Abstract
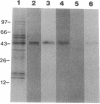
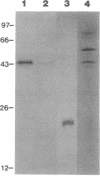
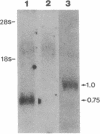
A monoclonal antibody (mAb L6) to a small-cell lung carcinoma surface antigen recognizes a common epitope of vasopressin-neurophysin and oxytocin-neurophysin in hypothalamic nuclei. We now report on the identification of a neurophysin-like precursor in human lung carcinoma (LX-1) cell membrane. mAb L6 immunoaffinity chromatography of solubilized membranes resulted in a single band of approximately 45 kDa. Western blot analysis demonstrated immunoreactivity of this band with mAb L6, anti-vasopressin, and an antibody to the vasopressin precursor, pro-pressophysin. N-terminal sequencing of this band demonstrated a 21-amino acid homology with the N terminus of human pro-pressophysin, and substitution of a Cys33 residue in the tumor antigen with Arg33. Absence of immunoreactivity with the antibodies described above in cytosolic extracts and culture medium suggests nonsecretion of processed or intact pro-pressophysin-like peptide. Northern analysis of LX-1 mRNA with a 30-mer to the C terminus of rat pro-pressophysin resulted in a band of approximately 1000 base pairs, 250 base pairs larger than hypothalamic message. In situ hybridization of LX-1 tumor-bearing nude rat brain with the same probe demonstrated specific hybridization in rat hypothalamus and xenografted tumor. These findings suggest expression of a pro-pressophysin-like protein in this tumor cell line that is preferentially targeted to the cell membrane.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodnar R. J., Truesdell L. S., Nilaver G. Potentiation of vasopressin analgesia in rats treated neonatally with monosodium glutamate. Peptides. 1985 Jul-Aug;6(4):621–626. doi: 10.1016/0196-9781(85)90163-9. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Brownstein M. J. Vasopressin, tissue-specific defects and the Brattleboro rat. Nature. 1984 Jul 5;310(5972):17–17. doi: 10.1038/310017a0. [DOI] [PubMed] [Google Scholar]
- Brakch N., Boussetta H., Rholam M., Cohen P. Processing endoprotease recognizes a structural feature at the cleavage site of peptide prohormones. The pro-ocytocin/neurophysin model. J Biol Chem. 1989 Sep 25;264(27):15912–15916. [PubMed] [Google Scholar]
- Breslow E. Chemistry and biology of the neurophysins. Annu Rev Biochem. 1979;48:251–274. doi: 10.1146/annurev.bi.48.070179.001343. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Cupo A., Rougon-Rapuzzi G., Pontarotti P. A., Delaage M. A. Quantitative detection and biochemical characterization of high-Mr forms of vasopressin in the rat hypothalamo--post-hypophysial system. Effect of water deprivation and rehydration. FEBS Lett. 1982 Oct 18;147(2):188–192. doi: 10.1016/0014-5793(82)81039-9. [DOI] [PubMed] [Google Scholar]
- Davis L. G., Arentzen R., Reid J. M., Manning R. W., Wolfson B., Lawrence K. L., Baldino F., Jr Glucocorticoid sensitivity of vasopressin mRNA levels in the paraventricular nucleus of the rat. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1145–1149. doi: 10.1073/pnas.83.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström I., Beaumier P. L., Hellström K. E. Antitumor effects of L6, an IgG2a antibody that reacts with most human carcinomas. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7059–7063. doi: 10.1073/pnas.83.18.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivell R., Richter D. The gene for the hypothalamic peptide hormone oxytocin is highly expressed in the bovine corpus luteum: biosynthesis, structure and sequence analysis. EMBO J. 1984 Oct;3(10):2351–2354. doi: 10.1002/j.1460-2075.1984.tb02139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Morton J. J., Padfield P. L., Forsling M. L. A radioimmunoassay for plasma arginine-vasopressin in man and dog: application to physiological and pathological states. J Endocrinol. 1975 Jun;65(3):411–424. doi: 10.1677/joe.0.0650411. [DOI] [PubMed] [Google Scholar]
- Nilaver G., Rosenbaum L. C., Hellström K. E., Hellström I., Neuwelt E. A. Identification of neurophysin immunoreactivity in hypothalamus by a monoclonal antibody to a carcinoma cell surface antigen. Neuroendocrinology. 1990 May;51(5):565–571. doi: 10.1159/000125392. [DOI] [PubMed] [Google Scholar]
- Nilaver G., Rosenbaum L. C., Van Tol H. H., Shannon E. M., Hagman H. M., Zimmerman E. A., Neuwelt E. A. Immunological studies with a monoclonal antibody suggest a different conformation of neurophysin in parvicellular neurons of rat hypothalamus. Brain Res. 1990 Oct 8;529(1-2):302–308. doi: 10.1016/0006-8993(90)90841-x. [DOI] [PubMed] [Google Scholar]
- North W. G., Ware J., Maurer L. H., Chahinian A. P., Perry M. Neurophysins as tumor markers for small cell carcinoma of the lung. A cancer and Leukemia Group B evaluation. Cancer. 1988 Oct 1;62(7):1343–1347. doi: 10.1002/1097-0142(19881001)62:7<1343::aid-cncr2820620717>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Ovejera A. A., Houchens D. P. Human tumor xenografts in athymic nude mice as a preclinical screen for anticancer agents. Semin Oncol. 1981 Dec;8(4):386–393. [PubMed] [Google Scholar]
- Rosenbaum L. C., Nilaver G., Hagman H. M., Neuwelt E. A. Detection of low-molecular-weight polypeptides on nitrocellulose with monoclonal antibodies. Anal Biochem. 1989 Dec;183(2):250–257. doi: 10.1016/0003-2697(89)90475-2. [DOI] [PubMed] [Google Scholar]
- Rosenior J. C., North W. G., Moore G. J. Putative precursors of vasopressin, oxytocin, and neurophysins in the rat hypothalamus. Endocrinology. 1981 Oct;109(4):1067–1072. doi: 10.1210/endo-109-4-1067. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Legg A., Pettican P. Vasopressin stimulation of mouse 3T3 cell growth. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1284–1287. doi: 10.1073/pnas.76.3.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff M. R., Pert C. B. Small cell carcinoma of the lung: macrophage-specific antigens suggest hemopoietic stem cell origin. Science. 1984 Sep 7;225(4666):1034–1036. doi: 10.1126/science.6089338. [DOI] [PubMed] [Google Scholar]
- Russell J. T., Brownstein M. J., Gainer H. Biosynthesis of vasopressin, oxytocin, and neurophysins: isolation and characterization of two common precursors (propressophysin and prooxyphysin). Endocrinology. 1980 Dec;107(6):1880–1891. doi: 10.1210/endo-107-6-1880. [DOI] [PubMed] [Google Scholar]
- Sausville E., Carney D., Battey J. The human vasopressin gene is linked to the oxytocin gene and is selectively expressed in a cultured lung cancer cell line. J Biol Chem. 1985 Aug 25;260(18):10236–10241. [PubMed] [Google Scholar]
- Schmale H., Heinsohn S., Richter D. Structural organization of the rat gene for the arginine vasopressin-neurophysin precursor. EMBO J. 1983;2(5):763–767. doi: 10.1002/j.1460-2075.1983.tb01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C., Newman R. A., Sutherland D. R., Asser U., Greaves M. F. A one-step purification of membrane proteins using a high efficiency immunomatrix. J Biol Chem. 1982 Sep 25;257(18):10766–10769. [PubMed] [Google Scholar]
- Sherman T. G., McKelvy J. F., Watson S. J. Vasopressin mRNA regulation in individual hypothalamic nuclei: a northern and in situ hybridization analysis. J Neurosci. 1986 Jun;6(6):1685–1694. doi: 10.1523/JNEUROSCI.06-06-01685.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Gómez C. M., Plummer T. H., Jr Deglycosylation of asparagine-linked glycans by peptide:N-glycosidase F. Biochemistry. 1985 Aug 13;24(17):4665–4671. doi: 10.1021/bi00338a028. [DOI] [PubMed] [Google Scholar]
- Thomas G., Thorne B. A., Thomas L., Allen R. G., Hruby D. E., Fuller R., Thorner J. Yeast KEX2 endopeptidase correctly cleaves a neuroendocrine prohormone in mammalian cells. Science. 1988 Jul 8;241(4862):226–230. doi: 10.1126/science.3291117. [DOI] [PubMed] [Google Scholar]
- Verbalis J. G., Robinson A. G. Characterization of neurophysin-vasopressin prohormones in human posterior pituitary tissue. J Clin Endocrinol Metab. 1983 Jul;57(1):115–123. doi: 10.1210/jcem-57-1-115. [DOI] [PubMed] [Google Scholar]
- Worley R. T., Pickering B. T. Non-neuronal cells of rat hypothalamus in dissociated cell culture. Evidence that neurophysin modulates growth and DNA synthesis of non-neuronal cells. Cell Tissue Res. 1984;237(1):161–168. doi: 10.1007/BF00229212. [DOI] [PubMed] [Google Scholar]