Abstract
Leukemia inhibitory factor (LIF) is required, but not sufficient, for pluripotent mouse embryonic stem (ES) cell expansion in vitro in the absence of serum or a feeder cell layer, suggesting that additional signals are provided by serum or feeders that are necessary to support self-renewal. Here we show that transgenic ES cell lines expressing Bcl-2, an antiapoptotic protein, continue to self-renew in serum- and feeder-free conditions when supplemented with LIF; even in the absence of bone morphogenic proteins. Bcl-2-expressing clones sustain the characteristics of undifferentiated, pluripotent ES cells during long-term culture, and maintain their potential to differentiate into mature cell types. These results suggest that LIF and Bcl-2 overexpression are sufficient to expand these mouse pluripotent stem cells in vitro.
Keywords: leukemia inhibitory factor, antiapoptotic protein, pluripotency
Stem cells are defined as cells that, at the single-cell level, are capable of self-renewal and differentiation to specialized cell types (1). ES cells are pluripotent stem cells derived from the inner cell mass of blastocysts (2) and can self-renew indefinitely in vitro in the presence of leukemia inhibitory factor (LIF) and FBS or mouse feeder layer cells, resulting in daughter cells that maintain their potential for multilineage differentiation (3, 4). When ES cells are maintained in serum- and feeder-free conditions, the number of undifferentiated cells quickly reaches a plateau and begins to decline after only a couple of passages (5), and cells with a non-ES cell morphology quickly arise in culture (6) despite the presence of LIF. Thus, additional factors provided by serum or feeders appear to be required to fully support the self-renewal of mouse ES cells.
Bone morphogenic proteins (BMPs) have been implicated as the factor contained in serum or provided by feeder layers that acts in concert with LIF to maintain undifferentiated mouse ES cells in vitro (7). It was recently suggested that BMPs can replace serum and feeder cell requirements in ES cell culture by activating the Smad pathway and inducing expression of the Id gene, a common target of Smad signaling (8) that appears to block differentiation by negatively regulating basic helix–loop–helix proteins (5). Although the exact mechanism by which BMP promotes self-renewal of ES cells is not certain, recent work suggests that it might also inhibit the mitogen-activated protein kinase (MAPK) pathway independent of Smads (7). Importantly, inhibition of p38 MAPK facilitates derivation of ES cells from blastocysts lacking Alk-3 (BMPRIA) (7), and ES cells can be derived from blastocysts lacking Smad4 (the common partner of all Smads; ref. 9), supporting the hypothesis that BMP acts by means of different mechanisms depending on the presence or absence of serum and feeders.
Considering the possibility that serum and feeder cells provide cell survival signals manifest as growth factors and cytokines (10) and that extrinsic survival signals are especially critical in low cell density conditions, where stimulation through autocrine and paracrine factors are minimal, ES cells likely become apoptotic in suboptimal culture conditions (i.e., in the absence of serum and feeder cells). At low cell density, ES cells infrequently generate pluripotent colonies. To analyze the effect of single cytokines, growth factors, and other molecules on the self-renewal and differentiation of ES cells, it would be optimal if cells could be protected from apoptotic cell death in serum- and feeder-free conditions. Although the use of N2- and B27- supplemented media to expand ES cells in serum- and feeder- free conditions improves viability and, thus, allows their survival even at low cell density conditions, LIF plus these supplements cannot support the self-renewal of ES cells unless the culture is further supplemented with BMP (5). Because N2 and B27 supplements contain hormones (corticosterone, progesterone, and T3) and retinyl acetate (a precursor of retinoic acid; refs. 11 and 12) and some of these components are used in ES cell differentiation protocols (13, 14), their presence complicates the analysis of the effects of single cytokines, growth factors, and other molecules on the self-renewal and differentiation of ES cells.
To prevent ES cells from undergoing apoptosis and to simplify the analysis of exogenous factors on the self-renewal and differentiation in the absence of serum and feeders, we established ES cell clones constitutively expressing human Bcl-2. The Bcl-2 family of antiapoptotic proteins has been implicated in the prevention of cell death by sequestering BH3-only proapoptotic proteins on the mitochondrial surface, thus antagonizing multidomain proapoptotic proteins (10). In the context of hematopoietic stem and progenitor cells, enforced expression of Bcl-2 helped separate the survival effects of certain cytokines from their roles in growth and differentiation (15–18) and enabled the examination of the effects of single growth factor and cytokine on the self-renewal and differentiation in vitro (19, 20).
Here we show that ES cells constitutively expressing Bcl-2 have a survival advantage in serum- and feeder-free conditions, and, interestingly, these Bcl-2 clones expand in an undifferentiated state in the absence of serum and feeders when supplemented with LIF.
Materials and Methods
Cell Culture. D3 ES cells (21) were maintained as described in ref. 22, and clones expressing human Bcl-2 were established by cotransfecting parental D3 ES cells with the human Bcl2–internal ribosome entry site–GFP transgene under control of the CAG promoter and a puromycin-resistance gene cassette. Bcl-2 ES cell clones were selected and expanded in the presence of puromycin. For serum-free cultures, ES cells were maintained in X-VIVO 15 (Cambrex, East Rutherford, NJ) supplemented with 2 mM GlutaMax-1 (GIBCO)/0.1 mM 2-mercaptoethanol/1,000 units/ml ESGRO (LIF; Chemicon International)/100 units/ml penicillin (GIBCO)/100 μg/ml streptomycin (GIBCO) on gelatin-coated dishes. Alternatively, Iscove's modified Dulbecco medium/F12 (1:1) (GIBCO) supplemented with 2 mM GlutaMax-1/0.1 mM 2-mercaptoethanol/0.1% polyvinyl alcohol (Sigma)/1× insulin-transferin-selenium-X (GIBCO)/1,000 units/ml ESGRO/100 units/ml penicillin/100 μg/ml streptomycin was used where indicated. Cells were dissociated with enzyme-free, Hanks'-based cell dissociation buffer (GIBCO). For MAPK inhibitors, 1 μM SB203580 (Calbiochem) and 12.5 μM PD98059 (Calbiochem) were used as described in refs. 7 and 23. To induce hematopoietic differentiation in vitro, cells were placed on ST2 (24) in MEM alpha media (GIBCO) supplemented with 10% FCS. On day 6 of differentiation, colonies were dissociated with 0.25% trypsin/0.5 mM EDTA (GIBCO) and replated onto freshly confluent ST2 cells. On day 14 of differentiation, nonadherent cells were harvested by gentle pipetting and replated onto OP9 feeder cells (25). On day 21, cells were harvested and analyzed by flow cytometry. For neuronal induction, cells were placed on AC11 stromal cells (26) and cultured in Iscove's modified Dulbecco medium supplemented with 2 mM GlutaMax-1/0.1 mM 2-mercaptoethanol/0.1 μM dexamethasone (Sigma)/15% FCS/100 units/ml penicillin/100 μg/ml streptomycin, and cells were analyzed for neuronal markers after 2 weeks of culture.
Flow Cytometry. For intracellular staining, cells were fixed in 1% paraformaldehyde/PBS for 20 min and then permeabilized and blocked by using 0.1% saponin/10% FCS for 20 min. Cells were then incubated with primary antibodies against human Bcl-2 (DAKO) or Oct-3/4 (BD Biosciences) for 1 h, washed, and then stained with phycoerythrin-conjugated secondary antibodies for 30 min. For hematopoietic analysis, cells were blocked with rat IgG for 20 min and then incubated with a mixture of the following phycoerythrin-conjugated antibodies for 20 min: M1/70 (Mac-1), 8C5 (Gr-1), and Ter119. Annexin V labeling was performed with allophycocyanin-conjugated annexin V (Molecular Probes) antibodies according to the manufacturer's instruction.
Immunocytochemistry. Cells were fixed in 4% paraformaldehyde for 15 min, permeabilized with 0.1% Triton X-100 in PBS for 5 min, and then blocked with 1% BSA in PBS for 15 min. Cells were incubated with primary antibodies against Oct-3/4, class III β-tubulin (TuJ1, Covance, Princeton), neuron-specific nuclear protein (Chemicon), or microtubule-associated protein-2 (Chemicon) overnight, washed, and then incubated with phycoerythrin- or Cy3-conjugated goat anti-mouse IgG secondary antibodies for 1 h.
PCR. Quantitative RT-PCR was performed by using total RNA isolated from parental murine D3 or Bcl-2 ES cells by using the RNeasy procedure (Qiagen, Valencia, CA), after treatment with DNase I for 15 min at room temperature. Reverse transcription was performed by using poly(dT) primers and the SuperScript firststrand synthesis system (Invitrogen). Amplification was performed by using the Nanog forward 5′-TCTGGGAACGCCTCATCAAT-3′ and reverse 5′-GGAGAGGCAGCCTCTGTGC-3′ primers, Rex-1 forward 5′-GCGACATTTTCTGGTGCACA-3′ and reverse 5′-TCGAACGTGCACTGATACGG-3′ primers, and GAPDH forward 5′-GGCAAATTCAACGGCACAGT-3′ and reverse 5′-TCGCTCCTGGAAGATGGTGAT-3′ primers with 40 cycles of two-step PCR (15 s at 95°C and 60 s at 60°C) after initial denaturation (95°C for 10 min) with a Prism 7000 Sequence Detector system (Applied Biosystems). Amplification of GAPDH mRNA was used to normalize reactions internally. Each sample was analyzed in duplicate, and results are expressed as the mean mRNA expression level ± SEM relative to parental D3 cells (n = 3). For genomic PCR, genomic DNA was isolated by using a DNeasy tissue kit (Qiagen). Amplification was performed by using the Sry forward 5′-CAGCCCTACAGCCACATGAT-3′ and reverse 5′-TTTAGCCCTCCGATGAGGC-3′ primers and Actin forward 5′-GTACCACAGGCATTGTGATG-3′ and reverse 5′-TAGTGATGACCTGGCCGTCA-3′ primers with 36 cycles of PCR (45 s at 94°C, 60 s at 56°C, and 90 s at 72°C) after initial denaturation (94°C for 5 min).
Chimeric Mice. Blastocysts were collected from superovulated C57BL/Ka females at embryonic day 3.5. Injected or noninjected blastocysts were transferred into the uterus of embryonic day 2.5 pseudopregnant BCBA/F1 females. All mice were maintained in Stanford University's Research Animal Facility in accordance with Stanford Administrative Panel for Laboratory Animal Care guidelines.
Western Blots. Cells were lysed in 50 mM Tris·HCl, pH 7.5/150 mM NaCl/1% Triton X-100/1% protease inhibitor mixture set III (Calbiochem)/1 mM EDTA/1 mM sodium orthovanadate/1 mM NaF, and proteins were separated by SDS/PAGE under denaturing conditions and transferred to an Immobilon-P membrane (Millipore). After blocking with 5% milk, the membrane was incubated with primary antibodies against phospho-Smad1/5/8 (Cell Signaling Technology, Beverly, MA), actin (Sigma), Bcl-2 (DAKO), Bcl-xL (BD Biosciences), Mcl-1 (Abgent, San Diego), or Bax (BD Biosciences); washed; and then incubated with horseradish peroxidase-conjugated secondary antibodies. Immunoreactive bands were visualized by using enhanced chemiluminescence (ECL, Amersham Pharmacia).
Results
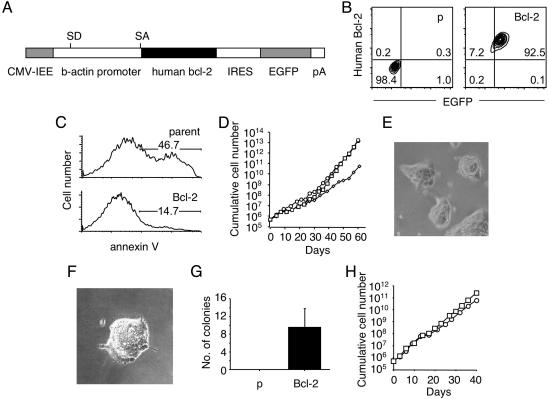
Serum- and Feeder-Independent Growth of ES Cells Overexpressing Bcl-2. To facilitate examination of cytokine, growth-factor and small-molecule effects on self-renewal and differentiation of ES cells in the absence of serum and feeders, we established ES cell clones overexpressing human Bcl-2. In these clones, human Bcl-2 expression was driven by the CAG promoter (human cytomegalovirus immediate-early enhancer and a modified chicken β-actin promoter) (27) and visualized by using bicistronic expression of EGFP (Figs. 1 A and B). In conventional culture conditions containing serum, ES cell clones expressing Bcl-2 and the parental ES cell line grew with similar kinetics. To confirm that Bcl-2 overexpression provides a survival advantage to the cells, parental and Bcl-2 ES cells were cultured in serum- and feeder-free conditions in X-Vivo media, which contains insulin, transferrin, and albumin as basic supplements for serum-free cultures but contains no other proteins or hormones. Apoptotic cells were then detected by annexin V staining. As shown in Fig. 1C, less Bcl-2 cells were positively labeled with annexin V versus parental ES cells, suggesting that Bcl-2 provides a survival advantage normally supported by factors included in serum or by feeder cells.
Fig. 1.
Serum- and feeder layer-independent growth of ES cell clones overexpressing Bcl-2. (A) Construction of CAG-human Bcl-2–internal ribosome entry site (IRES)–GFP plasmid. (B)(Right) Expression of human Bcl-2 (y axis) and GFP (x axis) in a representative Bcl-2 clone. (Left) The parental clone is shown as a control. (C) Annexin V staining. Parental (Upper) or Bcl-2 (Lower) ES cells were cultured in serum- and feeder-free conditions for 3 days, and the percentage of annexin V+ cells as visualized by flow cytometry is denoted. (D) Growth curve of three independent Bcl-2 ES cell clones in serum- and feeder-free conditions in X-Vivo media. (E) Colonies of a Bcl-2 clone growing independent of serum and feeders. (F) A representative colony formed under low-cell-density conditions. (G) Colony formation activity of parental (p) and Bcl-2 ES cells plated at a density of 200 cells per well (12-well plate). Colonies were counted 7 days later, and values are expressed as means ± SD. (H) Growth curve of two independent Bcl-2 clones in Iscove's modified Dulbecco medium/F12-based media.
Interestingly, Bcl-2 clones continued to proliferate in the presence of LIF alone in X-Vivo media, as demonstrated in Fig. 1D. In contrast, expansion of parental ES cells was not possible beyond a couple of passages, despite the presence of LIF. Although Bcl-2 transgenic ES cells grew slowly in the absence of serum and feeder layers compared with culture conditions containing serum, they expanded indefinitely (for at least 2 months). Bcl-2 clones expanded independent of serum, and feeders formed tightly packed colonies with indistinct cell boundaries (Fig. 1E), a hallmark of undifferentiated ES cells. Even at low cell density (50 cells per cm2), Bcl-2 cells could form tightly packed colonies with an undifferentiated appearance in the presence of LIF alone (Fig. 1F). After the input of 200 Bcl-2 ES cells per well, 9.5 ± 4.2 undifferentiated colonies were formed after 7 days of culture without serum and feeders (Fig. 1G), whereas parental ES cells never formed colonies at this clonal density (Fig. 1G), suggesting provision of survival signals by serum or feeders is critical at low cell density. Not only did Bcl-2-expressing ES cell clones expand in X-Vivo media, but they also grew in Iscove's modified Dulbecco medium/F-12 media containing insulin and transferrin but not containing other proteins or hormones other than LIF (Fig. 1H), which indicates that growth of Bcl-2 ES cell clones in serum- and feeder-free conditions was not specific to X-Vivo media.
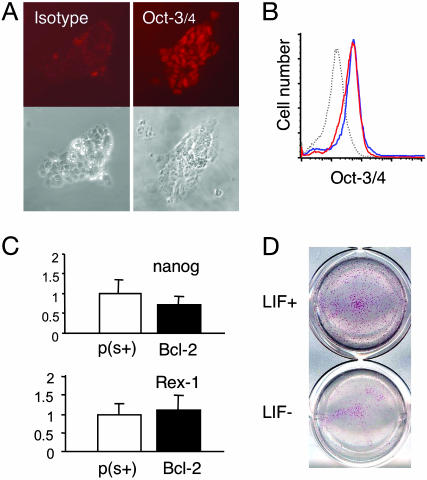
Serum- and Feeder-Free Expansion of Bcl-2 ES Cells Cultured with LIF. The tightly packed morphology of Bcl-2 ES cell clones in serum- and feeder-free conditions suggested maintenance in an undifferentiated state. To verify their “undifferentiated” status, molecular markers of undifferentiated ES cells were examined after extended serum- and feeder-free culture. Oct-3/4, the POU transcription factor required for the formation and maintenance of ES cells (28, 29), was detected by immunocytochemistry in Bcl-2 ES cell clones cultured in serum- and feeder-free conditions (Fig. 2A) and was comparable to parental ES cells cultured in serum as detected by FACS (Fig. 2B). Oct-3/4 gene expression, as determined by quantitative RT-PCR verified this finding (data not shown). Furthermore, gene expression of Nanog, a homeodomain protein required for ES cell pluripotency (30, 31), and Rex-1, a zinc-finger protein specifically expressed in pluripotent cells (32), was also similar in the parental ES cell line in conventional conditions and Bcl-2 ES cells cultured in the absence of serum and feeders (Fig. 2C). Bcl-2 clones cultured in serum- and feeder-free conditions also contained alkaline phosphatase activity (another marker of pluripotent cells of embryonic origin) (33) in the presence of LIF, but the clones lost this characteristic after the removal of LIF (Fig. 2D). These properties all suggest that Bcl-2-expressing ES cell clones expanded in the absence of serum and feeder layers are maintained in an undifferentiated state and that the requirement for supplemental LIF remains intact.
Fig. 2.
Expression of undifferentiated ES cell markers. (A) Cells cultured for 44 days after serum removal were stained with Oct-3/4 (Right) or isotype control antibody (Left). Shown are fluorescent (Upper) and phase contrast (Lower) images, respectively. (B) Bcl-2 ES cells cultured 45 days without serum and feeders (red line) or parental ES cells cultured with serum (blue line and dashed line) were stained with Oct-3/4 (red and blue lines) or isotype control antibody (dashed line). (C) The expression levels of nanog and Rex-1 were analyzed in parental D3 cells with serum [p(s+)] and Bcl-2 ES cells cultured for 49 days in serum- and feeder-free conditions (Bcl-2). Gene expression levels are shown after internal normalization with GAPDH and normalization against parental ES cells (value = 1). Values are expressed as means ± SEM. (D) Alkaline phosphatase activity was assessed in Bcl-2 ES cells grown in serum- and feeder-free conditions for 43 days before culture with (Upper) or without LIF (Lower) for 6 days in serum-free conditions before staining.
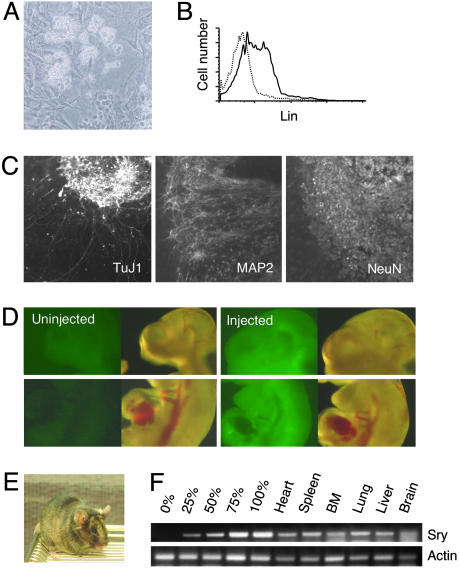
Pluripotency of Bcl-2 ES Cells Is Maintained in Serum- and Feeder-Free Conditions. To determine whether the multilineage potential of ES cells was sustained in Bcl-2 clones grown in the absence of serum and feeders, ES cell differentiation potential was investigated in vitro. Bcl-2 ES cells efficiently generated hematopoietic colonies when sequentially cultured on ST2 and OP9 stromal cell lines (22, 34) (Fig. 3A), and flow-cytometric analysis demonstrated the generation of cells with mature myeloid cell lineage markers (Fig. 3B). In addition, Bcl-2 ES cells efficiently generated neurons, as identified by staining with antibodies against class III β-tubulin, microtubule-associated protein-2, and neuron-specific nuclear protein after culture on AC11 stromal cells in the presence of dexamethasone, a synthetic corticosteroid (T.Y. and I.L.W., unpublished data) (Fig. 3C). These results indicate that Bcl-2 ES cell clones proliferating in serum- and feeder-free conditions maintain their multipotency.
Fig. 3.
Pluripotency of Bcl-2 ES cells growing independent of serum and feeders. (A) Phase contrast image of a hematopoietic cell cluster derived from Bcl-2 ES cells and generated on OP9 feeders after 41 days in serum- and feeder-free conditions. (B) Expression of hematopoietic markers (Lin), including Mac-1, Gr-1, and Ter119, as determined by flow cytometry. The dotted line shows isotype control antibody staining levels. (C) Bcl-2 ES cells were induced to differentiate on AC11 after 49 days of serum- and feeder-free culture and then analyzed for neuronal markers by immunofluorescence. MAP2, microtubule-associated protein-2; TuJ1, monoclonal antibody against neuronal class III β-tubulin; NeuN, neuron-specific nuclear protein. (D) Embryos derived from uninjected blastocysts (Left) or those injected with Bcl-2 ES cells (Right) cultured for 45 days without serum and feeders were analyzed for GFP expression (ES cell origin) on embryonic day 11.5. Photos of the head (Upper) and trunk (Lower) region were taken, and each image shows a fluorescent and bright field image. (E) Picture of a chimeric mouse generated from Bcl-2 ES cells cultured for 31 days without serum and feeders. Agouti coat color denotes Bcl-2 ES cell origin. (F) A female chimeric mouse was used to examine Bcl-2 ES cell contributions in various tissues. The Sry gene was used to detect male Bcl-2 ES cell-derived contribution. Standards are 0–100% male genomic DNA diluted in female genomic DNA, and actin is shown as a control.
To definitively determine whether Bcl-2-expressing ES cells expanded in the absence of serum and feeders fully maintain their pluripotency, we checked whether they could contribute to chimeric animals after being injected into blastocysts. Cells from a Bcl-2 clone cultured in the absence of serum and feeders were injected into blastocysts and transferred to the uteri of pseudopregnant mice. Mice on embryonic day 11.5 were checked for contribution of Bcl-2 ES cell-derived cells to the embryo by GFP expression, wherein green fluorescence was detected throughout the chimera (Fig. 3D). In addition, chimeric mice brought to term had Bcl-2 ES cell-derived (i.e., 129 background) agouti coat color (Fig. 3E). Tissue chimerism was investigated in female offspring (because Bcl-2 ES cells have male genotype), wherein the contribution of Bcl-2 ES cells was examined by quantification of Y chromosome genes on the female background. Semiquantitative PCR analysis for presence of the Sry gene (a Y chromosome gene) with genomic DNA from various tissues demonstrated an ≈30% contribution to all tissues analyzed, including the heart, spleen, bone marrow (mesoderm), lung, liver (endoderm), and brain (ectoderm) (Fig. 3F). To date, Bcl-2 chimeric mice have not developed tumors (6 months of age). Together, these results demonstrate the pluripotentiality of Bcl-2 ES cells maintained in the absence of serum and feeders.
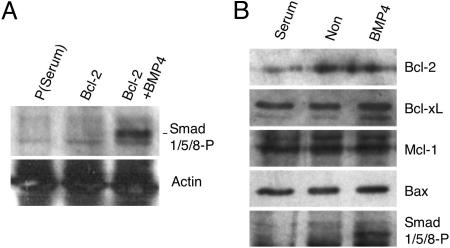
BMP-Independent Self-Renewal of Bcl-2 ES Cells. Ying et al. (5) recently reported that BMPs or growth and differentiation factors (GDFs) substitute serum and feeder requirements during the maintenance of ES cells (5); however, Bcl-2 ES cell clones can be maintained in serum- and feeder-free media containing only LIF, insulin, transferrin, and albumin as protein components. One possible explanation for the above discrepancy is that Bcl-2 clones secrete these effectors in an autocrine manner. Ying et al. (5) reported that BMP2, BMP4, and GDF6, but not TGF-β1 or activin, support the self-renewal of ES cells (5) and suggested that Smad1/5/8 are likely downstream targets of these effectors (8, 35, 36). To test whether BMP/GDF signaling acts in an autocrine fashion in our culture conditions, Western blot analysis was performed for phosphorylated Smad1/5/8. As shown in Fig. 4A, the activated forms of Smad1/5/8 were not detected in Bcl-2 ES cells in either serum- or feeder-free conditions, although they could respond to BMP4 stimulation (Fig. 4A). These results suggest that ES cells overexpressing Bcl-2 self-renew in the presence of LIF independent of BMP activity.
Fig. 4.
Western blot analysis of Smad proteins and Bcl-2 family members. (A) (Upper) Cell lysates of parental cells cultured in conventional conditions with serum [p(serum)] and a Bcl-2 clone in serum- and feeder-free conditions untreated (Bcl-2) or treated with BMP4 for 90 min (Bcl-2+BMP4) were analyzed for the presence of phospho-Smad1/5/8 by Western blotting. (Lower) The same membrane was stripped and reprobed with anti-actin antibody. (B) Western blot analysis of Bcl-2 family members was performed for parental ES cells in conventional conditions with serum and parental ES cells in serum- and feeder-free conditions without (Non) or with BMP4 for 6 h.
BMP and Serum Do Not Regulate Bcl-2 Family Expression. BMP- independent self-renewal of Bcl-2 clones prompted us to examine whether Bcl-2 family proteins are downstream targets of BMP signaling. Western blots were done to determine whether expression of antiapoptotic or proapoptotic Bcl-2 family members were up- or down-regulated upon BMP/GDF stimulation, respectively; however Bcl-2, Bcl-xL, and Mcl-1 antiapoptotic proteins were not up-regulated by BMP stimulation, nor was the Bax proapoptotic protein down-regulated by BMP stimulation (Fig. 4B). Importantly, addition of BMP did not improve the viability of the parental cell line cultured in serum- and feeder- free conditions. Thus, it appears unlikely that BMP confers a survival advantage to ES cells, and other factors in serum likely control ES cells survival by a mechanism independent of regulating the expression of Bcl-2 family members, because the removal of serum neither down-regulated Bcl-2, Bcl-xL, or Mcl-1 nor up-regulated Bax (Fig. 4B). It is more likely that factors in serum regulate Bcl-2 family members posttranslationally (e.g., by phosphorylation) to protect ES cells from apoptosis (10).
Does Bcl-2 Have Apoptosis-Independent Effects? LIF alone has been reported to be insufficient to block differentiation of ES cells, but BMPs in combination with LIF can efficiently block differentiation of ES cells (5). However, LIF is sufficient to support the self-renewal of Bcl-2 ES cells. One passage before unsuccessful propagation of parental ES cells we recognized differentiated cells (noncompact, round, or fibroblastic cells) appearing at the periphery of each colony (Fig. 5A). This phenomenon supports the possibility that Bcl-2 blocks differentiation; however, Bcl-2 ES cells differentiated in the absence of LIF (Fig. 2D), developed normally in in vitro differentiation assays (Fig. 3 A–C), and integrated normally during embryogenesis (Fig. 3 D–F). Silencing of the Bcl-2 transgene in these assays is unlikely, because GFP expression was always observed (Fig. 3D). Thus, if Bcl-2 actively blocks differentiation of ES cells, it must inhibit differentiation very specifically and may be contingent on LIF.
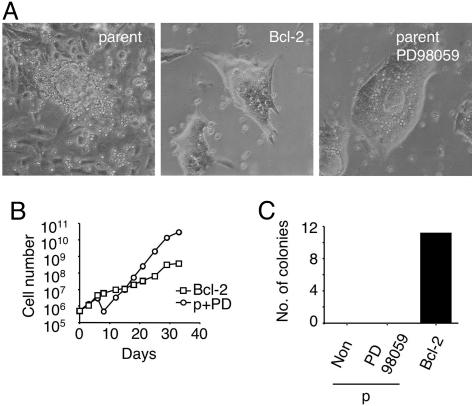
Fig. 5.
Effect of MAPK inhibitors on ES cell self-renewal. (A) Phase contrast image of parental ES cells cultured for 8 days (Left), Bcl-2 ES cells cultured for 14 days (Center), and parental ES cells cultured with PD98059 for 14 days (Right) in serum- and feeder-free conditions. (B) Growth curve of Bcl-2 ES cells (□) and parental ES cells cultured with 12.5 μM PD98059 (○) in serum- and feeder-free conditions. (C) Colony formation activity of parental (p) ES cells cultured without (Non) or with PD98059 and Bcl-2 ES cells at low cell density. Cultures were initiated with 200 cells per well (12-well plate), and colonies were enumerated 7 days later.
LIF is known to stimulate not only the signal transducer and activator of transcription (STAT) pathway but also the MAPK pathways (37). Although the signal transducer and activator of transcription 3 (STAT3) pathway mediates self-renewal of ES cells (38), the extracellular signal-regulated kinase (ERK)–MAPK pathway has been demonstrated to promote differentiation of ES cells (23, 39). LIF also activates p38 MAPK in ES cells (5), but the effect of this pathway on the self-renewal and differentiation of ES cells is not known. Qi et al. (7) recently reported that BMP4 supports self-renewal of ES cells by inhibiting ERK and p38 MAPK pathways. To investigate the role of these pathways in ES cell maintenance in serum- and feeder-free conditions, we blocked them by using specific inhibitors. Suppression of p38 function by SB203580 did not show any effect on the self-renewal and differentiation of ES cells in serum- and feeder-free conditions, suggesting that the p38 MAPK pathway does not affect self-renewal of ES cells. Intriguingly, inhibition of the ERK pathway in parental ES cells with the MAPK kinase-1/2 inhibitor PD98059 dramatically reduced the appearance of differentiated cells during culture (Fig. 5A) and supported extended growth in serum- and feeder-free conditions in the presence of LIF (Fig. 5B). Proliferation of Bcl-2 ES cells was slower than the parental cell line cultured in the presence of PD98059 (Fig. 5B); however, PD98059 could never support colony formation at low-cell-density conditions (Fig. 5C). These results suggest that ERK signaling promotes differentiation of ES cells and that the inhibition of this pathway allows self-renewal, but ES cells require Bcl-2 overexpression to survive at low cell density in the absence of extrinsic survival-promoting factors not yet identified.
Discussion
By generating ES cell lines overexpressing Bcl-2 that can be cultured in vitro in serum- and feeder-free conditions, we can effectively assess the effect of single growth factors, cytokines, and small molecules on the proliferation and differentiation of ES cells. In this study, we demonstrated that LIF is sufficient to support the self-renewal of ES cells in the absence of serum and feeders if cells are protected from apoptosis by Bcl-2 overexpression, thus indicating that survival, proliferation, and inhibition of differentiation can be maintained by signaling cascades initiated by LIF and mediated by Bcl-2 family members, respectively. Of course, it is possible that Bcl-2 has effects in addition to its known antiapoptotic functions that could allow our Bcl-2 ES clones to retain full ES activity in the absence of serum and feeder layers.
Bcl-2 ES cells survive well in the absence of serum and feeders, whereas parental ES cells become apoptotic and cannot be propagated in vitro (Fig. 1C). ES cells cultured in the presence of serum are likely protected from apoptosis through posttranslational phosphorylation modifications of Bcl-2 family members (10) rather than through transcriptional regulation of Bcl-2 family genes, because the gene expression (data not shown) and protein levels of Bcl-2 family members (Bcl-2, Bcl-xL, Mcl-1, and Bax) did not change significantly after the removal of serum (Fig. 4B). Importantly, Mcl-1 deficiency results in periimplantation embryonic lethality (40), and Mcl-1-null ES cells cannot be derived in vitro (J. Opferman and S. Korsmeyer, personal communication). Thus, Mcl-1 is likely to be the essential endogenous Bcl-2 family member expressed in ES cells, which is supported by our observation by quantitative RT-PCR that Mcl-1 expression is robust in mouse ES cells, whereas Bcl-2 and Bcl-xL expression is minimal. Nevertheless, endogenous Mcl-1 is not sufficient to support survival in serum- and feeder-free conditions.
The phosphatidylinositol 3-kinase (PI3K) pathway has been implicated in ES cell survival and proliferation largely based on studies of ES cells lacking PTEN, a lipid phosphatase that functions as negative regulator of the PI3K pathway. These cells have enhanced viability and proliferative activity (41). PTEN-null ES cells survive even in the absence of serum (41), and PTEN-null cells contain higher levels of inactive (phosphorylated) Bad, a BH3-only proapoptotic Bcl-2 family protein (41). Thus, activation of the PI3K pathway may enhance viability through inactivating proapoptotic Bcl-2 family members. This pathway might be a critical target of growth factors or cytokines present in serum or feeder layers (10). With regards to proliferation, LIF is presumably not important because Bcl-2 ES cells still proliferate in the absence of LIF in serum- and feeder-free conditions, although they also differentiate (Fig. 2D).
Our results suggest that LIF is sufficient to block the differentiation of ES cells under the condition that ES cells are protected from apoptosis. This contrasts the report of Ying et al. (5), which shows that LIF is not sufficient to keep ES cells undifferentiated. If Bcl-2 does act to block differentiation, it may do so very specifically under serum- and feeder-free conditions, because Bcl-2 ES cells differentiated normally in in vitro experiments and in vivo (Figs. 2D and 3). Interestingly, blocking the ERK pathway by using PD98059 reduced differentiation in wild-type ES cell culture and supported continuous growth in serum- and feeder-free conditions in the presence of LIF, although there was a crisis during early passages and PD98059 could not support colony formation at low cell density (Fig. 5). Whether the antiapoptotic activity of Bcl-2 is sufficient to support the self-renewal of ES cells will require careful examination, including the possibility that Bcl-2 blocks the ERK signaling pathway at some point.
Our results were recapitulated by two additional mouse ES cell lines overexpressing Bcl-2. and it would be intriguing if these results could be applied to ES cells of other species, including human. Although increased expression of Bcl-2 appears to predispose some cell types to neoplasia, increased expression of Bcl-2 alone is not oncogenic (42). Chimeric mice generated from human Bcl-2-expressing ES cells appear normal and remain tumor-free despite ubiquitous expression of the transgene as determined by GFP expression. Whether Bcl-2 ES cell-derived chimeras are more susceptible to tumors or whether the xenogeneic origin of the transgene is important regarding tumorigenesis is not known; however, previous human Bcl-2 transgenics generated in our laboratory (e.g., H2K-Bcl-2 mice) are also tumor-free and require additional hits before oncogenesis (43). Here, we clearly demonstrate that mouse ES cells overexpressing human Bcl-2 can be expanded in serum- and feeder-free conditions and maintain their pluripotentiality both in vitro and in vivo. These lines will allow precise dissection of the growth factors, cytokines, and other molecules that not only regulate self-renewal of ES cells themselves but also delineation of the factors that dictate differentiation toward specific lineages.
Acknowledgments
We thank Shin-Ichi Hayashi (Tottori University, Yonago, Japan) for cells and culture reagents, Jos Domen (Cellerant Therapeutics, Palo Alto, CA) for Bcl-2 plasmid, Takuya Sugiyama for helpful discussion, Libuse Jerabek for excellent laboratory management, and members of the I.L.W. laboratory and the Judy Shizuru laboratory for helpful advice and assistance. This work was supported by U. S. Public Health Service Grant CA 86065 (to I.L.W.). T.Y. and S.J.D. were supported by a Uehara Memorial Foundation Postdoctoral Fellowship and an American Cancer Society Edward-Albert Bielfelt Postdoctoral Fellowship, respectively.
Abbreviations: LIF, leukemia inhibitory factor; BMP, bone morphogenic protein; MAPK, mitogen-activated protein kinase; ERK, extracellular signal-regulated kinase.
References
- 1.Weissman, I. L. (2000) Cell 100, 157-168. [DOI] [PubMed] [Google Scholar]
- 2.Evans, M. J. & Kaufman, M. H. (1981) Nature 292, 154-156. [DOI] [PubMed] [Google Scholar]
- 3.Williams, R. L., Hilton, D. J., Pease, S., Willson, T. A., Stewart, C. L., Gearing, D. P., Wagner, E. F., Metcalf, D., Nicola, N. A. & Gough, N. M. (1988) Nature 336, 684-687. [DOI] [PubMed] [Google Scholar]
- 4.Smith, A. G., Heath, J. K., Donaldson, D. D., Wong, G. G., Moreau, J., Stahl, M. & Rogers, D. (1988) Nature 336, 688-690. [DOI] [PubMed] [Google Scholar]
- 5.Ying, Q. L., Nichols, J., Chambers, I. & Smith, A. (2003) Cell 115, 281-292. [DOI] [PubMed] [Google Scholar]
- 6.Johansson, B. M. & Wiles, M. V. (1995) Mol. Cell. Biol. 15, 141-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qi, X., Li, T. G., Hao, J., Hu, J., Wang, J., Simmons, H., Miura, S., Mishina, Y. & Zhao, G. Q. (2004) Proc. Natl. Acad. Sci. USA 101, 6027-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyazawa, K., Shinozaki, M., Hara, T., Furuya, T. & Miyazono, K. (2002) Genes Cells 7, 1191-1204. [DOI] [PubMed] [Google Scholar]
- 9.Sirard, C., de la Pompa, J. L., Elia, A., Itie, A., Mirtsos, C., Cheung, A., Hahn, S., Wakeham, A., Schwartz, L., Kern, S. E., et al. (1998) Genes Dev. 12, 107-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Opferman, J. T. & Korsmeyer, S. J. (2003) Nat. Immunol. 4, 410-415. [DOI] [PubMed] [Google Scholar]
- 11.Brewer, G. J., Torricelli, J. R., Evege, E. K. & Price, P. J. (1993) J. Neurosci. Res. 35, 567-576. [DOI] [PubMed] [Google Scholar]
- 12.Ito, Y. L., Zile, M., Ahrens, H. & DeLuca, H. F. (1974) J. Lipid Res. 15, 517-524. [PubMed] [Google Scholar]
- 13.Finley, M. F., Devata, S. & Huettner, J. E. (1999) Neurobiology 40, 271-287. [PubMed] [Google Scholar]
- 14.Yamane, T., Hayashi, S., Mizoguchi, M., Yamazaki, H. & Kunisada, T. (1999) Dev. Dyn. 216, 450-458. [DOI] [PubMed] [Google Scholar]
- 15.Fairbairn, L. J., Cowling, G. J., Reipert, B. M. & Dexter, T. M. (1993) Cell 74, 823-832. [DOI] [PubMed] [Google Scholar]
- 16.Lagasse, E. & Weissman, I. L. (1997) Cell 89, 1021-1031. [DOI] [PubMed] [Google Scholar]
- 17.Akashi, K., Kondo, M., von Freeden-Jeffry, U., Murray, R. & Weissman, I. L. (1997) Cell 89, 1033-1041. [DOI] [PubMed] [Google Scholar]
- 18.Kondo, M., Akashi, K., Domen, J., Sugamura, K. & Weissman, I. L. (1997) Immunity 7, 155-162. [DOI] [PubMed] [Google Scholar]
- 19.Domen, J. & Weissman, I. L. (2000) J. Exp. Med. 192, 1707-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reya, T., Duncan, A. W., Ailles, L., Domen, J., Scherer, D. C., Willert, K., Hintz, L., Nusse, R. & Weissman, I. L. (2003) Nature 423, 409-414. [DOI] [PubMed] [Google Scholar]
- 21.Doetschman, T. C., Eistetter, H., Katz, M., Schmidt, W. & Kemler, R. (1985) J. Embryol. Exp. Morphol. 87, 27-45. [PubMed] [Google Scholar]
- 22.Yamane, T., Kunisada, T., Yamazaki, H., Era, T., Nakano, T. & Hayashi, S. I. (1997) Blood 90, 3516-3523. [PubMed] [Google Scholar]
- 23.Burdon, T., Stracey, C., Chambers, I., Nichols, J. & Smith, A. (1999) Dev. Biol. 210, 30-43. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa, M., Nishikawa, S., Ikuta, K., Yamamura, F., Naito, M., Takahashi, K. & Nishikawa, S. (1988) EMBO J. 7, 1337-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kodama, H., Nose, M., Niida, S., Nishikawa, S. & Nishikawa, S. (1994) Exp. Hematol. 22, 979-984. [PubMed] [Google Scholar]
- 26.Whitlock, C. A., Tidmarsh, G. F., Muller-Sieburg, C. & Weissman, I. L. (1987) Cell 48, 1009-1021. [DOI] [PubMed] [Google Scholar]
- 27.Niwa, H., Yamamura, K. & Miyazaki, J. (1991) Gene 108, 193-199. [DOI] [PubMed] [Google Scholar]
- 28.Nichols, J., Zevnik, B., Anastassiadis, K., Niwa, H., Klewe-Nebenius, D., Chambers, I., Schöler, H. & Smith, A. (1998) Cell 95, 379-391. [DOI] [PubMed] [Google Scholar]
- 29.Niwa, H., Miyazaki, J. & Smith, A. G. (2000) Nat. Genet. 24, 372-376. [DOI] [PubMed] [Google Scholar]
- 30.Chambers, I., Colby, D., Robertson, M., Nichols, J., Lee, S., Tweedie, S. & Smith, A. (2003) Cell 113, 643-655. [DOI] [PubMed] [Google Scholar]
- 31.Mitsui, K., Tokuzawa, Y., Itoh, H., Segawa, K., Murakami, M., Takahashi, K., Maruyama, M., Maeda, M. & Yamanaka, S. (2003) Cell 113, 631-642. [DOI] [PubMed] [Google Scholar]
- 32.Rogers, M. B., Hosler, B. A. & Gudas, L. J. (1991) Development (Cambridge, U.K.) 113, 815-824. [DOI] [PubMed] [Google Scholar]
- 33.Matsui, Y., Zsebo, K. & Hogan, B. L. (1992) Cell 70, 841-847. [DOI] [PubMed] [Google Scholar]
- 34.Nakano, T., Kodama, H. & Honjo, T. (1994) Science 265, 1098-1101. [DOI] [PubMed] [Google Scholar]
- 35.Derynck, R. & Zhang, Y. E. (2003) Nature 425, 577-584. [DOI] [PubMed] [Google Scholar]
- 36.de Caestecker, M. (2004) Cytokine Growth Factor Rev. 15, 1-11. [DOI] [PubMed] [Google Scholar]
- 37.Kishimoto, T., Taga, T. & Akira, S. (1994) Cell 76, 253-262. [DOI] [PubMed] [Google Scholar]
- 38.Niwa, H., Burdon, T., Chambers, I. & Smith, A. (1998) Genes Dev. 12, 2048-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burdon, T., Smith, A. & Savatier, P. (2002) Trends Cell Biol. 12, 432-438. [DOI] [PubMed] [Google Scholar]
- 40.Rinkenberger, J. L., Horning, S., Klocke, B., Roth, K. & Korsmeyer, S. J. (2000) Genes Dev. 14, 23-27. [PMC free article] [PubMed] [Google Scholar]
- 41.Sun, H., Lesche, R., Li, D. M., Liliental, J., Zhang, H., Gao, J., Gavrilova, N., Mueller, B., Liu, X. & Wu, H. (1999) Proc. Natl. Acad. Sci. USA 96, 6199-6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jamieson, C. H. M., Passegue, E. & Weissman, I. L. (2004) in Stem cells in the Nervous System: Functional and Clinical Implications, ed. Gage, R. (Springer, Berlin), pp. 157-182.
- 43.Domen, J., Gandy, K. L. & Weissman, I. L. (1998) Blood 91, 2272-2282. [PubMed] [Google Scholar]