Abstract
Antagonists of growth hormone-releasing hormone (GHRH) exert antiproliferative effects directly on cancer cells, which are mediated by the tumoral GHRH receptors. However, the signal transduction pathways involved in antiproliferative effect of GHRH antagonists have not yet been elucidated. We used flow cytometry to investigate whether GHRH antagonist JV-1-38 can induce changes in the cytosolic free Ca2+ concentration leading to apoptosis in LNCaP human prostate cancer cells. JV-1-38 evoked prompt Ca2+ signal in a dose-dependent way (1–10 μM) and induced early stage of apoptosis in LNCaP human prostate cancer cells at a concentration effective in suppression of cell proliferation (10 μM) peaking after 3 h. Unexpectedly, agonist GHRH(1–29)NH2, which elevates cytosolic free Ca2+ concentration in pituitary somatotrophs at nanomolar concentrations, failed to induce Ca2+ signal or apoptosis even at a 10-fold higher concentration (100 μM). However, agonist GHRH(1–29)NH2 inhibited JV-1-38-induced Ca2+ signals in a dose-dependent way without affecting the antagonist-induced apoptosis. Peptides unrelated to GHRH did not induce Ca2+ signals in LNCaP human prostate cancer cells. EDTA (10 mM) or nifedipine (10 μM) significantly reduced the Ca2+ signal and early stage of apoptosis induced by JV-1-38, supporting the view that the increase in intracellular Ca2+ in response to JV-1-38 occurs primarily through extracellular Ca2+ entry through voltage-operated Ca2+ channels. In conclusion, GHRH antagonists activate tumoral GHRH receptors and are able to induce apoptosis in LNCaP human prostate cancer cells through a Ca2+-dependent pathway. Treatment with GHRH antagonists may offer a new approach to the therapy of prostate and other hormone-sensitive cancers.
Keywords: growth hormone-releasing hormone antagonist, growth hormone-releasing hormone receptor, calcium, cancer therapy
Growth hormone-releasing hormone (GHRH) is secreted by the hypothalamus and, upon binding to specific GHRH receptors (GHRHRs) in the pituitary, stimulates the synthesis and the release of GH. GH, in turn, induces the production of hepatic insulin-like growth factor I (IGF-I) (1–6), which is a known mitogen for various cell types and has been linked with malignant transformation, tumor progression, and metastasis of various cancers (reviewed in ref. 7). In vitro and in vivo studies using specific antagonists of GHRH revealed that, in addition to their action in suppressing cancer growth by interfering with the production of pituitary GH and IGF-I, GHRH antagonists act directly on cancer cells and strongly inhibit their proliferation (1, 8–15).
Although the expression of GHRH has been detected in various human normal and cancer cells, pituitary GHRHRs are virtually absent from almost every human nonpituitary tissue tested, including those in which GHRH had been previously shown to produce autocrine stimulation of cell proliferation (16–21).
We have demonstrated (22) that GHRH antagonists could act independently of receptors homologous to the GHRHR, such as those for vasoactive intestine peptide, pituitary adenylate cyclase-activating polypeptide, and others (23, 24). Moreover, recent evidence indicates that several splice variants of GHRHR are expressed in nonpituitary tissues, including primary cancers and established cell lines, whereas the pituitary GHRHR is not present (25–31). One of these splice variants, SV1, lacks only a portion of the extracellular part of the full-length receptor and therefore represents a form of the truncated receptor that possibly has a functional significance (26). We also have demonstrated that the ectopic expression of SV1 in 3T3 fibroblasts confers ligand binding and restores the responsiveness of the cells to GHRH antagonists, as reflected by the reduced rate of cell proliferation (32). In addition, tumoral GHRHR isoforms have been detected by immunohistochemical methods in primary breast cancers (33) by using a polyclonal antiserum against a polypeptide analog of segment 1–25 of the putative SV1 receptor protein (34), which differs from the sequence of pituitary GHRHR.
The intracellular signal transduction pathways involved in the receptor-mediated antiproliferative effects of GHRH antagonists on tumor cells have not yet been completely identified. We have demonstrated that, in addition to cAMP, other intracellular second messengers may participate in the signal transduction pathways of GHRH analogs mediated by tumoral GHRHR (35). The involvement of specific PKC isoforms, mitogen-activated protein kinase, and c-fos and c-jun oncogenes in the action of GHRH antagonists was recently shown (36, 37). In addition, a rapid increase in cytosolic free Ca2+ concentration after treatments with antitumor drugs may induce apoptosis in cancer cells by activating some elements of the apoptotic pathways including Ca2+-dependent nucleases that degrade chromosomal DNA (38). Extracellular Ca2+ has been reported to be required for GHRH to stimulate cAMP accumulation in pituitary cell preparations (39, 40), suggesting that Ca2+ also is a second messenger for GHRH, and that Ca2+ acts upstream or independently of cAMP in somatotrophs. Because most of the deduced amino acid sequence of the tumoral GHRHR is identical to that of pituitary GHRHR, including the C-terminal end, and all of the extra- and intracellular loops (26), Ca2+ could be a possible signal molecule for the antiproliferative actions of GHRH antagonists mediated by tumoral GHRHR.
Therefore, the aim of the present study was dual: (i) to investigate whether GHRH antagonists can influence intracellular Ca2+ concentration and induce apoptosis in LNCaP human prostate cancer cells by testing membrane alterations (externalization of phosphatidylserine) in the early stages of apoptosis; and (ii) to study whether Ca2+ influx is required for GHRH antagonist-induced apoptosis in LNCaP human prostate cancer cells.
Materials and Methods
Materials. Human GHRH(1–29)NH2 and GHRH antagonist JV-1-38 were synthesized by solid-phase method and purified as described in ref. 41. LHRH agonist [d-Trp-6]-LHRH and LHRH antagonist SB-75 (Cetrorelix), originally synthesized in the laboratory of A.V.S. (42, 43), were made by Debiopharm (Lausanne, Switzerland) and Asta Medica (Frankfurt am Main, Germany), respectively. Nifedipine, EDTA, DMSO, and propidium iodide (PI) were purchased from Sigma, and Fluo-3 acetoxymethyl ester (AM) and Annexin V-FITC were obtained from Molecular Probes and BD Pharmingen, respectively.
Cell Culture. LNCaP human prostate cancer cells (American Type Culture Collection) were cultured in RPMI medium 1640 supplemented with 10% FCS and a mixture of antibiotics and antimycotics (100 units/ml penicillin G, 100 μg/ml streptomycin, and 0.25 μM amphotericin). The cell culture media and reagents were purchased from Sigma. The cultures were maintained in a humidified atmosphere containing 95% air/5% CO2 at 37°C. The cells were passaged weekly and were routinely monitored for the presence of mycoplasma by using a test kit from Roche Molecular Biochemicals.
Flow Cytometry Analysis. The intracellular free Ca2+ levels and the early signal of apoptosis (externalization of phosphatidylserine) were measured by flow cytometry (FACSCalibur, Becton Dickinson) in a gated cell population at 526 nm (FL1 channel) by using Fluo-3 AM and Annexin V-FITC, respectively. Intracellular free calcium was measured by using Fluo-3 AM according to the protocol described by Minta et al. (44), with some modifications (45). Briefly, the fluorescence intensity of Fluo-3 AM dye was detected in the gated cell population at 526 nm (FL1 channel), which is proportional with the intracellular free calcium level (44). After measuring basal fluorescence at 526 nm, drugs were added to the tubes (each containing 105 cells per 500 μl loaded by Fluo-3 AM for 30 min at room temperature before analysis), and the measurement was continued for a further 400–600 s, thus making it possible to follow the alterations of intracellular free calcium level (FL1 fluorescence intensity) in time. Gates were created along the time axis of the activation dot plots at definite time points, and the mean fluorescence intensity at 526 nm was statistically analyzed from every gate. These values were corrected with the basal fluorescence intensities measured in the same sample before the addition of the activating agent and represented as fluorescence intensity ratios (y axis) (45).
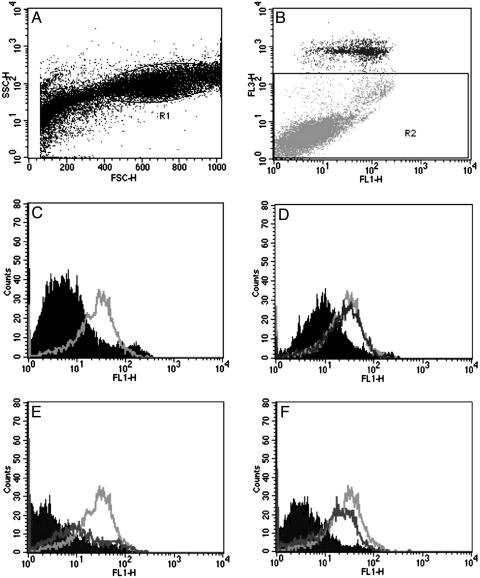
We used Annexin V-FITC/PI staining for the analysis of apoptotic cells described by Vermes et al. in ref. 46. At 24 h after seeding of 105 cells per well in culture medium, cells were treated with drugs for 30 min to 10 h in 500 μl of 2% FCS containing RPMI medium 1640. After trypsinization, cells were labeled with Annexin V-FITC according to the manufacturer's instructions, and PI was added to the samples immediately before flow cytometric measurement. We distinguished between live, early, and late apoptotic or necrotic cells based on their distribution on FL1/FL3 fluorescent dot plots. Data of late apoptotic cells (R2) were omitted on FL1 histogram plots.
Results
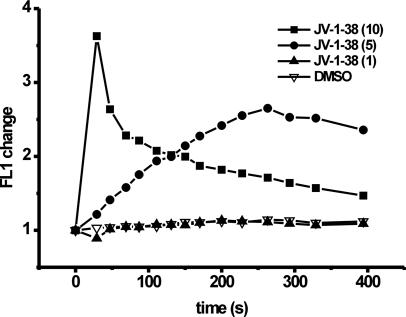
Time Scale of Dose-Dependent Effect of GHRH Antagonist JV-1-38 on Intracellular Free Ca2+ Concentration in LNCaP Human Prostate Cancer Cells. The basal intracellular free Ca2+ levels (basal Fluo-3 AM fluorescence) before exposure to JV-1-38 (1, 5, or 10 μM) were similar in all groups of cells (data not shown). Exposure to JV-1-38 (5 or 10 μM) resulted in a rapid monophasic elevation in FL1 fluorescence, indicative of an increase in the intracellular Ca2+ concentration, whereas 1 μM JV-1-38 was ineffective (Fig. 1). JV-1-38 at a concentration efficient for suppression of cell proliferation (10 μM) induced a 3.6-fold increase in the FL1 fluorescence intensity, peaking at 30 s postexposure to JV-1-38. This increase in intracellular Ca2+ was transient in nature and declined to basal levels after 10 min (data partially shown). However, at lower concentration (5 μM), the response to JV-1-38 was slower in onset, peaking at 260 s postexposure to JV-1-38 (Fig. 1). The magnitude of the response also was blunted (2.6-fold increase in FL1 fluorescence) (Fig. 1). All responses to JV-1-38 (5 or 10 μM) at all time points were significantly greater than those seen with the 0.5% DMSO vehicle, which had only a negligible effect on the FL1 fluorescence intensity (Fig. 1).
Fig. 1.
The time scale of the change in the fluorescence intensity ratio (FL1) of LNCaP human prostate cancer cells proportional with the cytosolic free Ca2+ level after exposure to either GHRH antagonist JV-1-38 (1–10 μM) or DMSO vehicle (0.5%). Results are representative of three sets of experiments.
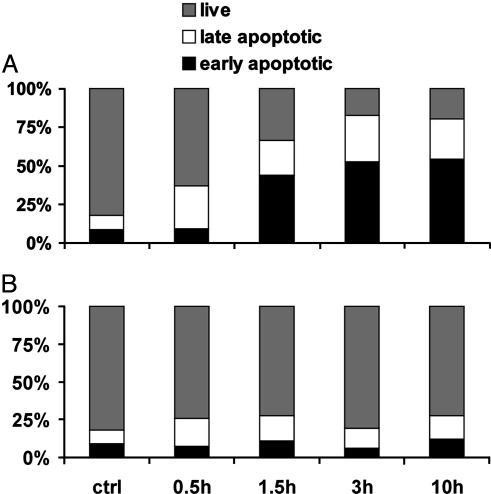
Time-Dependent Effect of GHRH Antagonist JV-1-38 on the Externalization of Phosphatidylserine in LNCaP Human Prostate Cancer Cells. GHRH antagonist JV-1-38 at a concentration effective for suppression of cell proliferation (10 μM) induced the externalization of phosphatidylserine in LNCaP human prostate cancer cells in a time-dependent manner (Fig. 2A). The percentage of early apoptotic cells has increased significantly (4.8-fold) after exposure to JV-1-38 for 90 min, whereas this GHRH antagonist was still ineffective on this cell population after 30 min. The effect of JV-1-38 has been saturated after treatment for 3 h (6.1-fold increase), and no further increase was observed in the percentage of early apoptotic cells after exposure for 10 h. In contrast, the 0.5% DMSO vehicle had only a negligible effect on the percentage of early apoptotic population of LNCaP human prostate cancer cells even after 10 h (Fig. 2B).
Fig. 2.
Time-dependent effect of JV-1-38 (10 μM) (A) and DMSO vehicle (0.5%) (B) on the percentage of live, early apoptotic, and late apoptotic populations of LNCaP human prostate cancer cells analyzed by flow cytometry using Annexin V-FITC and PI. Data are representative of at least three experiments in each case.
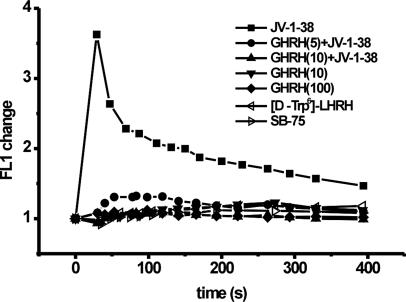
Effect of GHRH Antagonist JV-1-38 and GHRH(1–29)NH2Alone and in Combination, or Peptides Unrelated to GHRH on the Ca2+ Signal in LNCaP Human Prostate Cancer Cells. To test the tumoral GHRHR-specific effect of JV-1-38, we also studied the effect of a GHRH agonist GHRH(1–29)NH2 alone and in combination with JV-1-38 on intracellular free Ca2+ concentration in LNCaP human prostate cancer cells. In contrast to the stimulatory effect of GHRH antagonist JV-1-38 on cytosolic free Ca2+ concentration, exposure to agonist GHRH(1–29)NH2 alone did not change the cytosolic Ca2+ level in LNCaP human prostate cancer cells even at 10-fold higher concentration (100 μM) (Fig. 3). However, pretreatment of cells with 5 or 10 μM GHRH(1–29)NH2 for 10 min was able to inhibit JV-1-38-induced (10 μM) Ca2+-signal in a dose-dependent way, the resulting suppression being 64% and 100%, respectively (Fig. 3).
Fig. 3.
Effect of GHRH antagonist JV-1-38 (10 μM) and/or GHRH(1–29)NH2 (5–100 μM) or peptides unrelated to GHRH (LHRH agonist [d-Trp-6]-LHRH, LHRH antagonist SB-75, 10 μM) on the Ca2+ signal in LNCaP human prostate cancer cells measured by flow cytometry using Fluo-3 AM. In the case of combined treatments, cells were exposed to GHRH(1–29)NH2 10 min before treatment with JV-1-38. Results are representative of three sets of experiments.
To check the possible nonspecific effect of JV-1-38, LNCaP human prostate cancer cells also were treated with peptides other than GHRH analogs such as LHRH agonist [d-Trp-6]-LHRH or LHRH antagonist SB-75 at a 10 μM concentration (Fig. 3). None of these peptides unrelated to GHRH caused changes in intracellular free Ca2+ concentration in LNCaP human prostate cancer cells at the same high concentration (Fig. 3).
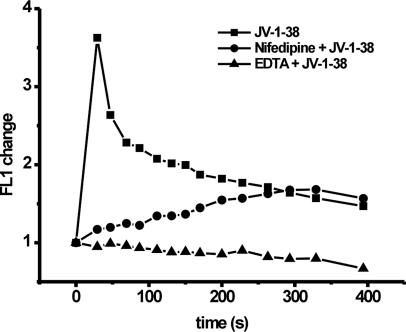
Effect of EDTA or Nifedipine on GHRH Antagonist JV-1-38-Induced Ca2+ Signal in LNCaP Human Prostate Cancer Cells. The addition of the Ca2+ chelator EDTA (10 mM) or the voltage-operated Ca2+ channel blocker nifedipine (10 μM) did not alter the cytosolic free Ca2+ concentration alone (data not shown). The stimulatory effect of GHRH antagonist JV-1-38 on Ca2+ influx was completely inhibited by EDTA (10 mM) (Fig. 4). Nifedipine (10 μM) also was able to reduce the prompt elevation of intracellular Ca2+ concentration induced by JV-1-38 (10 μM), but with shorter duration, because the cytosolic free Ca2+ level started to increase slowly, peaking after 300 s (Fig. 4).
Fig. 4.
Effect of GHRH antagonist JV-1-38 (10 μM) and EDTA (10 mM) or nifedipine (10 μM) on the Ca2+ signal in LNCaP human prostate cancer cells measured by flow cytometry using Fluo-3 AM. In the case of combined treatments, cells were exposed to either EDTA or nifedipine 10 min before treatment with JV-1-38. Results are representative of three sets of experiments.
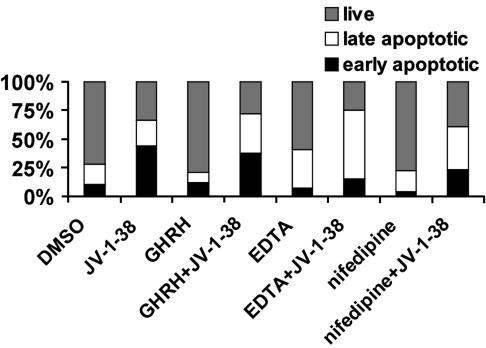
Effect of JV-1-38 Alone or After Pretreatment with GHRH, EDTA, or Nifedipine on the Percentage of Live, Early Apoptotic, and Late Apoptotic Populations of LNCaP Human Prostate Cancer Cells. LNCaP human prostate cancer cells were exposed to GHRH(1–29)NH2 (100 μM), EDTA (10 mM), or nifedipine (10 μM) for 30 min before the addition of GHRH antagonist JV-1-38 (10 μM) for 90 min (Figs. 5 and 6). The percentage of live, early, and late apoptotic/necrotic cell populations was analyzed by flow cytometry after Annexin V-FITC and PI labeling. Pretreatment with GHRH, EDTA, or nifedipine alone had only a negligible effect on the externalization of phosphatidylserine, an early marker of apoptosis (Figs. 5 and 6), whereas in the presence of EDTA, the ratio of late apoptotic/necrotic (Annexin V and PI double-positive) cells slightly increased (Fig. 6). Exposure to GHRH antagonist JV-1-38 (10 μM) for 90 min induced a remarkable increase (32.5%) in the population of early apoptotic cancer cells (Figs. 5C and 6). GHRH agonist GHRH(1–29)NH2 could not antagonize the stimulatory effect of JV-1-38 on the externalization of phosphatidylserine (Fig. 5D), whereas both EDTA (Fig. 5E) and nifedipine (Fig. 5F) reduced significantly the population of GHRH antagonist-induced early apoptotic cells by 77% and 62% (Fig. 6), respectively. The proportion of late apoptotic/necrotic cells was higher after all combined treatments, compared with exposures to the compounds alone (Fig. 6).
Fig. 5.
Effect of JV-1-38, GHRH(1–29)NH2, nifedipine, and EDTA alone or the combination of JV-1-38 with GHRH(1–29)NH2, nifedipine, or EDTA on the phosphatidylserine externalization in LNCaP human prostate cancer cells detected by flow cytometry. (A) Typical distribution of LNCaP cells based on their size (forward scatter, x axis) and granularity (side scatter, y axis). Further analysis took place on the R1 gated cells only. (B) Contour diagram of Annexin V-FITC/PI (FL1/FL3 channel) flow cytometry of LNCaP cells. Only those cells that are live or in the early stage of apoptosis (R2) are shown in C–F; necrotic or late apoptotic cells labeled by PI were omitted. (C–F) Typical experimental trace depicting the cellular fluorescence (FL1) of LNCaP cells exposed to 0.5% DMSO vehicle (C, filled curve) or 10 μM JV-1-38 (C, light gray curve); 100 μM GHRH(1–29)NH2 (D, filled curve) and/or 10 μM JV-1-38 (D, dark gray curve and light gray curve, respectively); 10 mM EDTA (E, filled curve) and/or 10 μM JV-1-38 (E, dark gray curve and light gray curve, respectively); or 10 μM nifedipine (F, filled curve) and/or 10 μM JV-1-38 (F, dark gray curve and light gray curve, respectively). Data are representative of at least three experiments in each case.
Fig. 6.
Effect of JV-1-38 (10 μM) alone for 90 min or after pretreatment with GHRH(1–29)NH2 (100μM), EDTA (10 mM), or nifedipine (10μM) for 30 min on the percentage of live, early apoptotic, and late apoptotic populations of LNCaP human prostate cancer cells analyzed by flow cytometry with Annexin V-FITC and PI. Data are representative of at least three experiments in each case.
Discussion
GHRH antagonists have been originally designed and synthesized to suppress GHRH-GH-IGF-I axis (1–3, 5, 6, 41, 47), because IGF-I is a known mitogen for various cell types and has been linked with malignant transformation, tumor progression, and metastasis of various cancers (7). In vitro and in vivo studies with specific GHRH antagonists revealed that, in addition to their action in suppressing cancer growth by interfering with the production of pituitary GH and hepatic IGF-I, GHRH antagonists act directly on cancer cells and strongly inhibit their proliferation (1, 8–15).
Although the expression of GHRH has been detected in various human normal and cancer cells, pituitary GHRHRs are virtually absent from almost every human nonpituitary tissue tested, including those in which GHRH had been previously shown to produce autocrine stimulation of cell proliferation (16–21). Recent demonstration of the presence of tumoral GHRHR as a splice variant of GHRHR can explain the potential direct target site mediating the antitumorigenic actions of GHRH antagonists on cancer cells (25–27). However, the exact mechanism underlying their antiproliferative actions and the signaling pathways of the tumoral GHRHR have not been elucidated completely.
It was assumed that the direct effects of GHRH antagonists on tumor growth were mediated by blocking the paracrine/autocrine actions of locally produced GHRH on tumoral GHRHR (8, 10, 11, 27, 29, 30, 33, 36). However, the accumulating evidence suggests that GHRH antagonists alone not only occupy but also activate tumoral GHRHR (35). In the present study, we demonstrated that GHRH antagonist JV-1-38 alone elevates intracellular free Ca2+ levels in LNCaP cancer cells in a dose-dependent way and induces externalization of phosphatidylserine as a marker of early apoptosis in a time-dependent way.
The stimulatory effect of JV-1-38 on Ca2+ signal seems to be tumoral GHRHR-specific, because GHRH(1–29)NH2 could antagonize it. Moreover, other peptides unrelated to GHRH, such as LHRH analogs, and agonist GHRH(1–29)NH2 alone were not effective in altering cytosolic free Ca2+ concentration at the concentrations tested.
We also demonstrated in this study that apoptosis induced by GHRH antagonist JV-1-38 requires an elevation of intracellular Ca2+ similar to other drug-induced apoptosis reported earlier (38). The increase in Ca2+ signal and the early apoptosis induced by JV-1-38 were extremely sensitive to removal of extracellular Ca2+ with EDTA and blockade of voltage-operated Ca2+ channel with nifedipine, indicating that the responses were mediated through extracellular Ca2+ entry.
All of the compounds tested, especially EDTA, caused some late apoptosis or necrosis detected by PI, but their effect was not dose-dependent or time-dependent, the degree of the effect varied between experiments, and the compounds did not influence significantly the proportions of early apoptotic and live cells. This nonspecific effect of these compounds was additive, because the proportion of late apoptotic/necrotic cells was higher after all combined treatments, compared with exposures to compounds alone.
The findings of the present study on tumoral GHRHR are in agreement with other results obtained from the signaling of pituitary GHRHR on somatotroph cells, because in both cases, the primary source of Ca2+ is from the extracellular environment (39, 40). The opposite effect of agonist GHRH(1–29)NH2 and antagonist JV-1-38 on tumoral GHRHR is surprising, compared with that on pituitary GHRHR, but can be explained by their different binding characteristics to tumoral and pituitary GHRHRs, which have distinct amino acid sequences (25, 26).
GHRH(1–29)NH2 very effectively inhibited Ca2+ response to JV-1-38; however, it failed to significantly suppress apoptosis induced by JV-1-38 in LNCaP human prostate cancer cells. This discrepancy could be explained by the longer time required for the externalization of phosphatidylserine than for Ca2+ signal and by the higher affinity of antagonist to the tumoral GHRHR than that of agonist. By the time of 90 min after the addition of GHRH antagonist, required for the manifestation of early apoptosis induced by JV-1-38, agonist GHRH(1–29)NH2 preincubated for 30 min, then incubated in combination with JV-1-38 could be displaced from the tumoral GHRHR by the GHRH antagonist. Therefore, the acute inhibitory effect of the GHRH agonist on Ca2+ signal may not extend until the onset of apoptosis. Alternatively, it is possible that the apoptotic effect of JV-1-38 is exerted in part by Ca2+-independent mechanisms that are less effectively counteracted by GHRH(1–29)NH2.
The data obtained showing an agonistic effect of JV-1-38 on Ca2+ signaling of tumoral GHRHR are consistent with our earlier results. GHRH antagonists at 10 μM concentration effectively suppressed the proliferation of MiaPaCa-2 human pancreatic cancer cells positive for tumoral GHRHR but negative for receptors for pituitary adenylate cyclase-activating polypeptide and vasoactive intestinal peptide. However, these antagonists caused a significant elevation in cAMP production in these cancer cells. In contrast, agonist GHRH(1–29)NH2 did not affect cAMP even at 100 μM (35). Our previous results also showed that GHRH antagonists were able to inhibit proliferation of those cells in which locally produced GHRH was neither present nor detectable (35). Another study found that the tumoral GHRHR possesses significant intrinsic, ligand-independent activity and stimulates cell proliferation in the absence of GHRH (48). These data suggest that JV-1-38 originally developed for antagonizing pituitary GHRHR also may act directly on tumoral GHRHR not only by blocking the effect of locally produced GHRH but by activating Ca2+ and cAMP signaling through the tumoral GHRHR as an agonist from a pharmacological and oncological point of view. Kineman pointed out in a commentary (49) that an increase, rather than a decrease, in cAMP production is associated with the inhibition of cell growth in most of the cancer cell lines tested. This conclusion supports our findings.
Thus, an increase in cAMP production and the elevation of intracellular free Ca2+ levels leading to apoptosis may be involved in the direct antiproliferative actions of GHRH antagonists. GHRH antagonists may offer a new approach to the therapy of prostate and other hormone sensitive cancers.
Acknowledgments
We thank Mrs. Judit Melczer for experimental assistance and Mrs. Eniko Nagy for technical help. This work was supported by Hungarian National Sciences Foundation Grant OTKA T 042713 (to Z.R.), grants from the Medical Research Service of the Department of Veterans Affairs and Zentaris (Frankfurt am Main, Germany) (to A.V.S.), and National Research and Development Programmes Grant 1/48/2001 (to P.N.).
Abbreviations: AM, acetoxymethyl ester; GH, growth hormone; GHRH, GH-releasing hormone; GHRHR, GHRH receptor; IGF-I, insulin-like growth factor I; LHRH, luteinizing hormone-releasing hormone; PI, propidium iodide.
References
- 1.Schally, A. V. & Varga, J. L. (1999) Trends Endocrinol. Metabol. 10, 383-391. [DOI] [PubMed] [Google Scholar]
- 2.Schally, A. V., Comaru-Schally, A. M., Plonowski, A., Nagy, A., Halmos, G. & Rekasi, Z. (2000) Prostate 45, 158-166. [DOI] [PubMed] [Google Scholar]
- 3.Schally, A. V., Comaru-Schally, A. M., Nagy, A., Kovacs, M., Szepeshazi, K., Plonowski, A., Varga, J. L. & Halmos, G. (2001) Front. Neuroendocrinol. 22, 248-291. [DOI] [PubMed] [Google Scholar]
- 4.Frohman, L. A. & Kineman, R. D. (2002) Trends Endocrinol. Metab. 13, 299-303. [DOI] [PubMed] [Google Scholar]
- 5.Schally, A. V. & Comaru-Schally, A. M. (2003) in Cancer Medicine, eds. Kufe, D. W., Pollock, R. E., Weichselbaum, R. R., Bast, R. C., Jr., Gansler, T. S., Holland, J. F. & Frei, E., III (BC Decker, Hamilton, ON, Canada), 6th Ed., pp. 911-926.
- 6.Schally, A. V., Szepeshazi, K., Nagy, A., Comaru-Schally, A. M. & Halmos, G. (2004) Cell. Mol. Life Sci. 61, 1042-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furstenberger, G. & Senn, H. J. (2002) Lancet Oncol. 3, 298-302. [DOI] [PubMed] [Google Scholar]
- 8.Kiaris, H., Schally, A. V., Varga, J. L., Groot, K. & Armatis, P. (1999) Proc. Natl. Acad. Sci. USA 96, 14894-14898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahan, Z., Varga, J. L., Schally, A. V., Rekasi, Z., Armatis, P., Chatzistamou, I., Czompoly, T. & Halmos, G. (2000) Breast Cancer Res. Treat. 60, 71-79. [DOI] [PubMed] [Google Scholar]
- 10.Chatzistamou, I., Schally, A. V., Varga, J. L., Groot, K., Busto, R., Armatis, P. & Halmos, G. (2001) Anticancer Drugs 12, 761-768. [DOI] [PubMed] [Google Scholar]
- 11.Chatzistamou, I., Schally, A. V., Varga, J. L., Groot, K., Armatis, P., Busto, R. & Halmos, G. (2001) J. Clin. Endocrinol. Metab. 86, 2144-2152. [DOI] [PubMed] [Google Scholar]
- 12.Braczkowski, R., Schally, A. V., Plonowski, A., Varga, J. L., Groot, K., Krupa, M. & Armatis, P. (2002) Cancer 95, 1735-1745. [DOI] [PubMed] [Google Scholar]
- 13.Busto, R., Schally, A. V., Varga, J. L., Garcia-Fernandez, M. O., Groot, K., Armatis, P. & Szepeshazi, K. (2002) Proc. Natl. Acad. Sci. USA 99, 11866-11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeitler, P. & Siriwardana, G. (2002) Endocrine 18, 85-90. [DOI] [PubMed] [Google Scholar]
- 15.Kiaris, H., Koutsilieris, M., Kalofoutis, A. & Schally, A. V. (2003) Expert Opin. Investig. Drugs 12, 1385-1394. [DOI] [PubMed] [Google Scholar]
- 16.Mayo, K. E. (1992) Mol. Endocrinol. 6, 1734-1744. [DOI] [PubMed] [Google Scholar]
- 17.Gaylinn, B. D., Harrison, J. K., Zysk, J. R., Lyons, C. E., Lynch, K. R. & Thorner, M. O. (1993) Mol. Endocrinol. 7, 77-84. [DOI] [PubMed] [Google Scholar]
- 18.Matsubara, S., Sato, M., Mizobuchi, M., Niimi, M. & Takahara, J. (1995) Endocrinology 136, 4147-4150. [DOI] [PubMed] [Google Scholar]
- 19.Mayo, K. E., Miller, T. L., De Almeida, V., Zheng, J. & Godfrey, P. A. (1996) Ann. N.Y. Acad. Sci. 805, 184-203. [DOI] [PubMed] [Google Scholar]
- 20.Boisvert, C., Pare, C., Veyrat-Durebex, C., Robert, A., Dubuisson, S., Morel, G. & Gaudreau, P. (2002) Endocrinology 143, 1475-1484. [DOI] [PubMed] [Google Scholar]
- 21.Boulanger, L., Girard, N., Strecko, J. & Gaudreau, P. (2002) Peptides 23, 1187-1194. [DOI] [PubMed] [Google Scholar]
- 22.Rekasi, Z., Varga, J. L., Schally, A. V., Halmos, G., Armatis, P., Groot, K. & Czompoly, T. (2000) Endocrinology 141, 2120-2128. [DOI] [PubMed] [Google Scholar]
- 23.Maruno, K., Absood, A. & Said, S. I. (1998) Proc. Natl. Acad. Sci. USA 95, 14373-14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherwood, N. M., Krueckl, S. L. & McRory, J. E. (2000) Endocr. Rev. 21, 619-670. [DOI] [PubMed] [Google Scholar]
- 25.Halmos, G., Schally, A. V., Varga, J. L., Plonowski, A., Rekasi, Z. & Czompoly, T. (2000) Proc. Natl. Acad. Sci. USA 97, 10555-10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rekasi, Z., Czompoly, T., Schally, A. V. & Halmos, G. (2000) Proc. Natl. Acad. Sci. USA 97, 10561-10566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chopin, L. K. & Herington, A. C. (2001) Prostate 49, 116-121. [DOI] [PubMed] [Google Scholar]
- 28.Busto, R., Schally, A. V., Braczkowski, R., Plonowski, A., Krupa, M., Groot, K., Armatis, P. & Varga, J. L. (2002) Regul. Peptides 108, 47-53. [DOI] [PubMed] [Google Scholar]
- 29.Halmos, G., Schally, A. V., Czompoly, T., Krupa, M., Varga, J. L. & Rekasi, Z. (2002) J. Clin. Endocrinol. Metab. 87, 4707-4714. [DOI] [PubMed] [Google Scholar]
- 30.Plonowski, A., Schally, A. V., Busto, R., Krupa, M., Varga, J. L. & Halmos, G. (2002) Peptides 23, 1127-1133. [DOI] [PubMed] [Google Scholar]
- 31.Plonowski, A., Schally, A. V., Letsch, M., Krupa, M., Hebert, F., Busto, R., Groot, K. & Varga, J. L. (2002) Prostate 52, 173-182. [DOI] [PubMed] [Google Scholar]
- 32.Kiaris, H., Schally, A. V., Busto, R., Halmos, G., Artavanis-Tsakonas, S. & Varga, J. L. (2002) Proc. Natl. Acad. Sci. USA 99, 196-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chatzistamou, I., Schally, A. V., Kiaris, H., Politi, E., Varga, J., Kanellis, G., Kalofoutis, A., Pafiti, A. & Koutselini, H. (2004) Eur. J. Endocrinol. 151, 391-396. [DOI] [PubMed] [Google Scholar]
- 34.Toller, G. L., Horvath, J. E., Schally, A. V., Halmos, G., Varga, J. L., Groot, K., Chism, D. & Zarandi, M. (2004) Proc. Natl. Acad. Sci. USA 101, 15160-15165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rekasi, Z., Varga, J. L., Schally, A. V., Plonowski, A., Halmos, G., Csernus, B., Armatis, P. & Groot, K. (2001) Peptides 22, 879-886. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Fernandez, M. O., Schally, A. V., Varga, J. L., Groot, K. & Busto, R. (2003) Breast Cancer Res. Treat. 77, 15-26. [DOI] [PubMed] [Google Scholar]
- 37.Kanashiro, C. A., Schally, A. V., Zarandi, M., Hammann, B. D. & Varga, J. L. (2004) Int. J. Cancer 112, 570-576. [DOI] [PubMed] [Google Scholar]
- 38.Hawkins, T. E., Das, D., Young, B. & Moss, S. E. (2002) Proc. Natl. Acad. Sci. USA 99, 8054-8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brazeau, P., Ling, N., Esch, F., Bohlen, P., Mougin, C. & Guillemin, R. (1982) Biochem. Biophys. Res. Commun. 109, 588-594. [DOI] [PubMed] [Google Scholar]
- 40.Lussier, B. T., French, B. M., Moor, B. C. & Kraicer, J. (1991) Endocrinology 128, 592-603. [DOI] [PubMed] [Google Scholar]
- 41.Varga, J. L., Schally, A. V., Csernus, V. J., Zarandi, M., Halmos, G., Groot, K. & Rekasi, Z. (1999) Proc. Natl. Acad. Sci. USA 96, 692-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coy, D. H., Vilchez-Martinez, J. A., Coy, E. J. & Schally, A. V. (1976) J. Med. Chem. 19, 423-425. [DOI] [PubMed] [Google Scholar]
- 43.Bajusz, S., Kovacs, M., Gazdag, M., Bokser, L., Karashima, T., Csernus, V. J., Janaky, T., Guoth, M. & Schally, A. V. (1988) Proc. Natl. Acad. Sci. USA 85, 1637-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minta, A., Kao, J. P. & Tsien, R. Y. (1989) J. Biol. Chem. 264, 8171-8178. [PubMed] [Google Scholar]
- 45.Boldizsar, F., Berki, T., Miseta, A. & Nemeth, P. (2002) Immunol. Lett. 82, 159-164. [DOI] [PubMed] [Google Scholar]
- 46.Vermes, I., Haanen, C., Steffens-Nakken, H. & Reutelingsperger, C. (1995) J. Immunol. Methods 184, 39-51. [DOI] [PubMed] [Google Scholar]
- 47.Zarandi, M., Horvath, J. E., Halmos, G., Pinski, J., Nagy, A., Groot, K., Rekasi, Z. & Schally, A. V. (1994) Proc. Natl. Acad. Sci. USA 91, 12298-12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kiaris, H., Chatzistamou, I., Schally, A. V., Halmos, G., Varga, J. L., Koutselini, H. & Kalofoutis, A. (2003) Proc. Natl. Acad. Sci. USA 100, 9512-9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kineman, R. D. (2000) Proc. Natl. Acad. Sci. USA 97, 532-534. [DOI] [PMC free article] [PubMed] [Google Scholar]