Abstract
T lymphocytes can be redirected to recognize a tumor target and harnessed to combat cancer by genetic introduction of T‐cell receptors of a defined specificity. This approach has recently mediated encouraging clinical responses in patients with cancers previously regarded as incurable. However, despite the great promise, T‐cell receptor gene therapy still faces a multitude of obstacles. Identification of epitopes that enable effective targeting of all the cells in a heterogeneous tumor while sparing normal tissues remains perhaps the most demanding challenge. Experience from clinical trials has revealed the dangers associated with T‐cell receptor gene therapy and highlighted the need for reliable preclinical methods to identify potentially hazardous recognition of both intended and unintended epitopes in healthy tissues. Procedures for manufacturing large and highly potent T‐cell populations can be optimized to enhance their antitumor efficacy. Here, we review the current knowledge gained from preclinical models and clinical trials using adoptive transfer of T‐cell receptor‐engineered T lymphocytes, discuss the major challenges involved and highlight potential strategies to increase the safety and efficacy to make T‐cell receptor gene therapy a standard‐of‐care for large patient groups.
Keywords: T-cell receptor, Gene therapy, Cancer, Immunotherapy, T cell
Highlights
T‐cell receptor (TCR) gene therapy can retarget T cells to eliminate cancer.
Selection of the target antigen is the key to success.
High‐affinity TCRs can be identified from non‐tolerized T cell repertoires.
High‐throughput screening for antigens and TCRs will increase applicability.
Improved in silico and in vitro preclinical assays will increase safety.
Abbreviations
- ACT
adoptive cell therapy
- allo-SCT
allogeneic stem cell transplantation
- APC
antigen-presenting cell
- BLAST
basic local alignment search tool
- CAR
chimeric antigen receptor
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- EGFR
epidermal growth factor receptor
- GVHD
graft-versus-host disease
- HLA
human leukocyte antigen
- HPA
Human Protein Atlas
- IL
interleukin
- ImmTAC
Immune-mobilizing monoclonal TCR Against Cancer
- MDSC
myeloid-derived suppressor cell
- MHC
major histocompatibility complex
- MiHA
minor histocompatibility antigen
- MPO
myeloperoxidase
- NK
natural killer cell
- NKT
natural killer T cell
- PBMC
peripheral blood mononuclear cell
- PD-1
programmed cell death protein 1
- pMHC
peptide-MHC complex
- SCT
stem cell transplantation
- TAA
tumor-associated antigen
- TCM
central memory T cell
- TCR
T-cell receptor
- TEM
effector memory T cell
- TE
effector T cell
- TH
helper T cell
- TIL
tumor infiltrating lymphocytes
- TN
naïve T cell
- Treg
regulatory T cell
- TSCM
stem cell memory T cell
- WT1
Wilms tumor antigen 1
1. Introduction
Solid evidence that the immune system can eradicate cancer came from studies of bone marrow transplantation from healthy donors to patients with hematological malignancies, first performed in the late 1950s by E. Donnall Thomas (Thomas et al., 1957). Although the transfer of donor‐derived T cells with up to 107 different and unknown specificities might not have received approval if suggested for the first time today, as proof of its curative potential this treatment still remains part of the standard therapeutic regimen for patients with leukemia or lymphoma. The transfer of a heterogeneous donor‐derived T‐cell repertoire to a human leukocyte antigen (HLA)‐matched donor is, however, frequently associated with severe adverse effects caused by T‐cell recognition of polymorphic antigens expressed in normal tissues of the patient. The occurrence of so‐called graft‐versus‐host disease (GVHD) thereby limits the application of this treatment (reviewed by Jedema and Falkenburg, 2015). This safety hazard is omitted by use of the endogenous T‐cell repertoire to target cancer. In a series of pioneering studies, Rosenberg and colleagues were the first to demonstrate that, following in vitro expansion and adoptive transfer back to the patient, tumor‐infiltrating lymphocytes (TIL) can efficiently treat malignant melanoma (Dudley et al., 2008, 1994, 2011, 1988). However, the efficacy of TILs in other types of cancers is uncertain (TIL therapy is reviewed by Geukes Foppen et al., 2015). Another approach that relies on the natural T‐cell repertoire is the blocking of negative immune checkpoints by therapeutic antibodies. Recent results indicate that checkpoint inhibition can induce remarkable clinical responses, including durable complete remissions (Hodi et al., 2010; Topalian et al., 2012; and reviewed in Lesokhin et al., 2015; Sharma and Allison, 2015; Sledzinska et al., 2015). The curative potential of TIL therapy as well as immune checkpoint inhibition is, however, likely limited by T‐cell tolerance to tumor. This tolerance can be circumvented by equipping large numbers of patient‐derived T cells with immune receptors recognizing a defined tumor‐associated antigen (TAA) with high affinity. Recently, adoptive transfer of T cells genetically modified to express chimeric antigen receptors (CARs) consisting of CD19‐specific antibodies grafted onto T‐cell receptor (TCR) signaling domains have successfully induced long‐lasting remissions in patients with hematological malignancies (Brentjens et al., 2011, 2013, 2013, 2012, 2013, 2015, 2014; and reviewed in Whilding and Maher, 2015). However, CARs are limited to engaging native cell surface molecules. In spite of an extensive search, it has proven difficult to identify membrane targets that are cancer‐specific or, in addition to tumor, are expressed only on cells that are expendable to the host (Lamers et al., 2013; Morgan et al., 2010). Thus, re‐directing T cells with TCRs, which in principle are capable of recognizing peptides from all cellular proteins, might be an attractive alternative to allow application of T‐cell gene therapy to a wider selection of cancers.
The first studies describing that TCR gene transfer can redirect T lymphocytes and provide them with antitumor reactivity in vitro (Clay et al., 1999) paved the way for studies demonstrating that both CD8 and CD4 T lymphocytes redirected by TCR gene transfer are functional and capable of mediating potent antitumor effects in vivo using mouse models (Chamoto et al., 2004, 2001, 2006, 2005, 2003, 2005). Immunotherapy based on TCR gene transfer rapidly followed. The first published clinical trial of TCR gene therapy was conducted with melanoma patients who received T lymphocytes targeting the melanoma/melanocyte differentiation antigen MART‐1, but relatively modest clinical responses were observed (Morgan et al., 2006) (Table 1). The TCR was derived from a TIL clone that was obtained from a melanoma patient who demonstrated a nearly complete regression of the metastatic cancer after adoptive cell therapy (ACT) with TILs (Hughes et al., 2005). A higher affinity TCR against the MART‐1 antigen derived from the same patient (Johnson et al., 2006) provided more potent antitumor responses, but on‐target toxicities in healthy tissues occurred (Johnson et al., 2009). An affinity‐enhanced TCR targeting the cancer/testis antigen NY‐ESO‐1 (Robbins et al., 2008) proved, however, safe and effective in therapy of melanoma, synovial cell sarcoma and myeloma, and 55%, 61% and 80% of treated patients had objective clinical responses, respectively (Rapoport et al., 2015, 2011, 2015) (Table 1).
Table 1.
Published clinical trials with TCR‐engineered T cells and preclinical studies conducted with the corresponding TCRs. When not otherwise indicated, the patients received non‐myeloablative but lymphodepleting preconditioning prior to and high dose IL‐2 administration following ACT. CR, complete response; PR, partial response.
| Intended target antigen | Cancer | TCR | Experience from clinical trials | Preclinical studies | |||
|---|---|---|---|---|---|---|---|
| Objective clinical response | Adverse effects related to TCR specificity | Reference | In vitro | In vivo | |||
| MART‐1 (MLANA) | Melanoma | Natural, human (DMF4) | 13% (4/31) | None | (Morgan et al., 2006) (Johnson et al., 2009) | T‐cell activation and killing of melanoma cells in HLA‐A*02 restricted manner (Hughes et al., 2005) | |
| Natural, human (DMF5) | 30% (6/20) | On‐target toxicity on normal melanocytes (Skin rash (14/20), Uveitis (11/20), Hearing impairment (10/20)) | (Johnson et al., 2009) | T‐cell activation and killing of melanoma cells in HLA‐A*02 restricted manner (Johnson et al., 2006) | |||
| No CR or PR at 3 months but stable disease in 7/13 patients | Skin rash (4/13), Acute respiratory distress (2/14) | (Chodon et al., 2014)1 | |||||
| Natural, human (1D3HMCys) | N/A | Mild skin rash (1/1) (Cytokine release syndrome and death) | (van den Berg et al., 2015) | Killing of melanoma cells in HLA‐A*02 restricted manner, no alloreactivity observed against a panel of cell lines expressing common HLA alleles (Jorritsma et al., 2007) | |||
| gp100 (PMEL) | Melanoma | Natural, murine | 19% (3/16) | On‐target toxicity on normal melanocytes (Skin rash (15/16), Uveitis (4/16), Hearing impairment (5/16)) | (Johnson et al., 2009) | T‐cell activation upon T2 cells loaded with gp100 but not irrelevant peptide, melanoma cell killing (Johnson et al., 2009) | |
| p53 (TP53) | Variety of solid tumors | Natural, murine | 10% (1/10) | Not reported | (Davis et al., 2010) | No correlation between T cell activation and p53 expression level in the tumor, some normal tissues also recognized at lower levels (Cohen et al., 2005; Theoret et al., 2008) | |
| CEA (CEACAM5) | Colorectal cancer | Affinity enhanced, murine | 33% (1/3) | Severe inflammatory colitis (3/3) due to on‐target toxicity in colon | (Parkhurst et al., 2011) | T‐cell activation by HLA‐A2 positive tumor cell lines in CEA specific manner, recognition of other CEA family members with lower affinity (Parkhurst et al., 2009) | |
| NY‐ESO‐1 (CTAG1B) | Melanoma | Affinity enhanced, human | 55% (11/20) | None | (Robbins et al., 2011) (Robbins et al., 2015) | T‐cell activation and killing of NY‐ESO‐1 positive but not NY‐ESO‐1 negative tumor cells (Robbins et al., 2008) | |
| Synovial cell sarcoma | 61% (11/18) | None | (Robbins et al., 2011) (Robbins et al., 2015) | ||||
| Myeloma | 80% (16/20) | None | (Rapoport et al., 2015)2 | ||||
| MAGE‐A3 (MAGEA3) | Melanoma, synovial cell sarcoma, esophageal cancer | Affinity enhanced, murine | 56% (5/9) | Central nervous system toxicities (Necrotizing leukoencephalopathy and death (2/9), Parkinson‐like symptoms (1/9), Aphasia (1/9)) due to recognition of MAGE‐A12 in the brain | (Morgan et al., 2013) | T‐cell activation by MAGE‐A3 positive but not MAGE‐A3 negative tumor cell lines in HLA‐A*02 restricted manner, cross‐recognition of MAGE‐A12 (and to a lesser extend ‐A2 and ‐A6) among MAGE‐A family (Chinnasamy et al., 2011) | |
| Melanoma, myeloma | Affinity enhanced, human | N/A | Cardiac toxicity and death (2/2) due to cross‐recognition of an unrelated epitope from Titin (TTN) | (Linette et al., 2013) (Cameron et al., 2013) | T‐cell activation and killing of MAGE‐A3 positive but not MAGE‐A3 negative tumor cell lines in HLA‐A*01 restricted manner; no recognition of a variety of primary cells; cross‐recognition of MAGE‐A6 and –B18 among related peptides derived from MAGE family (Cameron et al., 2013) | Efficacy in a xenogeneic mouse model of ovarian cancer (Cameron et al., 2013) | |
| MAGE‐A4 (MAGEA4) | Esophageal cancer | Natural, human | 0% (0/10) | None | (Kageyama et al., 2015)3 | T‐cell activation and killing of MAGE‐A4 positive tumor cell lines in HLA‐A*2402 dependent manner (Hiasa et al., 2008) | |
Protocol included MART‐1 peptide pulsed DC vaccination.
Protocol included conditioning with high dose melphalan, autologous stem cell infusion and lenalidomide maintenance, but did not include IL‐2.
Protocol included sequential MAGE‐A4 peptide vaccination, but did not include lymphodepletive preconditioning nor IL‐2 administration.
Studies targeting other antigens raised, however, concerns about the safety of TCR gene therapy (Linette et al., 2013; Morgan et al., 2013; Parkhurst et al., 2011) and demonstrated the need for developing preclinical methods to evaluate potential toxicities. Here, we discuss the increasing experience gained from preclinical and clinical studies with adoptive transfer of TCR gene‐modified T cells, and emerging solutions to address the safety concerns as well as the need for enhanced therapeutic efficacy. The major challenges include 1) identification of antigens for safe and effective tumor‐targeting, 2) identification of TCRs with optimal affinity and desired specificity, 3) development of preclinical assays and models that give information about safety and efficacy before clinical use, 4) improvement of ex vivo genetic engineering, cell selection and expansion procedures to generate a highly potent T‐cell infusion product, and 5) strategies to prepare the host to increase the persistence and therapeutic effect of the adoptively transferred T cells in vivo (Figure 1).
Figure 1.
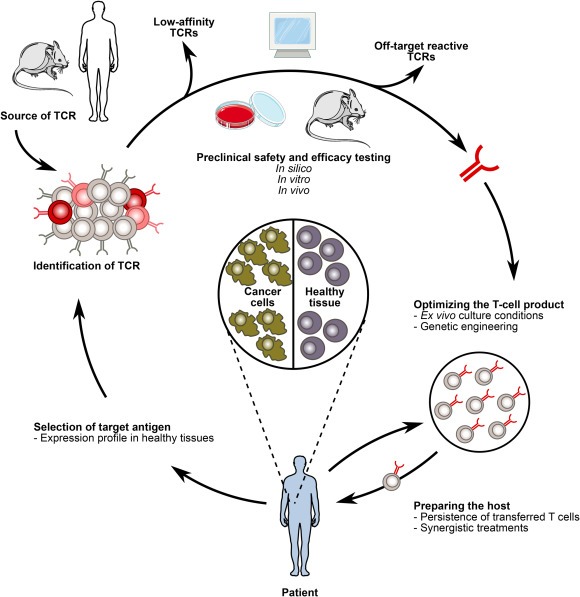
TCR gene therapy faces several challenges to fulfill its potential. 1. Selection of the target antigen is central for safe and efficacious TCR gene therapy. 2. An optimal affinity TCR specific for the selected target antigen should be isolated from the most suitable source. 3. Informative in silico, in vitro and in vivo preclinical methods should be developed to ensure safety and efficacy of the therapeutic TCR. 4. Clinical scale manufacturing procedures should be improved to deliver a highly potent infusion product. 5. The strategies for preparing the patient to enable proliferation, long‐term engraftment and optimal functionality of infused T cells can be optimized.
2. Identification of antigens for safe and effective tumor targeting
The choice of the target antigen forms the basis for the safety and efficacy of TCR‐based gene therapy, and as such remains the most central challenge in the development of this therapeutic strategy. Thorough knowledge of the expression profile of the selected target antigen is the key to success. A major question is which lessons can be learned with regard to on‐target toxicity from the clinical use of T cells genetically engineered with TCRs and CARs (off‐target toxicity is discussed in Sections 3 and 4). In retrospect, could any of the detrimental effects seen in some of these trials have been avoided with the knowledge we have today? And more importantly, can this knowledge be used to create strategies for target identification that reduce hazards in future trials?
An optimal TCR target would A) be expressed exclusively in tumor cells or, in addition to the tumor, be restricted to cell types that are non‐essential for the host to limit toxicity on healthy tissues, B) be present in tumor cells that are responsible for perpetuating growth in order to enable targeting of all tumor cells, C) be important for tumor cell survival to reduce the risk of tumor cell escape by downregulation of the target antigen, and D) be expressed in large patient groups and presented on frequent HLA molecules to enable therapy with “universal” TCRs. Clearly, very few of the antigens targeted thus far fulfill these criteria.
2.1. Targeting wild type proteins with cell type‐restricted expression
All TCRs and CARs that have been used in the clinic to date have exclusively targeted normal non‐mutated self‐antigens (thoroughly reviewed in Hinrichs and Restifo, 2013). Self‐antigens are attractive targets as they are expressed in all individuals, enabling the design of therapies for large patient groups. The major disadvantage is that self‐antigens are expressed also in healthy cells. To safely target a self‐antigen, its expression on healthy tissue must be limited to cells that are non‐essential to the host, at least during the time of treatment. This limitation is likely the reason why only a handful of targets have been utilized thus far. In fact, among the 20 ongoing TCR gene therapy studies listed on https://clinicaltrials.gov (search date 05/10/2015), only WT1 (Wilms tumor antigen 1) and thyroglobulin appear as target antigens that have not been published in a clinical setting before.
A prime example of safe and effective targeting of self is therapy of B‐cell malignancies with CAR T cells recognizing CD19 and anti‐CD20 antibodies, which have demonstrated that it is possible to live without normal B cells for prolonged periods of time (Solal‐Celigny, 2006). Also, targeting NY‐ESO‐1 with TCR‐transduced T cells was shown to be effective and safe (Rapoport et al., 2015, 2011, 2015), corresponding to the fact that the expression of NY‐ESO‐1 outside the tumor is limited to the major histocompatibility complex (MHC)‐negative normal cells in testis and placenta. In contrast, targeting several other self‐antigens has turned out to be problematic when high‐affinity immune receptors were used.
To what extent do we have reliable expression data in public databases that can be used to predict on‐target toxicities? For example, when high‐affinity TCRs reactive to MART‐1/Melan‐A (MLANA) and gp100 (PMEL) were used to treat melanoma, patients frequently experienced transient uveitis and hearing loss (Johnson et al., 2009). This is consistent with high expression of these antigens in retina reported in the Genevestigator (https://genevestigator.com/gv/) and BioGPS (http://biogps.org/) expression databases, and the well‐known presence of melanocytes in retina and inner ear. Similarly, autoimmune colitis caused by T cells genetically engineered with high‐affinity CEA‐specific TCRs (target sequence encoded by the CEACAM5 gene) (Parkhurst et al., 2011) was perhaps not surprising, given the known presence of this antigen in normal colonic mucosa (IST/Medisapiens (http://ist.medisapiens.com/), UniProt (http://www.uniprot.org/) and Human Protein Atlas (HPA) (http://www.proteinatlas.org/)). However, targeting the epitope in CEA did not result in reported adverse effects outside of colon although information in public databases indicate relatively high levels of CEACAM5 mRNA in a number of other tissues, including the respiratory tract (Genevestigator, IST/Medisapiens and HPA databases). This might indicate that some tissues can express target antigen without ensuing non‐tolerable toxicity.
Antibodies to ERBB2 were known to have cardiotoxic effects (Hervent and De Keulenaer, 2012) before the antigen was targeted by a CAR. This caused lethal effects in the only patient who was treated, probably due to reactivity in the lung (Morgan et al., 2010). Accordingly, ERBB2 mRNA expression levels in the heart and lung are reported to be above median in several databases. In contrast, the expression in the brain of the cancer/testis antigens MAGE‐A9 and MAGE‐A12 (Chinnasamy et al., 2011; Morgan et al., 2013) would have been difficult to predict based on currently available gene expression data. Both were unintentionally recognized by a TCR targeting MAGE‐A3. When searching for tissue expression of these genes in the IST/Medisapiens, none are listed, and no tissue staining data are available in HPA. However, BioGPS reports elevated levels of MAGE‐A9 mRNA in the trigeminal ganglion, and Genevestigator reports medium expression levels of both MAGE‐A9 and ‐A12 in brain tissue. A CAR targeting carboxy‐anhydrase‐IX (CAIX/CA9), an antigen frequently overexpressed in renal cell carcinoma, resulted in hepatotoxicity and cholangitis (Lamers et al., 2013). BioGPS and IST/Medisapiens report expression of CAIX/CA9 in many normal gastrointestinal tissues, particularly high in gastric mucosa and bile duct epithelial cells. However, no side effects were reported from gastric mucosa.
To summarize, the clinical studies performed highlight the need for a thorough understanding of the expression profile of the target epitope. Rigorous bioinformatic screening of expression databases, such as IST/Medisapiens, Genevestigator and BioGPS, containing information from thousands of samples across a wide variety of healthy tissues, is important (Figure 2). Consultation of more than one database provides a more comprehensive picture, since the number and quality of the datasets included in public databases vary. Even low levels of antigen expression in the brain or heart should raise a red flag, whereas higher levels might be tolerated in most other healthy organs. In fact, it is questionable whether the majority of antigens previously ranked as targets for potent ACT by the National Cancer Institute (Cheever et al., 2009) should be utilized therapeutically due to expression in multiple healthy tissues, as reviewed in (Hinrichs and Restifo, 2013). Thus, new strategies to search for better candidates among the roughly 23.000 available human proteins are warranted. However, searches for all genes specifically expressed in the tissue from which a tumor originates, while filtering away those expressed above defined threshold levels in others, is not straightforward. Moreover, data mining is complicated by the existence of multiple alternative identifiers for each gene. Reported lack of mRNA expression in healthy tissues in available databases does furthermore not preclude expression in rare, but vital, cell types. Micro‐array based expression data do furthermore not provide the sensitivity of RNA sequencing, which future expression databases may rely on. Treatment related factors such as inflammatory cytokines and lymphodepleting chemotherapy, might also have effects on the expression levels of the target antigen. Immunohistochemistry data on tissue arrays is critical, and is provided for some antigens in the HPA database, representing an important initiative (Uhlen et al., 2015). Since antibodies frequently cross‐react, it is however essential that the reagents are extensively validated. With better systems for high‐throughput antibody validation (Baker, 2015; Bradbury and Pluckthun, 2015; Holm et al., 2012), the quality of available immunohistochemistry data will gradually improve.
Figure 2.
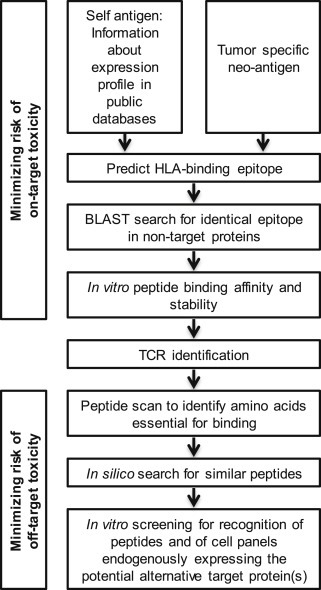
Suggested preclinical in silico and in vitro screening of antigen and cognate TCR to minimize on‐target and off‐target toxicity of TCR gene therapy.
2.2. Identifying candidate target peptides in self‐ and neo‐antigens
The quest for a safe and specific TCR target has only begun with the identification of a rational target protein. Next, peptides from the protein of interest that can bind defined HLA alleles and evoke a T‐cell response must be identified. Even if the bioinformatic screening indicates that the identified target antigen has the desired expression, this does not preclude existence of the candidate peptide epitope in other proteins. For example, the epitope recognized by the MAGE‐A3‐targeted TCR is also present in MAGE‐A9 (Morgan et al., 2013), and the epitope recognized by the NY‐ESO‐1‐targeted TCR is present in another cancer/testis antigen, LAGE‐1 (Rapoport et al., 2015). By running a BLAST search on peptide sequence homology in UniProt, homologous peptides derived from distinct proteins can be identified (Figure 2).
The identification of candidate epitopes in self‐antigens has traditionally relied on strategies for so‐called direct (or forward) and reverse immunology (reviewed in Hoppes et al., 2010; Kessler and Melief, 2007). Direct immunology departs from the reactive T‐cell clone, often patient‐derived T‐cell clones recognizing an unknown peptide on autologous HLA. The epitope can then be determined by different strategies, such as peptide elution from the MHC molecules and subsequent sequencing by mass spectrometry, screening on a tumor‐derived cDNA library expressed in antigen‐negative target cells followed by screening on peptide libraries, or screening against soluble pMHC (peptide‐MHC) libraries (HLA multimers). The low throughput of these assays may prohibit a systematic search for novel epitopes. Reverse immunology, on the other hand, departs from a known epitope, mostly synthetic peptide libraries tailored to bind a particular HLA‐allele by algorithms for in silico peptide prediction. The libraries are subsequently tested for their immunogenicity by induction of T cells against peptide‐pulsed antigen‐presenting cells (APCs). This approach allows a higher throughput. The main drawback is that false positives are common, as many of the responding T cells recognize epitopes that are not naturally processed in the target cells.
A comprehensive list of confirmed HLA‐class I epitopes from TAAs was recently compiled, including 230 proteins and 573 epitopes, of which 323 are melanoma‐associated (Andersen et al., 2012). Given that the first epitopes were identified in 1992, and that large efforts have been devoted to epitope discovery worldwide, the list is rather short. Mostly, target reactivity in autologous T‐cell repertoires has been used as readout for epitope discovery. It is likely that this represents a major limitation, since T cells that recognize self‐antigens with high affinity normally are depleted during negative selection in the thymus. A technology platform for high‐throughput discovery of immunogenic epitopes that are naturally processed was recently developed, using allo‐reactive T‐cell repertoires as tools for detection of self‐peptides presented on foreign HLA to avoid tolerance (Kumari et al., 2014). A large number of novel epitopes were discovered from the two leukemia‐associated differentiation antigens CD20 and myeloperoxidase (MPO), and T cells reactive with these epitopes efficiently and specifically killed primary target cells endogenously expressing the respective antigen, including leukemia cells. This study indicates that the self‐immunopeptidome is far more diverse than previously estimated and that such self‐antigens represent highly attractive targets for cancer immunotherapy.
In addition to self‐antigens, mutated gene products have emerged as an attractive group of targets (reviewed in Blankenstein et al., 2015; Schumacher and Schreiber, 2015). Neo‐antigens arise as a consequence of protein altering somatic missense mutations, or as products of abnormal fusion genes. Here, the expression pattern of the wild type protein is mostly irrelevant, as the neo‐antigen is tumor‐specific. However, neo‐antigens are also mostly patient‐specific, with the disadvantage that their utilization as targets for TCR‐based immunotherapy requires highly personalized strategies, which to date are not feasible with the current approaches. To move forward, high‐throughput methods for neo‐epitope and TCR identification are crucial.
The recent identification of neo‐epitopes targeted by T cells in patients has relied on computer algorithms that predict the binding affinity of peptides spanning the mutation to defined HLA alleles. These studies have convincingly shown that neo‐antigens are targeted both by CD8 and CD4 T cells in cancer patients (Cohen et al., 2015; Linnemann et al., 2015; Lu et al., 2013; Robbins et al., 2013; Tran et al., 2014), and that neo‐antigen reactive T cells constitute part of the responding T‐cell population in patients treated with immune checkpoint inhibition (Gubin et al., 2014; van Rooij et al., 2013). Nevertheless, it is striking that only few responses have been detected in spite of hundreds of identified mutations. A major question has been how reliably computer algorithms identify immunogenic epitopes. When utilizing allo‐reactive T cells as readout for epitope discovery in self‐antigens, it is worth noting that 74% of predicted epitopes were efficiently presented to cytotoxic T lymphocytes (CTLs) (Kumari et al., 2014). This indicates that algorithm‐based predictions reflect the self‐immunopeptidome more accurately than previously believed. In contrast, mass spectrometry is currently not sensitive enough to detect the full breadth of MHC‐bound peptides, although this might rapidly change with evolving technology. With the advent of high‐throughput sequencing, hundreds of expressed mutations can be rapidly identified and HLA‐binding candidate epitopes predicted. This, however, represents a considerable challenge with regard to throughput when determining T‐cell reactivity. In addition to the well‐established importance of high‐affinity binding to HLA (Harndahl et al., 2012), the immunogenicity of a peptide is determined by a multitude of factors, including level of antigen expression, peptide processing, trimming and transport in the cancer cells. These parameters are less well predicted by computer algorithms than binding of the peptide to HLA. Yet another important determinant of immunogenicity is the ability of the peptide to stabilize the pMHC complex, which can be evaluated in vitro (Harndahl et al., 2012; van der Burg et al., 1996). In fact, pMHC stability might be more important than binding affinity for the ability to stimulate immune responses (Harndahl et al., 2012).
3. Identifying T‐cell receptors with optimal affinity and desired specificity
Most TCRs can bind several pMHC complexes, which enables an individual to recognize a large variety of foreign peptide epitopes with a limited T‐cell repertoire (Mason, 1998; Wooldridge et al., 2012). In TCR gene therapy, recognition of epitopes other than the intended might lead to off‐target toxicity. The risk for toxicity depends on the quality of the negative selection that the TCR was exposed to, which is determined by the source of the TCR (Figure 3).
Figure 3.
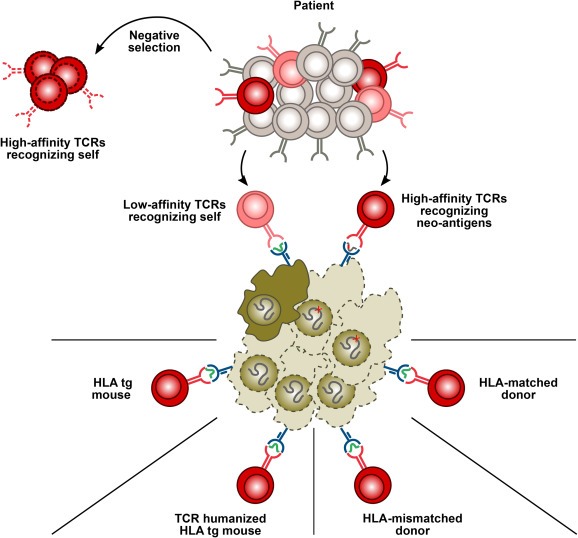
Identification of T‐cell receptors with desired specificity and optimal affinity. Maturing T cells undergo negative selection in the thymus during which T cells are exposed to the self‐peptidome presented on self‐MHC. T cells that recognize any of these peptide‐MHC complexes with high avidity are deleted. Thus, the autologous T‐cell repertoire (patient) is devoid of high‐avidity T cells recognizing self‐peptides. However, high affinity TCRs recognizing neo‐antigens in the context of self‐MHC can be found also in the autologous T‐cell repertoire. TCRs recognizing polymorphic antigens (minor histocompatibility antigens, MiHA) in the context of self‐MHC can be found in an HLA‐matched donor. High‐affinity TCRs recognizing self‐antigens can be obtained from non‐tolerized T‐cell repertoires that have not encountered the target peptide‐MHC complex during thymic selection. These include allogeneic TCRs from an HLA‐mismatched donor, human TCRs from TCR‐humanized HLA‐transgenic mice and murine TCRs from HLA transgenic mice.
Due to negative selection in the thymus, the autologous T‐cell repertoire is normally depleted for TCRs that recognize self‐epitopes with sufficiently high affinities to mediate clinical effects. This is evident from the low success rates obtained in clinical trials based on therapeutic vaccines with self‐epitopes (reviewed in Klebanoff et al., 2011; Rosenberg et al., 2004). The therapeutic efficacy of adoptively transferred T cells is, on the other hand, up to a certain threshold (Schmid et al., 2010) directly proportional to their avidity toward the desired target cells. The functional avidity of the interaction between a T cell and its target is predominantly dependent on the affinity and surface expression of the TCR, although co‐stimulatory adhesion molecules expressed by the T cell and ligands expressed by the target contribute (Johnson et al., 2006; Zeh et al., 1999). High affinity of the therapeutic TCR for its target, however, has to be weighed against potentially hazardous recognition of the target epitope when expressed outside the tumor tissue.
Autologous T‐cell repertoires are not tolerized to mutated peptide sequences during thymic selection. Thus, neo‐antigen‐specific TCRs are likely important for the clinical effects seen during treatment of melanoma with TILs (Cohen et al., 2015; Linnemann et al., 2015; Lu et al., 2013; Robbins et al., 2013; Tran et al., 2014), as well as with checkpoint‐inhibition (Gubin et al., 2014; van Rooij et al., 2013). Whether this immune response can be enhanced by equipping the patient's T cells with self‐derived neo‐antigen specific TCRs remains an exciting possibility. However, timelines and the logistic and financial requirements related to such an approach represent considerable hurdles. It is also not known to what degree the patient develops tolerance to neo‐antigens. To this end, a previous report demonstrated the presence of a T‐cell response to one out of 79 mutations in a patient with ovarian cancer, however the response disappeared with disease recurrence (Wick et al., 2014). Another study indicated that cancer shapes the immune response, as high‐avidity T cells recognizing a chronic myelogenous leukemia‐associated peptide were identified in patients in cytogenetic remission, whereas only low‐avidity T cells were found in patients with newly diagnosed disease (Molldrem et al., 2003).
3.1. TCRs from non‐tolerized human donors
In the setting of an HLA‐matched allogeneic stem cell transplantation (allo‐SCT), the donor T‐cell repertoire has normally not been exposed to polymorphic peptide sequences presented on recipient (identical to self) HLA. Such polymorphic peptides are encoded by single nucleotide polymorphisms that differ between donor and recipient, and are defined as minor histocompatibility antigens (MiHA) (reviewed by Jedema and Falkenburg, 2015). Consequently, MiHA‐specific T cells represent a source of high‐affinity TCRs with a high degree of specificity when expression of the MiHA is tissue‐restricted. Adoptive transfer of donor‐derived T cells genetically modified with a TCR recognizing the hematopoiesis‐specific MiHA HA‐1 is currently tested in a phase I trial including patients with hematological malignancies after allo‐SCT (Jedema and Falkenburg, 2015). The identification of tissue‐specific MiHAs restricted by commonly expressed HLA molecules has, however, proven challenging.
Since tolerance is self‐MHC restricted, high avidity allo‐reactive T cells recognizing tumor‐associated self‐antigens can be found in the T‐cell repertoire of an HLA‐mismatched individual (Gao et al., 2000, 2005, 1996, 1998, 2004, 2001). High affinity allogeneic TCRs targeting self‐antigens in the context of an HLA molecule foreign to the donor can be identified for a large repertoire of peptides to which the endogenous T‐cell repertoire is unresponsive (de Witte et al., 2006; Kumari et al., 2014). It was previously believed that allogeneic TCRs primarily react to the MHC molecule and therefore show extensive promiscuity with regard to peptide recognition (Huseby et al., 2005; Ignatowicz et al., 1996; Logunova et al., 2005). However, several recent studies have demonstrated allo‐reactive TCRs to possess a remarkable degree of peptide specificity (Abrahamsen et al., 2010, 2012, 2011, 2009, 2014, 2011, 2009, 1992, 2005, 2009 and reviewed in Felix and Allen, 2007; Housset and Malissen, 2003). To limit a dominant anti‐allogeneic immune response that obscures the detection of specific T cells, autologous dendritic cells (DCs) transfected with mRNA encoding a single foreign HLA molecule loaded with the target antigen can be used for efficient T‐cell priming (Stronen et al., 2009). T cells reactive for the desired target can then be directly identified by HLA multimers carrying the target peptide (Abrahamsen et al., 2010; Amir et al., 2011b; Kumari et al., 2014; Pittet et al., 2006; Wilde et al., 2009). Allogeneic TCRs have undergone negative selection against the whole human peptidome presented on up to 6 different MHC class I and 6 MHC class II molecules. This could potentially make allo‐reactive TCRs safer than TCRs derived from HLA transgenic mice, which have been selected against a single HLA molecule only, or affinity‐enhanced TCRs, which have not been selected against any human HLA molecule at all.
3.2. TCRs from humanized mice
Since the murine TCR repertoire is non‐tolerant to many human antigens, mice transgenic for an HLA‐molecule and immunized with the intended human target antigen provide an alternative source for identifying high affinity TCRs that recognize human self‐antigens (Chinnasamy et al., 2011; Cohen et al., 2005; Johnson et al., 2009; Parkhurst et al., 2009; Stanislawski et al., 2001; Theobald et al., 1995). Since mouse CD8 cannot efficiently interact with the human α3 domain of HLA, TCRs derived from HLA transgenic mice are often of high affinity and CD8‐independent in their recognition of the pMHC complex, and can redirect both CD8 CTLs and CD4 helper T (TH) cells to a desired tumor antigen (Kuball et al., 2005). A drawback with murine TCRs is the potential immunogenicity when transferred to humans. Development of antibodies against the variable, but not constant, parts, of natural murine TCRs was observed in a subset of responding as well as non‐responding patients treated with p53 or gp100 targeting T cells (Davis et al., 2010). However, the elicitation of a host immune response against the adoptively transferred T cells was not associated with shorter persistence of the transduced T cells or lesser clinical responses to the therapy. Anti‐mouse antibodies were also observed in 2 out of 3 patients treated with a CEA‐reactive TCR, although it is unclear whether this impacted the antitumor response (Parkhurst et al., 2011). Nevertheless, a host immune response against the therapeutic T cells remains a concern that can potentially affect clinical outcomes and thus limit the use of non‐human sequences.
Generation of a transgenic mouse carrying the entire human TCR α and β loci in addition to an HLA transgene has made it possible to screen a non‐tolerized human TCR repertoire in an antigen‐negative background to obtain human TCRs that recognize self‐antigens with high affinity (Li et al., 2010; Obenaus et al., 2015). MART‐1‐reactive CD8 T‐cell clones identified by this approach showed similar TCR usage as T‐cell clones identified from individuals with autoimmune vitiligo or melanoma. However, as in the case of murine TCRs, TCRs from a humanized mouse have passed negative selection only against one HLA molecule presenting the mouse peptidome and might thus possess an increased risk of cross‐reactivity to human antigens other than the intended target.
3.3. Affinity‐enhanced TCRs
Since the affinity of wild type TCRs is generally very low, in the micromolar range, the development of in vitro engineered, affinity‐enhanced TCRs seems a powerful and attractive approach to increase the efficacy of TCR gene therapy. Libraries covering different combinations of amino acid substitutions in a given TCR have been generated by random mutagenesis (Kieke et al., 1999) or structure‐based design (Haidar et al., 2009; Malecek et al., 2014; Pierce et al., 2014) and displayed on phage, yeast or mammalian T‐cell systems for screening of high affinity variants (Chervin et al., 2008; Holler et al., 2000; Kessels et al., 2000; Li et al., 2005b; Malecek et al., 2013). Single or dual amino acid substitutions in the antigen‐binding region of the TCR may increase the affinity even to the picomolar range. However, increasing TCR affinity above a certain threshold does not necessarily translate to increased functional response to target cells (Cameron et al., 2013; Thomas et al., 2011). An affinity‐enhanced TCR against NY‐ESO‐1 (Robbins et al., 2008) has provided encouraging tumor responses without adverse effects related to off‐target or on‐target toxicities (Rapoport et al., 2015, 2011, 2015). However, in vitro mutagenesis and selection of artificial, high‐affinity TCR variants that have not gone through natural negative selection bears the risk of loosing specificity and generating unpredictable cross‐reactivities to other self‐antigens (Cameron et al., 2013; Holler et al., 2003; Linette et al., 2013; Zhao et al., 2007).
4. Towards greater safety
Compared to radiation and chemotherapy, the current first line cancer treatments, cellular immunotherapy has the great potential to be precisely targeted with minimal impact on healthy tissues. However, fulfilling this potential requires reliable preclinical assays to evaluate possible recognition of unintended target cells. In addition to recognizing the intended target epitope in tissues outside the tumor (discussed in Section 2), a therapeutic TCR might be able to recognize pMHC complexes other than, but structurally similar to, the intended therapeutic target. Whether this recognition is clinically significant depends on the functional avidity of the TCR‐modified T cells for the unintended target cells and the expression profile of the alternative target antigen. Off‐target toxicity caused by cross‐recognition of a distinct peptide in the context of the target MHC has led to fatality in a recent clinical trial (Cameron et al., 2013; Linette et al., 2013). In theory, an additional possibility exists that a therapeutic TCR unpredictably recognizes a peptide in the context of a foreign MHC molecule. The fact that this phenomenon as of yet has not been reported in the clinic, might suggest that the risk for this type of cross‐reactivity is low.
4.1. Preclinical in vivo models in evaluating the safety of TCR gene therapy
Before proceeding to clinical trials new drugs are generally tested in a series of well‐defined preclinical in vivo assays. Murine tumor models have also been informative in revealing basic mechanisms and confirming the feasibility and efficacy of TCR gene therapy (Chamoto et al., 2004; Kessels et al., 2001; Morris et al., 2005). However, there is an increasing awareness that care should be taken in many situations when inferring results from murine models to the clinical setting. For instance, it was recently demonstrated that the major murine models of inflammation poorly reflect human inflammatory diseases (Seok et al., 2013). Murine tumor models, especially those based on transplantation, have limitations in replicating human cancer (reviewed in Olive and Tuveson, 2006). In vivo testing of cellular immunotherapy, and therapeutic TCRs in particular, might be even more complicated in animal models. This is due to the fact that 1) a TCR intended for therapeutic use must recognize antigen in the context of human MHC, 2) antigenic repertoires are species‐specific, and 3) incompatibility between murine and human MHC leads to xenogeneic GVHD from human T cells attacking murine cells (immunocompromised mouse) or rejection of infused human cells by the murine immune system (immunocompetent mouse). The adverse effects observed in recent clinical trials raise the question whether testing in preclinical models could have revealed the observed toxicities in advance, as in fact only one of the TCR gene therapy clinical trials performed was preceded by in vivo testing (Table 1).
Immunocompromised mice have for decades been used in cancer research, but a major breakthrough came with the development of the NOD/SCID/IL2R‐γ−/− mice, which allow for better engraftment of human cells (reviewed in Zhou et al., 2014b). Xenogeneic GVHD is reduced in a strain that additionally lacks β2 microglobulin and thus antigen‐presentation on MHC class I. While these strains can verify the efficacy of TCR‐transduced human peripheral blood mononuclear cells (PBMCs) in eradicating various implanted human tumors, they do not allow evaluation of simultaneous targeting of the antigen in healthy tissues as the expression of the human target antigen is restricted to the engrafted tumor cells.
Some information about the possibility of on‐target toxicity can be achieved in transgenic mice expressing the human antigen under the control of the endogenous or a physiologically relevant promoter or by targeting the mouse homolog of the human target antigen. Autoimmune colitis that occurred in patients treated with a TCR against CEA (Parkhurst et al., 2011) was also observed in CEA transgenic mice after adoptive transfer of T cells from CEA immunized wild type donor mice (Bos et al., 2008). Similarly, destruction of melanocytes observed in the eye of patients treated with T cells targeting melanocyte differentiation antigens (Johnson et al., 2009) also occurred in mice treated with gp100‐specific T cells along with a potent antitumor regimen (Palmer et al., 2008). In this model, the severity of ocular autoimmunity correlated with the efficacy of tumor eradication and, as in patients, was treatable with local administration of steroids without impairing the antitumor effect (Palmer et al., 2008).
However, for in vivo assessment of possible toxicities by intended therapeutic TCRs the antigens must be presented on the targeted HLA molecule. This can be achieved in immunocompromised mice transgenic for human HLA, which allow engraftment of TCR‐transduced human PBMCs. An immunocompetent alternative is to use mice transgenic for a chimeric MHC consisting of the antigen‐presenting part of human HLA fused to the co‐receptor‐interacting, transmembrane and intracellular domains of murine MHC (Newberg et al., 1996; Vitiello et al., 1991). This fusion molecule enables antigen‐presentation to mouse splenocytes transduced with the therapeutic TCR. Such models can provide some information on on‐target toxicity in situations where the tissue distribution and sequence of the mouse homolog of the human target epitope are identical between the species. This is the case with WT1. HLA‐A2 transgenic mice on the NOD/SCID background were used to demonstrate that PBMCs transduced with a WT1‐targeting TCR with increased functional avidity compared with PBMCs expressing the parental TCR did not induce toxicity to normal tissue despite greater expansion and improved persistence (Kuball et al., 2009). Such models can, however, not be used to assess possible off‐target reactivities against the whole range of the human peptidome. Since CARs do not require antigen‐presentation on HLA, non‐human primates, which more closely resemble humans in their antigen repertoire and gene expression profiles, might provide a relevant in vivo model for assessing safety of therapeutic CARs (Berger et al., 2015).
There are also examples where preclinical in vivo models demonstrate that results from murine experiments cannot be directly inferred to the situation observed in the clinic. Targeting human ERBB2 with a CAR in transgenic mice expressing the target antigen in brain and mammary gland did not result in autoimmune toxicity in these tissues, in spite of antitumor efficacy (Wang et al., 2010). Combination therapy of the ERBB2‐targeted CAR with anti‐PD‐1 (programmed cell death protein 1) antibodies enhanced the antitumor efficacy but still no autoimmune toxicities were observed in the same transgenic mouse model (John et al., 2013). However, fatal toxicity occurred in a clinical trial with a CAR targeting ERBB2, probably due to ERBB2 expression in lung epithelial cells (Morgan et al., 2010).
It is important to understand the limitations of in vivo models in providing information relevant to clinical safety of potential therapeutic TCRs. Despite the constant improvement of in vivo models that partly overcome the barriers of species‐specific MHC and xenoreactivity, differences in antigen repertoires between species will always prevent assessment of the full range of off‐target toxicities. Thus, carefully designed, comprehensive and biologically relevant in silico and in vitro strategies might provide more information than even the most sophisticated in vivo models.
4.2. In silico and in vitro strategies to predict the risk of TCR‐mediated off‐target toxicity
Generally, the TCRs that have proceeded to clinical trials have mostly been tested for their efficacy in recognizing and mediating killing of a limited set of cell lines, and occasionally primary cells, in a target antigen and/or HLA specific manner (Table 1). A recent study paved the way for a more stringent in vitro strategy to predict potential cross‐reactivities (Cameron et al., 2013). The approach was used to retrospectively identify the cause of cardiac toxicity by an HLA‐A1‐restricted MAGE‐A3‐targeted TCR as recognition of an alternative peptide derived from the muscle protein Titin. By sequential substitution of each position in the target peptide with an alanine or glycine the amino acid residues critical for TCR recognition were revealed. Peptides sharing this essential motif were identified by an in silico search (http://prosite.expasy.org/scanprosite/), loaded onto HLA‐A1 positive target cells and screened for recognition by the MAGE‐A3 TCR. Natural processing and recognition of endogenously expressed antigen was confirmed in physiologically relevant cell cultures (Cameron et al., 2013). This study demonstrates that assays such as peptide scanning and the screening of biologically relevant specialized cell cultures can reveal recognition of alternative peptides by candidate therapeutic TCRs (Figure 2). In another study each investigated TCR was able to recognize multiple peptides, but all identified peptides possessed recognition motifs closely resembling the known antigen of the corresponding TCR (Birnbaum et al., 2014). This suggests that the likelihood of degenerate recognition of completely unrelated peptides with no sequence homology to the intended target can be considered small.
4.3. Strategies to limit toxicities from TCR gene therapy
Pairing of the introduced TCR chains with the endogenous ones creates a possible safety concern by forming dimers with unpredictable reactivities (van Loenen et al., 2010). Such mispairing has lead to lethal consequences in a mouse model (Bendle et al., 2010), but has thus far not been observed in clinical trials (Rosenberg, 2010). Preferential dimerization of the introduced TCR chains can be promoted by use of constant regions from murine TCRs (Cohen et al., 2006), by introduction of an extra cysteine bridge between the exogenous TCR chains (Cohen et al., 2007; Kuball et al., 2007), by equimolar expression of both chains of the transgenic TCR (Szymczak et al., 2004) or by silencing the expression of the endogenous TCR. These approaches additionally enhance TCR surface expression and effector function. Downregulation of the endogenous TCR has been obtained by introducing sequences encoding small‐interfering RNAs (siRNA) into the vector coding for the TCR transgene, which itself escapes recognition by the siRNAs due to codon optimization (Ochi et al., 2011; Okamoto et al., 2009). Silencing of the endogenous TCR by RNA interference was shown to reduce lethal GVHD in a mouse model (Bunse et al., 2014). Disruption of the endogenous TCR expression has also efficiently been achieved by zinc finger nucleases (ZFNs) (Provasi et al., 2012) and transcription activator‐like effector nucleases (TALENs) (Berdien et al., 2014; Torikai et al., 2012). The novel CRISPR (clustered regularly interspaced short palindromic repeats) technology (reviewed in Doudna and Charpentier, 2014) might revolutionize genome editing also in T cells for adoptive immunotherapy. It has further been shown that expression of a dominant TCR intrinsically suppresses the surface expression of the endogenous one (Hart et al., 2008).
Transient expression of the therapeutic TCRs by mRNA electroporation (Barrett et al., 2011) or from non‐integrating expression vectors limits the exposure of the patient to the TCR. Although this approach does not prevent acute toxicities, it might represent a safer mode for testing of new TCRs than stable expression from viral vectors. However, it is questionable whether even repeated infusions of T cells transiently expressing therapeutic TCRs would provide sufficient clinical efficacy.
An emerging alternative to redirect T cells to eradicate cancer cells are ImmTACs (Immune‐mobilizing monoclonal TCRs Against Cancer), soluble chimeric proteins comprising an affinity enhanced monoclonal TCR recognizing a tumor‐associated epitope fused to a humanized CD3‐specific single‐chain antibody fragment for T‐cell activation (Liddy et al., 2012). These bi‐specific bridging molecules were shown to effectively engage the natural T‐cell activation pathway and to potently suppress tumor growth in mice (Bossi et al., 2014; Liddy et al., 2012). An ImmTAC specific for gp100 melanoma antigen is currently entering a phase II clinical trial (ClinicalTrials.gov identifiers NCT01211262 and NCT02535078). Due to their limited lifespan and generic nature these soluble TCR molecules might provide a safer alternative for initial testing of therapeutic TCRs than personalized TCR‐modified T cell products with potential permanent engraftment.
Dual targeting systems might provide an alternative strategy to reduce damage to healthy tissues that express the target antigen. T cells expressing a receptor that is suboptimally activated by recognition of the target antigen are functionally rescued in the tumor by a co‐stimulatory receptor engaging a second tumor antigen, but not in healthy tissues expressing either antigen alone (Kloss et al., 2013).
Conditional suicide genes introduced along with the therapeutic TCR can increase the safety of ACT by enabling selective destruction of the infused T cells in the event of toxicities. An inducible caspase 9 effectively eliminated more than 90% of the modified T cells in recipients of haploidentical SCT that developed GVHD (Di Stasi et al., 2011). Introduction of inert cell surface molecules that can be targeted by clinically approved monoclonal antibodies can mediate ablation of the modified cells through antibody‐dependent cellular cytotoxicity, and simultaneously serve as markers of transduction efficiency and in vivo cell tracking, as well as be utilized for ex vivo selection purposes. Suggested marker/suicide genes include a truncated epidermal growth factor receptor (EGFR) molecule (Wang et al., 2011) and a chimeric protein comprised of target epitopes from CD34 and CD20 (Philip et al., 2014). Although utilization of suicide genes might not provide sufficiently rapid reversal of acute toxicity on vital organs, they might prove beneficial in cases of toxicity on non‐essential organs.
A very recent possibility to pharmacologically control activation of CAR T cells by an “ON‐switch” might provide an attractive possibility that could be extended to TCR‐engineered T cells. A split CAR receptor that requires a heterodimerizing small molecule to assemble enables gradual titration of activity to required therapeutic levels (Wu et al., 2015). This new opportunity might provide the control required to safely and rapidly bring T cells genetically engineered with new immune receptors to the patient.
5. Optimizing the product
T cells undergo progressive differentiation from naïve T cells (TN) into stem cell‐like memory (TSCM), central memory (TCM), effector memory (TEM) and the terminally differentiated effector (TE) T cell subsets, with concomitant loss of proliferative and secondary lymphoid organ homing capacity while accumulating effector function (Buchholz et al., 2013; Gattinoni et al., 2011; Gerlach et al., 2013; Sallusto et al., 1999; and reviewed in Restifo and Gattinoni, 2013). Several clinical trials have demonstrated a clear correlation between the therapeutic efficacy of adoptively transferred T cells with their ability to expand and persist long‐term in vivo (Johnson et al., 2009; Morgan et al., 2006; Robbins et al., 2004; Rosenberg et al., 2011). This suggests that infusion of T cells at earlier stages of differentiation may have greater therapeutic potential, which is strongly supported by several preclinical models. Acquisition of an effector phenotype, though providing CD8 T cells with the ability to effectively lyse tumor cells in vitro, impaired their antitumor efficacy in vivo (Gattinoni et al., 2005b). T cell infusion products derived from TN cells (Hinrichs et al., 2009), TSCM (Cieri et al., 2013; Gattinoni et al., 2011) or TCM cell populations (Berger et al., 2008; Klebanoff et al., 2005; Yang et al., 2011) have been shown to possess increased proliferative capacity, persist longer and mediate superior antitumor responses compared to T cells derived from more differentiated populations (TEM and TE cells) in several in vivo models. Long‐term persistence of the adoptively transferred T cells requires homing to secondary lymphoid organs, where they proliferate and replenish the pool of circulating T cells, which then enter the tumor sites (Klebanoff et al., 2005). T‐cell homing to secondary lymphoid organs is mediated by CCR7 and CD62L, which are lost during maturation to TEM cells. These observations suggest that protocols for genetic modification and ex vivo expansion of T cells should be optimized to yield T‐cell infusion products with predominantly TSCM and TCM phenotype to ensure homing, long‐term persistence and clinical efficacy.
5.1. Delivering the gene while limiting differentiation
TCR‐modified T lymphocytes for ACT are most commonly generated from an unselected heterogeneous pool of PBMCs, from which T cells are activated by crosslinking anti‐CD3 and/or anti‐CD28 antibodies, transduced with γ‐retroviral or lentiviral vectors encoding the gene of interest and expanded ex vivo in the presence of the cytokine interleukin‐2 (IL‐2).
Gamma‐retroviral vectors are most widely used in the clinical protocols for the delivery of TCRs (Chodon et al., 2014, 2010, 2014, 2009, 2015, 2006, 2013, 2011, 2011, 2015). Lentiviral vectors, relative to retroviral vectors, have the advantage that they can transduce non‐dividing cells (Naldini et al., 1996), which might enable production of gene‐modified T lymphocytes in less differentiated memory states (Bobisse et al., 2009, 2010, 2008, 2010). They have been used in clinical trials without adverse effects related to gene transfer (Kalos et al., 2011; Rapoport et al., 2015). Lentiviral vectors might also have a higher efficiency for genetic engineering of human (but not murine) T cells and be more resistant to gene silencing as compared to γ‐retroviral vectors (reviewed in Morgan and Kakarla, 2014).
IL‐2 effectively stimulates T‐cell proliferation but also promotes differentiation of CD8 T cells into TEM and TE states (Cha et al., 2010; Hinrichs et al., 2008). Several modifications to the prevailing ex vivo expansion protocols of T cells have been suggested to enable in vitro proliferation while limiting differentiation. Expansion of T cells in the presence of homeostatic cytokines, such as IL‐7 and IL‐15 (Cha et al., 2010; Cieri et al., 2013; Gomez‐Eerland et al., 2014; Xu et al., 2014), IL‐21 (Chapuis et al., 2013; Hinrichs et al., 2008; Li et al., 2005a), IL‐12 (Rubinstein et al., 2015) or IL‐12 together with IL‐7 or IL‐21 (Yang et al., 2013), have been suggested superior to IL‐2 in inducing a TSCM/TCM phenotype. This phenotype is associated with increased capacity to expand, survive and persist in vivo, to migrate into secondary lymphoid organs, and to perform antitumor activity in mouse tumor models. An alternative possibility to limit differentiation of cultured T cells is pharmacological manipulation of signaling pathways associated with cellular differentiation. Induction of Wnt‐β‐catenin signaling was shown to arrest differentiation of CD8 T cells and to promote generation of the TSCM phenotype (Gattinoni et al., 2009, 2011). Moreover, pharmacologic inhibition of AKT enabled expansion of TILs with properties characteristic of a memory T‐cell phenotype, leading to enhanced in vivo persistence and antitumor efficacy (Crompton et al., 2015). T cells with potent antitumor effects have also been generated from TCR‐transduced hematopoietic stem cells (Vatakis et al., 2013) and induced pluripotent stem cells (Lei et al., 2011).
5.2. CD8 versus CD4 T cells
Whereas the primary focus of cancer immunotherapy has traditionally been on the CD8 CTLs, the contribution of CD4 T cells to tumor immunity and to therapeutic efficacy of ACT is poorly understood and might be more important than thus far appreciated. Whereas CD4 regulatory T cells (Treg) suppressed antitumor immune responses, CD4 TH cells augmented CD8 CTL activity in a mouse model (Antony et al., 2005). Tumor Treg are associated with reduced survival in human ovarian carcinoma (Curiel et al., 2004), endogenous peripheral Treg are negatively associated with clinical response to ACT (Yao et al., 2012) and the proportion of CD4 T cells in the infusion product inversely correlated with tumor regression in TIL treated melanoma patients (Prieto et al., 2010). Neo‐antigen reactive CD4 T cells can be found in human tumors (Linnemann et al., 2015; Tran et al., 2014). T‐cell populations highly enriched for neo‐antigen reactive CD4 TH1 cells efficiently mediated tumor regression in a patient with cholangiocarcinoma (Tran et al., 2014) and NY‐ESO‐1 specific CD4 T cell clones have been shown to mediate a durable clinical remission in a melanoma patient (Hunder et al., 2008). These results suggest that CD4 TH cells, in addition to augmenting CD8 CTL activity, might also convey a direct effector function in tumor eradication (reviewed in Haabeth et al., 2014). This is supported by the observation that tumor‐specific cytotoxic CD4 TH1 cells are able to eradicate established tumors in mouse models even in the absence of CD8 T, B, natural killer (NK) and NKT cells (Quezada et al., 2010; Xie et al., 2010). However, the presence of CD4 T cells in TIL infusion product seems not to be necessary for objective responses in melanoma patients (Dudley et al., 2010).
Very few MHC class II restricted epitopes from TAAs have been identified. Therefore, the concomitant re‐directing of CD4 and CD8 T cells with TCRs specific for MHC class I‐restricted epitopes has emerged as a viable alternative strategy. By this approach, CD8 CTL function and induction of long‐term memory can be augmented (Chhabra et al., 2008; Frankel et al., 2010; Kessels et al., 2006; Kuball et al., 2005; Morris et al., 2005; Ray et al., 2010; Willemsen et al., 2005). The optimal ratios of CD4 and CD8 subsets in T‐cell infusion products remain to be determined. Development of clinical‐grade cell selection methods would advance the manufacture of therapeutic T‐cell infusion products enriched for T cells at earlier stages of differentiation, having an ideal composition of CD8 and CD4 subsets and depleted of inhibitory Treg.
5.3. Modifying the TCR to improve expression
The level of the TCR on the cell surface makes an essential contribution to the functional avidity of the T cell towards its target. The expression level of the introduced TCR is efficiently increased by optimization of the codon usage in the TCR transgene (de Witte et al., 2008; Kerkar et al., 2011b; Scholten et al., 2006). The concentration of CD3, particularly the ζ component, is limiting for the assembly and surface expression of the TCR complex. Transduction of T cells with the CD3 chains alongside the TCR lead to enhanced surface expression (Ahmadi et al., 2011). Certain structural modifications in the constant parts of the TCR have been shown to increase the expression level, stability and signaling efficiency of the introduced TCR and to simultaneously decrease pairing with the endogenous TCR chains. Changing the constant parts of the human TCR chains, or essential amino acid residues therein, into their murine counterparts leads to preferential pairing of the introduced TCR chains and to a more stable association with the CD3 complex (Bialer et al., 2010; Cohen et al., 2006; Goff et al., 2010; Sommermeyer and Uckert, 2010). Minimally instead of completely murinized TCRs have the advantage of being potentially less immunogenic (Bialer et al., 2010; Sommermeyer and Uckert, 2010). Introduction of an additional disulfide bond between the constant domains of the TCR leads to higher surface expression levels of the introduced TCR (Cohen et al., 2007; Kuball et al., 2007; van Loenen et al., 2010). Furthermore, removal of defined N‐glycosylation sites in the TCR constant regions was shown to enhance the functional avidity of transduced T cells (Kuball et al., 2009).
6. Preparing the host
6.1. Lymphodepletion
Non‐myeloablative lymphodepletion, either by radiotherapy and/or chemotherapy, can significantly prolong the persistence of adoptively transferred T cells in vivo and profoundly improve the efficacy of adoptive T‐cell therapies (Dudley et al., 2002, 2002, 2005, 2008, 2004). The necessity of lymphodepleting pretreatment for the therapeutic efficacy of ACT has also been demonstrated in mouse models (de Witte et al., 2008; Dummer et al., 2002; Rosenberg et al., 1986). A recent clinical trial with MAGE‐A4‐reactive TCR‐engineered T cells in patients with esophageal cancer was performed with a regimen not including lymphodepletive pretreatment nor IL‐2 administration, but rather sequential MAGE‐A4 vaccinations (Kageyama et al., 2015). No tumor regression was observed even though the adoptively transferred T cells persisted long‐term in circulation, trafficked to tumor sites and maintained ex vivo tumor reactivity (Kageyama et al., 2015). The results suggest that lymphodepletion contributes not only to T‐cell expansion and survival but has additional beneficial mechanisms. The beneficial effect of lymphodepletion might depend on i) elimination of immunosuppressive host cells, such as Treg and myeloid‐derived suppressor cells (MDSCs) (North, 1982; Yao et al., 2012), ii) on increased availability of homeostatic cytokines, such as IL‐7 and IL‐15, due to reduced competition from endogenous lymphocytes (Dudley et al., 2008; Gattinoni et al., 2005a) and iii) on activation of APCs by release of tumor antigens, increasing systemic levels of inflammatory cytokines as well as promoting translocation of microbial products across damaged mucosal membranes (Huang et al., 2011; Paulos et al., 2007; Radojcic et al., 2010). However, lymphodepletion by itself might not be a prerequisite for successful adoptive immunotherapy. It has been suggested that targeted therapies that replicate the key elements of lymphopenia, depletion of Treg with or without simultaneous introduction of IL‐7, could be superior to lymphodepletion in enhancing the antitumor effects of adoptive immunotherapy without the toxicities associated with lymphodepletion (Cui et al., 2009; Matsushita et al., 2008).
6.2. IL‐2 administration and alternative approaches to improve expansion and persistence of adoptively transferred T cells
Systemic administration of recombinant IL‐2 is included in most clinical protocols to support the expansion and persistence of adoptively transferred T cells. However, it is known to cause significant toxicity, which limits its use to a few days after ACT. Attempts to increase the proliferation and persistence of the transferred T cells without the toxic effects of systemic IL‐2 have been made by introducing genes into the T cells that code for cytokines, their receptors, or factors that inhibit apoptosis or provide cytokine independent autocrine growth signals. Transduction of the IL‐2 gene into TILs, however, did not increase their persistence in vivo or their clinical effect (Heemskerk et al., 2008). Forced expression of IL‐7 receptor was able to restore the responsiveness of terminally differentiated T cells to IL‐7 (Vera et al., 2009), but the effect of such approaches in vivo remains to be elucidated. Co‐expression of the anti‐apoptotic proteins Bcl‐2 or Bcl‐xL along with a tumor specific TCR prevented apoptosis of the modified T cells upon IL‐2 withdrawal both in vitro and in vivo without altering the phenotype of the T cells (Kalbasi et al., 2010).
Mitochondrial respiration through reactive oxygen species signaling and the mTOR pathway appear critical for antigen‐specific T‐cell activation and memory development (Araki et al., 2009; Sena et al., 2013; van der Windt et al., 2012). Manipulation of these pathways might promote persistence and effector function of adoptively transferred T cells (Okoye et al., 2015; Pearce et al., 2009).
6.3. Enhancing the antitumor efficacy of TCR‐transduced T cells
The function of adoptively transferred T cells can further be supported by simultaneous targeting of the immunosuppressive tumor microenvironment or by introduction of tumor‐homing receptors to promote T‐cell localization into the tumor. Forced expression of the chemokine receptor CCR4 in otherwise CCR4‐negative effector T cells was shown to enhance their migration to CCL17 producing tumors and to improve their antitumor effect after adoptive transfer into a mouse lymphoma model (Di Stasi et al., 2009). The TRUCK (T cells redirected for universal cytokine killing) concept utilizes redirected T cells to release transgenic pro‐inflammatory cytokines to recruit other immune cells into the tumor (reviewed in Chmielewski et al., 2014). Secretion of IL‐12 improved the therapeutic efficacy of tumor‐reactive T cells by triggering a programmatic change in the otherwise immunosuppressive bone marrow‐derived tumor stromal cells to promote tumor regression, while toxicity of systemic IL‐12 administration was avoided (Kerkar et al., 2010, 2011). In a mouse model of prostate cancer, the immunosuppressive effect of TGF‐β could efficiently be neutralized by expression of a dominant negative form of TGF‐β receptor II by the adoptively transferred T cells (Bendle et al., 2013). In another study secretion of a Toll‐like receptor agonist by the tumor‐reactive T cells generated a co‐stimulatory signal that augmented their antitumor activity, characterized by increased tumor infiltration of the T cells, reduced numbers of PD‐1+ T cells and MDSCs, and changes in the chemokine/cytokine profiles of the tumors (Geng et al., 2015). However, the regulatory pathways controlling T‐cell function in the immunosuppressive tumor microenvironment are incompletely understood. A recently developed in vivo shRNA screen might be useful in revealing novel immunotherapy targets (Zhou et al., 2014a).
7. Conclusions and reflections
The adoptive transfer of T cells genetically modified to express TCRs has the potential to generate a population of tumor‐specific T cells not naturally present in the patient. This represents a promising therapeutic option for the large patient groups who do not respond well to TIL therapy or to checkpoint inhibition, which rely on the endogenous T‐cell repertoires. Although T cells genetically modified with immune receptors have provided encouraging clinical results, it seems clear that the antigens targeted in clinical trials thus far are insufficient, or even inappropriate, for the majority of cancer patients. Thus, new and better strategies for identification of novel targets should be prioritized to advance the field and make TCR‐based gene therapy widely applicable. This should involve unbiased identification of candidate targets by high‐throughput screening platforms. Moreover, systems for careful in vitro characterization of the reactivity of therapeutic TCR candidates guided by computational predictions will be instrumental to avoid toxicities, while current in vivo models seem to provide limited information in this regard. Targeting of neo‐antigens with personalized TCR‐based strategies has emerged as an exciting tumor‐specific possibility, although the logistic, regulatory and financial hurdles are considerable, as reviewed elsewhere (June et al., 2015). The timelines needed from diagnosis to TCR identification with current approaches might furthermore limit this therapeutic option to a small patient group with slowly progressing disease. To further add complexity, more than one TCR might be needed to minimize tumor escape, whether targeting neo‐ or self‐antigens. Results from clinical trials combining different immunotherapeutic strategies or immunotherapy with other treatment modalities seem very promising in terms of synergistic effects (Kolstad et al., 2015; Postow et al., 2015). This might also apply to TCR‐based therapy, which for example might be potentiated by simultaneous checkpoint inhibition, or preceding chemo‐ or radiotherapies. Here, mouse models can provide important mechanistic insights. Moreover, the fact that clinical successes have boosted the interest among pharmaceutical companies, should further speed up clinical testing of new TCRs and application of new concepts. Although TCR‐based gene therapy remains a highly personalized treatment modality, its efficacy might promote more widespread application in the near future.
Acknowledgments
We thank Muhammad Ali, Maxi‐Lu Böschen, Jodie Goodridge and Mateusz Walczak for critical comments on the manuscript and Erlend Strønen for help with creating the figures. The work in the author's laboratory is supported by the Research Council of Norway (grants 193283 and 234000/o30), the Regional Health Authorities South‐Eastern Norway, K. G. Jebsen Foundation (Center for Cancer Immunotherapy), University of Oslo, Oslo University Hospital Radiumhospitalet and the Norwegian Cancer Society.
Karpanen Terhi, Olweus Johanna, (2015), T‐cell receptor gene therapy — ready to go viral?, Molecular Oncology, 9, doi: 10.1016/j.molonc.2015.10.006.
This is a contribution to the special issue edited by Johanna Olweus, Cancer Immunotherapy.
Contributor Information
Terhi Karpanen, Email: Terhi.Karpanen@rr-research.no.
Johanna Olweus, Email: Johanna.Olweus@medisin.uio.no.
References
- Abrahamsen, I.W. , Stronen, E. , Walchli, S. , Johansen, J.N. , Kjellevoll, S. , Kumari, S. , Komada, M. , Gaudernack, G. , Tjonnfjord, G. , Toebes, M. , Schumacher, T.N. , Lund-Johansen, F. , Olweus, J. , 2010. Targeting B cell leukemia with highly specific allogeneic T cells with a public recognition motif. Leukemia. 24, 1901–1909. [DOI] [PubMed] [Google Scholar]
- Abrahamsen, I.W. , Kjellevoll, S. , Greve-Isdahl, M. , Mensali, N. , Walchli, S. , Kumari, S. , Loland, B.F. , Egeland, T. , Kolstad, A. , Olweus, J. , 2012. T cells raised against allogeneic HLA-A2/CD20 kill primary follicular lymphoma and acute lymphoblastic leukemia cells. Int. J. Cancer. 130, 1821–1832. [DOI] [PubMed] [Google Scholar]
- Ahmadi, M. , King, J.W. , Xue, S.A. , Voisine, C. , Holler, A. , Wright, G.P. , Waxman, J. , Morris, E. , Stauss, H.J. , 2011. CD3 limits the efficacy of TCR gene therapy in vivo. Blood. 118, 3528–3537. [DOI] [PubMed] [Google Scholar]
- Amir, A.L. , van der Steen, D.M. , Hagedoorn, R.S. , Kester, M.G. , van Bergen, C.A. , Drijfhout, J.W. , de Ru, A.H. , Falkenburg, J.H. , van Veelen, P.A. , Heemskerk, M.H. , 2011. Allo-HLA-reactive T cells inducing graft-versus-host disease are single peptide specific. Blood. 118, 6733–6742. [DOI] [PubMed] [Google Scholar]
- Amir, A.L. , van der Steen, D.M. , van Loenen, M.M. , Hagedoorn, R.S. , de Boer, R. , Kester, M.D. , de Ru, A.H. , Lugthart, G.J. , van Kooten, C. , Hiemstra, P.S. , Jedema, I. , Griffioen, M. , van Veelen, P.A. , Falkenburg, J.H. , Heemskerk, M.H. , 2011. PRAME-specific Allo-HLA-restricted T cells with potent antitumor reactivity useful for therapeutic T-cell receptor gene transfer. Clin. Cancer Res. 17, 5615–5625. [DOI] [PubMed] [Google Scholar]
- Andersen, R.S. , Thrue, C.A. , Junker, N. , Lyngaa, R. , Donia, M. , Ellebaek, E. , Svane, I.M. , Schumacher, T.N. , Thor Straten, P. , Hadrup, S.R. , 2012. Dissection of T-cell antigen specificity in human melanoma. Cancer Res. 72, 1642–1650. [DOI] [PubMed] [Google Scholar]
- Antony, P.A. , Piccirillo, C.A. , Akpinarli, A. , Finkelstein, S.E. , Speiss, P.J. , Surman, D.R. , Palmer, D.C. , Chan, C.C. , Klebanoff, C.A. , Overwijk, W.W. , Rosenberg, S.A. , Restifo, N.P. , 2005. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J. Immunol. 174, 2591–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki, K. , Turner, A.P. , Shaffer, V.O. , Gangappa, S. , Keller, S.A. , Bachmann, M.F. , Larsen, C.P. , Ahmed, R. , 2009. mTOR regulates memory CD8 T-cell differentiation. Nature. 460, 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, M. , 2015. Reproducibility crisis: blame it on the antibodies. Nature. 521, 274–276. [DOI] [PubMed] [Google Scholar]
- Barrett, D.M. , Zhao, Y. , Liu, X. , Jiang, S. , Carpenito, C. , Kalos, M. , Carroll, R.G. , June, C.H. , Grupp, S.A. , 2011. Treatment of advanced leukemia in mice with mRNA engineered T cells. Hum. Gene Ther. 22, 1575–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendle, G.M. , Linnemann, C. , Hooijkaas, A.I. , Bies, L. , de Witte, M.A. , Jorritsma, A. , Kaiser, A.D. , Pouw, N. , Debets, R. , Kieback, E. , Uckert, W. , Song, J.Y. , Haanen, J.B. , Schumacher, T.N. , 2010. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat. Med. 16, 565–570. [DOI] [PubMed] [Google Scholar]
- Bendle, G.M. , Linnemann, C. , Bies, L. , Song, J.Y. , Schumacher, T.N. , 2013. Blockade of TGF-beta signaling greatly enhances the efficacy of TCR gene therapy of cancer. J. Immunol. 191, 3232–3239. [DOI] [PubMed] [Google Scholar]
- Berdien, B. , Mock, U. , Atanackovic, D. , Fehse, B. , 2014. TALEN-mediated editing of endogenous T-cell receptors facilitates efficient reprogramming of T lymphocytes by lentiviral gene transfer. Gene Ther. 21, 539–548. [DOI] [PubMed] [Google Scholar]
- Berger, C. , Jensen, M.C. , Lansdorp, P.M. , Gough, M. , Elliott, C. , Riddell, S.R. , 2008. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J. Clin. Invest. 118, 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, C. , Sommermeyer, D. , Hudecek, M. , Berger, M. , Balakrishnan, A. , Paszkiewicz, P.J. , Kosasih, P.L. , Rader, C. , Riddell, S.R. , 2015. Safety of targeting ROR1 in primates with chimeric antigen receptor-modified T cells. Cancer Immunol. Res. 3, 206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialer, G. , Horovitz-Fried, M. , Ya'acobi, S. , Morgan, R.A. , Cohen, C.J. , 2010. Selected murine residues endow human TCR with enhanced tumor recognition. J. Immunol. 184, 6232–6241. [DOI] [PubMed] [Google Scholar]
- Birnbaum, M.E. , Mendoza, J.L. , Sethi, D.K. , Dong, S. , Glanville, J. , Dobbins, J. , Ozkan, E. , Davis, M.M. , Wucherpfennig, K.W. , Garcia, K.C. , 2014. Deconstructing the peptide-MHC specificity of T cell recognition. Cell. 157, 1073–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenstein, T. , Leisegang, M. , Uckert, W. , Schreiber, H. , 2015. Targeting cancer-specific mutations by T cell receptor gene therapy. Curr. Opin. Immunol. 33C, 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobisse, S. , Rondina, M. , Merlo, A. , Tisato, V. , Mandruzzato, S. , Amendola, M. , Naldini, L. , Willemsen, R.A. , Debets, R. , Zanovello, P. , Rosato, A. , 2009. Reprogramming T lymphocytes for melanoma adoptive immunotherapy by T-cell receptor gene transfer with lentiviral vectors. Cancer Res. 69, 9385–9394. [DOI] [PubMed] [Google Scholar]
- Bos, R. , van Duikeren, S. , Morreau, H. , Franken, K. , Schumacher, T.N. , Haanen, J.B. , van der Burg, S.H. , Melief, C.J. , Offringa, R. , 2008. Balancing between antitumor efficacy and autoimmune pathology in T-cell-mediated targeting of carcinoembryonic antigen. Cancer Res. 68, 8446–8455. [DOI] [PubMed] [Google Scholar]
- Bossi, G. , Buisson, S. , Oates, J. , Jakobsen, B.K. , Hassan, N.J. , 2014. ImmTAC-redirected tumour cell killing induces and potentiates antigen cross-presentation by dendritic cells. Cancer Immunol. Immunother. 63, 437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury, A. , Pluckthun, A. , 2015. Reproducibility: standardize antibodies used in research. Nature. 518, 27–29. [DOI] [PubMed] [Google Scholar]
- Brentjens, R.J. , Riviere, I. , Park, J.H. , Davila, M.L. , Wang, X. , Stefanski, J. , Taylor, C. , Yeh, R. , Bartido, S. , Borquez-Ojeda, O. , Olszewska, M. , Bernal, Y. , Pegram, H. , Przybylowski, M. , Hollyman, D. , Usachenko, Y. , Pirraglia, D. , Hosey, J. , Santos, E. , Halton, E. , Maslak, P. , Scheinberg, D. , Jurcic, J. , Heaney, M. , Heller, G. , Frattini, M. , Sadelain, M. , 2011. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 118, 4817–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens, R.J. , Davila, M.L. , Riviere, I. , Park, J. , Wang, X. , Cowell, L.G. , Bartido, S. , Stefanski, J. , Taylor, C. , Olszewska, M. , Borquez-Ojeda, O. , Qu, J. , Wasielewska, T. , He, Q. , Bernal, Y. , Rijo, I.V. , Hedvat, C. , Kobos, R. , Curran, K. , Steinherz, P. , Jurcic, J. , Rosenblat, T. , Maslak, P. , Frattini, M. , Sadelain, M. , 2013. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 5, 177ra138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz, V.R. , Flossdorf, M. , Hensel, I. , Kretschmer, L. , Weissbrich, B. , Graf, P. , Verschoor, A. , Schiemann, M. , Hofer, T. , Busch, D.H. , 2013. Disparate individual fates compose robust CD8+ T cell immunity. Science. 340, 630–635. [DOI] [PubMed] [Google Scholar]
- Bunse, M. , Bendle, G.M. , Linnemann, C. , Bies, L. , Schulz, S. , Schumacher, T.N. , Uckert, W. , 2014. RNAi-mediated TCR knockdown prevents autoimmunity in mice caused by mixed TCR dimers following TCR gene transfer. Mol. Ther. 22, 1983–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron, B.J. , Gerry, A.B. , Dukes, J. , Harper, J.V. , Kannan, V. , Bianchi, F.C. , Grand, F. , Brewer, J.E. , Gupta, M. , Plesa, G. , Bossi, G. , Vuidepot, A. , Powlesland, A.S. , Legg, A. , Adams, K.J. , Bennett, A.D. , Pumphrey, N.J. , Williams, D.D. , Binder-Scholl, G. , Kulikovskaya, I. , Levine, B.L. , Riley, J.L. , Varela-Rohena, A. , Stadtmauer, E.A. , Rapoport, A.P. , Linette, G.P. , June, C.H. , Hassan, N.J. , Kalos, M. , Jakobsen, B.K. , 2013. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci. Transl. Med. 5, 197ra103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha, E. , Graham, L. , Manjili, M.H. , Bear, H.D. , 2010. IL-7 + IL-15 are superior to IL-2 for the ex vivo expansion of 4T1 mammary carcinoma-specific T cells with greater efficacy against tumors in vivo. Breast Cancer Res. Treat. 122, 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamoto, K. , Tsuji, T. , Funamoto, H. , Kosaka, A. , Matsuzaki, J. , Sato, T. , Abe, H. , Fujio, K. , Yamamoto, K. , Kitamura, T. , Takeshima, T. , Togashi, Y. , Nishimura, T. , 2004. Potentiation of tumor eradication by adoptive immunotherapy with T-cell receptor gene-transduced T-helper type 1 cells. Cancer Res. 64, 386–390. [DOI] [PubMed] [Google Scholar]
- Chapuis, A.G. , Ragnarsson, G.B. , Nguyen, H.N. , Chaney, C.N. , Pufnock, J.S. , Schmitt, T.M. , Duerkopp, N. , Roberts, I.M. , Pogosov, G.L. , Ho, W.Y. , Ochsenreither, S. , Wolfl, M. , Bar, M. , Radich, J.P. , Yee, C. , Greenberg, P.D. , 2013. Transferred WT1-reactive CD8+ T cells can mediate antileukemic activity and persist in post-transplant patients. Sci. Transl. Med. 5, 174ra127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever, M.A. , Allison, J.P. , Ferris, A.S. , Finn, O.J. , Hastings, B.M. , Hecht, T.T. , Mellman, I. , Prindiville, S.A. , Viner, J.L. , Weiner, L.M. , Matrisian, L.M. , 2009. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin. Cancer Res. 15, 5323–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervin, A.S. , Aggen, D.H. , Raseman, J.M. , Kranz, D.M. , 2008. Engineering higher affinity T cell receptors using a T cell display system. J. Immunol. Methods. 339, 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra, A. , Yang, L. , Wang, P. , Comin-Anduix, B. , Das, R. , Chakraborty, N.G. , Ray, S. , Mehrotra, S. , Yang, H. , Hardee, C.L. , Hollis, R. , Dorsky, D.I. , Koya, R. , Kohn, D.B. , Ribas, A. , Economou, J.S. , Baltimore, D. , Mukherji, B. , 2008. CD4+CD25- T cells transduced to express MHC class I-restricted epitope-specific TCR synthesize Th1 cytokines and exhibit MHC class I-restricted cytolytic effector function in a human melanoma model. J. Immunol. 181, 1063–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnasamy, N. , Wargo, J.A. , Yu, Z. , Rao, M. , Frankel, T.L. , Riley, J.P. , Hong, J.J. , Parkhurst, M.R. , Feldman, S.A. , Schrump, D.S. , Restifo, N.P. , Robbins, P.F. , Rosenberg, S.A. , Morgan, R.A. , 2011. A TCR targeting the HLA-A*0201-restricted epitope of MAGE-A3 recognizes multiple epitopes of the MAGE-A antigen superfamily in several types of cancer. J. Immunol. 186, 685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielewski, M. , Hombach, A.A. , Abken, H. , 2014. Of CARs and TRUCKs: chimeric antigen receptor (CAR) T cells engineered with an inducible cytokine to modulate the tumor stroma. Immunol. Rev. 257, 83–90. [DOI] [PubMed] [Google Scholar]
- Chodon, T. , Comin-Anduix, B. , Chmielowski, B. , Koya, R.C. , Wu, Z. , Auerbach, M. , Ng, C. , Avramis, E. , Seja, E. , Villanueva, A. , McCannel, T.A. , Ishiyama, A. , Czernin, J. , Radu, C.G. , Wang, X. , Gjertson, D.W. , Cochran, A.J. , Cornetta, K. , Wong, D.J. , Kaplan-Lefko, P. , Hamid, O. , Samlowski, W. , Cohen, P.A. , Daniels, G.A. , Mukherji, B. , Yang, L. , Zack, J.A. , Kohn, D.B. , Heath, J.R. , Glaspy, J.A. , Witte, O.N. , Baltimore, D. , Economou, J.S. , Ribas, A. , 2014. Adoptive transfer of MART-1 T-cell receptor transgenic lymphocytes and dendritic cell vaccination in patients with metastatic melanoma. Clin. Cancer Res. 20, 2457–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieri, N. , Camisa, B. , Cocchiarella, F. , Forcato, M. , Oliveira, G. , Provasi, E. , Bondanza, A. , Bordignon, C. , Peccatori, J. , Ciceri, F. , Lupo-Stanghellini, M.T. , Mavilio, F. , Mondino, A. , Bicciato, S. , Recchia, A. , Bonini, C. , 2013. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood. 121, 573–584. [DOI] [PubMed] [Google Scholar]
- Clay, T.M. , Custer, M.C. , Sachs, J. , Hwu, P. , Rosenberg, S.A. , Nishimura, M.I. , 1999. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J. Immunol. 163, 507–513. [PubMed] [Google Scholar]
- Cohen, C.J. , Zheng, Z. , Bray, R. , Zhao, Y. , Sherman, L.A. , Rosenberg, S.A. , Morgan, R.A. , 2005. Recognition of fresh human tumor by human peripheral blood lymphocytes transduced with a bicistronic retroviral vector encoding a murine anti-p53 TCR. J. Immunol. 175, 5799–5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, C.J. , Zhao, Y. , Zheng, Z. , Rosenberg, S.A. , Morgan, R.A. , 2006. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 66, 8878–8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, C.J. , Li, Y.F. , El-Gamil, M. , Robbins, P.F. , Rosenberg, S.A. , Morgan, R.A. , 2007. Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Res. 67, 3898–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, C.J. , Gartner, J.J. , Horovitz-Fried, M. , Shamalov, K. , Trebska-McGowan, K. , Bliskovsky, V.V. , Parkhurst, M.R. , Ankri, C. , Prickett, T.D. , Crystal, J.S. , Li, Y.F. , El-Gamil, M. , Rosenberg, S.A. , Robbins, P.F. , 2015. Isolation of neoantigen-specific T cells from tumor and peripheral lymphocytes. J. Clin. Invest. 125, 3981–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton, J.G. , Sukumar, M. , Roychoudhuri, R. , Clever, D. , Gros, A. , Eil, R.L. , Tran, E. , Hanada, K. , Yu, Z. , Palmer, D.C. , Kerkar, S.P. , Michalek, R.D. , Upham, T. , Leonardi, A. , Acquavella, N. , Wang, E. , Marincola, F.M. , Gattinoni, L. , Muranski, P. , Sundrud, M.S. , Klebanoff, C.A. , Rosenberg, S.A. , Fearon, D.T. , Restifo, N.P. , 2015. Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res. 75, 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, Y. , Zhang, H. , Meadors, J. , Poon, R. , Guimond, M. , Mackall, C.L. , 2009. Harnessing the physiology of lymphopenia to support adoptive immunotherapy in lymphoreplete hosts. Blood. 114, 3831–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel, T.J. , Coukos, G. , Zou, L. , Alvarez, X. , Cheng, P. , Mottram, P. , Evdemon-Hogan, M. , Conejo-Garcia, J.R. , Zhang, L. , Burow, M. , Zhu, Y. , Wei, S. , Kryczek, I. , Daniel, B. , Gordon, A. , Myers, L. , Lackner, A. , Disis, M.L. , Knutson, K.L. , Chen, L. , Zou, W. , 2004. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 10, 942–949. [DOI] [PubMed] [Google Scholar]
- Davis, J.L. , Theoret, M.R. , Zheng, Z. , Lamers, C.H. , Rosenberg, S.A. , Morgan, R.A. , 2010. Development of human anti-murine T-cell receptor antibodies in both responding and nonresponding patients enrolled in TCR gene therapy trials. Clin. Cancer Res. 16, 5852–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Witte, M.A. , Coccoris, M. , Wolkers, M.C. , van den Boom, M.D. , Mesman, E.M. , Song, J.Y. , van der Valk, M. , Haanen, J.B. , Schumacher, T.N. , 2006. Targeting self-antigens through allogeneic TCR gene transfer. Blood. 108, 870–877. [DOI] [PubMed] [Google Scholar]
- de Witte, M.A. , Jorritsma, A. , Kaiser, A. , van den Boom, M.D. , Dokter, M. , Bendle, G.M. , Haanen, J.B. , Schumacher, T.N. , 2008. Requirements for effective antitumor responses of TCR transduced T cells. J. Immunol. 181, 5128–5136. [DOI] [PubMed] [Google Scholar]
- Di Stasi, A. , De Angelis, B. , Rooney, C.M. , Zhang, L. , Mahendravada, A. , Foster, A.E. , Heslop, H.E. , Brenner, M.K. , Dotti, G. , Savoldo, B. , 2009. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 113, 6392–6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stasi, A. , Tey, S.K. , Dotti, G. , Fujita, Y. , Kennedy-Nasser, A. , Martinez, C. , Straathof, K. , Liu, E. , Durett, A.G. , Grilley, B. , Liu, H. , Cruz, C.R. , Savoldo, B. , Gee, A.P. , Schindler, J. , Krance, R.A. , Heslop, H.E. , Spencer, D.M. , Rooney, C.M. , Brenner, M.K. , 2011. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 365, 1673–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudna, J.A. , Charpentier, E. , 2014. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 346, 1258096 [DOI] [PubMed] [Google Scholar]
- Dudley, M.E. , Wunderlich, J.R. , Robbins, P.F. , Yang, J.C. , Hwu, P. , Schwartzentruber, D.J. , Topalian, S.L. , Sherry, R. , Restifo, N.P. , Hubicki, A.M. , Robinson, M.R. , Raffeld, M. , Duray, P. , Seipp, C.A. , Rogers-Freezer, L. , Morton, K.E. , Mavroukakis, S.A. , White, D.E. , Rosenberg, S.A. , 2002. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 298, 850–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley, M.E. , Wunderlich, J.R. , Yang, J.C. , Hwu, P. , Schwartzentruber, D.J. , Topalian, S.L. , Sherry, R.M. , Marincola, F.M. , Leitman, S.F. , Seipp, C.A. , Rogers-Freezer, L. , Morton, K.E. , Nahvi, A. , Mavroukakis, S.A. , White, D.E. , Rosenberg, S.A. , 2002. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J. Immunother. 25, 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley, M.E. , Wunderlich, J.R. , Yang, J.C. , Sherry, R.M. , Topalian, S.L. , Restifo, N.P. , Royal, R.E. , Kammula, U. , White, D.E. , Mavroukakis, S.A. , Rogers, L.J. , Gracia, G.J. , Jones, S.A. , Mangiameli, D.P. , Pelletier, M.M. , Gea-Banacloche, J. , Robinson, M.R. , Berman, D.M. , Filie, A.C. , Abati, A. , Rosenberg, S.A. , 2005. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol. 23, 2346–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley, M.E. , Yang, J.C. , Sherry, R. , Hughes, M.S. , Royal, R. , Kammula, U. , Robbins, P.F. , Huang, J. , Citrin, D.E. , Leitman, S.F. , Wunderlich, J. , Restifo, N.P. , Thomasian, A. , Downey, S.G. , Smith, F.O. , Klapper, J. , Morton, K. , Laurencot, C. , White, D.E. , Rosenberg, S.A. , 2008. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol. 26, 5233–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley, M.E. , Gross, C.A. , Langhan, M.M. , Garcia, M.R. , Sherry, R.M. , Yang, J.C. , Phan, G.Q. , Kammula, U.S. , Hughes, M.S. , Citrin, D.E. , Restifo, N.P. , Wunderlich, J.R. , Prieto, P.A. , Hong, J.J. , Langan, R.C. , Zlott, D.A. , Morton, K.E. , White, D.E. , Laurencot, C.M. , Rosenberg, S.A. , 2010. CD8+ enriched “young” tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin. Cancer Res. 16, 6122–6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dummer, W. , Niethammer, A.G. , Baccala, R. , Lawson, B.R. , Wagner, N. , Reisfeld, R.A. , Theofilopoulos, A.N. , 2002. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J. Clin. Invest. 110, 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix, N.J. , Allen, P.M. , 2007. Specificity of T-cell alloreactivity. Nat. Rev. Immunol. 7, 942–953. [DOI] [PubMed] [Google Scholar]
- Frankel, T.L. , Burns, W.R. , Peng, P.D. , Yu, Z. , Chinnasamy, D. , Wargo, J.A. , Zheng, Z. , Restifo, N.P. , Rosenberg, S.A. , Morgan, R.A. , 2010. Both CD4 and CD8 T cells mediate equally effective in vivo tumor treatment when engineered with a highly avid TCR targeting tyrosinase. J. Immunol. 184, 5988–5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, L. , Bellantuono, I. , Elsasser, A. , Marley, S.B. , Gordon, M.Y. , Goldman, J.M. , Stauss, H.J. , 2000. Selective elimination of leukemic CD34(+) progenitor cells by cytotoxic T lymphocytes specific for WT1. Blood. 95, 2198–2203. [PubMed] [Google Scholar]
- Gao, L. , Downs, A.M. , Stauss, H.J. , 2005. Immunotherapy with CTL restricted by nonself MHC. Methods Mol. Med. 109, 215–228. [DOI] [PubMed] [Google Scholar]
- Gattinoni, L. , Finkelstein, S.E. , Klebanoff, C.A. , Antony, P.A. , Palmer, D.C. , Spiess, P.J. , Hwang, L.N. , Yu, Z. , Wrzesinski, C. , Heimann, D.M. , Surh, C.D. , Rosenberg, S.A. , Restifo, N.P. , 2005. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med. 202, 907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni, L. , Klebanoff, C.A. , Palmer, D.C. , Wrzesinski, C. , Kerstann, K. , Yu, Z. , Finkelstein, S.E. , Theoret, M.R. , Rosenberg, S.A. , Restifo, N.P. , 2005. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J. Clin. Invest. 115, 1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni, L. , Zhong, X.S. , Palmer, D.C. , Ji, Y. , Hinrichs, C.S. , Yu, Z. , Wrzesinski, C. , Boni, A. , Cassard, L. , Garvin, L.M. , Paulos, C.M. , Muranski, P. , Restifo, N.P. , 2009. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat. Med. 15, 808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni, L. , Lugli, E. , Ji, Y. , Pos, Z. , Paulos, C.M. , Quigley, M.F. , Almeida, J.R. , Gostick, E. , Yu, Z. , Carpenito, C. , Wang, E. , Douek, D.C. , Price, D.A. , June, C.H. , Marincola, F.M. , Roederer, M. , Restifo, N.P. , 2011. A human memory T cell subset with stem cell-like properties. Nat. Med. 17, 1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng, D. , Kaczanowska, S. , Tsai, A. , Younger, K. , Ochoa, A. , Rapoport, A.P. , Ostrand-Rosenberg, S. , Davila, E. , 2015. TLR5 ligand-secreting T cells reshape the tumor microenvironment and enhance antitumor activity. Cancer Res. 75, 1959–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach, C. , Rohr, J.C. , Perie, L. , van Rooij, N. , van Heijst, J.W. , Velds, A. , Urbanus, J. , Naik, S.H. , Jacobs, H. , Beltman, J.B. , de Boer, R.J. , Schumacher, T.N. , 2013. Heterogeneous differentiation patterns of individual CD8+ T cells. Science. 340, 635–639. [DOI] [PubMed] [Google Scholar]
- Geukes Foppen, M.H. , Donia, M. , Svane, I.M. , Haanen, J.B.A.G. , 2015. Tumor-infiltrating lymphocytes for the treatment of metastatic cancer. Mol. Oncol. 9, 1918–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff, S.L. , Johnson, L.A. , Black, M.A. , Xu, H. , Zheng, Z. , Cohen, C.J. , Morgan, R.A. , Rosenberg, S.A. , Feldman, S.A. , 2010. Enhanced receptor expression and in vitro effector function of a murine-human hybrid MART-1-reactive T cell receptor following a rapid expansion. Cancer Immunol. Immunother. 59, 1551–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Eerland, R. , Nuijen, B. , Heemskerk, B. , van Rooij, N. , van den Berg, J.H. , Beijnen, J.H. , Uckert, W. , Kvistborg, P. , Schumacher, T.N. , Haanen, J.B. , Jorritsma, A. , 2014. Manufacture of gene-modified human T-cells with a memory stem/central memory phenotype. Hum. Gene Ther. Methods. 25, 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupp, S.A. , Kalos, M. , Barrett, D. , Aplenc, R. , Porter, D.L. , Rheingold, S.R. , Teachey, D.T. , Chew, A. , Hauck, B. , Wright, J.F. , Milone, M.C. , Levine, B.L. , June, C.H. , 2013. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 368, 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubin, M.M. , Zhang, X. , Schuster, H. , Caron, E. , Ward, J.P. , Noguchi, T. , Ivanova, Y. , Hundal, J. , Arthur, C.D. , Krebber, W.J. , Mulder, G.E. , Toebes, M. , Vesely, M.D. , Lam, S.S. , Korman, A.J. , Allison, J.P. , Freeman, G.J. , Sharpe, A.H. , Pearce, E.L. , Schumacher, T.N. , Aebersold, R. , Rammensee, H.G. , Melief, C.J. , Mardis, E.R. , Gillanders, W.E. , Artyomov, M.N. , Schreiber, R.D. , 2014. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 515, 577–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haabeth, O.A. , Tveita, A.A. , Fauskanger, M. , Schjesvold, F. , Lorvik, K.B. , Hofgaard, P.O. , Omholt, H. , Munthe, L.A. , Dembic, Z. , Corthay, A. , Bogen, B. , 2014. How do CD4(+) T cells detect and eliminate tumor cells that either lack or express MHC class II molecules?. Front. Immunol. 5, 174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidar, J.N. , Pierce, B. , Yu, Y. , Tong, W. , Li, M. , Weng, Z. , 2009. Structure-based design of a T-cell receptor leads to nearly 100-fold improvement in binding affinity for pepMHC. Proteins. 74, 948–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harndahl, M. , Rasmussen, M. , Roder, G. , Dalgaard Pedersen, I. , Sorensen, M. , Nielsen, M. , Buus, S. , 2012. Peptide-MHC class I stability is a better predictor than peptide affinity of CTL immunogenicity. Eur. J. Immunol. 42, 1405–1416. [DOI] [PubMed] [Google Scholar]
- Hart, D.P. , Xue, S.A. , Thomas, S. , Cesco-Gaspere, M. , Tranter, A. , Willcox, B. , Lee, S.P. , Steven, N. , Morris, E.C. , Stauss, H.J. , 2008. Retroviral transfer of a dominant TCR prevents surface expression of a large proportion of the endogenous TCR repertoire in human T cells. Gene Ther. 15, 625–631. [DOI] [PubMed] [Google Scholar]
- Heemskerk, B. , Liu, K. , Dudley, M.E. , Johnson, L.A. , Kaiser, A. , Downey, S. , Zheng, Z. , Shelton, T.E. , Matsuda, K. , Robbins, P.F. , Morgan, R.A. , Rosenberg, S.A. , 2008. Adoptive cell therapy for patients with melanoma, using tumor-infiltrating lymphocytes genetically engineered to secrete interleukin-2. Hum. Gene Ther. 19, 496–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervent, A.S. , De Keulenaer, G.W. , 2012. Molecular mechanisms of cardiotoxicity induced by ErbB receptor inhibitor cancer therapeutics. Int. J. Mol. Sci. 13, 12268–12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiasa, A. , Hirayama, M. , Nishikawa, H. , Kitano, S. , Nukaya, I. , Yu, S.S. , Mineno, J. , Kato, I. , Shiku, H. , 2008. Long-term phenotypic, functional and genetic stability of cancer-specific T-cell receptor (TCR) alphabeta genes transduced to CD8+ T cells. Gene Ther. 15, 695–699. [DOI] [PubMed] [Google Scholar]
- Hinrichs, C.S. , Restifo, N.P. , 2013. Reassessing target antigens for adoptive T-cell therapy. Nat. Biotechnol. 31, 999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs, C.S. , Spolski, R. , Paulos, C.M. , Gattinoni, L. , Kerstann, K.W. , Palmer, D.C. , Klebanoff, C.A. , Rosenberg, S.A. , Leonard, W.J. , Restifo, N.P. , 2008. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 111, 5326–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs, C.S. , Borman, Z.A. , Cassard, L. , Gattinoni, L. , Spolski, R. , Yu, Z. , Sanchez-Perez, L. , Muranski, P. , Kern, S.J. , Logun, C. , Palmer, D.C. , Ji, Y. , Reger, R.N. , Leonard, W.J. , Danner, R.L. , Rosenberg, S.A. , Restifo, N.P. , 2009. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc. Natl. Acad. Sci. U. S. A. 106, 17469–17474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi, F.S. , O'Day, S.J. , McDermott, D.F. , Weber, R.W. , Sosman, J.A. , Haanen, J.B. , Gonzalez, R. , Robert, C. , Schadendorf, D. , Hassel, J.C. , Akerley, W. , van den Eertwegh, A.J. , Lutzky, J. , Lorigan, P. , Vaubel, J.M. , Linette, G.P. , Hogg, D. , Ottensmeier, C.H. , Lebbe, C. , Peschel, C. , Quirt, I. , Clark, J.I. , Wolchok, J.D. , Weber, J.S. , Tian, J. , Yellin, M.J. , Nichol, G.M. , Hoos, A. , Urba, W.J. , 2010. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holler, P.D. , Holman, P.O. , Shusta, E.V. , O'Herrin, S. , Wittrup, K.D. , Kranz, D.M. , 2000. In vitro evolution of a T cell receptor with high affinity for peptide/MHC. Proc. Natl. Acad. Sci. U. S. A. 97, 5387–5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holler, P.D. , Chlewicki, L.K. , Kranz, D.M. , 2003. TCRs with high affinity for foreign pMHC show self-reactivity. Nat. Immunol. 4, 55–62. [DOI] [PubMed] [Google Scholar]
- Holm, A. , Wu, W. , Lund-Johansen, F. , 2012. Antibody array analysis of labelled proteomes: how should we control specificity?. Nat. Biotechnol. 29, 578–585. [DOI] [PubMed] [Google Scholar]
- Hoppes, R. , Ekkebus, R. , Schumacher, T.N. , Ovaa, H. , 2010. Technologies for MHC class I immunoproteomics. J. Proteomics. 73, 1945–1953. [DOI] [PubMed] [Google Scholar]
- Housset, D. , Malissen, B. , 2003. What do TCR-pMHC crystal structures teach us about MHC restriction and alloreactivity?. Trends Immunol. 24, 429–437. [DOI] [PubMed] [Google Scholar]
- Huang, J. , Wang, Q.J. , Yang, S. , Li, Y.F. , El-Gamil, M. , Rosenberg, S.A. , Robbins, P.F. , 2011. Irradiation enhances human T-cell function by upregulating CD70 expression on antigen-presenting cells in vitro. J. Immunother. 34, 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, M.S. , Yu, Y.Y. , Dudley, M.E. , Zheng, Z. , Robbins, P.F. , Li, Y. , Wunderlich, J. , Hawley, R.G. , Moayeri, M. , Rosenberg, S.A. , Morgan, R.A. , 2005. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum. Gene Ther. 16, 457–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunder, N.N. , Wallen, H. , Cao, J. , Hendricks, D.W. , Reilly, J.Z. , Rodmyre, R. , Jungbluth, A. , Gnjatic, S. , Thompson, J.A. , Yee, C. , 2008. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N. Engl. J. Med. 358, 2698–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huseby, E.S. , White, J. , Crawford, F. , Vass, T. , Becker, D. , Pinilla, C. , Marrack, P. , Kappler, J.W. , 2005. How the T cell repertoire becomes peptide and MHC specific. Cell. 122, 247–260. [DOI] [PubMed] [Google Scholar]
- Ignatowicz, L. , Kappler, J. , Marrack, P. , 1996. The repertoire of T cells shaped by a single MHC/peptide ligand. Cell. 84, 521–529. [DOI] [PubMed] [Google Scholar]
- Jedema, I. , Falkenburg, F. , 2015. Allo-reactive T cells for the treatment of hematological malignancies. Mol. Oncol. 9, 1894–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John, L.B. , Devaud, C. , Duong, C.P. , Yong, C.S. , Beavis, P.A. , Haynes, N.M. , Chow, M.T. , Smyth, M.J. , Kershaw, M.H. , Darcy, P.K. , 2013. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin. Cancer Res. 19, 5636–5646. [DOI] [PubMed] [Google Scholar]
- Johnson, L.A. , Heemskerk, B. , Powell, D.J. , Cohen, C.J. , Morgan, R.A. , Dudley, M.E. , Robbins, P.F. , Rosenberg, S.A. , 2006. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J. Immunol. 177, 6548–6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, L.A. , Morgan, R.A. , Dudley, M.E. , Cassard, L. , Yang, J.C. , Hughes, M.S. , Kammula, U.S. , Royal, R.E. , Sherry, R.M. , Wunderlich, J.R. , Lee, C.C. , Restifo, N.P. , Schwarz, S.L. , Cogdill, A.P. , Bishop, R.J. , Kim, H. , Brewer, C.C. , Rudy, S.F. , VanWaes, C. , Davis, J.L. , Mathur, A. , Ripley, R.T. , Nathan, D.A. , Laurencot, C.M. , Rosenberg, S.A. , 2009. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 114, 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorritsma, A. , Gomez-Eerland, R. , Dokter, M. , van de Kasteele, W. , Zoet, Y.M. , Doxiadis, I.I. , Rufer, N. , Romero, P. , Morgan, R.A. , Schumacher, T.N. , Haanen, J.P. , 2009. Selecting highly affine and well-expressed TCRs for gene therapy of melanoma. Blood. 110, 3564–3572. [DOI] [PubMed] [Google Scholar]
- June, C.H. , Riddell, S.R. , Schumacher, T.N. , 2015. Adoptive cellular therapy: a race to the finish line. Sci. Transl. Med. 7, 280ps287 [DOI] [PubMed] [Google Scholar]
- Kageyama, S. , Ikeda, H. , Miyahara, Y. , Imai, N. , Ishihara, M. , Saito, K. , Sugino, S. , Ueda, S. , Ishikawa, T. , Kokura, S. , Naota, H. , Ohishi, K. , Shiraishi, T. , Inoue, N. , Tanabe, M. , Kidokoro, T. , Yoshioka, H. , Tomura, D. , Nukaya, I. , Mineno, J. , Takesako, K. , Katayama, N. , Shiku, H. , 2015. Adoptive transfer of MAGE-A4 T-cell receptor gene-transduced lymphocytes in patients with recurrent esophageal cancer. Clin. Cancer Res. 21, 2268–2277. [DOI] [PubMed] [Google Scholar]
- Kalbasi, A. , Shrimali, R.K. , Chinnasamy, D. , Rosenberg, S.A. , 2010. Prevention of interleukin-2 withdrawal-induced apoptosis in lymphocytes retrovirally cotransduced with genes encoding an antitumor T-cell receptor and an antiapoptotic protein. J. Immunother. 33, 672–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalos, M. , Levine, B.L. , Porter, D.L. , Katz, S. , Grupp, S.A. , Bagg, A. , June, C.H. , 2011. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 3, 95ra73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkar, S.P. , Muranski, P. , Kaiser, A. , Boni, A. , Sanchez-Perez, L. , Yu, Z. , Palmer, D.C. , Reger, R.N. , Borman, Z.A. , Zhang, L. , Morgan, R.A. , Gattinoni, L. , Rosenberg, S.A. , Trinchieri, G. , Restifo, N.P. , 2010. Tumor-specific CD8+ T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. Cancer Res. 70, 6725–6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkar, S.P. , Goldszmid, R.S. , Muranski, P. , Chinnasamy, D. , Yu, Z. , Reger, R.N. , Leonardi, A.J. , Morgan, R.A. , Wang, E. , Marincola, F.M. , Trinchieri, G. , Rosenberg, S.A. , Restifo, N.P. , 2011. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J. Clin. Invest. 121, 4746–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkar, S.P. , Sanchez-Perez, L. , Yang, S. , Borman, Z.A. , Muranski, P. , Ji, Y. , Chinnasamy, D. , Kaiser, A.D. , Hinrichs, C.S. , Klebanoff, C.A. , Scott, C.D. , Gattinoni, L. , Morgan, R.A. , Rosenberg, S.A. , Restifo, N.P. , 2011. Genetic engineering of murine CD8+ and CD4+ T cells for preclinical adoptive immunotherapy studies. J. Immunother. 34, 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels, H.W. , van Den Boom, M.D. , Spits, H. , Hooijberg, E. , Schumacher, T.N. , 2000. Changing T cell specificity by retroviral T cell receptor display. Proc. Natl. Acad. Sci. U. S. A. 97, 14578–14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels, H.W. , Wolkers, M.C. , van den Boom, M.D. , van der Valk, M.A. , Schumacher, T.N. , 2001. Immunotherapy through TCR gene transfer. Nat. Immunol. 2, 957–961. [DOI] [PubMed] [Google Scholar]
- Kessels, H.W. , Schepers, K. , van den Boom, M.D. , Topham, D.J. , Schumacher, T.N. , 2006. Generation of T cell help through a MHC class I-restricted TCR. J. Immunol. 177, 976–982. [DOI] [PubMed] [Google Scholar]
- Kessler, J.H. , Melief, C.J. , 2007. Identification of T-cell epitopes for cancer immunotherapy. Leukemia. 21, 1859–1874. [DOI] [PubMed] [Google Scholar]
- Kieke, M.C. , Shusta, E.V. , Boder, E.T. , Teyton, L. , Wittrup, K.D. , Kranz, D.M. , 1999. Selection of functional T cell receptor mutants from a yeast surface-display library. Proc. Natl. Acad. Sci. U. S. A. 96, 5651–5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff, C.A. , Gattinoni, L. , Torabi-Parizi, P. , Kerstann, K. , Cardones, A.R. , Finkelstein, S.E. , Palmer, D.C. , Antony, P.A. , Hwang, S.T. , Rosenberg, S.A. , Waldmann, T.A. , Restifo, N.P. , 2005. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc. Natl. Acad. Sci. U. S. A. 102, 9571–9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff, C.A. , Acquavella, N. , Yu, Z. , Restifo, N.P. , 2011. Therapeutic cancer vaccines: are we there yet?. Immunol. Rev. 239, 27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloss, C.C. , Condomines, M. , Cartellieri, M. , Bachmann, M. , Sadelain, M. , 2013. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat. Biotechnol. 31, 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer, J.N. , Dudley, M.E. , Feldman, S.A. , Wilson, W.H. , Spaner, D.E. , Maric, I. , Stetler-Stevenson, M. , Phan, G.Q. , Hughes, M.S. , Sherry, R.M. , Yang, J.C. , Kammula, U.S. , Devillier, L. , Carpenter, R. , Nathan, D.A. , Morgan, R.A. , Laurencot, C. , Rosenberg, S.A. , 2012. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 119, 2709–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer, J.N. , Dudley, M.E. , Carpenter, R.O. , Kassim, S.H. , Rose, J.J. , Telford, W.G. , Hakim, F.T. , Halverson, D.C. , Fowler, D.H. , Hardy, N.M. , Mato, A.R. , Hickstein, D.D. , Gea-Banacloche, J.C. , Pavletic, S.Z. , Sportes, C. , Maric, I. , Feldman, S.A. , Hansen, B.G. , Wilder, J.S. , Blacklock-Schuver, B. , Jena, B. , Bishop, M.R. , Gress, R.E. , Rosenberg, S.A. , 2013. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 122, 4129–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer, J.N. , Dudley, M.E. , Kassim, S.H. , Somerville, R.P. , Carpenter, R.O. , Stetler-Stevenson, M. , Yang, J.C. , Phan, G.Q. , Hughes, M.S. , Sherry, R.M. , Raffeld, M. , Feldman, S. , Lu, L. , Li, Y.F. , Ngo, L.T. , Goy, A. , Feldman, T. , Spaner, D.E. , Wang, M.L. , Chen, C.C. , Kranick, S.M. , Nath, A. , Nathan, D.A. , Morton, K.E. , Toomey, M.A. , Rosenberg, S.A. , 2015. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 33, 540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolstad, A. , Kumari, S. , Walczak, M. , Madsbu, U. , Hagtvedt, T. , Bogsrud, T.V. , Kvalheim, G. , Holte, H. , Aurlien, E. , Delabie, J. , Tierens, A. , Olweus, J. , 2015. Sequential intranodal immunotherapy induces antitumor immunity and correlated regression of disseminated follicular lymphoma. Blood. 125, 82–89. [DOI] [PubMed] [Google Scholar]
- Kronig, H. , Hofer, K. , Conrad, H. , Guilaume, P. , Muller, J. , Schiemann, M. , Lennerz, V. , Cosma, A. , Peschel, C. , Busch, D.H. , Romero, P. , Bernhard, H. , 2009. Allorestricted T lymphocytes with a high avidity T-cell receptor towards NY-ESO-1 have potent anti-tumor activity. Int. J. Cancer. 125, 649–655. [DOI] [PubMed] [Google Scholar]
- Kuball, J. , Schmitz, F.W. , Voss, R.H. , Ferreira, E.A. , Engel, R. , Guillaume, P. , Strand, S. , Romero, P. , Huber, C. , Sherman, L.A. , Theobald, M. , 2005. Cooperation of human tumor-reactive CD4+ and CD8+ T cells after redirection of their specificity by a high-affinity p53A2.1-specific TCR. Immunity. 22, 117–129. [DOI] [PubMed] [Google Scholar]
- Kuball, J. , Dossett, M.L. , Wolfl, M. , Ho, W.Y. , Voss, R.H. , Fowler, C. , Greenberg, P.D. , 2007. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood. 109, 2331–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuball, J. , Hauptrock, B. , Malina, V. , Antunes, E. , Voss, R.H. , Wolfl, M. , Strong, R. , Theobald, M. , Greenberg, P.D. , 2009. Increasing functional avidity of TCR-redirected T cells by removing defined N-glycosylation sites in the TCR constant domain. J. Exp. Med. 206, 463–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari, S. , Walchli, S. , Fallang, L.E. , Yang, W. , Lund-Johansen, F. , Schumacher, T.N. , Olweus, J. , 2014. Alloreactive cytotoxic T cells provide means to decipher the immunopeptidome and reveal a plethora of tumor-associated self-epitopes. Proc. Natl. Acad. Sci. U. S. A. 111, 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers, C.H. , Sleijfer, S. , van Steenbergen, S. , van Elzakker, P. , van Krimpen, B. , Groot, C. , Vulto, A. , den Bakker, M. , Oosterwijk, E. , Debets, R. , Gratama, J.W. , 2013. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol. Ther. 21, 904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, F. , Zhao, B. , Haque, R. , Xiong, X. , Budgeon, L. , Christensen, N.D. , Wu, Y. , Song, J. , 2011. In vivo programming of tumor antigen-specific T lymphocytes from pluripotent stem cells to promote cancer immunosurveillance. Cancer Res. 71, 4742–4747. [DOI] [PubMed] [Google Scholar]
- Lesokhin, A.M. , Callahan, M.K. , Postow, M.A. , Wolchok, J.D. , 2015. On being less tolerant: enhanced cancer immunosurveillance enabled by targeting checkpoints and agonists of T cell activation. Sci. Transl. Med. 7, 280sr281 [DOI] [PubMed] [Google Scholar]
- Li, Y. , Bleakley, M. , Yee, C. , 2005. IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response. J. Immunol. 175, 2261–2269. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Moysey, R. , Molloy, P.E. , Vuidepot, A.L. , Mahon, T. , Baston, E. , Dunn, S. , Liddy, N. , Jacob, J. , Jakobsen, B.K. , Boulter, J.M. , 2005. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat. Biotechnol. 23, 349–354. [DOI] [PubMed] [Google Scholar]
- Li, L.P. , Lampert, J.C. , Chen, X. , Leitao, C. , Popovic, J. , Muller, W. , Blankenstein, T. , 2010. Transgenic mice with a diverse human T cell antigen receptor repertoire. Nat. Med. 16, 1029–1034. [DOI] [PubMed] [Google Scholar]
- Liddy, N. , Bossi, G. , Adams, K.J. , Lissina, A. , Mahon, T.M. , Hassan, N.J. , Gavarret, J. , Bianchi, F.C. , Pumphrey, N.J. , Ladell, K. , Gostick, E. , Sewell, A.K. , Lissin, N.M. , Harwood, N.E. , Molloy, P.E. , Li, Y. , Cameron, B.J. , Sami, M. , Baston, E.E. , Todorov, P.T. , Paston, S.J. , Dennis, R.E. , Harper, J.V. , Dunn, S.M. , Ashfield, R. , Johnson, A. , McGrath, Y. , Plesa, G. , June, C.H. , Kalos, M. , Price, D.A. , Vuidepot, A. , Williams, D.D. , Sutton, D.H. , Jakobsen, B.K. , 2012. Monoclonal TCR-redirected tumor cell killing. Nat. Med. 18, 980–987. [DOI] [PubMed] [Google Scholar]
- Linette, G.P. , Stadtmauer, E.A. , Maus, M.V. , Rapoport, A.P. , Levine, B.L. , Emery, L. , Litzky, L. , Bagg, A. , Carreno, B.M. , Cimino, P.J. , Binder-Scholl, G.K. , Smethurst, D.P. , Gerry, A.B. , Pumphrey, N.J. , Bennett, A.D. , Brewer, J.E. , Dukes, J. , Harper, J. , Tayton-Martin, H.K. , Jakobsen, B.K. , Hassan, N.J. , Kalos, M. , June, C.H. , 2013. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 122, 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnemann, C. , van Buuren, M.M. , Bies, L. , Verdegaal, E.M. , Schotte, R. , Calis, J.J. , Behjati, S. , Velds, A. , Hilkmann, H. , Atmioui, D.E. , Visser, M. , Stratton, M.R. , Haanen, J.B. , Spits, H. , van der Burg, S.H. , Schumacher, T.N. , 2015. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat. Med. 21, 81–85. [DOI] [PubMed] [Google Scholar]
- Logunova, N.N. , Viret, C. , Pobezinsky, L.A. , Miller, S.A. , Kazansky, D.B. , Sundberg, J.P. , Chervonsky, A.V. , 2005. Restricted MHC-peptide repertoire predisposes to autoimmunity. J. Exp. Med. 202, 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y.C. , Yao, X. , Li, Y.F. , El-Gamil, M. , Dudley, M.E. , Yang, J.C. , Almeida, J.R. , Douek, D.C. , Samuels, Y. , Rosenberg, S.A. , Robbins, P.F. , 2013. Mutated PPP1R3B is recognized by T cells used to treat a melanoma patient who experienced a durable complete tumor regression. J. Immunol. 190, 6034–6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malecek, K. , Zhong, S. , McGary, K. , Yu, C. , Huang, K. , Johnson, L.A. , Rosenberg, S.A. , Krogsgaard, M. , 2013. Engineering improved T cell receptors using an alanine-scan guided T cell display selection system. J. Immunol. Methods. 392, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malecek, K. , Grigoryan, A. , Zhong, S. , Gu, W.J. , Johnson, L.A. , Rosenberg, S.A. , Cardozo, T. , Krogsgaard, M. , 2014. Specific increase in potency via structure-based design of a TCR. J. Immunol. 193, 2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, D. , 1998. A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunol. Today. 19, 395–404. [DOI] [PubMed] [Google Scholar]
- Matsushita, N. , Pilon-Thomas, S.A. , Martin, L.M. , Riker, A.I. , 2008. Comparative methodologies of regulatory T cell depletion in a murine melanoma model. J. Immunol. Methods. 333, 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude, S.L. , Frey, N. , Shaw, P.A. , Aplenc, R. , Barrett, D.M. , Bunin, N.J. , Chew, A. , Gonzalez, V.E. , Zheng, Z. , Lacey, S.F. , Mahnke, Y.D. , Melenhorst, J.J. , Rheingold, S.R. , Shen, A. , Teachey, D.T. , Levine, B.L. , June, C.H. , Porter, D.L. , Grupp, S.A. , 2014. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molldrem, J.J. , Lee, P.P. , Kant, S. , Wieder, E. , Jiang, W. , Lu, S. , Wang, C. , Davis, M.M. , 2003. Chronic myelogenous leukemia shapes host immunity by selective deletion of high-avidity leukemia-specific T cells. J. Clin. Invest. 111, 639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, R.A. , Kakarla, S. , 2014. Genetic modification of T cells. Cancer J. 20, 145–150. [DOI] [PubMed] [Google Scholar]
- Morgan, R.A. , Dudley, M.E. , Wunderlich, J.R. , Hughes, M.S. , Yang, J.C. , Sherry, R.M. , Royal, R.E. , Topalian, S.L. , Kammula, U.S. , Restifo, N.P. , Zheng, Z. , Nahvi, A. , de Vries, C.R. , Rogers-Freezer, L.J. , Mavroukakis, S.A. , Rosenberg, S.A. , 2006. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 314, 126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, R.A. , Yang, J.C. , Kitano, M. , Dudley, M.E. , Laurencot, C.M. , Rosenberg, S.A. , 2010. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 18, 843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, R.A. , Chinnasamy, N. , Abate-Daga, D. , Gros, A. , Robbins, P.F. , Zheng, Z. , Dudley, M.E. , Feldman, S.A. , Yang, J.C. , Sherry, R.M. , Phan, G.Q. , Hughes, M.S. , Kammula, U.S. , Miller, A.D. , Hessman, C.J. , Stewart, A.A. , Restifo, N.P. , Quezado, M.M. , Alimchandani, M. , Rosenberg, A.Z. , Nath, A. , Wang, T. , Bielekova, B. , Wuest, S.C. , Akula, N. , McMahon, F.J. , Wilde, S. , Mosetter, B. , Schendel, D.J. , Laurencot, C.M. , Rosenberg, S.A. , 2013. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 36, 133–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, E.C. , Tsallios, A. , Bendle, G.M. , Xue, S.A. , Stauss, H.J. , 2005. A critical role of T cell antigen receptor-transduced MHC class I-restricted helper T cells in tumor protection. Proc. Natl. Acad. Sci. U. S. A. 102, 7934–7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini, L. , Blomer, U. , Gallay, P. , Ory, D. , Mulligan, R. , Gage, F.H. , Verma, I.M. , Trono, D. , 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 272, 263–267. [DOI] [PubMed] [Google Scholar]
- Newberg, M.H. , Smith, D.H. , Haertel, S.B. , Vining, D.R. , Lacy, E. , Engelhard, V.H. , 1996. Importance of MHC class 1 alpha2 and alpha3 domains in the recognition of self and non-self MHC molecules. J. Immunol. 156, 2473–2480. [PubMed] [Google Scholar]
- North, R.J. , 1982. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J. Exp. Med. 155, 1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obenaus, M. , Leitao, C. , Leisegang, M. , Chen, X. , Gavvovidis, I. , van der Bruggen, P. , Uckert, W. , Schendel, D.J. , Blankenstein, T. , 2015. Identification of human T-cell receptors with optimal affinity to cancer antigens using antigen-negative humanized mice. Nat. Biotechnol. 33, 402–407. [DOI] [PubMed] [Google Scholar]
- Ochi, T. , Fujiwara, H. , Okamoto, S. , An, J. , Nagai, K. , Shirakata, T. , Mineno, J. , Kuzushima, K. , Shiku, H. , Yasukawa, M. , 2011. Novel adoptive T-cell immunotherapy using a WT1-specific TCR vector encoding silencers for endogenous TCRs shows marked antileukemia reactivity and safety. Blood. 118, 1495–1503. [DOI] [PubMed] [Google Scholar]
- Okamoto, S. , Mineno, J. , Ikeda, H. , Fujiwara, H. , Yasukawa, M. , Shiku, H. , Kato, I. , 2009. Improved expression and reactivity of transduced tumor-specific TCRs in human lymphocytes by specific silencing of endogenous TCR. Cancer Res. 69, 9003–9011. [DOI] [PubMed] [Google Scholar]
- Okoye, I. , Wang, L. , Pallmer, K. , Richter, K. , Ichimura, T. , Haas, R. , Crouse, J. , Choi, O. , Heathcote, D. , Lovo, E. , Mauro, C. , Abdi, R. , Oxenius, A. , Rutschmann, S. , Ashton-Rickardt, P.G. , 2015. The protein LEM promotes CD8+ T cell immunity through effects on mitochondrial respiration. Science. 348, 995–1001. [DOI] [PubMed] [Google Scholar]
- Olive, K.P. , Tuveson, D.A. , 2006. The use of targeted mouse models for preclinical testing of novel cancer therapeutics. Clin. Cancer Res. 12, 5277–5287. [DOI] [PubMed] [Google Scholar]
- Palmer, D.C. , Chan, C.C. , Gattinoni, L. , Wrzesinski, C. , Paulos, C.M. , Hinrichs, C.S. , Powell, D.J. , Klebanoff, C.A. , Finkelstein, S.E. , Fariss, R.N. , Yu, Z. , Nussenblatt, R.B. , Rosenberg, S.A. , Restifo, N.P. , 2008. Effective tumor treatment targeting a melanoma/melanocyte-associated antigen triggers severe ocular autoimmunity. Proc. Natl. Acad. Sci. U. S. A. 105, 8061–8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst, M.R. , Joo, J. , Riley, J.P. , Yu, Z. , Li, Y. , Robbins, P.F. , Rosenberg, S.A. , 2009. Characterization of genetically modified T-cell receptors that recognize the CEA:691-699 peptide in the context of HLA-A2.1 on human colorectal cancer cells. Clin. Cancer Res. 15, 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst, M.R. , Yang, J.C. , Langan, R.C. , Dudley, M.E. , Nathan, D.A. , Feldman, S.A. , Davis, J.L. , Morgan, R.A. , Merino, M.J. , Sherry, R.M. , Hughes, M.S. , Kammula, U.S. , Phan, G.Q. , Lim, R.M. , Wank, S.A. , Restifo, N.P. , Robbins, P.F. , Laurencot, C.M. , Rosenberg, S.A. , 2011. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol. Ther. 19, 620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulos, C.M. , Wrzesinski, C. , Kaiser, A. , Hinrichs, C.S. , Chieppa, M. , Cassard, L. , Palmer, D.C. , Boni, A. , Muranski, P. , Yu, Z. , Gattinoni, L. , Antony, P.A. , Rosenberg, S.A. , Restifo, N.P. , 2007. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J. Clin. Invest. 117, 2197–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce, E.L. , Walsh, M.C. , Cejas, P.J. , Harms, G.M. , Shen, H. , Wang, L.S. , Jones, R.G. , Choi, Y. , 2009. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 460, 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perro, M. , Tsang, J. , Xue, S.A. , Escors, D. , Cesco-Gaspere, M. , Pospori, C. , Gao, L. , Hart, D. , Collins, M. , Stauss, H. , Morris, E.C. , 2010. Generation of multi-functional antigen-specific human T-cells by lentiviral TCR gene transfer. Gene Ther. 17, 721–732. [DOI] [PubMed] [Google Scholar]
- Philip, B. , Kokalaki, E. , Mekkaoui, L. , Thomas, S. , Straathof, K. , Flutter, B. , Marin, V. , Marafioti, T. , Chakraverty, R. , Linch, D. , Quezada, S.A. , Peggs, K.S. , Pule, M. , 2014. A highly compact epitope-based marker/suicide gene for easier and safer T-cell therapy. Blood. 124, 1277–1287. [DOI] [PubMed] [Google Scholar]
- Pierce, B.G. , Hellman, L.M. , Hossain, M. , Singh, N.K. , Vander Kooi, C.W. , Weng, Z. , Baker, B.M. , 2014. Computational design of the affinity and specificity of a therapeutic T cell receptor. PLoS Comput. Biol. 10, e1003478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittet, M.J. , Gati, A. , Le Gal, F.A. , Bioley, G. , Guillaume, P. , de Smedt, M. , Plum, J. , Speiser, D.E. , Cerottini, J.C. , Dietrich, P.Y. , Romero, P. , Zippelius, A. , 2006. Ex vivo characterization of allo-MHC-restricted T cells specific for a single MHC-peptide complex. J. Immunol. 176, 2330–2336. [DOI] [PubMed] [Google Scholar]
- Postow, M.A. , Chesney, J. , Pavlick, A.C. , Robert, C. , Grossmann, K. , McDermott, D. , Linette, G.P. , Meyer, N. , Giguere, J.K. , Agarwala, S.S. , Shaheen, M. , Ernstoff, M.S. , Minor, D. , Salama, A.K. , Taylor, M. , Ott, P.A. , Rollin, L.M. , Horak, C. , Gagnier, P. , Wolchok, J.D. , Hodi, F.S. , 2015. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 372, 2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto, P.A. , Durflinger, K.H. , Wunderlich, J.R. , Rosenberg, S.A. , Dudley, M.E. , 2010. Enrichment of CD8+ cells from melanoma tumor-infiltrating lymphocyte cultures reveals tumor reactivity for use in adoptive cell therapy. J. Immunother. 33, 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provasi, E. , Genovese, P. , Lombardo, A. , Magnani, Z. , Liu, P.Q. , Reik, A. , Chu, V. , Paschon, D.E. , Zhang, L. , Kuball, J. , Camisa, B. , Bondanza, A. , Casorati, G. , Ponzoni, M. , Ciceri, F. , Bordignon, C. , Greenberg, P.D. , Holmes, M.C. , Gregory, P.D. , Naldini, L. , Bonini, C. , 2012. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat. Med. 18, 807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada, S.A. , Simpson, T.R. , Peggs, K.S. , Merghoub, T. , Vider, J. , Fan, X. , Blasberg, R. , Yagita, H. , Muranski, P. , Antony, P.A. , Restifo, N.P. , Allison, J.P. , 2010. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J. Exp. Med. 207, 637–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radojcic, V. , Bezak, K.B. , Skarica, M. , Pletneva, M.A. , Yoshimura, K. , Schulick, R.D. , Luznik, L. , 2010. Cyclophosphamide resets dendritic cell homeostasis and enhances antitumor immunity through effects that extend beyond regulatory T cell elimination. Cancer Immunol. Immunother. 59, 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport, A.P. , Stadtmauer, E.A. , Binder-Scholl, G.K. , Goloubeva, O. , Vogl, D.T. , Lacey, S.F. , Badros, A.Z. , Garfall, A. , Weiss, B. , Finklestein, J. , Kulikovskaya, I. , Sinha, S.K. , Kronsberg, S. , Gupta, M. , Bond, S. , Melchiori, L. , Brewer, J.E. , Bennett, A.D. , Gerry, A.B. , Pumphrey, N.J. , Williams, D. , Tayton-Martin, H.K. , Ribeiro, L. , Holdich, T. , Yanovich, S. , Hardy, N. , Yared, J. , Kerr, N. , Philip, S. , Westphal, S. , Siegel, D.L. , Levine, B.L. , Jakobsen, B.K. , Kalos, M. , June, C.H. , 2015. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat. Med. 21, 914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray, S. , Chhabra, A. , Chakraborty, N.G. , Hegde, U. , Dorsky, D.I. , Chodon, T. , von Euw, E. , Comin-Anduix, B. , Koya, R.C. , Ribas, A. , Economou, J.S. , Rosenberg, S.A. , Mukherji, B. , 2010. MHC-I-restricted melanoma antigen specific TCR-engineered human CD4+ T cells exhibit multifunctional effector and helper responses, in vitro. Clin. Immunol. 136, 338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restifo, N.P. , Gattinoni, L. , 2013. Lineage relationship of effector and memory T cells. Curr. Opin. Immunol. 25, 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins, P.F. , Dudley, M.E. , Wunderlich, J. , El-Gamil, M. , Li, Y.F. , Zhou, J. , Huang, J. , Powell, D.J. , Rosenberg, S.A. , 2004. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J. Immunol. 173, 7125–7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins, P.F. , Li, Y.F. , El-Gamil, M. , Zhao, Y. , Wargo, J.A. , Zheng, Z. , Xu, H. , Morgan, R.A. , Feldman, S.A. , Johnson, L.A. , Bennett, A.D. , Dunn, S.M. , Mahon, T.M. , Jakobsen, B.K. , Rosenberg, S.A. , 2008. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J. Immunol. 180, 6116–6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins, P.F. , Morgan, R.A. , Feldman, S.A. , Yang, J.C. , Sherry, R.M. , Dudley, M.E. , Wunderlich, J.R. , Nahvi, A.V. , Helman, L.J. , Mackall, C.L. , Kammula, U.S. , Hughes, M.S. , Restifo, N.P. , Raffeld, M. , Lee, C.C. , Levy, C.L. , Li, Y.F. , El-Gamil, M. , Schwarz, S.L. , Laurencot, C. , Rosenberg, S.A. , 2011. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 29, 917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins, P.F. , Lu, Y.C. , El-Gamil, M. , Li, Y.F. , Gross, C. , Gartner, J. , Lin, J.C. , Teer, J.K. , Cliften, P. , Tycksen, E. , Samuels, Y. , Rosenberg, S.A. , 2013. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat. Med. 19, 747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins, P.F. , Kassim, S.H. , Tran, T.L. , Crystal, J.S. , Morgan, R.A. , Feldman, S.A. , Yang, J.C. , Dudley, M.E. , Wunderlich, J.R. , Sherry, R.M. , Kammula, U.S. , Hughes, M.S. , Restifo, N.P. , Raffeld, M. , Lee, C.C. , Li, Y.F. , El-Gamil, M. , Rosenberg, S.A. , 2015. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-Reactive T-cell receptor: long-term follow-up and correlates with response. Clin. Cancer Res. 21, 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg, S.A. , 2010. Of mice, not men: no evidence for graft-versus-host disease in humans receiving T-cell receptor-transduced autologous T cells. Mol. Ther. 18, 1744–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg, S.A. , Spiess, P. , Lafreniere, R. , 1986. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 233, 1318–1321. [DOI] [PubMed] [Google Scholar]
- Rosenberg, S.A. , Yannelli, J.R. , Yang, J.C. , Topalian, S.L. , Schwartzentruber, D.J. , Weber, J.S. , Parkinson, D.R. , Seipp, C.A. , Einhorn, J.H. , White, D.E. , 1994. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J. Natl. Cancer Inst. 86, 1159–1166. [DOI] [PubMed] [Google Scholar]
- Rosenberg, S.A. , Yang, J.C. , Restifo, N.P. , 2004. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 10, 909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg, S.A. , Yang, J.C. , Sherry, R.M. , Kammula, U.S. , Hughes, M.S. , Phan, G.Q. , Citrin, D.E. , Restifo, N.P. , Robbins, P.F. , Wunderlich, J.R. , Morton, K.E. , Laurencot, C.M. , Steinberg, S.M. , White, D.E. , Dudley, M.E. , 2011. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 17, 4550–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein, M.P. , Su, E.W. , Suriano, S. , Cloud, C.A. , Andrijauskaite, K. , Kesarwani, P. , Schwartz, K.M. , Williams, K.M. , Johnson, C.B. , Li, M. , Scurti, G.M. , Salem, M.L. , Paulos, C.M. , Garrett-Mayer, E. , Mehrotra, S. , Cole, D.J. , 2015. Interleukin-12 enhances the function and anti-tumor activity in murine and human CD8 T cells. Cancer Immunol. Immunother. 64, 539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadovnikova, E. , Stauss, H.J. , 1996. Peptide-specific cytotoxic T lymphocytes restricted by nonself major histocompatibility complex class I molecules: reagents for tumor immunotherapy. Proc. Natl. Acad. Sci. U. S. A. 93, 13114–13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadovnikova, E. , Jopling, L.A. , Soo, K.S. , Stauss, H.J. , 1998. Generation of human tumor-reactive cytotoxic T cells against peptides presented by non-self HLA class I molecules. Eur. J. Immunol. 28, 193–200. [DOI] [PubMed] [Google Scholar]
- Sallusto, F. , Lenig, D. , Forster, R. , Lipp, M. , Lanzavecchia, A. , 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 401, 708–712. [DOI] [PubMed] [Google Scholar]
- Savage, P. , Gao, L. , Vento, K. , Cowburn, P. , Man, S. , Steven, N. , Ogg, G. , McMichael, A. , Epenetos, A. , Goulmy, E. , Stauss, H.J. , 2004. Use of B cell-bound HLA-A2 class I monomers to generate high-avidity, allo-restricted CTLs against the leukemia-associated protein Wilms tumor antigen. Blood. 103, 4613–4615. [DOI] [PubMed] [Google Scholar]
- Schmid, D.A. , Irving, M.B. , Posevitz, V. , Hebeisen, M. , Posevitz-Fejfar, A. , Sarria, J.C. , Gomez-Eerland, R. , Thome, M. , Schumacher, T.N. , Romero, P. , Speiser, D.E. , Zoete, V. , Michielin, O. , Rufer, N. , 2010. Evidence for a TCR affinity threshold delimiting maximal CD8 T cell function. J. Immunol. 184, 4936–4946. [DOI] [PubMed] [Google Scholar]
- Scholten, K.B. , Kramer, D. , Kueter, E.W. , Graf, M. , Schoedl, T. , Meijer, C.J. , Schreurs, M.W. , Hooijberg, E. , 2006. Codon modification of T cell receptors allows enhanced functional expression in transgenic human T cells. Clin. Immunol. 119, 135–145. [DOI] [PubMed] [Google Scholar]
- Schumacher, T.N. , Schreiber, R.D. , 2015. Neoantigens in cancer immunotherapy. Science. 348, 69–74. [DOI] [PubMed] [Google Scholar]
- Sena, L.A. , Li, S. , Jairaman, A. , Prakriya, M. , Ezponda, T. , Hildeman, D.A. , Wang, C.R. , Schumacker, P.T. , Licht, J.D. , Perlman, H. , Bryce, P.J. , Chandel, N.S. , 2013. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 38, 225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok, J. , Warren, H.S. , Cuenca, A.G. , Mindrinos, M.N. , Baker, H.V. , Xu, W. , Richards, D.R. , McDonald-Smith, G.P. , Gao, H. , Hennessy, L. , Finnerty, C.C. , Lopez, C.M. , Honari, S. , Moore, E.E. , Minei, J.P. , Cuschieri, J. , Bankey, P.E. , Johnson, J.L. , Sperry, J. , Nathens, A.B. , Billiar, T.R. , West, M.A. , Jeschke, M.G. , Klein, M.B. , Gamelli, R.L. , Gibran, N.S. , Brownstein, B.H. , Miller-Graziano, C. , Calvano, S.E. , Mason, P.H. , Cobb, J.P. , Rahme, L.G. , Lowry, S.F. , Maier, R.V. , Moldawer, L.L. , Herndon, D.N. , Davis, R.W. , Xiao, W. , Tompkins, R.G. , 2013. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. U. S. A. 110, 3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, P. , Allison, J.P. , 2015. The future of immune checkpoint therapy. Science. 348, 56–61. [DOI] [PubMed] [Google Scholar]
- Simpson, A.A. , Mohammed, F. , Salim, M. , Tranter, A. , Rickinson, A.B. , Stauss, H.J. , Moss, P.A. , Steven, N.M. , Willcox, B.E. , 2011. Structural and energetic evidence for highly peptide-specific tumor antigen targeting via allo-MHC restriction. Proc. Natl. Acad. Sci. U. S. A. 108, 21176–21181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sledzinska, A. , Menger, L. , Bergerhoff, K. , Peggs, K.S. , Quezada, S.A. , 2015. Negative immune checkpoints on T lymphocytes and their relevance to cancer immunotherapy. Mol. Oncol. 9, 1936–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solal-Celigny, P. , 2006. Safety of rituximab maintenance therapy in follicular lymphomas. Leuk. Res. 30, (Suppl. 1) S16–S21. [DOI] [PubMed] [Google Scholar]
- Sommermeyer, D. , Uckert, W. , 2010. Minimal amino acid exchange in human TCR constant regions fosters improved function of TCR gene-modified T cells. J. Immunol. 184, 6223–6231. [DOI] [PubMed] [Google Scholar]
- Stanislawski, T. , Voss, R.H. , Lotz, C. , Sadovnikova, E. , Willemsen, R.A. , Kuball, J. , Ruppert, T. , Bolhuis, R.L. , Melief, C.J. , Huber, C. , Stauss, H.J. , Theobald, M. , 2001. Circumventing tolerance to a human MDM2-derived tumor antigen by TCR gene transfer. Nat. Immunol. 2, 962–970. [DOI] [PubMed] [Google Scholar]
- Stronen, E. , Abrahamsen, I.W. , Gaudernack, G. , Walchli, S. , Munthe, E. , Buus, S. , Johansen, F.E. , Lund-Johansen, F. , Olweus, J. , 2009. Dendritic cells engineered to express defined allo-HLA peptide complexes induce antigen-specific cytotoxic T cells efficiently killing tumour cells. Scand. J. Immunol. 69, 319–328. [DOI] [PubMed] [Google Scholar]
- Szymczak, A.L. , Workman, C.J. , Wang, Y. , Vignali, K.M. , Dilioglou, S. , Vanin, E.F. , Vignali, D.A. , 2004. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat. Biotechnol. 22, 589–594. [DOI] [PubMed] [Google Scholar]
- Tahara, H. , Fujio, K. , Araki, Y. , Setoguchi, K. , Misaki, Y. , Kitamura, T. , Yamamoto, K. , 2003. Reconstitution of CD8+ T cells by retroviral transfer of the TCR alpha beta-chain genes isolated from a clonally expanded P815-infiltrating lymphocyte. J. Immunol. 171, 2154–2160. [DOI] [PubMed] [Google Scholar]
- Theobald, M. , Biggs, J. , Dittmer, D. , Levine, A.J. , Sherman, L.A. , 1995. Targeting p53 as a general tumor antigen. Proc. Natl. Acad. Sci. U. S. A. 92, 11993–11997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoret, M.R. , Cohen, C.J. , Nahvi, A.V. , Ngo, L.T. , Suri, K.B. , Powell, D.J. , Dudley, M.E. , Morgan, R.A. , Rosenberg, S.A. , 2008. Relationship of p53 overexpression on cancers and recognition by anti-p53 T cell receptor-transduced T cells. Hum. Gene Ther. 19, 1219–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, E.D. , Lochte, H.L. , Lu, W.C. , Ferrebee, J.W. , 1957. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N. Engl. J. Med. 257, 491–496. [DOI] [PubMed] [Google Scholar]
- Thomas, S. , Xue, S.A. , Bangham, C.R. , Jakobsen, B.K. , Morris, E.C. , Stauss, H.J. , 2011. Human T cells expressing affinity-matured TCR display accelerated responses but fail to recognize low density of MHC-peptide antigen. Blood. 118, 319–329. [DOI] [PubMed] [Google Scholar]
- Topalian, S.L. , Solomon, D. , Avis, F.P. , Chang, A.E. , Freerksen, D.L. , Linehan, W.M. , Lotze, M.T. , Robertson, C.N. , Seipp, C.A. , Simon, P. , 1988. Immunotherapy of patients with advanced cancer using tumor-infiltrating lymphocytes and recombinant interleukin-2: a pilot study. J. Clin. Oncol. 6, 839–853. [DOI] [PubMed] [Google Scholar]
- Topalian, S.L. , Hodi, F.S. , Brahmer, J.R. , Gettinger, S.N. , Smith, D.C. , McDermott, D.F. , Powderly, J.D. , Carvajal, R.D. , Sosman, J.A. , Atkins, M.B. , Leming, P.D. , Spigel, D.R. , Antonia, S.J. , Horn, L. , Drake, C.G. , Pardoll, D.M. , Chen, L. , Sharfman, W.H. , Anders, R.A. , Taube, J.M. , McMiller, T.L. , Xu, H. , Korman, A.J. , Jure-Kunkel, M. , Agrawal, S. , McDonald, D. , Kollia, G.D. , Gupta, A. , Wigginton, J.M. , Sznol, M. , 2012. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torikai, H. , Reik, A. , Liu, P.Q. , Zhou, Y. , Zhang, L. , Maiti, S. , Huls, H. , Miller, J.C. , Kebriaei, P. , Rabinovitch, B. , Lee, D.A. , Champlin, R.E. , Bonini, C. , Naldini, L. , Rebar, E.J. , Gregory, P.D. , Holmes, M.C. , Cooper, L.J. , 2012. A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood. 119, 5697–5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran, E. , Turcotte, S. , Gros, A. , Robbins, P.F. , Lu, Y.C. , Dudley, M.E. , Wunderlich, J.R. , Somerville, R.P. , Hogan, K. , Hinrichs, C.S. , Parkhurst, M.R. , Yang, J.C. , Rosenberg, S.A. , 2014. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 344, 641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udaka, K. , Tsomides, T.J. , Eisen, H.N. , 1992. A naturally occurring peptide recognized by alloreactive CD8+ cytotoxic T lymphocytes in association with a class I MHC protein. Cell. 69, 989–998. [DOI] [PubMed] [Google Scholar]
- Uhlen, M. , Fagerberg, L. , Hallstrom, B.M. , Lindskog, C. , Oksvold, P. , Mardinoglu, A. , Sivertsson, A. , Kampf, C. , Sjostedt, E. , Asplund, A. , Olsson, I. , Edlund, K. , Lundberg, E. , Navani, S. , Szigyarto, C.A. , Odeberg, J. , Djureinovic, D. , Takanen, J.O. , Hober, S. , Alm, T. , Edqvist, P.H. , Berling, H. , Tegel, H. , Mulder, J. , Rockberg, J. , Nilsson, P. , Schwenk, J.M. , Hamsten, M. , von Feilitzen, K. , Forsberg, M. , Persson, L. , Johansson, F. , Zwahlen, M. , von Heijne, G. , Nielsen, J. , Ponten, F. , 2015. Proteomics. Tissue-based map of the human proteome. Science. 347, 1260419 [DOI] [PubMed] [Google Scholar]
- van den Berg, J.H. , Gomez-Eerland, R. , van de Wiel, B. , Hulshoff, L. , van den Broek, D. , Bins, A. , Tan, H.L. , Harper, J.V. , Hassan, N.J. , Jakobsen, B.K. , Jorritsma, A. , Blank, C.U. , Schumacher, T.N. , Haanen, J.B. , 2015. Case Report of a Fatal Serious Adverse Event Upon Administration of T Cells Transduced With a MART-1-specific T-cell Receptor. Mol. Ther. 23, 1541–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Burg, S.H. , Visseren, M.J. , Brandt, R.M. , Kast, W.M. , Melief, C.J. , 1996. Immunogenicity of peptides bound to MHC class I molecules depends on the MHC-peptide complex stability. J. Immunol. 156, 3308–3314. [PubMed] [Google Scholar]
- van der Windt, G.J. , Everts, B. , Chang, C.H. , Curtis, J.D. , Freitas, T.C. , Amiel, E. , Pearce, E.J. , Pearce, E.L. , 2012. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 36, 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loenen, M.M. , de Boer, R. , Amir, A.L. , Hagedoorn, R.S. , Volbeda, G.L. , Willemze, R. , van Rood, J.J. , Falkenburg, J.H. , Heemskerk, M.H. , 2010. Mixed T cell receptor dimers harbor potentially harmful neoreactivity. Proc. Natl. Acad. Sci. U. S. A. 107, 10972–10977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij, N. , van Buuren, M.M. , Philips, D. , Velds, A. , Toebes, M. , Heemskerk, B. , van Dijk, L.J. , Behjati, S. , Hilkmann, H. , El Atmioui, D. , Nieuwland, M. , Stratton, M.R. , Kerkhoven, R.M. , Kesmir, C. , Haanen, J.B. , Kvistborg, P. , Schumacher, T.N. , 2013. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J. Clin. Oncol. 31, e439–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatakis, D.N. , Arumugam, B. , Kim, S.G. , Bristol, G. , Yang, O. , Zack, J.A. , 2013. Introduction of exogenous T-cell receptors into human hematopoietic progenitors results in exclusion of endogenous T-cell receptor expression. Mol. Ther. 21, 1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera, J.F. , Hoyos, V. , Savoldo, B. , Quintarelli, C. , Giordano Attianese, G.M. , Leen, A.M. , Liu, H. , Foster, A.E. , Heslop, H.E. , Rooney, C.M. , Brenner, M.K. , Dotti, G. , 2009. Genetic manipulation of tumor-specific cytotoxic T lymphocytes to restore responsiveness to IL-7. Mol. Ther. 17, 880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello, A. , Marchesini, D. , Furze, J. , Sherman, L.A. , Chesnut, R.W. , 1991. Analysis of the HLA-restricted influenza-specific cytotoxic T lymphocyte response in transgenic mice carrying a chimeric human-mouse class I major histocompatibility complex. J. Exp. Med. 173, 1007–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L.X. , Westwood, J.A. , Moeller, M. , Duong, C.P. , Wei, W.Z. , Malaterre, J. , Trapani, J.A. , Neeson, P. , Smyth, M.J. , Kershaw, M.H. , Darcy, P.K. , 2010. Tumor ablation by gene-modified T cells in the absence of autoimmunity. Cancer Res. 70, 9591–9598. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Chang, W.C. , Wong, C.W. , Colcher, D. , Sherman, M. , Ostberg, J.R. , Forman, S.J. , Riddell, S.R. , Jensen, M.C. , 2011. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 118, 1255–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whilding, L.M. , Maher, J. , 2015. CAR T-cell immunotherapy: the path from the by-road to the freeway?. Mol. Oncol. 9, 1994–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelegg, A.M. , Oosten, L.E. , Jordan, S. , Kester, M. , van Halteren, A.G. , Madrigal, J.A. , Goulmy, E. , Barber, L.D. , 2005. Investigation of peptide involvement in T cell allorecognition using recombinant HLA class I multimers. J. Immunol. 175, 1706–1714. [DOI] [PubMed] [Google Scholar]
- Wick, D.A. , Webb, J.R. , Nielsen, J.S. , Martin, S.D. , Kroeger, D.R. , Milne, K. , Castellarin, M. , Twumasi-Boateng, K. , Watson, P.H. , Holt, R.A. , Nelson, B.H. , 2014. Surveillance of the tumor mutanome by T cells during progression from primary to recurrent ovarian cancer. Clin. Cancer Res. 20, 1125–1134. [DOI] [PubMed] [Google Scholar]
- Wilde, S. , Sommermeyer, D. , Frankenberger, B. , Schiemann, M. , Milosevic, S. , Spranger, S. , Pohla, H. , Uckert, W. , Busch, D.H. , Schendel, D.J. , 2009. Dendritic cells pulsed with RNA encoding allogeneic MHC and antigen induce T cells with superior antitumor activity and higher TCR functional avidity. Blood. 114, 2131–2139. [DOI] [PubMed] [Google Scholar]
- Willemsen, R. , Ronteltap, C. , Heuveling, M. , Debets, R. , Bolhuis, R. , 2005. Redirecting human CD4+ T lymphocytes to the MHC class I-restricted melanoma antigen MAGE-A1 by TCR alphabeta gene transfer requires CD8alpha. Gene Ther. 12, 140–146. [DOI] [PubMed] [Google Scholar]
- Wooldridge, L. , Ekeruche-Makinde, J. , van den Berg, H.A. , Skowera, A. , Miles, J.J. , Tan, M.P. , Dolton, G. , Clement, M. , Llewellyn-Lacey, S. , Price, D.A. , Peakman, M. , Sewell, A.K. , 2012. A single autoimmune T cell receptor recognizes more than a million different peptides. J. Biol. Chem. 287, 1168–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, C.Y. , Roybal, K.T. , Puchner, E.M. , Onuffer, J. , Lim, W.A. , 2015. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science. 350, aab4077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Y. , Akpinarli, A. , Maris, C. , Hipkiss, E.L. , Lane, M. , Kwon, E.K. , Muranski, P. , Restifo, N.P. , Antony, P.A. , 2010. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J. Exp. Med. 207, 651–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y. , Zhang, M. , Ramos, C.A. , Durett, A. , Liu, E. , Dakhova, O. , Liu, H. , Creighton, C.J. , Gee, A.P. , Heslop, H.E. , Rooney, C.M. , Savoldo, B. , Dotti, G. , 2014. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood. 123, 3750–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, S.A. , Gao, L. , Hart, D. , Gillmore, R. , Qasim, W. , Thrasher, A. , Apperley, J. , Engels, B. , Uckert, W. , Morris, E. , Stauss, H. , 2005. Elimination of human leukemia cells in NOD/SCID mice by WT1-TCR gene-transduced human T cells. Blood. 106, 3062–3067. [DOI] [PubMed] [Google Scholar]
- Yang, S. , Cohen, C.J. , Peng, P.D. , Zhao, Y. , Cassard, L. , Yu, Z. , Zheng, Z. , Jones, S. , Restifo, N.P. , Rosenberg, S.A. , Morgan, R.A. , 2008. Development of optimal bicistronic lentiviral vectors facilitates high-level TCR gene expression and robust tumor cell recognition. Gene Ther. 15, 1411–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, S. , Dudley, M.E. , Rosenberg, S.A. , Morgan, R.A. , 2010. A simplified method for the clinical-scale generation of central memory-like CD8+ T cells after transduction with lentiviral vectors encoding antitumor antigen T-cell receptors. J. Immunother. 33, 648–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, S. , Gattinoni, L. , Liu, F. , Ji, Y. , Yu, Z. , Restifo, N.P. , Rosenberg, S.A. , Morgan, R.A. , 2011. In vitro generated anti-tumor T lymphocytes exhibit distinct subsets mimicking in vivo antigen-experienced cells. Cancer Immunol. Immunother. 60, 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, S. , Ji, Y. , Gattinoni, L. , Zhang, L. , Yu, Z. , Restifo, N.P. , Rosenberg, S.A. , Morgan, R.A. , 2013. Modulating the differentiation status of ex vivo-cultured anti-tumor T cells using cytokine cocktails. Cancer Immunol. Immunother. 62, 727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, X. , Ahmadzadeh, M. , Lu, Y.C. , Liewehr, D.J. , Dudley, M.E. , Liu, F. , Schrump, D.S. , Steinberg, S.M. , Rosenberg, S.A. , Robbins, P.F. , 2012. Levels of peripheral CD4(+)FoxP3(+) regulatory T cells are negatively associated with clinical response to adoptive immunotherapy of human cancer. Blood. 119, 5688–5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeh, H.J. , Perry-Lalley, D. , Dudley, M.E. , Rosenberg, S.A. , Yang, J.C. , 1999. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J. Immunol. 162, 989–994. [PubMed] [Google Scholar]
- Zhao, Y. , Bennett, A.D. , Zheng, Z. , Wang, Q.J. , Robbins, P.F. , Yu, L.Y. , Li, Y. , Molloy, P.E. , Dunn, S.M. , Jakobsen, B.K. , Rosenberg, S.A. , Morgan, R.A. , 2007. High-affinity TCRs generated by phage display provide CD4+ T cells with the ability to recognize and kill tumor cell lines. J. Immunol. 179, 5845–5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, P. , Shaffer, D.R. , Alvarez Arias, D.A. , Nakazaki, Y. , Pos, W. , Torres, A.J. , Cremasco, V. , Dougan, S.K. , Cowley, G.S. , Elpek, K. , Brogdon, J. , Lamb, J. , Turley, S.J. , Ploegh, H.L. , Root, D.E. , Love, J.C. , Dranoff, G. , Hacohen, N. , Cantor, H. , Wucherpfennig, K.W. , 2014. In vivo discovery of immunotherapy targets in the tumour microenvironment. Nature. 506, 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Q. , Facciponte, J. , Jin, M. , Shen, Q. , Lin, Q. , 2014. Humanized NOD-SCID IL2rg-/- mice as a preclinical model for cancer research and its potential use for individualized cancer therapies. Cancer Lett. 344, 13–19. [DOI] [PubMed] [Google Scholar]


