cancer cells. J. Biol. Chem.. 285, 32657–32670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli, U.K. , 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Li, Z. , Kustikova, O.S. , Kamino, K. , Neumann, T. , Rhein, M. , Grassman, E. , Fehse, B. , Baum, C. , 2007. Insertional mutagenesis by replication-deficient retroviral vectors encoding the large T oncogene. Ann. N. Y. Acad. Sci.. [DOI] [PubMed] [Google Scholar]
- Lord, C.J. , Ashworth, A. , 2013. Mechanisms of resistance to therapies targeting BRCA-mutant cancers. Nat. Med.. 19, 1381–1388. [DOI] [PubMed] [Google Scholar]
- Loser, D.A. , Shibata, A. , Shibata, A.K. , Woodbine, L.J. , Jeggo, P.A. , Chalmers, A.J. , 2010. Sensitization to radiation and alkylating agents by inhibitors of poly(ADP-ribose) polymerase is enhanced in cells deficient in DNA double-strand break repair. Mol. Cancer Ther.. 9, 1775–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour, W.Y. , Schumacher, S. , Rosskopf, R. , Rhein, T. , Schmidt-Petersen, F. , Gatzemeier, F. , Haag, F. , Borgmann, K. , Willers, H. , Dahm-Daphi, J. , 2008. Hierarchy of nonhomologous end-joining, single-strand annealing and gene conversion at site-directed DNA double-strand breaks. Nucleic Acids Res.. 36, 4088–4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour, W.Y. , Rhein, T. , Dahm-Daphi, J. , 2010. The alternative end-joining pathway for repair of DNA double-strand breaks requires PARP1 but is not dependent upon microhomologies. Nucleic Acids Res.. 38, 6065–6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour, W.Y. , Borgmann, K. , Petersen, C. , Dikomey, E. , Dahm-Daphi, J. , 2013. The absence of Ku but not defects in classical non-homologous end-joining is required to trigger PARP1-dependent end-joining. DNA Repair (Amst). 12, 1134–1142. [DOI] [PubMed] [Google Scholar]
- Muller, C. , Monferran, S. , Gamp, A.C. , Calsou, P. , Salles, B. , 2001. Inhibition of Ku heterodimer DNA end binding activity during granulocytic differentiation of human promyelocytic cell lines. Oncogene. 20, 4373–4382. [DOI] [PubMed] [Google Scholar]
- Nussenzweig, A. , Nussenzweig, M.C. , 2007. A backup DNA repair pathway moves to the forefront. Cell. 131, 223–225. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy, J. , Osborne, C. , Pippen, J.E. , Yoffe, M. , Patt, D. , Rocha, C. , Koo, I.C. , Sherman, B.M. , Bradley, C. , 2011. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N. Engl. J. Med.. 364, 205–214. [DOI] [PubMed] [Google Scholar]
- Ossovskaya, V. , Koo, I.C. , Kaldjian, E.P. , Alvares, C. , Sherman, B.M. , 2010. Upregulation of poly (ADP-ribose) polymerase-1 (PARP1) in triple-negative breast Cancer and other primary human tumor types. Genes & Cancer. I, 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell, C. , Mikropoulos, C. , Kaye, S.B. , Nutting, C.M. , Bhide, S.A. , Newbold, K. , Harrington, K.J. , 2010. Pre-clinical and clinical evaluation of PARP inhibitors as tumour-specific radiosensitisers. Cancer Treat Rev.. 36, 566–575. [DOI] [PubMed] [Google Scholar]
- Pucci, S. , Mazzarelli, P. , Rabitti, C. , Giai, M. , Gallucci, M. , Flammia, G. , Alcini, A. , Altomare, V. , Fazio, V.M. , 2001. Tumor specific modulation of KU70/80 DNA binding activity in breast and bladder human tumor biopsies. Oncogene. 20, 739–747. [DOI] [PubMed] [Google Scholar]
- Rehman, F.L. , Lord, C.J. , Ashworth, A. , 2010. Synthetic lethal approaches to breast cancer therapy. Nat. Rev. Clin. Oncol.. 7, 718–724. [DOI] [PubMed] [Google Scholar]
- Senra, J.M. , Telfer, B.A. , Cherry, K.E. , McCrudden, C.M. , Hirst, D.G. , O'Connor, M.J. , Wedge, S.R. , Stratford, I.J. , 2011. Inhibition of PARP-1 by olaparib (AZD2281) increases the radiosensitivity of a lung tumor xenograft. Mol. Cancer Ther.. 10, 1949–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, K.H. , Kang, M.K. , Kim, R.H. , Kameta, A. , Baluda, M.A. , Park, N.H. , 2006. Abnormal DNA end-joining activity in human head and neck cancer. Int. J. Mol. Med.. 17, 917–924. [PubMed] [Google Scholar]
- Tutt, A.N. , Lord, C.J. , McCabe, N. , Farmer, H. , Turner, N. , Martin, N.M. , Jackson, S.P. , Smith, G.C. , Ashworth, A. , 2005. Exploiting the DNA repair defect in BRCA mutant cells in the design of new therapeutic strategies for cancer. Cold Spring Harb. Symp. Quant. Biol.. 70, 139–148. [DOI] [PubMed] [Google Scholar]
- Walker, J.R. , Corpina, R.A. , Goldberg, J. , 2001. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature. 412, 607–614. [DOI] [PubMed] [Google Scholar]
- Wang, H. , Perrault, A.R. , Takeda, Y. , Qin, W. , Iliakis, G. , 2003. Biochemical evidence for Ku-independent backup pathways of NHEJ. Nucleic Acids Res.. 31, 5377–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. , Wu, W. , Rosidi, B. , Zhang, L. , Wang, H. , Iliakis, G. , 2006. PARP-1 and Ku compete for repair of DNA double strand breaks by distinct NHEJ pathways. Nucleic Acids Res.. 34, 6170–6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q. , Gao, F. , May, W.S. , Zhang, Y. , Flagg, T. , Deng, X. , 2008. Bcl2 negatively regulates DNA double-strand-break repair through a nonhomologous end-joining pathway. Mol. Cell. 29, 488–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following is the supplementary data related to this article:
Supplementary data


