Abstract
Targeted therapy is currently under intensive investigation due to the resistance of liver cancer to cytotoxic chemotherapies. Dissecting the molecular events that drive the progression of liver cancer and defining specific targets are urgently needed to develop efficient tailored therapies. Cell membrane gp96 (mgp96) has been implicated in tumor growth and malignancy. Here, we explored the functional and clinical relevance of mgp96 in liver cancer. We found that elevated mgp96 abundance was associated with tumor metastasis and recurrence in patients with primary liver tumors. Decreased KDELR1 levels in hepatoma cells contribute to cell membrane translocation of the normally ER‐resident gp96. Urokinase‐type plasminogen activator receptor (uPAR) was identified as a mgp96 client protein, and mgp96 stabilized uPAR protein. Our clinical results proved that elevated mgp96 abundance is positively correlated with uPAR expression levels in liver tumors. We further provided evidence that targeting mgp96 with siRNA or a specific mAb that blocked the mgp96‐uPAR interaction led to inhibited cell growth, survival, and invasion in vitro, as well as the suppression of liver tumor growth and metastasis in vivo. mgp96 promotes liver cancer progression through increasing the protein stability and signaling of uPAR, and may be a new promising target for suppressing uPAR‐mediated tumor growth and metastasis in liver cancer.
Keywords: mgp96, uPAR, Liver cancer, MAPK, DFS
Highlights
mgp96 expression is attributed to the decreased KDELR1 levels in hepatoma cells.
Elevated mgp96 level is associated with poor prognosis in primary HCC patients.
mgp96 regulates cell proliferation, survival, and invasion through uPAR.
Targeting mgp96 inhibits uPAR‐driven cell growth, survival, and invasion.
Abbreviations
- ADAMTS9
a disintegrin-like and metalloprotease domain with thrombospondin type 1 motifs 9
- AIMP1
aminoacyl tRNA synthetase complex-interacting multifunctional protein 1
- AKT
v-akt murine thymoma viral oncogene homolog
- mTOR
mammalian target of Rapamycin
- AP1
activator protein 1
- AP2
activator protein 2
- DFS
disease-free survival
- ECM
extracellular matrix
- EGFR
epidermal growth factor receptor
- ENDO180
endocytic receptor 180
- ER
endoplasmic reticulum
- ERK
extracellular signal-regulated kinases
- FAK
focal adhesion kinase
- gp96
glycoprotein 96
- HCC
hepatocellular carcinoma
- HER2
human epidermal growth factor receptor 2
- HIF1α
hypoxia-inducible factor 1α
- HSP
heat shock protein
- IGF
insulin-like growth factor
- ISX
intestine-specific homeobox
- JAK-STAT
Janus kinase/signal transducers and activators of transcription
- KDELR1
KDEL (Lys-Asp-Glu-Leu) endoplasmic reticulum protein retention receptor 1
- LAMP1
lysosomal-associated membrane protein 1
- LRP-1
low density lipoprotein receptor-related protein 1
- mAb
monoclonal antibody
- MAPK
mitogen-activated protein kinase
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PAI-1
plasminogen activator inhibitor-1
- PBF
PTTG1 binding factor
- PCR
polymerase chain reaction
- PDGFR
platelet-derived growth factor receptor
- PI3K
phosphoinositide-3-kinase
- shRNA
short hairpin RNA
- siRNA
small interfering RNA
- SP1
specificity protein 1
- uPA
urokinase-type plasminogen activator
- uPAR
urokinase-type plasminogen activator receptor
- VEGFR
vascular endothelial growth factor receptor
1. Introduction
Worldwide, liver cancer ranks as the sixth most common cancer in incidence and the third most common cause of cancer mortality (Pez et al., 2013; Nakagawa and Maeda, 2012). The development of liver cancer involves long‐term and multistep neoplastic transformation. Under conditions of chronic viral infection, the persistent cycle of necro‐inflammation and hepatocyte regeneration promotes genetic mutations and oxidative DNA damage in hepatocytes and survival of initiated cells (Nakagawa and Maeda, 2012; Goldstein and Li, 2009). Multiple signaling pathways and factors are involved in hepatocarcinogenesis and liver cancer progression, including the NF‐κB, JAK‐STAT, Raf/MAPK/ERK, Wnt‐β‐catenin, and PI3K/AKT/mTOR pathways, as well as the proinflammatory homeobox gene (ISX), insulin‐like growth factor (IGF), urokinase‐type plasminogen activator (uPA) and its receptor (uPAR), vascular endothelial growth factor receptor (VEGFR), platelet‐derived growth factor receptor (PDGFR), epidermal growth factor receptor (EGFR), and PTTG1 binding factor (PBF) (Pez et al., 2013, 2012, 2013, 2013, 2004).
Due to its potent anti‐apoptotic activity and unique regenerative characteristics, liver cancer is generally resistant to cytotoxic chemotherapies (Epstein and Leung, 2007). Additionally, liver cancer is a highly heterogeneous disease displaying temporal regulation of complex signaling networks. Aside from sorafenib, which is an anti‐angiogenic inhibitor of several kinases (e.g., Raf, VEGFR, and PDGFR), no targeted therapy is effective for treating patients with hepatocellular carcinoma (HCC) (Kudo, 2011). Given that currently defined signaling pathways in liver cancer possess broad functions and reciprocal crosstalk, uncovering the potential specific targets and blocking positive feedback loops of regenerative pathways may be a more efficient way to develop liver cancer treatments.
Heat shock protein (HSP) gp96 is an endoplasmic reticulum (ER)‐resident member of the HSP90 family. It mainly functions as a molecular chaperone that participates in the folding and extension of target proteins and guides their assembly and maturation (Marzec et al., 2012; Hong et al., 2013). The normally ER‐resident gp96 translocates to the cell membrane under certain circumstances. For example, some microbial stimuli such as Listeria monocytogenes and Escherichia coli K1 infections up‐regulate the cell membrane expression of gp96 (Martins et al., 2012; Mittal and Prasadarao, 2011; Cabanes et al., 2005). Moreover, cell membrane expression of gp96 has also been observed in some types of tumor cells and is associated with tumor malignancy (Melendez et al., 2006; Altmeyer et al., 1996). Aminoacyl tRNA synthetase complex‐interacting multifunctional protein 1 (AIMP1) may play a critical role in regulating cell membrane expression of gp96 by affecting the interaction between gp96 and KDELR1 in the ER (Han et al., 2007; Kim et al., 2010). mgp96 displays different roles in different contexts, such as a bacterial receptor during infections (Martins et al., 2012; Mittal and Prasadarao, 2011) and the incentive for autoimmune diseases (Dai et al., 2007). In tumor cells, mgp96 binds to HER2 (Chavany et al., 1996; Patel et al., 2013) and metalloprotease pro‐a disintegrin‐like and metalloproteinase domain with thrombospondin type 1 motifs 9 (pro‐ADAMTS9) (Koo and Apte, 2010), to sustain the stability or modulate the processing of these membrane molecules, leading to increased tumor growth and angiogenesis.
In this study, we examined the cell membrane expression status of gp96 in hepatoma cells and liver cancer tissues. Then, we identified mgp96‐associated proteins in hepatoma cells and further explored the impact of interactions between mgp96 and its client proteins on cell growth, which may offer a new therapeutic strategy for liver cancer treatment.
2. Materials and methods
2.1. Cell lines, viruses, antibodies, and reagents
All the human liver cell lines (L02, Chang Liver, Huh7, HepG2, SK‐Hep‐1) were obtained from ATCC. The recombinant adenoviruses, ad‐mgp96 expressing the mgp96, and control adenoviruses ad‐pDC312 were created by our lab. gp96 polyclonal antibody and protein G were purchased from Santa Cruz Biotechnology. The uPAR monoclonal and polyclonal antibodies were obtained from R&D systems. gp96 mAb was produced by our lab. All the signaling pathway antibodies were purchased from Cell Signaling Technology. All other antibodies were obtained from Zhongshan Goldenbridge Biotechnology. The protein cross‐linkers DTSSP and BS3 were purchased from Thermo Scientific. Glutathione Sepharose 4B was from GE Healthcare Life Sciences. Cycloheximide (CHX) was from Beyotime Institute of Biotechnology.
2.2. Tumorigenicity and metastasis assays in vivo
Model 1 – six‐week‐old female BALB/c nude mice were randomly divided into two groups (n = 6/group) and injected s.c. in the right hind flank with 1 × 107 SK‐Hep‐1‐gp96‐RNAi or SK‐Hep‐1‐luc‐RNAi cells. Tumor growth was monitored every 3 days, and tumor size was calculated with the formula: Tv = (L × W2)/2. Mice were sacrificed 30 days after injection for tumor weight evaluation.
Model 2 – six‐week‐old female BALB/c nude mice were injected s.c. in the right hind flank with 1 × 107 SK‐Hep‐1 or Huh7 cells. Tumor growth was monitored every 3 days. Mice were randomly divided to two groups (n = 5/group) when tumors reached a volume of ∼100 mm3. Mice were treated with gp96 mAb or control antibody (2 mg/kg) via intraperitoneal injection twice a week. Three weeks later, mice were sacrificed for tumor weight evaluation.
Model 3 – SK‐Hep‐1‐gp96‐RNAi or SK‐Hep‐1‐luc‐RNAi cells were washed with Ca2+‐ and Mg2+‐free Hank's solution, and 5 × 106 cells (in 50 μl Matrigel) were subserosally injected into the left lobe of the livers of female SCID mice (n = 6/group). All mice were sacrificed for metastasis assays when they appeared distressed (about 7 weeks). Lungs were fixed in 10% neutral formalin for H&E staining. The metastatic sites were counted in 10 random fields by light microscope. The test was repeated twice by two independent viewers.
Model 4 – SK‐Hep‐1 or Huh7 cells were washed with Ca2+‐ and Mg2+‐free Hank's solution, and 5 × 106 cells (in 50 μl Matrigel) were subserosally injected into the left lobe of the livers of female SCID mice. Three weeks after injection, mice were randomly divided into two groups (n = 6/group) and treated with gp96 mAb or control antibody (2 mg/kg) via intraperitoneal injection twice a week for three weeks. Mice were sacrificed for metastasis assays when they appeared distressed. Lungs were fixed in 10% neutral formalin for H&E staining. The metastatic sites were counted in 10 random fields by light microscope. The test was repeated twice by two independent viewers.
Mice were maintained and cared for in strict compliance with the guidelines of the Institute of Microbiology, Chinese Academy of Sciences of Research Ethics Committee. All procedures were approved by the Research Ethics Committee.
2.3. Patient samples and immunohistochemistry (IHC)
Paraffin‐embedded liver tumor samples and corresponding tumor‐adjacent tissues obtained from 88 patients between 2006 and 2013 (302 Military Hospital of China) were used for this study. The clinical characteristics of the studied subjects are listed in Table 1. IHC was performed as described (Wang et al., 2012). Cell membrane gp96 and uPAR straining were categorized as high (score 2 + or 3+) and low (score 0 or 1+) expression on the basis of the staining intensity and immunoreactive cell percentage according to a widely used scoring method (slightly modified) (Hicks and Tubbs, 2005). The scoring is described as follows: Score 0 (−), no staining; Score 1+, faint and incomplete cell membrane staining of hepatocytes; Score 2+, moderate complete membrane staining in at least 50% of hepatocytes; Score 3+, strong complete membrane staining in more than 50% of hepatocytes. The assessments were scored by two independent observers in a blinded manner.
Table 1.
The associations of mgp96 expression with clinicopathologic characteristics in patients with primary HCC.
| Characteristics | mgp96 expression | P | |
|---|---|---|---|
| Low (n = 26) | High (n = 62) | ||
| Age (years) | 0.862 | ||
| <50 | 12.5% (11/88) | 28.4 (25/88) | |
| ≥50 | 17.0% (15/88) | 42.0% (37/88) | |
| Gender | 0.806 | ||
| Male | 27.3% (24/88) | 65.9% (58/88) | |
| Female | 2.3% (2/88) | 4.5% (4/88) | |
| Tumor size | 0.127 | ||
| <3 cm | 11.4% (10/88) | 15.9% (14/88) | |
| ≥3 cm | 18.2% (16/88) | 54.5% (48/88) | |
| Tumor number | 0.522 | ||
| =1 | 19.3% (17/88) | 40.9% (36/88) | |
| >1 | 10.2% (9/88) | 29.6% (26/88) | |
| HBs Ag | 0.022 | ||
| Positive | 19.3% (17/88) | 62.5% (55/88) | |
| Negative | 10.2% (9/88) | 8% (7/88) | |
| T stage | 0.019 | ||
| I–II | 6.8% (6/88) | 35.2% (31/88) | |
| III–IV | 22.7% (20/88) | 35.2% (31/88) | |
| Differentiation grade | 0.041 | ||
| Well differentiated | 6.8% (6/88) | 4.5% (4/88) | |
| Moderately differentiated | 18.2% (16/88) | 43.2% (38/88) | |
| Poorly differentiated | 4.5% (4/88) | 22.7% (20/88) | |
| Extrahepatic spread | 0.042 | ||
| Yes | 15.9% (14/88) | 53.4% (47/88) | |
| No | 13.6% (12/88) | 17.0% (15/88) | |
| Recurrence | 0.01 | ||
| Positive | 15.9% (14/88) | 56.8% (50/88) | |
| Negative | 13.6% (12/88) | 13.6% (12/88) | |
2.4. Methods
The methods of Western blot and co‐IP, cross‐linking, mass spectrometry, GST pull‐down, flow cytometry, confocal microscopy, cell proliferation and apoptosis, invasion, establishment of stable shRNA transfectant, statistical analysis are described in the Supporting Information.
3. Results
3.1. Overexpression of mgp96 is associated with poor prognosis in patients with primary liver tumors
To address the clinical relevance of gp96 in liver cancer, we analyzed mgp96 levels in 88 patients with primary liver tumors. We found that mgp96 was highly expressed in up to 70.5% (62 of 88) of liver tumors (Figure 1A). Compared to patients with low mgp96 expression, cases with high mgp96 expression had lower tumor stage, differentiation grades, and higher extrahepatic tumor spread rate and recurrent rate (all P < 0.05) (Table 1). In addition, mgp96 expression was also associated with HBV infection (P = 0.022). There were no statistical differences in gender, age, tumor size, or tumor number between patients with low mgp96 expression and those with high mgp96 expression. As shown in Figure 1B and C, patients with high mgp96 levels had a much shorter DFS, as well as advanced tumoral disease (tumor stage and extrahepatic spread) and higher recurrence rate than those with low mgp96 expression, implying that elevated mgp96 expression in liver cancer may be associated with poor prognosis.
Figure 1.

mgp96 was overexpressed in liver cancer tissues and hepatoma cells. (A) IHC analysis of mgp96 expression in primary liver tumors. Arrows indicate mgp96 positive cells. Scale bar, 20 μm. (B) Kaplan–Meier survival analysis of mgp96 expression for DFS in patients with primary liver tumors. (C) Tumor stage (left), extrahepatic spread (middle) and recurrence rate (right) were analyzed in patients with primary HCC. (D) Flow cytometric analysis of mgp96 levels in normal liver cells and hepatoma cells. Cells stained with control IgG served as a control. (E) Levels of KDELR1 protein were detected by Western blot. (F) Flow cytometric analysis of the expression of mgp96 in L02 and Chang Liver cells treated with KDELR1 siRNA or control siRNA (mock) for 48 h. Cells stained with control IgG served as a control.
Human normal liver cell lines L02 and Chang Liver, as well as hepatoma cell lines Huh7, HepG2, and SK‐Hep‐1, were used to analyze mgp96 expression. mgp96 was highly expressed on hepatoma cells but not on normal liver cells as determined by flow cytometry (Figure 1D). Expression of KDELR1 was then assessed which is essential for the retention of gp96 in the ER. We found that KDELR1 levels were much higher in L02 and Chang Liver cells with low mgp96, than in Huh7, HepG2, and SK‐Hep‐1 cells with high mgp96 (Figure 1E). Moreover, KDELR1 knock‐down by RNAi resulted in an obvious increase in mgp96, indicating that the cell membrane expression of the normally ER‐resident gp96 may be attributed to the decreased KDELR1 levels in hepatoma cells (Figure 1F).
3.2. mgp96 interacts with uPAR on the cell membrane of hepatoma cells, increases its stability, and enhances cell proliferation, survival, and invasion
Cross‐linking and co‐IP were performed with an anti‐gp96 antibody on cell membrane of SK‐Hep‐1 cells, and candidate proteins that interacted with mgp96 were analyzed by SDS‐PAGE and mass spectrometry (Figure 2A). Among these proteins, uPAR, a membrane receptor that plays a key role in cancer invasion and metastasis (Smith and Marshall, 2010; Li et al., 2010), was chosen for further study. The interaction between uPAR and mgp96 was confirmed by co‐IP (Figure 2B), in vitro GST pull‐down (Figure 2C), and cross‐linking (Figure 2D) assays. Cell membrane protein integrin α5, which does not bind to gp96 (Staron et al., 2010), served as a negative control, indicating that the interaction between mgp96 and uPAR was specific. Moreover, confocal microscopy analysis of SK‐Hep‐1 and Huh7 cells revealed that mgp96 extensively co‐localized with uPAR on the cell membrane (Figure 2E). To determine the region of mgp96 involved in the mgp96‐uPAR interaction, a variety of truncated fragments of mgp96 were expressed. As shown in Figure 2F, the C‐terminal domain of mgp96, C243 (aa 540–782), interacts with uPAR.
Figure 2.
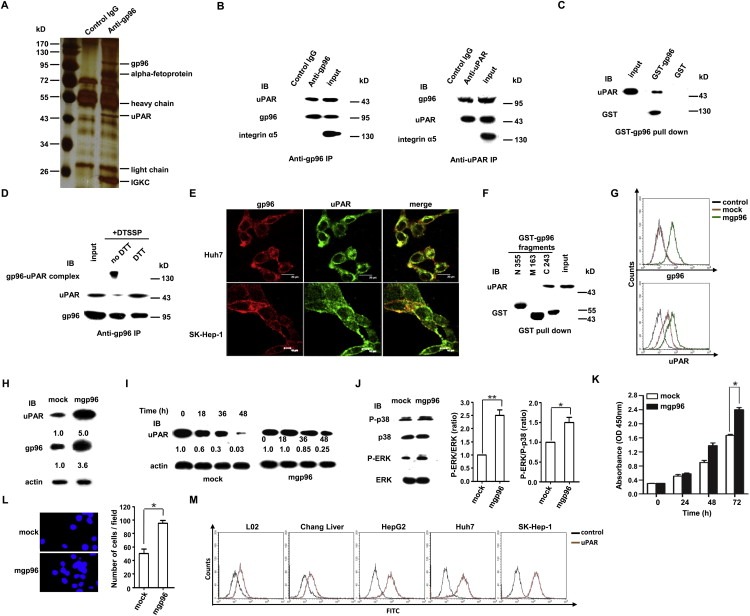
mgp96 binds to uPAR on the surface of hepatoma cells, increases its stability, and enhances cell growth and invasion. (A) SK‐Hep‐1 cell membrane proteins were biotinylated and captured with Streptavidin Agarose Resins. Captured proteins were immunoprecipitated with anti‐gp96 antibody or control IgG and separated by SDS‐PAGE for mass spectrometry analysis. mgp96‐associated proteins identified by mass spectrometry are shown. (B) Co‐IP assay with the anti‐gp96 or anti‐uPAR polyclonal antibodies in SK‐Hep‐1 cells. Integrin α5 served as a negative control. (C and F) In vitro GST pull‐down assays were performed to test for interactions between purified GST‐gp96 (C) or GST‐gp96 fragments (N355, M163 and C243) (F) and uPAR. (D) Co‐IP with the anti‐gp96 polyclonal antibody in SK‐Hep‐1 cells cross‐linked with DTSSP. (E) Detection of cell membrane gp96 and uPAR by confocal microscopy in unpermeabilized SK‐Hep‐1 and Huh‐7 cells. Scale bar, 20 μm (G–L) L02 cells were infected with ad‐mgp96 or ad‐pDC312 (mock) adenoviruses. (G) Detection of cell membrane gp96 and uPAR levels by FACS. Cells stained with control IgG served as a control. (H) Western blot analysis of total gp96 and uPAR levels. (I) Western blot analysis of uPAR levels. After infection (48 h), cells were treated with 50 μg/ml CHX for the time as indicated. (J) Western blot analysis of the impact of mgp96 overexpression on the MAPK signaling pathway. (K and L) Cell proliferation and invasion were determined by CCK‐8 (K) and transwell (L) assays, respectively. (M) Flow cytometric analysis of membrane uPAR levels in normal liver cells and hepatoma cells. Cells stained with control IgG served as a control. Data are presented as the mean ± SD from three independent experiments. *P < 0.05 and **P < 0.01 compared to mock.
We next examined the effect of mgp96 on uPAR expression and signaling. Our results revealed that overexpression of mgp96 caused a dramatic increase in cell membrane (∼4‐fold) (Figure 2G) and total uPAR levels (Figure 2H) and a significant enhancement of uPAR protein stability (Figure 2I). Overexpression of mgp96 increased the phosphorylated level of ERK and the ratio of P‐ERK to P‐p38 (Figure 2J), as well as cell proliferation (Figure 2K) and invasion (Figure 2L). In addition, high expression of cell membrane uPAR was observed in hepatoma cells whereas only low to moderate uPAR expression was observed in normal liver cells (Figure 2M), indicating that high levels of cell membrane uPAR may at least partly attribute to increased mgp96 expression.
3.3. Overexpression of mgp96 correlates with uPAR levels in primary liver tumors
uPAR levels were then analyzed in 88 patients with primary liver tumors (Figure 3A). We found that uPAR expression was significantly correlated with mgp96 levels (P < 0.01) (Figure 3B). Patients with high mgp96 levels also displayed much higher uPAR levels than patients with low mgp96 levels in tumors (P < 0.01) (Figure 3C). As shown in Figure 3D, patients with high mgp96 and uPAR levels had a much shorter DFS than those with low mgp96 and uPAR expression (P = 0.0001), however, there was no significant difference in DFS between patients with high mgp96 and low uPAR levels and those with low mgp96 and uPAR expression (P = 0.074), indicating that the effect of mgp96 overexpression on patients' DFS may be dependent on uPAR status. Moreover, mgp96 extensively co‐localized with uPAR on the cell membrane of liver cancer tissues (Figure 3E).
Figure 3.
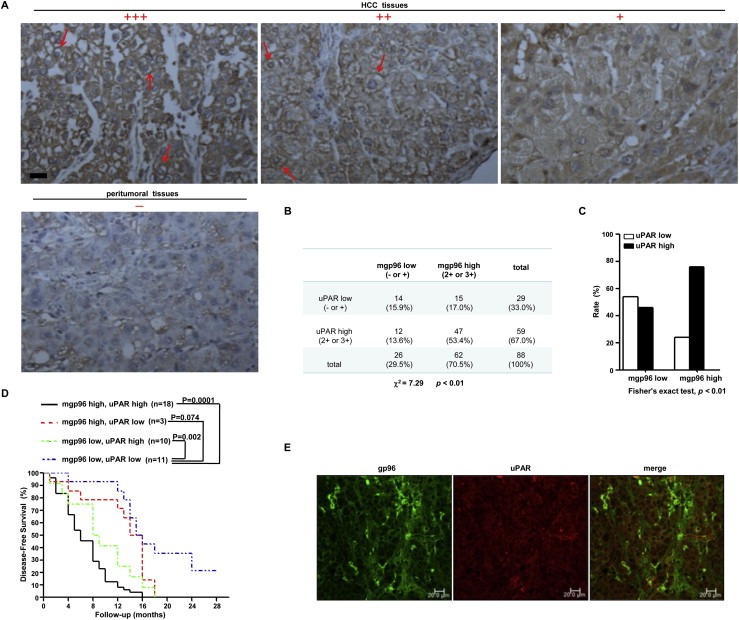
mgp96 expression correlates with uPAR levels in patients with primary liver tumors. (A) IHC analysis of uPAR expression in primary tumors. Arrows indicate uPAR positive cells. Scale bar, 20 μm. (B) Correlation between cell membrane gp96 and uPAR levels was analyzed using the Pearson χ2 test. (C) Distribution of uPAR expression levels in tumors with high or low mgp96. (D) Kaplan–Meier survival analysis of cell membrane gp96 and uPAR expression for DFS in patients with primary liver tumors. (E) Confocal microscopy analysis of cell membrane gp96 and uPAR expression in liver cancer tissues. Scale bar, 20 μm.
3.4. gp96 knockdown suppresses cell growth, survival, and invasion through uPAR inhibition
As seen in Figure 4A and B, knockdown of gp96 by RNAi significantly reduced cell membrane (∼80%) and total uPAR levels. However, no significant change in uPAR mRNA levels was observed (Supplementary Fig. S1). Instead, a more sharp, time‐dependent decrease in uPAR protein was observed in gp96 siRNA‐treated cells compared to mock‐treated cells (Figure 4C), indicating that the protein stability of uPAR was greatly decreased by gp96 depletion. In contrast, no obvious change in gp96 expression was observed under uPAR knockdown (Figure 4D), indicating that uPAR has no effect on gp96 expression. As expected, gp96 knockdown led to decreased ERK phosphorylation and a decreased ratio of P‐ERK to P‐p38 (Figure 4E), as well as the suppression of cell proliferation (Figure 4F), survival (Figure 4G), and invasion (Figure 4H). Notably, the inhibitory effects on cell proliferation, survival, and invasion by gp96 depletion could be rescued by overexpression of uPAR (Supplementary Fig. S2), indicating that cell membrane gp96 regulates cell growth and invasion through uPAR.
Figure 4.

gp96 knockdown decreases uPAR levels and suppresses cell growth and invasion. (A and B) SK‐Hep‐1 and Huh7 cells were treated with gp96 siRNA or control siRNA (mock) for 48 h. Cell membrane gp96 and uPAR levels were determined by flow cytometry (A). Cells stained with control IgG served as a control. Total gp96 and uPAR levels were detected by Western blot (B). (C) Western blot detection of uPAR expression in SK‐Hep‐1 cells. Cells were transfected with gp96 siRNA or control siRNA (mock). After the transfection (24 h), cells were treated with 50 μg/ml CHX for the time as indicated. (D) Total gp96 and uPAR levels were detected by Western blot in SK‐Hep‐1 cells treated with uPAR siRNA or control siRNA (mock) for 48 h. (E) Western blot assessing the impact of gp96 siRNA on uPAR signaling in SK‐Hep‐1 cells. (F–H) SK‐Hep‐1 cells were transfected with gp96 siRNA or control siRNA (mock). Cell proliferation was detected by CCK‐8 assay (F). Cell apoptosis was analyzed by FACS (G). Transwell assay was performed to detect cell invasion (H). (I–K) SK‐Hep‐1 cells were transfected with uPAR siRNA and/or gp96 siRNA, or control siRNA (mock). Cell proliferation, apoptosis, and invasion were determined by CCK‐8 (I), FACS (J), and transwell (K) assays, respectively. Data are presented as the mean ± SD from three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 compared to mock.
In addition, the effect of gp96 on cell proliferation (Figure 4I), survival (Figure 4J), and invasion (Figure 4K) was largely abolished by simultaneous transfection of uPAR siRNA in SK‐Hep‐1 cells (all P > 0.05). Similar results were also observed in Huh7 cells (all P > 0.05) (Supplementary Fig. S3). Taken together, these results indicate that gp96 regulates cell proliferation, survival, and invasion through uPAR.
3.5. Antitumor activity of an anti‐gp96 mAb by blocking the mgp96‐uPAR interaction
Multiple monoclonal antibodies (mAbs) against gp96 have been generated by our lab (Zhang et al., 2011). Cross‐linking and co‐IP assays demonstrated that treatment with a gp96 mAb only for 8 h, which did not have obvious effect on uPAR levels yet (Figure 5A), significantly decreased levels of the gp96‐uPAR complex (Figure 5B). This indicates that gp96 mAb can effectively block the association of mgp96 with uPAR. Treatment with the gp96 mAb for 48 h led to a decrease in cell membrane uPAR by 67% (Figure 5C), which may be due to its blocking the association between gp96 and uPAR. Thus, the gp96 mAb blocked the MAPK signaling pathway mediated by uPAR (Figure 5D). As shown in Figure 5E–G, the gp96 mAb pronouncedly suppressed the growth, survival, and invasion of SK‐Hep‐1 cells. Similar results were also observed in Huh7 cells (Supplementary Fig. S4). Similar to gp96 knockdown, the inhibitory effects on cell proliferation, survival, and invasion by gp96 mAb could be rescued by overexpression of uPAR (Supplementary Fig. S5), indicating that gp96 mAg inhibits cell growth and invasion through regulation of uPAR.
Figure 5.

Blockage of the mgp96‐uPAR interaction with an anti‐gp96 mAb suppresses uPAR‐mediated cell growth, survival, and invasion. SK‐Hep‐1 cells were treated with gp96 mAb or control antibody (50 μg/ml) for 48 h, except in A and B (8 h), E and H (72 h). (A and C) Flow cytometric analysis of cell membrane gp96 and uPAR abundance. Cells stained with control IgG served as a control. (B) Cross‐linking and co‐IP analysis of the mgp96‐uPAR complex with the anti‐gp96 polyclonal antibody in cells cross‐linked with BS3. (D) Western blot detection of the impact of the gp96 mAb on the MAPK signaling pathway. (E–G) Cell proliferation, apoptosis, and invasion were assessed by CCK‐8 (E), FACS (F), and transwell (G) assays, respectively. (H and I) CCK‐8 (H) and transwell (I) analysis of cell growth and invasion in SK‐Hep‐1 cells treated with uPAR siRNA and/or gp96 mAb. Data are presented as the mean ± SD from three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001.
Of note, the inhibitory effect of the gp96 mAb on cell proliferation (Figure 5H) and invasion (Figure 5I) was almost eliminated by simultaneous transfection of uPAR siRNA (all P > 0.05), indicating that the gp96 mAb suppressed cell proliferation, survival, and invasion by decreasing uPAR levels.
3.6. Targeting mgp96 induces the regression of liver cancer xenografts and lessens lung metastasis of orthotopic liver cancer in vivo
To determine whether targeting mgp96 is an effective strategy to inhibit liver tumor growth and metastasis in vivo, we generated a stable gp96 knockdown cell line, SK‐Hep‐1‐gp96‐RNAi. In concert with the in vitro results, tumor growth was significantly slowed in SK‐Hep‐1‐gp96‐RNAi xenograft nude mice compared to the mock control (P < 0.01) (Figure 6A). gp96 depletion resulted in decreased tumor weight by 63.6% (P < 0.01) (Figure 6B). Meanwhile, mice lung metastasis model was established by subserosal injections of SK‐Hep‐1‐gp96‐RNAi cells into the livers of SCID mice. As seen in Figure 6C, lung metastasis was dramatically lessened by 69% (P < 0.01) under gp96 depletion.
Figure 6.

Targeting mgp96 with RNAi and mAb leads to inhibited liver tumor growth and lung metastasis in mice. (A and B) BALB/c nude mice (n = 6/group) were injected s.c. with SK‐Hep‐1‐gp96‐RNAi or SK‐Hep‐1‐luc‐RNAi (mock) cells (model 1). (A) Tumor volume was monitored every 3 days. (B) Representative images of tumor growth (left) and mean tumor weights (right) were analyzed after the mice were sacrificed at day 30. (C) Lung metastasis was analyzed 7 weeks after subserosal injection of SK‐Hep‐1‐gp96‐RNAi or SK‐Hep‐1‐luc‐RNAi (mock) cells into the left lobe of the livers of SCID mice (n = 6/group) (model 3). Representative images of lung metastasis (left). Mean number of lung metastases (right). (D and E) BALB/c nude mice (n = 5/group) were injected s.c. with SK‐Hep‐1 cells, and treated with gp96 mAb or control antibody (2 mg/kg) when tumors reached a volume of ∼100 mm3 (model 2). (D) Tumor volume was monitored every 3 days. (E) Representative images of tumor growth (left) and mean tumor weights (right) were analyzed after 24 days of gp96 mAb treatment. (F) Lung metastasis of SK‐Hep‐1 cells in response to gp96 mAb treatment (2 mg/kg) in SCID mice subserosally injected with tumor cells into the livers (n = 6/group) (model 4). Representative images (left) and mean number (right) of lung metastases after 3 weeks of gp96 mAb treatment. (G and H) BALB/c nude mice (n = 5/group) were injected s.c. with Huh7 cells, and treated with gp96 mAb or control antibody (2 mg/kg) when tumors reached a volume of ∼100 mm3 (model 2). (G) Tumor volume was monitored every 3 days. (H) Representative images of tumor growth (left) and mean tumor weights (right) were analyzed after 24 days of gp96 mAb treatment. (I) Lung metastasis of Huh7 cells in response to gp96 mAb treatment (2 mg/kg) in SCID mice subserosally injected with tumor cells into the livers (n = 6/group) (model 4). Representative images (left) and mean number (right) of lung metastases after 3 weeks of gp96 mAb treatment. These experiments were repeated at least twice with comparable results. **P < 0.01.
Finally, the therapeutic effect of gp96 mAb against liver cancer was determined using SK‐Hep‐1 xenograft nude mice and lung metastasis mice models. We found that treatment with gp96 mAb slowed tumor growth (P < 0.01) (Figure 6D) and reduced tumor burdens by 46.2% (P < 0.01) in xenograft nude mice (Figure 6E). Moreover, gp96 mAb treatment significantly lessened lung metastasis by 64% (P < 0.01) (Figure 6F). Similar results were also observed in Huh7 xenograft nude mice (Figure 6G–I). No obvious toxicity of gp96 mAb was observed in mice.
4. Discussion
In the present study, we investigated the functional and clinical relevance of mgp96 in liver cancer. We observed that elevated mgp96 expression is significantly correlated with tumor metastasis, a shorter DFS, and poor prognosis in liver cancer patients. Using proteomic profiling of mgp96‐associated proteins, we identified uPAR as a mgp96 client protein and found that mgp96 directly binds to uPAR on the cell membrane. Next, we demonstrated that mgp96 stabilizes uPAR and thereby increases uPAR levels and downstream signaling. The gp96 mAb which blocks the mgp96‐uPAR interaction inhibits uPAR‐driven cell growth, survival, and invasion both in vitro and in vivo. These results validate that mgp96 is a potential therapeutic target for liver cancer.
uPAR, also known as CD87, is a single‐chain membrane‐bound protein with a glycosylphosphatidylinositol (GPI)‐anchor. It serves as an important regulator of extracellular matrix (ECM) proteolysis and cell–ECM interactions. uPAR promotes plasminogen activation by binding uPA, and plays an essential role in the proteolytic degradation of ECM, which may favor cancer invasion and metastasis. Additionally, uPAR functions through coordinated interaction with its transmembrane co‐receptors such as integrins, G protein‐coupled receptors, and growth factor receptors. uPAR signaling through these co‐receptors involves diverse signaling pathways, including mitogen‐activated protein kinase (MAPK)/ERK, PI3K/AKT, JAK/STAT, Rac, Src, and focal adhesion kinase (FAK), leading to enhanced cell proliferation, migration, invasion, and angiogenesis (Smith and Marshall, 2010; Noh et al., 2013; Blasi and Sidenius, 2010). Inhibiting the cell signaling functions of uPAR by targeting it with anti‐uPAR mAbs (Bauer et al., 2005) and inhibition of interactions between uPAR and its ligands (e.g., uPA and vitronectin) or co‐receptors (e.g., EGFR) (Huang et al., 2012; Sun et al., 2008; Ghamande et al., 2008) therefore provide an attractive strategy for cancer therapy in both animal models and clinical trials. In this study, we observed that elevated mgp96 expression is positively associated with tumor metastasis and recurrence in liver cancer patients. Furthermore, we found that mgp96 interacts with uPAR, and this interaction stabilizes the uPAR protein, thereby increasing uPAR signaling and promoting cell proliferation, survival, and invasion. Targeting mgp96 with the specific mAb that blocks mgp96 binding to uPAR inhibited liver tumor growth and metastasis in mouse xenograft models, suggesting that the mgp96‐uPAR interaction is a valid drug target.
uPAR expression is frequently increased in liver cancer and many other cancers, as well as in tumor‐associated stromal cells. Elevated uPAR levels are associated with an aggressive tumor phenotype, tumor invasion, and recurrence (Dubuisson et al., 2000; Zheng et al., 2000; Morita et al., 1997). Our previous studies show that the gene amplification and overexpression of HER2 and uPAR occur most frequently in the same individual tumor cells, and there may be a positive feedback loop between HER2 and uPAR (Li et al., 2010; Meng et al., 2006). Aside from uPAR gene amplification, uPAR expression is regulated by transcription factors such as activator protein 1 (AP1), hypoxia‐inducible factor 1α (HIF1α), NF‐κB, specificity protein 1 (SP1) and SP2, and by post‐transcriptional mechanisms mediated by mRNA binding proteins, which may drive sustained uPAR expression in tumors (Smith and Marshall, 2010; Noh et al., 2013). Cell membrane uPAR is regulated by endocytosis, recycling, and lysosomal degradation, which maintain the amount of cell membrane uPAR available for signaling. Upon binding to the uPA‐plasminogen activator inhibitor‐1 (PAI‐1) complex, uPAR undergoes clathrin‐dependent endocytosis and recycling, a process dependent or independent on the association with low density lipoprotein receptor‐related protein 1 (LRP1) (Li et al., 2010; Czekay et al., 2001). uPAR also associates with uPAR‐associated protein (uPARAP)/endocytic receptor 180 (ENDO180), which leads it to lysosomal degradation (Smith and Marshall, 2010; Noh et al., 2013; Nykjaer et al., 1998). In contrast, our results show that mgp96 binds to uPAR and increases its stability, leading to its accumulation on the cell membrane. This may be attributed to decreased endocytosis and subsequent lysosomal degradation (Cortese et al., 2008). Further, the uPAR levels are also correlated with mgp96 expression in liver tumors (Figure 3). The results presented here therefore reveal that uPAR expression levels are positively regulated by mgp96. As PAI‐1, LRP1, and ENDO180 play a complex role in the internalization and recycling of uPAR, the exact role of mgp96 in this context awaits further investigation.
Interestingly, higher expression of mgp96 was observed in HBV‐infected liver cancer patients than uninfected ones (Table 1), which is consistent to our previous study showing that HBV × protein promotes gp96 expression through NF‐κB activation (Fan et al., 2013). As chronic HBV infection is a major etiological factor in liver cancer development, our study provides the potential of targeting mgp96 as an effective strategy to treat liver cancer under HBV infection. In addition, the possible regulation of gp96 translocation to cell membrane by KDELR1 in liver cancer deserves further investigation.
Furthermore, it would be worthwhile to investigate whether elevated expression of mgp96 contributes to the intrinsic anti‐apoptotic, drug‐resistant, and potently regenerative properties of liver cancer, which are the major obstacles to the development of efficient therapies.
Considering the key role of uPAR in liver cancer invasion and metastasis, and the emerging roles of mgp96 in the regulation of cancer metastasis and angiogenesis, the regulation of uPAR expression by mgp96 presented here has several implications. First, our study represents an effort to address the underlying mechanism of elevated uPAR levels in liver cancer. Second, our results demonstrate that mgp96 may function as a scaffolding protein to increase uPAR stability on the cell membrane. More importantly, the cell membrane localization of the normally ER‐resident gp96 may serve as a potential predictive factor of poor prognosis in liver cancer. Given that mgp96 exists predominantly in malignant but not in normal cells, our study therefore supports the notion that the inhibition of mgp96 activity might provide a novel therapeutic approach for liver cancer.
Authors' contributions
Conceived and designed the experiments: SM, JH. Performed the experiments: JH, XL, CL, LS. Analyzed the data: JH, SM. Contributed reagents/materials/analysis tools: YZ, JZ. Wrote the paper: JH, SM.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Financial support
This work was supported by a grant from Major State Basic Research Development Program of China (973 Program) (No. 2014CB542602) and grants from the National Natural Science Foundation of China (31230026, 81321063, 81102018, 81471960), and a grant from Key Projects in the National Science & Technology Program (2013ZX10002001‐003‐003).
Supporting information
The following are the Supplementary data related to this article:
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Acknowledgments
We thank Libo Zhao and Liping Luo for the technical support and advice in animal test.
Supplementary data 1.
1.1.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.molonc.2015.03.004.
Hou Junwei, Li Xin, Li Changfei, Sun Lu, Zhao Yulai, Zhao Jingmin, Meng Songdong, (2015), Plasma membrane gp96 enhances invasion and metastatic potential of liver cancer via regulation of uPAR, Molecular Oncology, 9, doi: 10.1016/j.molonc.2015.03.004.
Contributor Information
Jingmin Zhao, Email: jmzhao302@163.com.
Songdong Meng, Email: mengsd@im.ac.cn.
References
- Altmeyer, A. , Maki, R.G. , Feldweg, A.M. , Heike, M. , Protopopov, V.P. , Masur, S.K. , Srivastava, P.K. , 1996. Tumor-specific cell surface expression of the-KDEL containing, endoplasmic reticular heat shock protein gp96. Int. J. Cancer 69, 340–349. [DOI] [PubMed] [Google Scholar]
- Bauer, T.W. , Liu, W. , Fan, F. , Camp, E.R. , Yang, A. , Somcio, R.J. , Bucana, C.D. , Callahan, J. , Parry, G.C. , Evans, D.B. , Boyd, D.D. , Mazar, A.P. , Ellis, L.M. , 2005. Targeting of urokinase plasminogen activator receptor in human pancreatic carcinoma cells inhibits c-Met- and insulin-like growth factor-I receptor-mediated migration and invasion and orthotopic tumor growth in mice. Cancer Res. 65, 7775–7781. [DOI] [PubMed] [Google Scholar]
- Blasi, F. , Sidenius, N. , 2010. The urokinase receptor: focused cell surface proteolysis, cell adhesion and signaling. FEBS Lett. 584, 1923–1930. [DOI] [PubMed] [Google Scholar]
- Cabanes, D. , Sousa, S. , Cebriá, A. , Lecuit, M. , García-del Portillo, F. , Cossart, P. , 2005. Gp96 is a receptor for a novel Listeria monocytogenes virulence factor, Vip, a surface protein. EMBO J. 24, 2827–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavany, C. , Mimnaugh, E. , Miller, P. , Bitton, R. , Nguyen, P. , Trepel, J. , Whitesell, L. , Schnur, R. , Moyer, J. , Neckers, L. , 1996. p185erbB2 binds to GRP94 in vivo. Dissociation of the p185erbB2/GRP94 heterocomplex by benzoquinone ansamycins precedes depletion of p185erbB2. J. Biol. Chem. 271, 4974–4977. [DOI] [PubMed] [Google Scholar]
- Cortese, K. , Sahores, M. , Madsen, C.D. , Tacchetti, C. , Blasi, F. , 2008. Clathrin and LRP-1 -independent constitutive endocytosis and recycling of uPAR. PLoS One 3, e3730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czekay, R.P. , Kuemmel, T.A. , Orlando, R.A. , Farquhar, M.G. , 2001. Direct binding of occupied urokinase receptor (uPAR) to LDL receptor-related protein is required for endocytosis of uPAR and regulation of cell surface urokinase activity. Mol. Biol. Cell 12, 1467–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, J. , Liu, B. , Ngoi, S.M. , Sun, S. , Vella, A.T. , Li, Z. , 2007. TLR4 hyperresponsiveness via cell surface expression of heat shock protein gp96 potentiates suppressive function of regulatory T cells. J. Immunol. 178, 3219–3225. [DOI] [PubMed] [Google Scholar]
- Dubuisson, L. , Monvoisin, A. , Nielsen, B.S. , Le Bail, B. , Bioulac-Sage, P. , Rosenbaum, J. , 2000. Expression and cellular localization of the urokinase-type plasminogen activator and its receptor in human hepatocellular carcinoma. J. Pathol. 190, 190–195. [DOI] [PubMed] [Google Scholar]
- Epstein, R.J. , Leung, T.W. , 2007. Reversing hepatocellular carcinoma progression by using networked biological therapies. Clin. Cancer Res. 13, 11–17. [DOI] [PubMed] [Google Scholar]
- Fan, H. , Yan, X. , Zhang, Y. , Zhang, X. , Gao, Y. , Xu, Y. , Wang, F. , Meng, S. , 2013. Increased expression of Gp96 by HBx-induced NF-κB activation feedback enhances hepatitis B virus production. PLoS One 8, e65588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamande, S.A. , Silverman, M.H. , Huh, W. , Behbakht, K. , Ball, G. , Cuasay, L. , Würtz, S.O. , Brunner, N. , Gold, M.A. , 2008. A phase 2, randomized, double-blind, placebo-controlled trial of clinical activity and safety of subcutaneous A6 in women with asymptomatic CA125 progression after first-line chemotherapy of epithelial ovarian cancer. Gynecol. Oncol. 111, 89–94. [DOI] [PubMed] [Google Scholar]
- Goldstein, M.G. , Li, Z. , 2009. Heat-shock proteins in infection-mediated inflammation- induced tumorigenesis. J. Hematol. Oncol. 2, 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, J.M. , Park, S.G. , Liu, B. , Park, B.J. , Kim, J.Y. , Jin, C.H. , Song, Y.W. , Li, Z. , Kim, S. , 2007. Aminoacyl-tRNA synthetase-interacting multifunctional protein 1/p43 controls endoplasmic reticulum retention of heat shock protein gp96: its pathological implications in lupus-like autoimmune diseases. Am. J. Pathol. 170, 2042–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks, D.G. , Tubbs, R.R. , 2005. Assessment of the HER2 status in breast cancer by fluorescence in situ hybridization: a technical review with interpretive guidelines. Hum. Pathol. 36, 250–261. [DOI] [PubMed] [Google Scholar]
- Hong, F. , Liu, B. , Chiosis, G. , Gewirth, D.T. , Li, Z. , 2013. α7 helix region of αI domain is crucial for integrin binding to endoplasmic reticulum chaperone gp96: a potential therapeutic target for cancer metastasis. J. Biol. Chem. 288, 18243–18248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, S.H. , Wang, L.T. , Lee, K.T. , Chen, Y.L. , Liu, K.Y. , Suen, J.L. , Chai, C.Y. , Wang, S.N. , 2013. Proinflammatory homeobox gene, ISX, regulates tumor growth and survival in hepatocellular carcinoma. Cancer Res. 73, 508–518. [DOI] [PubMed] [Google Scholar]
- Hsu, W.H. , Chen, C.N. , Huang, H.I. , Lai, Y.L. , Teng, C.Y. , Kuo, W.H. , 2012. Urokinase induces stromal cell-derived factor-1 expression in human hepatocellular carcinoma cells. J. Cell Physiol. 227, 697–704. [DOI] [PubMed] [Google Scholar]
- Huang, J. , Li, Y.M. , Massague, J. , Sicheneder, A. , Vallera, D.A. , Hall, W.A. , 2012. Intracerebral infusion of the bispecific targeted toxin DTATEGF in a mouse xenograft model of a human metastatic non-small cell lung cancer. J. Neurooncol. 109, 229–238. [DOI] [PubMed] [Google Scholar]
- Kim, G. , Han, J.M. , Kim, S. , 2010. Toll-like receptor 4-mediated c-Jun N-terminal kinase activation induces gp96 cell surface expression via AIMP1 phosphorylation. Biochem. Biophys. Res. Commun. 397, 100–105. [DOI] [PubMed] [Google Scholar]
- Koo, B.H. , Apte, S.S. , 2010. Cell-surface processing of the metalloprotease pro-ADAMTS9 is influenced by the chaperone GRP94/gp96. J. Biol. Chem. 285, 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo, M. , 2011. Signaling pathway and molecular-targeted therapy for hepatocellular carcinoma. Dig. Dis. 29, 289–302. [DOI] [PubMed] [Google Scholar]
- Li, C. , Cao, S. , Liu, Z. , Ye, X. , Chen, L. , Meng, S. , 2010. RNAi-mediated downregulation of uPAR synergizes with targeting of HER2 through ERK pathway in breast cancer cells. Int. J. Cancer 127, 1507–1516. [DOI] [PubMed] [Google Scholar]
- Li, C. , Wang, Y. , Wang, S. , Wu, B. , Hao, J. , Fan, H. , Ju, Y. , Ding, Y. , Chen, L. , Chu, X. , Liu, W. , Ye, X. , Meng, S. , 2013. HBV mRNAs-mediated miR-122 inhibition up-regulates PTTG1-binding protein which promotes HCC tumor growth and cell invasion. J. Virol. 87, 2193–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins, M. , Custódio, R. , Camejo, A. , Almeida, M.T. , Cabanes, D. , Sousa, S. , 2012. Listeria monocytogenes triggers the cell surface expression of Gp96 protein and interacts with its N terminus to support cellular infection. J. Biol. Chem. 287, 43083–43093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzec, M. , Eletto, D. , Argon, Y. , 2012. GRP94: an HSP90-like protein specialized for protein folding and quality control in the endoplasmic reticulum. Biochim. Biophys. Acta 1823, 774–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez, K. , Wallen, E.S. , Edwards, B.S. , Mobarak, C.D. , Bear, D.G. , Moseley, P.L. , 2006. Heat shock protein 70 and glycoprotein 96 are differentially expressed on the surface of malignant and nonmalignant breast cells. Cell Stress Chaperones 11, 334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, S. , Tripathy, D. , Shete, S. , Ashfaq, R. , Saboorian, H. , Haley, B. , Frenkel, E. , Euhus, D. , Leitch, M. , Osborne, C. , Clifford, E. , Perkins, S. , Beitsch, P. , Khan, A. , Morrison, L. , Herlyn, D. , Terstappen, L.W. , Lane, N. , Wang, J. , Uhr, J. , 2006. uPAR and HER-2 gene status in individual breast cancer cells from blood and tissues. Proc. Natl. Acad. Sci. USA 103, 17361–17365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal, R. , Prasadarao, N.V. , 2011. gp96 expression in neutrophils is critical for the onset of Escherichia coli K1 (RS218) meningitis. Nat. Commun. 2, 552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita, Y. , Hayashi, Y. , Wang, Y. , Kanamaru, T. , Suzuki, S. , Kawasaki, K. , Ohta, K. , Yamamoto, M. , Saitoh, Y. , Itoh, H. , Doe, W.F. , 1997. Expression of urokinase-type plasminogen activator receptor in hepatocellular carcinoma. Hepatology 25, 856–861. [DOI] [PubMed] [Google Scholar]
- Nakagawa, H. , Maeda, S. , 2012. Inflammation- and stress-related signaling pathways in hepatocarcinogenesis. World J. Gastroenterol. 18, 4071–4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh, H. , Hong, S. , Huang, S. , 2013. Role of urokinase receptor in tumor progression and development. Theranostics 3, 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykjaer, A. , Christensen, E.I. , Vorum, H. , Hager, H. , Petersen, C.M. , Røigaard, H. , Min, H.Y. , Vilhardt, F. , Møller, L.B. , Kornfeld, S. , Gliemann, J. , 1998. Mannose 6-phosphate/insulin-like growth factor-II receptor targets the urokinase receptor to lysosomes via a novel binding interaction. J. Cell Biol. 141, 815–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, P.D. , Yan, P. , Seidler, P.M. , Patel, H.J. , Sun, W. , Yang, C. , Que, N.S. , Taldone, T. , Finotti, P. , Stephani, R.A. , Gewirth, D.T. , Chiosis, G. , 2013. Paralog-selective Hsp90 inhibitors define tumor-specific regulation of HER2. Nat. Chem. Biol. 9, 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pez, F. , Lopez, A. , Kim, M. , Wands, J.R. , Caron de Fromentel, C. , Merle, P. , 2013. Wnt signaling and hepatocarcinogenesis: molecular targets for the development of innovative anticancer drugs. J. Hepatol. 59, 1107–1117. [DOI] [PubMed] [Google Scholar]
- Pikarsky, E. , Porat, R.M. , Stein, I. , Abramovitch, R. , Amit, S. , Kasem, S. , Gutkovich-Pyest, E. , Urieli-Shoval, S. , Galun, E. , Ben-Neriah, Y. , 2004. NF-kappa B functions as a tumor promoter in inflammation-associated cancer. Nature 431, 461–466. [DOI] [PubMed] [Google Scholar]
- Smith, H.W. , Marshall, C.J. , 2010. Regulation of cell signaling by uPAR. Nat. Rev. Mol. Cell Biol. 11, 23–36. [DOI] [PubMed] [Google Scholar]
- Staron, M. , Yang, Y. , Liu, B. , Li, J. , Shen, Y. , Zúñiga-Pflücker, J.C. , Aguila, H.L. , Goldschneider, I. , Li, Z. , 2010. gp96, an endoplasmic reticulum master chaperone for integrins and Toll-like receptors, selectively regulates early T and B lymphopoiesis. Blood 115, 2380–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Q. , Xu, Q. , Dong, X. , Cao, L. , Huang, X. , Hu, Q. , Hua, Z.C. , 2008. A hybrid protein comprising ATF domain of pro-UK and VAS, an angiogenesis inhibitor, is a potent candidate for targeted cancer therapy. Int. J. Cancer 123, 942–950. [DOI] [PubMed] [Google Scholar]
- Wang, S. , Qiu, L. , Yan, X. , Jin, W. , Wang, Y. , Chen, L. , Wu, E. , Ye, X. , Gao, G.F. , Wang, F. , Chen, Y. , Duan, Z. , Meng, S. , 2012. Loss of microRNA 122 expression in patients with hepatitis B enhances hepatitis B virus replication through cyclin G(1)-modulated P53 activity. Hepatology 55, 730–741. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Cao, S. , Meng, S. , Gao, G.F. , 2011. A strategy to produce monoclonal antibodies against gp96 by prime-boost regimen using endogenous protein and E. coli heterologously-expressed fragment. J. Cent. South Univ. Technol. 18, 1857–1864. [Google Scholar]
- Zheng, Q. , Tang, Z.Y. , Xue, Q. , Shi, D.R. , Song, H.Y. , Tang, H.B. , 2000. Invasion and metastasis of hepatocellular carcinoma in relation to urokinase-type plasminogen activator, its receptor and inhibitor. J. Cancer Res. Clin. Oncol. 126, 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following are the Supplementary data related to this article:
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data


