Abstract
Wnts comprise a family of secreted signaling proteins that regulate diverse developmental processes. Activation of Wnt signaling by Wnt10b inhibits differentiation of preadipocytes and blocks adipose tissue development; however, the effect of Wnt10b on other mesenchymal lineages has not been defined. To explore the physiological role of Wnt signaling in bone development, we analyzed FABP4-Wnt10b mice, which express the Wnt10b transgene in marrow. Femurs from FABP4-Wnt10b mice have almost four times as much bone in the distal metaphyses and are mechanically stronger. These mice maintain elevated bone mass at least through 23 months of age. In addition, FABP4-Wnt10b mice are protected from the bone loss characteristic of estrogen deficiency. We used pharmacological and genetic approaches to demonstrate that canonical Wnt signaling stimulates osteoblastogenesis and inhibits adipogenesis of bipotential mesenchymal precursors. Wnt10b shifts cell fate toward the osteoblast lineage by induction of the osteoblastogenic transcription factors Runx2, Dlx5, and osterix and suppression of the adipogenic transcription factors C/EBPα and PPARγ. One mechanism whereby Wnt10b promotes osteoblastogenesis is suppression of PPARγ expression. Finally, Wnt10b-/- mice have decreased trabecular bone and serum osteocalcin, confirming that Wnt10b is an endogenous regulator of bone formation.
Keywords: adipogenesis, development, stem cells
Mesenchymal progenitors can differentiate into a number of cell types, including adipocytes and osteoblasts (1). One factor that regulates the reciprocal relationship between these lineages is Wnt signaling, which inhibits adipogenesis and stimulates osteoblastogenesis. Activation of Wnt signaling blocks preadipocyte differentiation by inhibiting expression of the adipogenic transcription factors C/EBPα and PPARγ (2–5). The endogenous inhibitory Wnt signal may be initiated by Wnt10b, which is expressed in preadipocytes and stromal vascular cells but not in adipocytes (2, 3). Expression of Wnt10b from the FABP4 promoter decreases accumulation of white adipose tissue by ≈50% and completely blocks the development of brown fat (6, 7). Furthermore, FABP4-Wnt10b mice resist diet-induced obesity and the associated glucose intolerance.
The first indication that Wnt signaling plays a critical role in bone formation came from human studies where inactivating mutations in the Wnt coreceptor LRP5 were shown to cause osteoporosis (8). These findings were supported by the observation that LRP5-/- mice also have low bone mass (9). Furthermore, gain-of-function mutations in LRP5 that increase Wnt signaling result in higher bone density in humans and mice (10, 11). Consistent with the effects of LRP5 on bone mass being mediated through canonical Wnt signaling, activation of this pathway in vitro results in the expression of alkaline phosphatase, an early osteoblast marker (12–14). Although these and other studies suggest that endogenous Wnt signaling regulates osteoblastogenesis and bone formation (15), a specific Wnt or Wnts responsible for activation of this pathway in marrow have not been identified.
We report herein that FABP4-Wnt10b mice have increased bone mass and strength and that they resist the loss of bone that occurs with aging or estrogen deficiency. The expression of Wnt10b in mesenchymal progenitors induces the expression of osteoblastogenic transcription factors Runx2, Dlx5, and osterix and strongly stimulates osteoblastogenesis. In addition, Wnt10b inhibits the adipogenic transcription factors C/EBPα and PPARγ and blocks adipogenesis. Suppression of PPARγ is one mechanism whereby Wnt10b induces osteoblastogenesis. Compelling evidence supportingWnt10b as an endogenous regulator of bone mass comes from analyses of Wnt10b-/- mice, which have decreased trabecular bone mass and serum osteocalcin.
Methods
Animals. Animal studies were approved by the University Committee on the Use and Care of Animals, and mice were cared for by the Unit for Laboratory Animal Medicine. FABP4-Wnt10b mice were generated and characterized as described (6, 7). Wnt10b-/- mice were created by Timothy Lane (University of California, Los Angeles) and Philip Leder (Harvard University, Cambridge, MA).
Radiography, Microcomputerized Tomography (μCT), and Mechanical Testing. For microradiographic analysis, anesthetized mice were analyzed with Faxitron Specimen Radiography System Model MX-20 (Faxitron, Wheeling, IL). Femoral μCT was measured as described (16) by using the Stereology function of GE Medical Systems microview software. Material properties of femurs were evaluated by using the 810 Material Test System (Servo-hydraulic, Eden Prairie, MN), as described (16).
Ovariectomy. Wild-type and FABP4-Wnt10b mice were ovariectomized or sham-operated at 3 months of age, as described (16). Mice were killed 4 weeks later, and hind limbs were processed for μCT analysis.
Serology. Blood was collected at time of kill, and serum was prepared. ELISAs for osteocalcin (Biomedical Technologies, Stoughton, MA) and TRAP5b (SBA Sciences, Turku, Finland) were performed according to the manufacturer's protocols.
Cell Culture. Marrow-derived ST2 cells were incubated at 37°C and 5% CO2 in α-MEM supplemented with 10% FCS (Bio-Whittaker) and 25 μg/ml ascorbic acid (Sigma–Aldrich). For osteoblastogenesis, cells were cultured on 0.1% gelatin-coated plates in the same medium supplemented with 10 mM β-glycerophosphate (Sigma–Aldrich). Where indicated, troglitazone (5 μM) was added to activate PPARγ. Induction of adipogenesis and staining with Oil red O was as described (17, 18). The GSK3 inhibitor, CHIR99021, was a generous gift from Chiron. Alkaline phosphatase activity was evaluated by using a kit (86-C, Sigma–Aldrich). Mineralization of β-glycerophosphate-treated cells was assessed by staining with 1% Alizarin red after fixation in 50% ethanol (19). Mineralized calcium was evaluated by von Kossa staining (20).
Retroviral Infection. Delivery of genes by retrovirus was as described (18). Retroviral vectors for Wnt1, Wnt10b, β-catenin, dnTCF4, and Wnt5a have been described (2, 21). pMSCV and pMSCV-PPARγ were provided by Evan Rosen (Harvard Medical School) (22).
Expression of RNA. Expression of mRNAs was estimated by quantitative PCR as described (6). All primers were verified by using ST2 cDNA, and sequences are available upon request.
Results
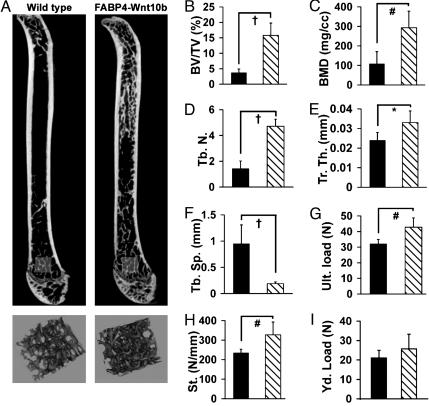
Wnt10b Increases Bone Mass and Strength. Initial characterization of FABP4-Wnt10b mice revealed expression of Wnt10b from the FABP4 promoter not only in white and brown adipose tissue but also in bone marrow (6). To determine whether expression of Wnt10b in marrow alters bone, skeletons of FABP4-Wnt10b mice were evaluated. Analysis of femurs from FABP4-Wnt10b mice with μCT at 18-μm resolution showed increased trabecular bone throughout the endocortical compartment (Fig. 1A), without substantial changes in cortical bone. Effects of Wnt10b on bone mass are not restricted to femur, because FABP4-Wnt10b mice have increased bone at the tibia, hip, and vertebrae (Fig. 5, which is published as supporting information on the PNAS web site) and the humerus (data not shown). This increase in bone mass is observed in both sexes and is present as early as 8 weeks of age. Three-dimensional analyses of a 1-mm3 region of the distal femoral metaphyses (Fig. 1 A) revealed a 4-fold increase in bone volume/total volume (Fig. 1B) and a similar rise in bone mineral density (Fig. 1C). This increase in bone is the result of a 3-fold increase in trabecular number (Fig. 1D) and an increase in trabecular thickness (Fig. 1E), with a corresponding decrease in trabecular spacing (Fig. 1F). Analysis of a 3-mm midcortical segment revealed an increase in bone cross-sectional area, cortical thickness, and bending moments; however, diaphyseal analysis was complicated by high trabecular content (data not shown).
Fig. 1.
Increased bone mass and strength in FABP4-Wnt10b mice. (A) μCT of femurs from wild-type and FABP4-Wnt10b mice (Upper). Three-dimensional reconstructions of metaphyseal trabeculae from regions highlighted (Lower). Morphometric properties of 1 mm3 of distal femur from wild-type (▪) and FABP4-Wnt10b ( ) mice at 6 months of age were analyzed for bone volume fraction (B; BV/TV, %), bone mineral density (C; BMD), trabecular number (D; Tr. N.), trabecular thickness (E; Tb. Th.), trabecular spacing (F; Tb. Sp.), ultimate load (G), stiffness (H), and yield load (I). Statistical significance for each measurement was evaluated with Student's t test: P < 0.05 (*), < 0.01 (#), < 0.001 (†), with n = 6 males for each genotype.
) mice at 6 months of age were analyzed for bone volume fraction (B; BV/TV, %), bone mineral density (C; BMD), trabecular number (D; Tr. N.), trabecular thickness (E; Tb. Th.), trabecular spacing (F; Tb. Sp.), ultimate load (G), stiffness (H), and yield load (I). Statistical significance for each measurement was evaluated with Student's t test: P < 0.05 (*), < 0.01 (#), < 0.001 (†), with n = 6 males for each genotype.
To assess the quality of bone in Wnt10b mice, femurs from control and FABP4-Wnt10b mice were subjected to mechanical testing by four-point bending analyses. FABP4-Wnt10b mice have increased ultimate load (Fig. 1G) and stiffness (Fig. 1H) without a significant change in other properties, including yield load (Fig. 1I) and energy and displacement ratio (data not shown). In addition, material properties between genotypes were not different. Thus, Wnt10b signaling increases trabecular bone mass, which in turn enhances bending strength without adversely impacting bone material properties.
FABP4-Wnt10b Mice Resist Loss of Bone Associated with Aging. To evaluate whether FABP4-Wnt10b mice resist the changes in bone architecture that occur with aging, femurs from mice at 23 months of age were analyzed by μCT. Quantification of a 1-mm3 region of trabecular bone in distal femur indicated that bone volume fraction in wild-type mice was only slightly decreased with age (Table 1). Although the number of trabeculi was decreased by ≈60%, there was an increase in thickness of remaining trabeculi that compensated for this loss. In contrast, the bone volume fraction of FABP4-Wnt10b mice increased >2-fold from 6 to 23 months, mostly because of increased trabecular thickness (Table 1). Visualization of bone architecture by μCT and histology further supports the observation that aged FABP4-Wnt10b mice maintain elevated levels of trabecular bone compared with age-matched controls (Fig. 6, which is published as supporting information on the PNAS web site). Thus, the elevated bone mass of FABP4-Wnt10b mice is maintained throughout life.
Table 1. μCT of femurs from WT and FABP4-Wnt10b mice at 23 mo of age (mean ± SD).
| Properties | WT | % change (from 6 mo) | FABP4·Wnt10b | % change (from 6 mo) |
|---|---|---|---|---|
| BV/TV; % | 2.9 ± 2.4 | -21.6 | 34.8 ± 20* | +121.6† |
| Tb. n. | 0.57 ± 0.44 | -60.1† | 5.2 ± .9* | +10.6 |
| Tb.Th., mm | 0.045 ± 0.017 | +84.4† | 0.066 ± 0.037 | +101 |
| Tb.Sp.; mm | 5.7 ± 5.2 | +500† | 0.15 ± 0.09* | -20.2 |
| BMD; mg/cc | 100 ± 32 | -6.4 | 424 ± 191* | +45.2 |
Percent change in morphometric properties compared with 6 months of age. BV/TV, bone volume fraction; BMD, bone mineral density; Tb. n., trabecular number; Tb.Th., trabecular thickness; Tb.Sp., trabecular spacing.
Difference between genotype at 23 months P < 0.05
Difference between 6 and 23 months of age within a genotype P < 0.06
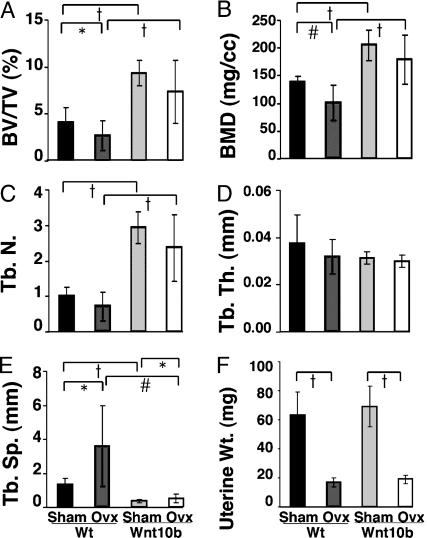
Wnt10b Protects Against Bone Loss Due to Ovariectomy. To determine whether Wnt10b protects against bone loss associated with estrogen deficiency, wild-type and transgenic females at 9 weeks of age were sham-operated or ovariectomized. Four weeks later, mice were killed, and effects on femoral architecture were analyzed by μCT. Female FABP4-Wnt10b mice have a similar phenotype to that observed in male mice (Fig. 1), with increased bone volume fraction, bone mineral density, and trabecular number (Fig. 2 A–C). Ovariectomy of wild-type mice caused a decrease in bone volume/total volume and bone mineral density, primarily because of a loss of trabecular number. In contrast, ovariectomy of FABP4-Wnt10b mice did not significantly change bone volume/total volume, bone mineral density, or trabecular number (Fig. 2 A–C). In female mice, trabecular thickness was unaltered by genotype or by surgery (Fig. 2D). As expected, uteri of wild-type and FABP4-Wnt10b mice were atrophied after ovariectomy (Fig. 2E). Although there may be some bone loss in FABP4-Wnt10b mice after ovariectomy, increased trabecular bone mass protects these mice against osteopenia associated with 4 weeks of estrogen deficiency.
Fig. 2.
Wnt10b expression maintains bone in ovarectomized mice. (A) μCT of femurs from 4-month wild-type and FABP4-Wnt10b mice were either sham-operated or ovariectomized at 3 months of age. Morphometric variables analyzed include bone volume/total volume (B; BV/TV), trabecular number (C; Tb. N.), trabecular thickness (D; Tb. Th.), trabecular spacing (E; Tb.Sp.), and uterine weight (F). Statistical significance for each measurement was evaluated with Student's t test: P < 0.05 (*), < 0.01 (#), with n = 6–8 mice for each treatment.
Wnt Signaling Stimulates Osteoblastogenesis and Inhibits Adipogenesis of Bipotential Marrow Stromal Cells. Increased trabecular bone mass in FABP4-Wnt10b mice could be due to a direct effect of Wnt10b in marrow. Alternatively, increased trabecular number could be an indirect effect of reduced serum leptin (6), which acts through a hypothalamic circuit to influence bone formation (23, 24). To assess whether Wnt signaling directly stimulates osteoblastogenesis, bipotential ST2 cells were treated with CHIR99021, an inhibitor of GSK3 that stabilizes cytosolic β-catenin in cultured cells (3). Exposure of ST2 cells to CHIR99021 induces osteoblastogenesis, as indicated by elevated alkaline phosphatase activity (Fig. 7A, which is published as supporting information on the PNAS web site), and increases mineralization, as assessed by Alizarin red staining. CHIR99021 also inhibits adipogenesis, as visualized by Oil red O staining (Fig. 7B).
Although the effects of CHIR99021 are consistent with canonical Wnt signaling directly inducing osteoblast differentiation, the central role of GSK3 in several growth factor pathways leaves open the possibility that CHIR99021 stimulates osteoblastogenesis through another mechanism. To address this issue, activation of canonical Wnt signaling was initiated by infection of ST2 cells with retroviruses that express Wnt1, Wnt10b, or dominant stable β-catenin. When compared with controls, these cell lines had increased alkaline phosphatase activity at confluent density and underwent rapid osteoblastogenesis and mineralization when cultured in osteogenic media (Fig. 8 A and B, which is published as supporting information on the PNAS web site). Control ST2 cells have exceedingly low levels of alkaline phosphatase and mineralization under these conditions, and this background osteoblastogenesis was inf luenced neither by dnTCF4 nor by activation of noncanonical signaling with Wnt5a. Furthermore, activation of noncanonical Wnt signaling with Wnt5a had no effect on adipogenesis of ST2 cells, whereas activation of the canonical pathway with Wnt10b or Wnt1 completely inhibited adipocyte differentiation (Fig. 8C). Similar results are observed in C3H10T1/2 cells (data not shown). Taken together, these results demonstrate that canonical Wnt signaling promotes osteoblastogenesis and inhibits adipogenesis, further supporting the hypothesis that expression of Wnt10b in marrow directly increases bone mass in FABP4-Wnt10b mice.
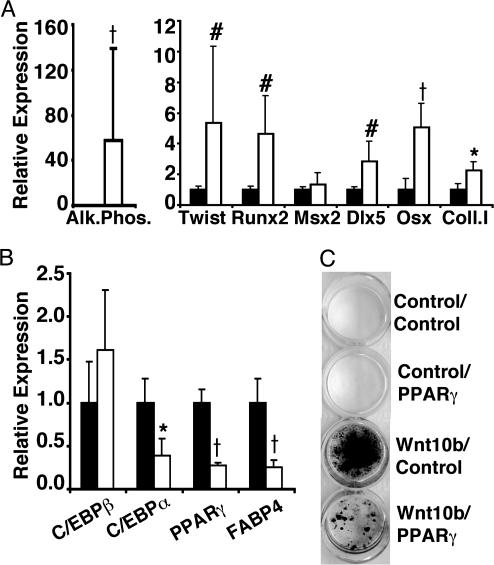
To assess mechanisms whereby Wnt10b promotes osteoblastogenesis, RNA was isolated from control and Wnt10b-expressing ST2 cells 2 days after confluence. Consistent with the increase in alkaline phosphatase activity observed in Fig. 8A, quantitative PCR revealed that Wnt10b cells express high levels of alkaline phosphatase mRNA (Fig. 3A). Moreover, Wnt10b induced expression of osteoblast transcription factors, Runx2, Dlx5, and osterix, but Wnt10b did not consistently increase Msx2. Type I collagen, a major component of the extracellular matrix of active osteoblasts, is also elevated by Wnt10b (Fig. 3A). Although Wnt signaling increases expression of twist in a number of cell types (21, 25), induction of twist in ST2 cells is expected to oppose osteoblastogenic effects of Wnt10b (26). As predicted, expression of Wnt10b in ST2 cells did not suppress C/EBPβ, but it inhibited expression of the major adipogenic transcription factors, C/EBPα, and PPARγ, as well as the adipocyte gene, FABP4 (Fig. 3B). Thus, Wnt10b signaling alters cell fate of pluripotent mesenchymal precursors by shifting the balance of transcription factors to favor osteoblastogenesis.
Fig. 3.
Wnt10b promotes osteoblastogenesis of pluripotent mesenchymal precursors. ST2 cells were infected with control or Wnt10b retroviruses. Two days postconfluence, RNA was isolated for quantitative PCR analysis of osteoblast genes: alkaline phosphatase (Alk. Phos.); Twist, Runx2, Msx2, Dlx5, osterix (Osx); and type I collagen (Coll I) (A) and adipocyte genes: C/EBPβ, C/EBPα, PPARγ, and FABP4 (B). Statistical differences from controls were evaluated with Student's t test: P < 0.05 (*), < 0.01 (#), < 0.001 (†), with n = 3 mice. Results are representative of at least four independent experiments. (C) Control and Wnt10b cells were infected with control and PPARγ retroviruses. Confluent cells were incubated in osteogenic media supplemented with troglitazone (5 μM). Mineral deposition was assessed on day 6 with von Kossa stain.
Reduced PPARγ expression and activity is associated with increased osteoblast mineralization and bone formation (27, 28). To assess whether suppression of PPARγ by Wnt10b is a mechanism whereby Wnt signaling promotes osteoblastogenesis, we infected control and Wnt10b cells with control or PPARγ-expressing retroviruses and treated these cells for 6 days with osteogenic media supplemented with the PPARγ ligand, troglitazone. As assessed by von Kossa staining, ST2 cells with empty retroviral vectors failed to mineralize (Fig. 3C). Likewise, controls cells infected with a retrovirus expressing PPARγ did not undergo osteoblastogenesis but instead differentiated into adipocytes (data not shown). Interestingly, although cells infected with Wnt10b and control vectors underwent mineralization, ST2 cells that expressed Wnt10b and PPARγ had greatly reduced osteoblastogenesis (Fig. 3C), and some cells were converted into adipocytes (data not shown). Thus, suppression of PPARγ is required for Wnt10b to induce osteoblastogenesis.
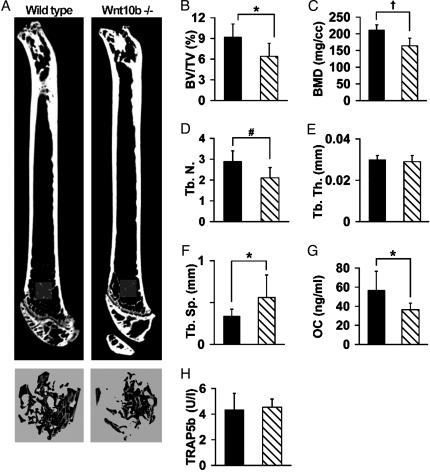
Reduced Bone Mass in Femurs of Wnt10b-/- Mice. Although differences in bone mass observed in mice and humans with mutations in LRP5 suggest that at least one of the 19 Wnts signals in marrow to regulate trabecular bone mass, the identity of the specific Wnt involved in this process remains unknown. One possibility is Wnt10b, which is expressed in marrow and activates canonical Wnt signaling (2, 29). To determine whether signaling by endogenous Wnt10b regulates bone mass, we analyzed mice with a deletion of the Wnt10b ORF. Femurs from 8-week Wnt10b-/- females were analyzed by μCT. Three-dimensional analysis of the distal metaphyses revealed a 30% reduction in bone volume/total volume and bone mineral density in these mice (Fig. 4 A–C). This loss is attributed to a decrease in trabecular number with a concomitant increase in trabecular spacing (Fig. 4 D and E). Quantitative analyses of age-matched wild-type and Wnt10b-/- femora by histomorphometry provided independent support for these observations (data not shown). In addition to decreased bone mass in the femur, Wnt10b-/- mice also have decreased bone volume fraction in proximal tibia (data not shown). The comparable reduction in bone volume fraction and other bone variables in Wnt10b-/- and LRP5-/- mice suggests that the bone phenotype in LRP5-/- mice may reflect a loss of endogenous Wnt10b signaling (29). Reduced levels of serum osteocalcin without changes in serum TRAP5b suggest that Wnt10b-/- mice have decreased osteoblast number or function rather than increased number of osteoclasts or bone resorption (Fig. 4 G and H). Taken together, these experiments suggest that Wnt10b is an endogenous regulator of bone mass.
Fig. 4.
Reduced trabecular bone in femurs of Wnt10b-/- mice. (A) μCT of femurs from wild-type and Wnt10b-/- mice (Upper). Three-dimensional reconstructions of metaphyseal trabeculae from regions highlighted (Lower). Morphometric properties of distal femur (1 mm3) from wild-type (▪) and Wnt10b null ( ) mice. Properties analyzed include bone volume fraction (B;BV/TV, %), bone mineral density (C; BMD), trabecular number (D; Tr. N.), trabecular thickness (E; Tb. Th.), and trabecular spacing (F; Tb. Sp.). Serum levels of osteocalcin (G) and TRAP5b activity (H) in wild-type (▪) and Wnt10b null (
) mice. Properties analyzed include bone volume fraction (B;BV/TV, %), bone mineral density (C; BMD), trabecular number (D; Tr. N.), trabecular thickness (E; Tb. Th.), and trabecular spacing (F; Tb. Sp.). Serum levels of osteocalcin (G) and TRAP5b activity (H) in wild-type (▪) and Wnt10b null ( ) mice. Statistical significance for each measurement was evaluated with Student's t test: P < 0.05 (*), < 0.01 (#), and <0.001 (†), with n = 6 mice for each genotype.
) mice. Statistical significance for each measurement was evaluated with Student's t test: P < 0.05 (*), < 0.01 (#), and <0.001 (†), with n = 6 mice for each genotype.
Discussion
A relationship between bone and fat is established at both developmental and physiological levels. During development, mesenchymal precursor cells can differentiate into adipocytes, osteoblasts, and other cell types in vitro, and a reciprocal relationship exists such that increased osteoblast differentiation is associated with decreased adipocyte differentiation (30). This relationship is also observed in vivo, where osteopetrosis is associated with fewer marrow adipocytes, and osteopenia or osteoporosis due to aging or disease is accompanied by increased marrow adipocytes (30, 31). Canonical Wnt signaling is one of the mechanisms controlling the development of precursor cells into osteoblasts or adipocytes (Figs. 7 and 8). Other investigators have reported that activation of Wnt signaling with lithium chloride or Wnt3a induces early stages of osteoblastogenesis, including expression of alkaline phosphatase (12, 14, 32). However, we have observed that activation of canonical Wnt signaling by pharmacological or genetic means is sufficient to stimulate the entire program of osteoblastogenesis, including expression of osteoblast genes and matrix mineralization.
The discovery that obese mice have increased bone provided direct evidence for a physiological relationship between adipose tissue and bone (23). This connection is established through leptin, which is released by adipocytes and signals through the hypothalamus and sympathetic nervous system to inhibit bone formation. FABP4-Wnt10b mice have an ≈50% decrease in whole body fat and a proportional decline in leptin (6). However, this decrease in serum leptin is unlikely to account for the 4-fold increase in bone mass, because complete loss of leptin in ob/ob mice causes only a 2-fold increase in bone volume fraction (23). Further support for a direct effect of Wnt10b on bone formation comes from Wnt10b-/- mice, which have serum leptin levels indistinguishable from wild-type mice yet a 25–30% decrease in bone volume fraction, bone mineral density, and trabecular number compared with wild-type controls (Fig. 4). Thus, although there may be some effect of leptin deficiency on bone formation in FABP4-Wnt10b mice, it is likely that Wnt10b increases bone mass primarily through local effects in marrow.
Our experiments indicate that suppression of PPARγ by Wnt signaling is part of the mechanism whereby Wnt10b inhibits adipogenesis and stimulates osteoblastogenesis. (Fig. 3). In addition to an integral role in adipogenesis and adipose tissue development (33, 34), PPARγ inhibits osteoblastogenesis in vitro and bone formation in vivo, perhaps by decreasing Wnt10b expression and inhibiting Runx2 activity (35–38). Furthermore, PPARγ-/- embryonic stem cells and PPARγ+/- mice have increased matrix mineralization and bone formation, respectively (27), suggesting that suppression of PPARγ is sufficient to promote osteoblastogenesis. However, in our experiments with ST2 cells, inhibition of PPARγ with pharmacological inhibitors was not sufficient to stimulate differentiation (data not shown), suggesting that induction of one or more osteoblastogenic transcription factors (e.g., Runx2, Dlx5, or osterix; Fig. 3) is also required for the actions of Wnt10b. For example, induction of collagen type I in Wnt10b-expressing cells may be due to the increase in Dlx5 (Fig. 3), which has been shown to activate the collagen type I promoter (39). Interestingly, Wnt10b does not consistently induce Msx2, even though Msx2 inhibits adipogenesis and promotes osteoblastogenesis (40, 41). Taken together, these data suggest that Wnt signaling influences the differentiation potential of mesenchymal precursors by repressing PPARγ and increasing expression of key osteoblastogenic transcription factors.
We have also found that expression of Wnt10b in marrow increases trabecular bone mass and strength (Fig. 1). FABP4-Wnt10b mice resist loss of bone due to estrogen deficiency (Fig. 2). Furthermore, they have increased bone volume/total volume and trabecular parameters throughout life (Table 1 and Fig. 6). Decreased trabecular bone and serum osteocalcin in Wnt10b-/- mice provide strong evidence that Wnt10b is an endogenous regulator of bone formation (Fig. 4). Although Wnt10b may influence bone mass through increased osteoblast differentiation, replication, and/or activity, it could also decrease osteoblast apoptosis (21). Based upon prior work in LRP5-/-, LRP5 G171V, and sFRP1-/- mice, Wnt10b is likely to influence osteoblast differentiation, replication, and apoptosis (9, 11, 15). Uncovering mechanisms whereby Wnt signaling influences development of bone and adipose tissue is important for our understanding and potential treatment of osteoporosis and other human diseases.
Supplementary Material
Acknowledgments
We thank Jacklyn Kriegl and David Fisher for μCT scans and Philip Leder (Harvard Medical School) for his considerable contribution to making Wnt10b-/- mice. This work was supported by grants from the National Institutes of Health [DK51563 and DK62876 (to O.A.M.) and RR00161 and AR49682 (to K.D.H.)]. Other support was from the Diabetes Research and Training Center (P60 DK20572), the Nathan Shock Mutant and Transgenic Rodent Core, and the Core Center for Musculoskeletal Disorders (P30AR046024). Fellowships to C.N.B. were from Tissue Engineering and Regeneration Training Grant DE07057 and the American Physiological Society Porter Fellowship. K.A.L. was supported by a mentor-based postdoctoral fellowship from the American Diabetes Association.
Author contributions: C.N.B., K.D.H., and O.A.M. designed research; C.N.B., K.A.L., W.S.W., L.J.S., and K.D.H. performed research; T.F.L. and K.D.H. contributed new reagents/analytic tools; C.N.B., K.A.L., W.S.W., L.J.S., K.D.H., and O.A.M. analyzed data; and C.N.B. and O.A.M. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: μCT, microcomputerized tomography.
References
- 1.Nuttall, M. E. & Gimble, J. M. (2004) Curr. Opin. Pharmacol. 4, 290-294. [DOI] [PubMed] [Google Scholar]
- 2.Ross, S. E., Hemati, N., Longo, K. A., Bennett, C. N., Lucas, P. C., Erickson, R. L. & MacDougald, O. A. (2000) Science 289, 950-953. [DOI] [PubMed] [Google Scholar]
- 3.Bennett, C. N., Ross, S. E., Longo, K. A., Bajnok, L., Hemati, N., Johnson, K. W., Harrison, S. D. & MacDougald, O. A. (2002) J. Biol. Chem. 277, 30998-31004. [DOI] [PubMed] [Google Scholar]
- 4.Kennell, J. A., O'Leary, E. E., Gummow, B. M., Hammer, G. D. & MacDougald, O. A. (2003) Mol. Cell. Biol. 23, 5366-5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moldes, M., Zuo, Y., Morrison, R. F., Silva, D., Park, B. H., Liu, J. & Farmer, S. R. (2003) Biochem. J. 376, 607-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longo, K. A., Wright, W. S., Kang, S., Gerin, I., Chiang, S. H., Lucas, P. C., Opp, M. R. & MacDougald, O. A. (2004) J. Biol. Chem. 279, 35503-35509. [DOI] [PubMed] [Google Scholar]
- 7.Kang, S., Bajnok, L., Longo, K. A., Petersen, R. K., Hanson, J. B., Kristiansen, K., MacDougald, O. A. (2004) Mol. Cell. Biol. 25, 1272-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong, Y., Slee, R. B., Fukai, N., Rawadi, G., Roman-Roman, S., Reginato, A. M., Wang, H., Cundy, T., Glorieux, F. H., Lev, D., et al. (2001) Cell 107, 513-523. [DOI] [PubMed] [Google Scholar]
- 9.Kato, M., Patel, M. S., Levasseur, R., Lobov, I., Chang, B. H., Glass, D. A., 2nd, Hartmann, C., Li, L., Hwang, T. H., Brayton, C. F., et al. (2002) J. Cell Biol. 157, 303-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyden, L. M., Mao, J., Belsky, J., Mitzner, L., Farhi, A., Mitnick, M. A., Wu, D., Insogna, K. & Lifton, R. P. (2002) N. Engl. J. Med. 346, 1513-1521. [DOI] [PubMed] [Google Scholar]
- 11.Babij, P., Zhao, W., Small, C., Kharode, Y., Yaworsky, P. J., Bouxsein, M. L., Reddy, P. S., Bodine, P. V., Robinson, J. A., Bhat, B., et al. (2003) J. Bone Miner. Res. 18, 960-974. [DOI] [PubMed] [Google Scholar]
- 12.Bain, G., Muller, T., Wang, X. & Papkoff, J. (2003) Biochem. Biophys. Res. Commun. 301, 84-91. [DOI] [PubMed] [Google Scholar]
- 13.Kitagaki, J., Iwamoto, M., Liu, J. G., Tamamura, Y., Pacifci, M. & Enomoto-Iwamoto, M. (2003) Osteoarthr. Cartilage 11, 36-43. [DOI] [PubMed] [Google Scholar]
- 14.Rawadi, G., Vayssiere, B., Dunn, F., Baron, R. & Roman-Roman, S. (2003) J. Bone Miner. Res. 18, 1842-1853. [DOI] [PubMed] [Google Scholar]
- 15.Bodine, P. V., Zhao, W., Kharode, Y. P., Bex, F. J., Lambert, A. J., Goad, M. B., Gaur, T., Stein, G. S., Lian, J. B. & Komm, B. S. (2004) Mol. Endocrinol. 18, 1222-1237. [DOI] [PubMed] [Google Scholar]
- 16.Hankenson, K. D., Bain, S. D., Kyriakides, T. R., Smith, E. A., Goldstein, S. A. & Bornstein, P. (2000) J. Bone Miner. Res. 15, 851-862. [DOI] [PubMed] [Google Scholar]
- 17.Hemati, N., Ross, S. E., Erickson, R. L., Groblewski, G. E. & MacDougald, O. A. (1997) J. Biol. Chem. 272, 25913-25919. [DOI] [PubMed] [Google Scholar]
- 18.Erickson, R. L., Hemati, N., Ross, S. E. & MacDougald, O. A. (2001) J. Biol. Chem. 276, 16348-16355. [DOI] [PubMed] [Google Scholar]
- 19.Hankenson, K. D. & Bornstein, P. (2002) J. Bone Miner. Res. 17, 415-425. [DOI] [PubMed] [Google Scholar]
- 20.Bills, C. E., Eisenberg, H. & Pallante, S. L. (1974) Johns Hopkins Med. J. 128, 194-207. [PubMed] [Google Scholar]
- 21.Longo, K. A., Kennell, J. A., Ochocinska, M. J., Ross, S. E., Wright, W. S. & MacDougald, O. A. (2002) J. Biol. Chem. 277, 38239-38244 [DOI] [PubMed] [Google Scholar]
- 22.Rosen, E. D., Hsu, C. H., Wang, X., Sakai, S., Freeman, M. W., Gonzalez, F. J. & Spiegelman, B. M. (2002) Genes Dev. 16, 22-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ducy, P., Amling, M., Takeda, S., Priemel, M., Schilling, A. F., Beil, F. T., Shen, J., Vinson, C., Rueger, J. M. & Karsenty, G. (2000) Cell 100, 197-207. [DOI] [PubMed] [Google Scholar]
- 24.Takeda, S., Elefteriou, F., Levasseur, R., Liu, X., Zhao, L., Parker, K. L., Armstrong, D., Ducy, P. & Karsenty, G. (2002) Cell 111, 305-317. [DOI] [PubMed] [Google Scholar]
- 25.Howe, L. R., Watanabe, O., Leonard, J. & Brown, A. M. (2003) Cancer Res. 63, 1906-1913. [PubMed] [Google Scholar]
- 26.Bialek, P., Kern, B., Yang, X., Schrock, M., Sosic, D., Hong, N., Wu, H., Yu, K., Ornitz, D. M., Olson, E. N., Justice, M. J. & Karsenty, G. (2004) Dev. Cell 6, 423-435. [DOI] [PubMed] [Google Scholar]
- 27.Akune, T., Ohba, S., Kamekura, S., Yamaguchi, M., Chung, U. I., Kubota, N., Terauchi, Y., Harada, Y., Azuma, Y., Nakamura, K., et al. (2004) J. Clin. Invest. 113, 846-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cock, T. A., Back, J., Elefteriou, F., Karsenty, G., Kastner, P., Chan, S. & Auwerx, J. (2004) EMBO Rep. 5, 1007-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reya, T., O'Riordan, M., Okamura, R., Devaney, E., Willert, K., Nusse, R. & Grosschedl, R. (2000) Immunity 13, 15-24. [DOI] [PubMed] [Google Scholar]
- 30.Nuttall, M. E. & Gimble, J. M. (2000) Bone 27, 177-184. [DOI] [PubMed] [Google Scholar]
- 31.Kajkenova, O., Lecka-Czernik, B., Gubrij, I., Hauser, S. P., Takahashi, K., Parfitt, A. M., Jilka, R. L., Manolagas, S. C. & Lipschitz, D. A. (1997) J. Bone Miner. Res. 12, 1772-1779. [DOI] [PubMed] [Google Scholar]
- 32.Derfoul, A., Carlberg, A. L., Tuan, R. S. & Hall, D. J. (2004) Differentiation 72, 209-223. [DOI] [PubMed] [Google Scholar]
- 33.Rangwala, S. M. & Lazar, M. A. (2000) Annu. Rev. Nutr. 20, 535-559. [DOI] [PubMed] [Google Scholar]
- 34.Rosen, E. D., Walkey, C. J., Puigserver, P. & Spiegelman, B. M. (2000) Genes Dev. 14, 1293-1307. [PubMed] [Google Scholar]
- 35.Lecka-Czernik, B., Gubrij, I., Moerman, E. J., Kajkenova, O., Lipschitz, D. A., Manolagas, S. C. & Jilka, R. L. (1999) J. Cell Biochem. 74, 357-371. [PubMed] [Google Scholar]
- 36.Lecka-Czernik, B., Moerman, E. J., Grant, D. F., Lehmann, J. M., Manolagas, S. C. & Jilka, R. L. (2002) Endocrinology 143, 2376-2384. [DOI] [PubMed] [Google Scholar]
- 37.Jeon, M. J., Kim, J. A., Kwon, S. H., Kim, S. W., Park, K. S., Park, S. W., Kim, S. Y. & Shin, C. S. (2003) J. Biol. Chem. 278, 23270-23277. [DOI] [PubMed] [Google Scholar]
- 38.Rzonca, S. O., Suva, L. J., Gaddy, D., Montague, D. C. & Lecka-Czernik, B. (2004) Endocrinology 145, 401-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tadic, T., Dodig, M., Erceg, I., Marijanovic, I., Mina, M., Kalajzic, Z., Velonis, D., Kronenberg, M. S., Kosher, R. A., Ferrari, D., et al. (2002) J. Bone Miner. Res. 17, 1008-1014. [DOI] [PubMed] [Google Scholar]
- 40.Cheng, S. L., Shao, J. S., Charlton-Kachigian, N., Loewy, A. P. & Towler, D. A. (2003) J. Biol. Chem. 278, 45969-45977. [DOI] [PubMed] [Google Scholar]
- 41.Ichida, F., Nishimura, R., Hata, K., Matsubara, T., Ikeda, F., Hisada, K., Yatani, H., Cao, X., Komori, T., Yamaguchi, A. & Yoneda, T. (2004) J. Biol. Chem. 279, 34015-34022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.