Abstract
Peroxynitrite contributes to the pathogenesis of various neurodegenerative disorders through multiple mechanisms and is thought to mediate secondary neuronal cell death after spinal cord injury (SCI). Here we establish that physiologically relevant levels of uric acid (UA), a selective inhibitor of certain peroxynitrite-mediated reactions, block the toxic effects of peroxynitrite on primary spinal cord neurons in vitro. Furthermore, administration of UA at the onset of SCI in a mouse model inhibits several pathological changes in the spinal cord including general tissue damage, nitrotyrosine formation, lipid peroxidation, activation of poly(ADP-ribose) polymerase, and neutrophil invasion. More importantly, UA treatment improves functional recovery from the injury. Taken together, our findings support the concept that peroxynitrite contributes to the pathophysiology of secondary damage after SCI. They also raise the possibility that elevating UA levels may provide a therapeutic approach for the treatment of SCI as well as other neurological diseases with a peroxynitrite-mediated pathological component.
Keywords: blood–brain barrier, neutrophils, peroxynitrite, spinal cord neurons, cytotoxicity
A cascade of pathophysiological processes rapidly follows mechanical trauma to the spinal cord, resulting in secondary neuronal damage that can significantly exacerbate the original injury (1). An acute inflammatory response at the site of the initial lesion is at least partly responsible for this secondary spinal cord pathology (e.g., refs. 2–4). Among the inflammatory cells recruited to the injured area are macrophages/microglia and neutrophils that can mediate tissue damage by producing a variety of cytotoxic factors including reactive nitrogen species (3–7). Several studies have implicated peroxynitrite, a molecule generated when nitric oxide and superoxide combine, in secondary neuronal damage after spinal cord injury (SCI) (5–7). Not only has evidence of peroxynitrite production been detected in spinal cord tissues from rats after traumatic injury (5–7), but administration of a peroxynitrite donor directly into the rat spinal cord has been shown to cause neuronal cell death and neurological deficit (8). In addition, a number of previous reports have demonstrated that peroxynitrite is toxic for neurons, including primary spinal cord neurons, and neuronal cell lines in vitro (9–13).
Peroxynitrite is known to mediate several potentially destructive chemical reactions, including tyrosine nitration and lipid peroxidation (14). Mitochondrial respiration is directly inhibited by peroxynitrite and is an early marker of its cytotoxic effects (9, 14). In addition, peroxynitrite causes DNA strand breakage, which activates the enzyme poly(ADP-ribose) polymerase (PARP), which in turn triggers a cascade of processes that often lead to cell death (14). Because increased nitrotyrosine formation, lipid peroxidation, and PARP activation have all been associated with secondary damage in spinal cord trauma (5–7, 14–20), it is possible that secondary neurological deficits in SCI could be limited by interfering with either the production or activity of peroxynitrite.
We have previously demonstrated that uric acid (UA), a selective inhibitor of certain peroxynitrite-mediated chemical reactions (21), is therapeutic in experimental allergic encephalomyelitis (EAE) (e.g., refs. 22–24), a neurodegenerative disease model. There is evidence that UA protects different neuronal cell populations from peroxynitrite-mediated toxicity (11, 12). However, an additional aspect of the protective effect of UA in EAE is evidently directed at CNS inflammation, because UA treatment prevents the loss of blood–brain barrier (BBB) integrity that occurs in the disease, thereby inhibiting inflammatory cell infiltration (24–27). Consequently, raising UA levels may impact secondary pathology in SCI by directly preventing peroxynitrite-mediated cell toxicity or interfering with the acute inflammatory response if there is a peroxynitrite-dependent component. To examine these two hypotheses further, we first determined whether physiologically relevant levels of UA protect spinal cord neurons from the toxic effects of peroxynitrite in vitro and then assessed the effects of UA treatment in a mouse model of SCI.
Materials and Methods
Preparation and Culture of Spinal Cord Neurons. Spinal cord neuronal cultures were prepared according to the method of Toborek et al. (28) with minor modifications. Briefly, spinal cords from 13- to 14-day-old fetal mice were minced and incubated for 30 min at 37°C in a buffered solution containing 0.67 mg/ml papain, then titrated in 40 μg/ml DNase in MEM supplemented with 10% FBS and 10% horse serum. The cell suspension was seeded in poly-l-lysine-coated plates at a density of 1.5 × 106 cells per 35-mm-diameter dish, and 7 h later the medium was replaced with Neurobasal medium containing B-27 supplement (minus antioxidants) (GIBCO), 2 mM glutamine, 100 μg/ml gentamicin, and 50 μg/ml fungizone. Cultures were maintained at 37°C in 5% CO2. Three days later, 1.4 × 10-5 M uridine and 5.4 × 10-5 M fluorodeoxyuridine was added to the cultures to inhibit the proliferation of nonneuronal cells. Experiments were carried out on replicate cultures derived from single platings of spinal cord neurons after 14 days of culture.
Cell Treatment and Viability Assessment. Peroxynitrite (100–500 μM) diluted in PBS, pH 8.3, was added to spinal cord neuron cultures in 1/10 of the volume of the wells. Control samples were treated with an equal volume of PBS, pH 8.3. Replicate cultures were treated with UA (100–1,000 μM) for 10 min before the addition of peroxynitrite. Cell viability was assessed by measuring the reduction of 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide (MTT) to formazan, an indicator of mitochondrial respiration, as well as the release of the cytoplasmic enzyme lactate dehydrogenase (LDH) into medium, an index of the loss of cell membrane integrity, as described (29).
Mouse SCI Model. Male Adult CD1 mice (25–30 g, Harlan Nossan, Milan) were housed and cared for in compliance with Italian regulations on the protection of animals used for experimental and other scientific purposes (Italian regulation code D.M. 116192) as well as with European Economic Community regulations (Official Journal of the European Communities L 358/1, December 18, 1986). Mice were anesthetized with chloral hydrate (40 μg per kg of body weight). A longitudinal incision was made on the midline of the back, exposing the paravertebral muscles, which were dissected away to uncover vertebrae T5–T8. The spinal cord was exposed via a four-level T6–T7 laminectomy, and SCI was produced by extradural compression of the spinal cord for 1 min by using an aneurysm clip with a closing force of 24 g. Postoperatively, animals were administered 1.0 ml of saline s.c. to replace the blood volume lost during the surgery. During recovery from anesthesia, the mice were placed on a warm heating pad and covered with a warm towel. Mice were housed singly in a temperature-controlled room at 27°C for a survival period of 10 days with food and water provided ad libitum. During this time, the animals' bladders were manually voided twice a day until the mice were able to regain normal bladder function. Sham-injured animals were subjected to all procedures except extradural compression.
Treatment. Mice were randomly allocated into the following groups: (i) SCI plus saline, subjected to SCI and given saline vehicle i.p. 30 min before and 6 h after injury (n = 40 mice); (ii) SCI plus UA, subjected to SCI and given UA (500 mg per kg of body weight in saline, i.p.) 30 min before and 6 h after injury (n = 40 mice); (iii) sham plus saline, subjected to a T6–T7 laminectomy and given saline i.p. 30 min before and 6 h after laminectomy (n = 40 mice); (iv) sham plus UA, subjected to a T6–T7 laminectomy and given UA (500 mg per kg of body weight in saline, i.p.) 30 min before and 6 h after laminectomy (n = 50 mice). This dose of UA raises serum UA levels in mice from ≈0.5 mg/dl to ≈3 mg/dl with a half-life of ≈2 h.
Grading of Motor Disturbance. The motor function of the mice was graded once a day for 10 days after injury by using the modified murine Basso, Beattie, and Bresnahan hind limb locomotor rating scale (30), summarized as follows. No hind limb movement is scored as 0. Slight (<50% range of motion) movement of one or two joints is 1. Extensive (>50% range of motion) movement of one joint and slight movement of one other joint is 2. Extensive movement of two joints is 3. Slight movement of all three joints is 4. Extensive movement of one joint and slight movement of two, extensive movement of two joints and slight movement of one, or extensive movement of all three joints are scored as 5, 6, and 7 respectively. Sweeping without weight support or plantar placement and no weight support is 8. Plantar placement with weight support in stance only or dorsal stepping with weight support is 9. Occasional (<50% of the time) weight-supported plantar steps and no front/hind limb coordination is 10. Frequent (50–94% of the time) to consistent (>95% of the time) weight-supported plantar steps with no, occasional, or frequent coordination are scored as 11, 12, and 13, respectively. Scores of 14–19 (in parentheses) are given to animals with consistent weight-supported plantar steps and consistent coordination and the following: a predominantly rotated paw position during locomotion or frequent plantar stepping and occasional dorsal stepping (14); no or occasional toe clearance and parallel paw position at initial contact (15); frequent toe clearance, parallel paw position at initial contact, and either rotated or parallel paw position at lift-off (16 and 17, respectively); consistent toe clearance and parallel paw position at initial contact but rotated or parallel paw position at lift-off (18 and 19, respectively). Finally, mice with consistent plantar stepping, coordinated gait, and toe clearance that predominantly have a parallel paw position at initial contact and lift-off and that have trunk instability are scored as 20, whereas similar animals with trunk stability are scored as 21. Tail position was not incorporated into the outcome scale because its relationship with neurological function in the mice is inconsistent.
Histology and Immunohistochemistry. Spinal cord tissues were collected 24 h after the trauma and fixed in 4% paraformaldehyde solution (in 0.1 M PBS). For histology, the tissues were dehydrated by graded ethanol and embedded in Paraplast (Sherwood Medical Industries, Mahwah, NJ). Tissue sections (5 μm) were deparaffinized with xylene and graded alcohol and then stained with hematoxylin/eosin and examined by light microscopy (Dialux 22, Leitz). Nitrotyrosine, as an index of peroxynitrite formation and/or nitrosative stress, and poly-(ADP-ribose), as a marker of PARP activation, were localized in 8-μm sections from paraffin-embedded spinal cord tissue by using anti-nitrotyrosine and anti-PARP antibodies (DBA, Milan) and an immunoperoxidase technique with a chromogen diaminobenzidine substrate as previously described (31).
Measurement of Lipid Peroxidation and Myeloperoxidase (MPO) Activity. Malondialdehyde (MDA) levels, an indicator of lipid peroxidation, were determined in spinal cord tissues 1 h after SCI by using a previously described assay based on the absorbance of the products of a thiobarbituric acid reaction at 650 nm (32). The levels of MDA in each sample were calculated by comparison with standards and expressed as μM MDA per 100 mg of tissue. The extent of polymorphonuclear leukocyte accumulation in spinal cord tissues was estimated 4 h after SCI by measuring MPO activity using a colorimetric method as previously described (33). MPO activity was defined as the quantity of enzyme degrading 1 μmol of peroxide per min at 37°C and is expressed in milliunits/g wet tissue.
Statistical Analysis. All results are expressed as the mean ± SEM of replicates. Data sets were analyzed by either one- or two-way analysis of variance (ANOVA) followed by a post hoc Dunnett's test or by Student's unpaired t test. Motor scale data were analyzed by using the Mann–Whitney U test. In each case, P < 0.05 was considered significant.
Results
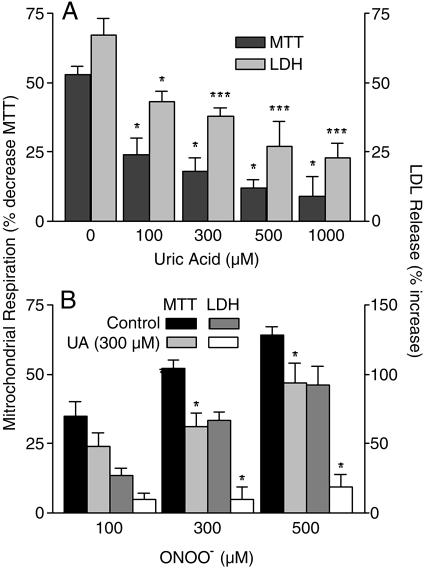
UA Protects Primary Spinal Cord Neurons from Peroxynitrite-Induced Cytotoxicity. To confirm whether UA inhibits the deleterious effects of peroxynitrite on spinal cord neurons, various concentrations of UA were added to cultures of primary spinal cord neurons before the addition of 300 μM peroxynitrite, and the effects on mitochondrial respiration and LDH release were assessed. UA protected spinal cord neurons from the toxic effects of peroxynitrite in a dose-dependent manner with concentrations as low as 100 μM providing significant protection (P < 0.05) (Fig. 1A). Because the precise concentrations of peroxynitrite that spinal cord neurons may be exposed to in SCI are difficult to predict, we next determined the protective capacity of 300 μM UA, a level approximating that of normal human serum (200–350 μM), against a range of peroxynitrite concentrations. Fig. 1B shows that, regardless of the amount of peroxynitrite added, 300 μM UA inhibits both the decline in mitochondrial respiration and, particularly, the enhanced release of LDH.
Fig. 1.
UA protects spinal cord neurons from the deleterious effects of peroxynitrite. Spinal cord neuron cultures either were untreated or pretreated with UA (100–1,000 μM) for 10 min and then exposed to authentic peroxynitrite (100–500 μM) for 1 h. Mitochondrial respiration and LDH release were then assessed as detailed in Materials and Methods. (A) The results for treatment with different concentrations of UA and 300 μM peroxynitrite are presented as percentage of control (mean ± SEM), where n = 5–9 replicate cultures. *, P < 0.05; ***, P < 0.001 compared with the 0 μM UA group by one-way ANOVA with a post hoc Dunnett's test. (B) The results for treatment with 300 μM UA and different concentrations of peroxynitrite are expressed as percentage of control (mean ± SEM), where n = 6–9 replicate cultures. *, P < 0.05, compared with the untreated group by Student's unpaired t test.
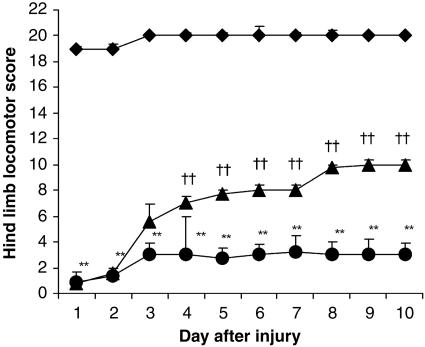
UA Treatment Enhances Recovery of Motor Function After SCI. To determine whether UA may protect against peroxynitrite-mediated secondary neuronal damage in SCI, mice were treated with UA 30 min before and 6 h after injury, and their recovery of motor function was assessed over 10 days by using a modified Basso, Beattie, and Bresnahan hind limb locomotor rating scale (Fig. 2). Although motor function was only slightly impaired in sham-injured mice, animals undergoing SCI had extensive deficits in hind limb movement. In the first 2 days after SCI, there was no difference in the extent of locomotor dysfunction between the UA- and saline-treated groups of mice, demonstrating that all animals experienced the same degree of injury. However, by 4 days postinjury, motor recovery was significantly improved in UA-treated mice compared with animals that had received saline vehicle (P < 0.001). Over the remainder of the study, the UA-treated animals continued to regain motor function while the saline controls did not. At the end of the study period, 10 days postinjury, the UA-treated mice were able to support their weight while saline-treated animals exhibited only limited joint movement.
Fig. 2.
UA treatment enhances motor function recovery in clip-injured mice. Adult CD1 mice were subjected to SCI and given either saline (circles) or UA (500 mg/kg) (triangles) i.p. 30 min before and 6 h after injury as described in Materials and Methods. Sham-operated control mice (diamonds) were subjected only to laminectomy. The motor function of the mice was graded once per day for 10 days by using the modified murine Basso, Beattie, and Bresnahan hind-limb locomotor score. Results are presented as the mean score ± SEM (n = 15 mice per group). **, P < 0.001 compared with the sham-operated group by the Mann–Whitney U test. ††, P < 0.001 compared with the SCI plus saline group by the Mann–Whitney U test.
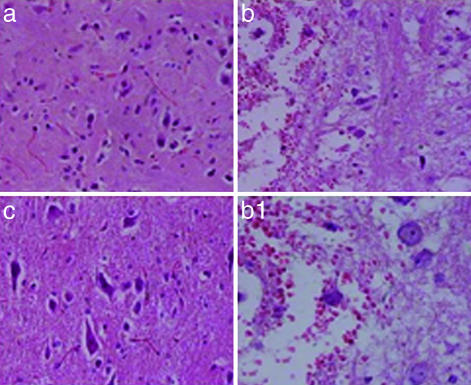
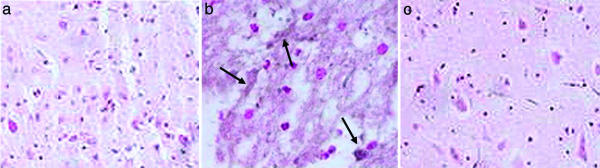
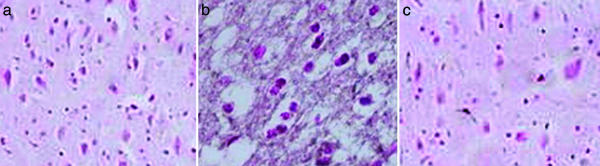
UA Treatment Inhibits Histopathological Changes and Decreases the Levels of Nitrotyrosine and Poly(ADP-Ribose) in Spinal Cord Tissues of SCI Mice. Compression injury to the spinal cord causes marked, perilesional histopathological changes within 24 h that can be readily seen by comparing the sections in Fig. 3 a (sham-operated control) and b (SCI, saline-treated). Evidence of edema as well as alterations in the white matter are apparent. However, such changes did not occur in spinal cord tissue from a compression-injured mouse treated with UA (Fig. 3c). In addition to the general pathological changes, nitrotyrosine, a product of peroxynitrite, can be detected by immunohistochemistry in the area of the lesion in SCI mice given saline but not in sham-operated controls or UA-treated animals (Fig. 4). Similarly, UA administration suppressed the formation of poly(ADP-ribose), an indicator of PARP activity, in the area of the lesion in SCI mice (Fig. 5).
Fig. 3.
UA treatment prevents the histological damage normally associated with SCI. Adult CD1 mice were subjected to compression-induced SCI and treated with UA or saline as detailed in the legend to Fig. 2. Spinal cord biopsies were collected 24 h after SCI, and sections from sham-operated (a), saline-treated (b and b1), and UA-treated (c) SCI mice were stained with hematoxylin/eosin as described in Materials and Methods and photographed (a, b, and c, ×200; b1, ×450).
Fig. 4.
UA administration prevents nitrotyrosine formation after SCI. CD1 mice were subjected to SCI and treated with UA or saline as detailed in the legend to Fig. 2. Spinal cord tissues were collected 24 h after trauma and fixed in paraformaldehyde. Nitrotyrosine, a marker of peroxynitrite reactivity, was assessed in paraffin-embedded sections of spinal cord tissue from sham-operated (a), saline-treated (b; arrows indicate accumulations of nitrotyrosine), and UA-treated (c) SCI mice by immunohistochemistry as described in Materials and Methods and photographed (×200).
Fig. 5.
UA administration reduces PARP activation after SCI. CD1 mice were subjected to compression-induced SCI and treated with UA or saline as described in the legend to Fig. 2. Spinal cord tissues were collected 24 h after trauma and fixed in paraformaldehyde. Immunohistochemical analysis of poly(ADP-ribose) as a marker of PARP activity was performed in paraffin-embedded sections from the spinal cord tissues of sham-operated (a), saline-treated (b), and UA-treated (c) SCI mice as described in Materials and Methods and photographed (×200).
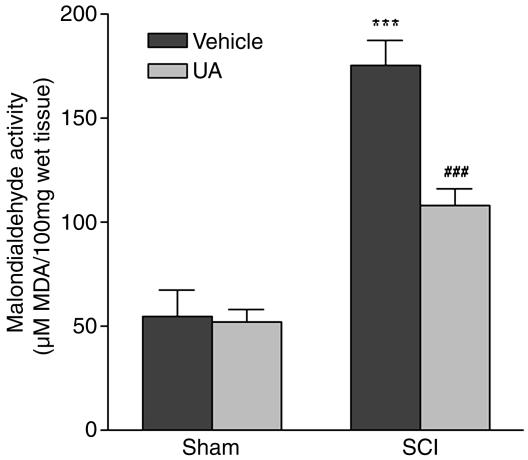
UA Treatment Inhibits Lipid Peroxidation in SCI. To determine whether UA treatment affects lipid peroxidation in SCI, we measured MDA, an end product of lipid peroxidation, in the spinal cords of control mice and SCI mice administered saline or UA (Fig. 6). Whereas the baseline MDA levels in spinal cord tissues from sham-operated animals were unaffected by UA administration, the substantial elevation in spinal cord MDA levels observed after SCI was significantly reduced by UA treatment (Fig. 6).
Fig. 6.
Lipid peroxidation is reduced in spinal cord tissues from SCI mice by UA. CD1 mice were subjected to compression-induced SCI and treated with UA or saline as described in the legend to Fig. 2. Spinal cord tissues were collected 1 h after SCI, and levels of MDA were determined as described in Materials and Methods as an indicator of lipid peroxidation. Results are expressed as μM MDA per 100 g of wet tissue (mean ± SEM) (n = 15 mice per group). ***, P < 0.001 compared with the sham-operated group by Student's unpaired t test. ###, P < 0.001 compared with the SCI plus saline group by Student's unpaired t test.
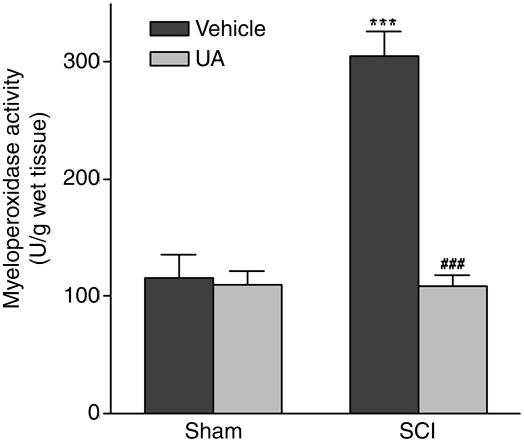
UA Administration Limits Neutrophil Infiltration After SCI. UA treatment inhibits BBB permeability changes and prevents inflammatory cell infiltration into the CNS in a variety of models of CNS inflammation (24–27, 34, 35). Consequently, it is possible that UA protects against secondary damage in SCI by limiting the acute inflammatory response. Hence, we assessed the effects of UA treatment on neutrophil infiltration in SCI by evaluating MPO activity in spinal cord tissues (Fig. 7). MPO activity was detected at low levels in spinal cord tissues of sham-treated mice, and UA administration did not alter basal spinal cord levels of MPO. By comparison, spinal cord MPO activity was significantly increased after SCI (P < 0.001). However, when the SCI mice were treated with UA, MPO activity was maintained at control values (Fig. 7).
Fig. 7.
Neutrophil infiltration after SCI is inhibited by UA treatment. CD1 mice were subjected to compression-induced SCI and treated with UA or saline as described in the legend to Fig. 2. Spinal cord tissues were collected 4 h after SCI, and tissue levels of MPO were determined as an indicator of neutrophil accumulation. Results are expressed as unit per gram of wet tissue (mean ± SEM) (n = 15 mice per group). ***, P < 0.001 compared with the sham-operated group by Student's unpaired t test. ###,P < 0.001 compared with the SCI plus saline group by Student's unpaired t test.
Discussion
Acute inflammatory changes in spinal cord tissue have been implicated in a secondary injury cascade that results in a considerably greater loss of function than that expected from the initiating mechanical damage. Peroxynitrite, one of a number of toxic factors produced in the spinal cord tissues after SCI (5–7), likely contributes to secondary neuronal damage through pathways resulting from the chemical modification of cellular proteins and lipids. However, studies in EAE and several models of CNS infection suggest that peroxynitrite may be an important mediator of the CNS inflammatory response through effects manifested in enhanced BBB or blood–spinal cord barrier permeability (24–27, 34, 35). Moreover, inhibition of iNOS, the rate-limiting enzyme in the production of peroxynitrite in an inflammatory response, has been demonstrated to be therapeutic in SCI (36).
To probe the pathological contributions of peroxynitrite to secondary damage after SCI, we have used UA, a selective inhibitor of certain peroxynitrite-mediated chemistries including tyrosine nitration (37, 38) but not lipid peroxidation (38, 39). UA has been extensively used as a neuroprotective agent and has been shown to inhibit CNS inflammation and BBB permeability changes in several models (24–27, 34, 35). As expected, UA can protect spinal cord neurons from peroxynitrite-induced cytotoxicity in vitro (Fig. 1). More importantly, the administration of UA to raise the normally low serum UA levels of mice at the time of injury, as well as shortly afterward, was sufficient to promote a significant and sustained improvement in recovery from a spinal cord compression injury (Fig. 2). Accompanying this therapeutic effect, the extensive pathological changes observed in the lesion area of SCI animals administered saline did not occur in similarly injured mice treated with UA.
Several indices of the peroxynitrite-mediated pathological changes that normally acutely follow SCI are reduced in the spinal cords of UA-treated mice. These include nitrated tyrosine residues, PARP activity, and evidence of lipid peroxidation. Tyrosine nitration, which occurs in areas of active cell death in the spinal cords of SCI rats (5–7), can interfere with protein function and may therefore negatively impact cell metabolism and survival (40). PARP activity is believed to contribute to neuronal cell death in a variety of neurological conditions (41), including traumatic brain injury (42, 43) and SCI (5), as a consequence of energy failure (14) or through modification of the activity of various proteins by poly(ADP-ribosylation) (44). Lipid peroxidation can cause cell death through different pathways including disruption of cell signaling or altering mitochondrial function (36) and is thought to be one of the main contributors to secondary damage after CNS trauma (9). For example, inhibitors of lipid peroxidation have been shown to promote functional recovery in animal models of SCI (16–19).
The chemistries possible in vivo among peroxynitrite, its potential targets, and UA are complex and not fully resolved. UA effectively blocks peroxynitrite-mediated tyrosine nitration in vitro by a reaction with second-order kinetics (21). On the other hand, UA enhances the oxidation of low-density lipoproteins by peroxynitrite in vitro (39) and has variable effects on lipid peroxidation by other radicals (45). In an EAE model, where peroxynitrite makes a major contribution to pathology, UA treatment inhibits tyrosine nitration in lesions (24) but fails to significantly reduce lipid peroxidation (38). These observations raise the possibility that the chemistries responsible for lipid peroxidation as a consequence of SCI may not be susceptible to inactivation by UA. If this is the case, the protective effects of UA may be directed at preventing the accumulation of neutrophils and activated cells of the macrophage/microglia lineage at the site of the injury and thereby the local production of a range of pathological factors.
In our previous studies of UA treatment in models of CNS inflammation, the maintenance of BBB integrity proved to be a key element of the therapeutic response, preventing neurotoxic cells and factors from entering CNS tissues (e.g., refs. 24, 27, and 35). Disruption of the blood–spinal cord barrier is an early event after spinal cord trauma (46), with significant permeability changes detected as early as 35 min after injury (47). In addition, it has been demonstrated that inhibition of iNOS reduces the disruption of the blood–spinal cord barrier, neutrophil accumulation, and neuronal cell death after contusion SCI (36). This suggested to us that peroxynitrite may trigger the induction of blood–spinal cord barrier permeability changes in SCI, as is evidently the case in other models (24, 26, 34, 35). The foremost protective effect of UA in SCI would therefore be manifested in the suppression of the acute inflammatory response. Indeed, UA treatment prevented the elevation of MPO activity seen in the spinal cords of SCI mice, indicating that the accumulation of neutrophils in the tissue was inhibited. The concept that the inactivation of peroxynitrite by UA protects against secondary damage after SCI by interfering with the acute inflammatory response is supported by the lack of histopathological changes seen in the area of the mechanical lesion in UA-treated SCI mice.
Unlike EAE and Borna disease virus infection, where the CNS inflammatory responses are driven by an adaptive immune response of antigen-specific T cells and activated macrophages, innate immunity and neutrophils play a major role in the acute inflammatory response after SCI (4). Nevertheless, our results demonstrate that these disparate pathological processes share a common, peroxynitrite-mediated component, which we conclude is a mechanism responsible for the loss of BBB and blood–spinal cord barrier integrity in a variety of neurodegenerative conditions.
The finding that UA administration prevents secondary pathological events in compression-induced SCI and promotes recovery of motor function in an animal model raises the issue of whether UA could be used therapeutically in humans after SCI. Because of inactivation of the urate oxidase gene, humans naturally have higher levels of UA than most lower animals (48, 49). However, UA levels in humans are variable, with lower than average levels perhaps representing a risk factor for several neurodegenerative diseases including multiple sclerosis (23, 50–52) and Parkinson's (53, 54) and Alzheimer's (55–57) diseases. It is possible that secondary damage after SCI could be more extensive in individuals with lower UA levels, through both reduced control of cell invasion into the tissues and greater peroxynitrite-mediated neuronal cell death. Moreover, it is unlikely that normal circulating UA levels are sufficient to protect spinal cord tissues against the amounts of peroxynitrite that may be produced by an acute inflammatory response. Therefore, the transient elevation of serum UA as soon as possible after spinal cord trauma [for example, by the oral administration of inosine (58, 59)] may have some benefit.
Acknowledgments
This work was supported in part by grants from the Paralyzed Veterans of America Spinal Cord Research Foundation (to G.S.S.), National Multiple Sclerosis Society Grant RG 2896A/5 (to D.C.H.), and Programmi di Ricerca di Interesse Nazionale/Ministero dell'Istruzione, dell'Università e della Ricerca (to S.C.), and by a grant to the Biotechnology Foundation Laboratories from the Commonwealth of Pennsylvania.
Author contributions: D.C.H. designed research; G.S.S., S.C., and T.G. performed research; S.C. contributed new reagents/analytic tools; G.S.S., S.C., T.G., and D.C.H. analyzed data; G.S.S. and D.C.H. wrote the paper; and H.K. contributed to the direction of the work through discussions with the corresponding author and facilitated some of the experiments.
Abbreviations: SCI, spinal cord injury; PARP, poly(ADP-ribose) polymerase; UA, uric acid; EAE, experimental allergic encephalomyelitis; BBB, blood–brain barrier; LDH, lactate dehydrogenase; MDA, malondialdehyde.
References
- 1.Anderson, D. K. & Hall, E. D. (1993) Ann. Emerg. Med. 22, 987-992. [DOI] [PubMed] [Google Scholar]
- 2.Popovich, P. G., Wei, P. & Stokes, B. T. (1997) J. Comp. Neurol. 377, 443-464. [DOI] [PubMed] [Google Scholar]
- 3.Taoka, Y., Okajima, K., Uchiba, M., Murakami, K., Kushimoto, S., Johno, M., Naruo, M., Okabe, H. & Takatsuki, K. (1997) Neuroscience 79, 1177-1182. [DOI] [PubMed] [Google Scholar]
- 4.Carlson, S. L., Parrish, M. E., Springer, J. E., Doty, K. & Dossett, L. (1998) Exp. Neurol. 151, 77-88. [DOI] [PubMed] [Google Scholar]
- 5.Scott, G. S., Jakeman, L. B., Stokes, B. T. & Szabó, C. (1999) Ann. Neurol. 45, 120-124. [PubMed] [Google Scholar]
- 6.Liu, D., Ling, X., Wen, J. & Liu, J. (2000) J. Neurochem. 75, 2144-2154. [DOI] [PubMed] [Google Scholar]
- 7.Xu, J., Kim, G.-M., Chen, S., Yan, P., Ahmed, S. H., Ku, G., Beckman, J. S., Xu, X. M. & Hsu, C. Y. (2001) J. Neurotrauma 18, 523-532. [DOI] [PubMed] [Google Scholar]
- 8.Bao, F. & Liu, D. (2002) Neuroscience 115, 839-849. [DOI] [PubMed] [Google Scholar]
- 9.Bolaños, J. P., Heales, S. J., Land, J. M. & Clark, J. B. (1995) J. Neurochem. 64, 1965-1972. [DOI] [PubMed] [Google Scholar]
- 10.Cookson, M. R., Ince, P. G. & Shaw, P. J. (1998) J. Neurochem. 70, 501-508. [DOI] [PubMed] [Google Scholar]
- 11.Yu, Z. F., Bruce-Keller, A. J., Goodman, Y. & Mattson, M. P. (1998) J. Neurosci. Res. 53, 613-625. [DOI] [PubMed] [Google Scholar]
- 12.Terwel, D., Nieland, L. J. M., Schutte, B., Reutelingsperger, C. P., Ramaekers, F. C. & Steinbusch, H. W. (2000) Eur. J. Pharmacol. 400, 19-33. [DOI] [PubMed] [Google Scholar]
- 13.Scott, G. S., Szabó, C. & Hooper, D. C. (2004) J. Neurotrauma 21, 1255-1263. [DOI] [PubMed] [Google Scholar]
- 14.Szabó, C. (2003) Toxicol. Lett. 140/141, 105-112. [DOI] [PubMed] [Google Scholar]
- 15.Braughler, J. M. & Hall, E. D. (1992) J. Neurotrauma 9, S1-S7. [PubMed] [Google Scholar]
- 16.Holtz, A. & Gerdin, B. (1992) Neurol. Res. 14, 49-52. [DOI] [PubMed] [Google Scholar]
- 17.Diaz-Ruiz, A., Rios, C., Duarte, I., Correa, D., Guizar-Sahagun, G., Grijalva, I. & Ibarra, A. (1999) Neurosci. Lett. 266, 61-64. [DOI] [PubMed] [Google Scholar]
- 18.Bao, F., Chen, Y., Dekaban, G. A. & Weaver, L. C. (2004) J. Neurochem. 88, 1335-1344. [DOI] [PubMed] [Google Scholar]
- 19.Amar, A. P. & Levy, M. L. (1999) Neurosurgery 44, 1027-1039. [DOI] [PubMed] [Google Scholar]
- 20.Joosten, E. A. & Houweling, D. A. (2004) NeuroReport 15, 1163-1166. [DOI] [PubMed] [Google Scholar]
- 21.Squadrito, G. L., Cueto, R., Splenser, A. E., Valavanidis, A., Zhang, H., Uppu, R. M.& Pryor, W. A. (2000) Arch. Biochem. Biophys. 376, 333-337. [DOI] [PubMed] [Google Scholar]
- 22.Hooper, D. C., Bagasra, O., Marini, J. C., Zborek, A., Ohnishi, S. T., Kean, R., Champion, J. M., Sarker, A. B., Bobroski, L., Farber, J. L., et al. (1997) Proc. Natl. Acad. Sci. USA 94, 2528-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hooper, D. C., Spitsin, S., Kean, R. B., Champion, J. M., Dickson, G. M., Chaudhry, I. & Koprowski, H. (1998) Proc. Natl. Acad. Sci. USA 95, 675-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hooper, D. C., Scott, G. S., Zborek, A., Mikheeva, T., Kean, R. B., Koprowski, H. & Spitsin, S. V. (2000) FASEB J. 14, 691-698. [DOI] [PubMed] [Google Scholar]
- 25.Spitsin, S. V., Scott, G. S., Kean, R. B., Mikheeva, T. & Hooper, D. C. (2000) Neurosci. Lett. 292, 137-141. [DOI] [PubMed] [Google Scholar]
- 26.Kean, R. B., Spitsin, S. V., Mikheeva, T., Scott, G. S. & Hooper, D. C. (2000) J. Immunol. 165, 6511-6518. [DOI] [PubMed] [Google Scholar]
- 27.Scott, G. S., Kean, R. B., Fabis, M. J., Mikheeva, T., Brimer, C. M., Phares, T. W., Spitsin, S. V. & Hooper, D. C. (2004) J. Neuroimmunol. 155, 32-42. [DOI] [PubMed] [Google Scholar]
- 28.Toborek, M., Malecki, A., Garrido, R., Mattson, M. P., Hennig, B. & Young, B. (1999) J. Neurochem. 73, 684-692. [DOI] [PubMed] [Google Scholar]
- 29.Scott, G. S., Virág, L., Szabó, C. & Hooper, D. C. (2003) Glia 41, 105-116. [DOI] [PubMed] [Google Scholar]
- 30.Joshi, M. & Fehlings, M. G. (2002) J. Neurotrauma 19, 175-190. [DOI] [PubMed] [Google Scholar]
- 31.Cuzzocrea, S., Pisano, B., Dugo, L., Ianaro, A., Maffia, P., Patel, N. S., Di Paola, R., Ialenti, A., Genovese, T., Chatterjee, P.K., et al. (2004) Eur. J. Pharmacol. 483, 79-93. [DOI] [PubMed] [Google Scholar]
- 32.Ohkawa, H., Ohishi, N. & Yagi, K. (1979) Anal. Biochem. 95, 351-358. [DOI] [PubMed] [Google Scholar]
- 33.Mullane, K. M., Kraemer, R. & Smith, B. (1985) J. Pharmacol. Methods 14, 157-167. [DOI] [PubMed] [Google Scholar]
- 34.Kastenbauer, S., Koedel, U., Becker, B. F. & Pfister, H. W. (2001) Eur. J. Pharmacol. 425, 149-152. [DOI] [PubMed] [Google Scholar]
- 35.Hooper, D. C., Kean, R. B., Scott, G. S., Spitsin, S. V., Mikheeva, T., Morimoto, K., Bette, M., Rohrenbeck, A. M., Dietzschold, B. & Weihe, E. (2001) J. Immunol. 167, 3470-3477. [DOI] [PubMed] [Google Scholar]
- 36.Pearse, D. D., Chatzipanteli, K., Marcillo, A. E., Bunge, M. B. & Dietrich, W. D. (2003) J. Neuropathol. Exp. Neurol. 62, 1096-1107. [DOI] [PubMed] [Google Scholar]
- 37.Whiteman, M., Ketsawatsakul, U. & Halliwell, B. (2002) Ann. N.Y. Acad. Sci. 962, 242-259. [DOI] [PubMed] [Google Scholar]
- 38.Spitsin, S. V., Scott, G. S., Mikheeva, T., Zborek, A., Kean, R. B., Brimer, C. M., Koprowski, H. & Hooper, D. C. (2002) Free Radical Biol. Med. 33, 1363-1371. [DOI] [PubMed] [Google Scholar]
- 39.Santos, C. X., Anjos, E. I. & Augusto, O. (1999) Arch. Biochem. Biophys. 372, 285-294. [DOI] [PubMed] [Google Scholar]
- 40.Nakazawa, H., Fukuyama, N., Takizawa, S., Tsuji, C., Yoshitake, M. & Ishid, H. (2000) Free Radical Res. 33, 771-784. [DOI] [PubMed] [Google Scholar]
- 41.Skaper, S. D. (2003) Ann. N.Y. Acad. Sci. 993, 217-228. [DOI] [PubMed] [Google Scholar]
- 42.Whalen, M. J., Clark, R. S., Dixon, C. E., Robichaud, P., Marion, D. W., Vagni, V., Graham, S., Virag, L., Hasko, G., Stachlewitz, R., et al. (2000) Acta Neurochir. Suppl. 76, 61-64. [DOI] [PubMed] [Google Scholar]
- 43.LaPlaca, M. C., Zhang, J., Raghupathi, R., Li, J. H., Smith, F., Bareyre, F. M., Snyder, S. H., Graham, D. I. & McIntosh, T. K. (2001) J. Neurotrauma 18, 369-376. [DOI] [PubMed] [Google Scholar]
- 44.Burkle, A. (2001) BioEssays 23, 795-806. [DOI] [PubMed] [Google Scholar]
- 45.Muraoka, S. & Miura, T. (2003) Pharmacol. Toxicol. 93, 284-289. [DOI] [PubMed] [Google Scholar]
- 46.Popovich, P. G., Horner, P. J., Mullin, B. B. & Stokes, B. T. (1996) Exp. Neurol. 142, 258-275. [DOI] [PubMed] [Google Scholar]
- 47.Whetstone, W. D., Hsu, J. Y., Eisenberg, M., Werb, Z. & Noble-Haeusslein, L. J. (2003) J. Neurosci. Res. 74, 227-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeldandi, A. V., Yeldandi, V., Kumar, S., Murthy, C. V., Wang, X. D., Alvares, K., Rao, M. S. & Reddy, J. K. (1991) Gene 109, 281-284. [DOI] [PubMed] [Google Scholar]
- 49.Wu, X. W., Muzny, D. M., Lee, C. C. & Catskey, C. T. (1992) J. Mol. Evol. 34, 78-84. [DOI] [PubMed] [Google Scholar]
- 50.Spitsin, S. V., Hooper, D. C., Mikheeva, T. & Koprowski, H. (2001) Mult. Scler. 7, 165-166. [DOI] [PubMed] [Google Scholar]
- 51.Drulovic, J., Dujmovic, I., Stojsavljevic, N., Mesaros, S., Miljkovic, D., Peric, V., Dragutinovic, G., Marinkoviv, J., Levic, Z. & Stojkovic, M. M. (2001) J. Neurol. 248, 121-126. [DOI] [PubMed] [Google Scholar]
- 52.Tonev, G., Milici, B., Toncev, S. & Samardzic, G. (2002) Eur. J. Neurol. 9, 221-226. [DOI] [PubMed] [Google Scholar]
- 53.Church, W. H. & Ward, V. L. (1994) Brain Res. Bull. 33, 419-25. [DOI] [PubMed] [Google Scholar]
- 54.Davis, J. W., Grandinetti, A., Waslien, C. I., Ross, G. W., White, L. R. & Morens, D. M. (1996) Am. J. Epidemiol. 144, 480-484. [DOI] [PubMed] [Google Scholar]
- 55.Tohgi, H., Abe, T., Takahashi, S. & Kikuchi, T. (1993) J. Neural. Transm. Park. Dis. Dement. Sect. 6, 119-126. [DOI] [PubMed] [Google Scholar]
- 56.Maesaka, J. K., Wolf-Klein, G., Piccione, J. M. & Ma, C. M. (1993) J. Am. Geriatr. Soc. 41, 501-506. [DOI] [PubMed] [Google Scholar]
- 57.Rinaldi, P., Polidori, M. C., Metastasio, A., Mariani, E., Mattioli, P., Cherubini, A., Catani, M., Cecchetti, R., Senin, U. & Mecocci, P. (2003) Neurobiol. Aging 24, 915-919. [DOI] [PubMed] [Google Scholar]
- 58.Scott, G. S., Spitsin, S. V., Kean, R. B., Mikheeva, T., Koprowski, H. & Hooper, D. C. (2002) Proc. Natl. Acad. Sci. USA 99, 16303-16308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koprowski, H., Spitsin, S. V. & Hooper, D. C. (2001) Ann. Neurol. 49, 139 (lett.). [DOI] [PubMed] [Google Scholar]